Assessment of the Characters of a Novel Phosphoric Acid and Mineral-Comodified Biochar Composite and Its Potential Application in Saline–Alkali Soil Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified Biochar Composites
2.3. Physicochemical Properties of the Modified Biochar Composites
2.4. Thermal Stability of the Modified Biochar Composites
2.5. Structural of the Modified Biochar Composites
2.6. Elemental Analysis of the Modified Biochar Composites
2.7. Pot Experiments
2.8. Statistical Analysis
3. Results and Discussion
3.1. Structural Analysis of the Modified Biochar Composites
3.2. Stability Analysis of the Modified Biochar Composites
3.3. Physicochemical Properties Analysis of the Modified Biochar Composites
3.4. Nutrient Content of the Modified Biochar Composites
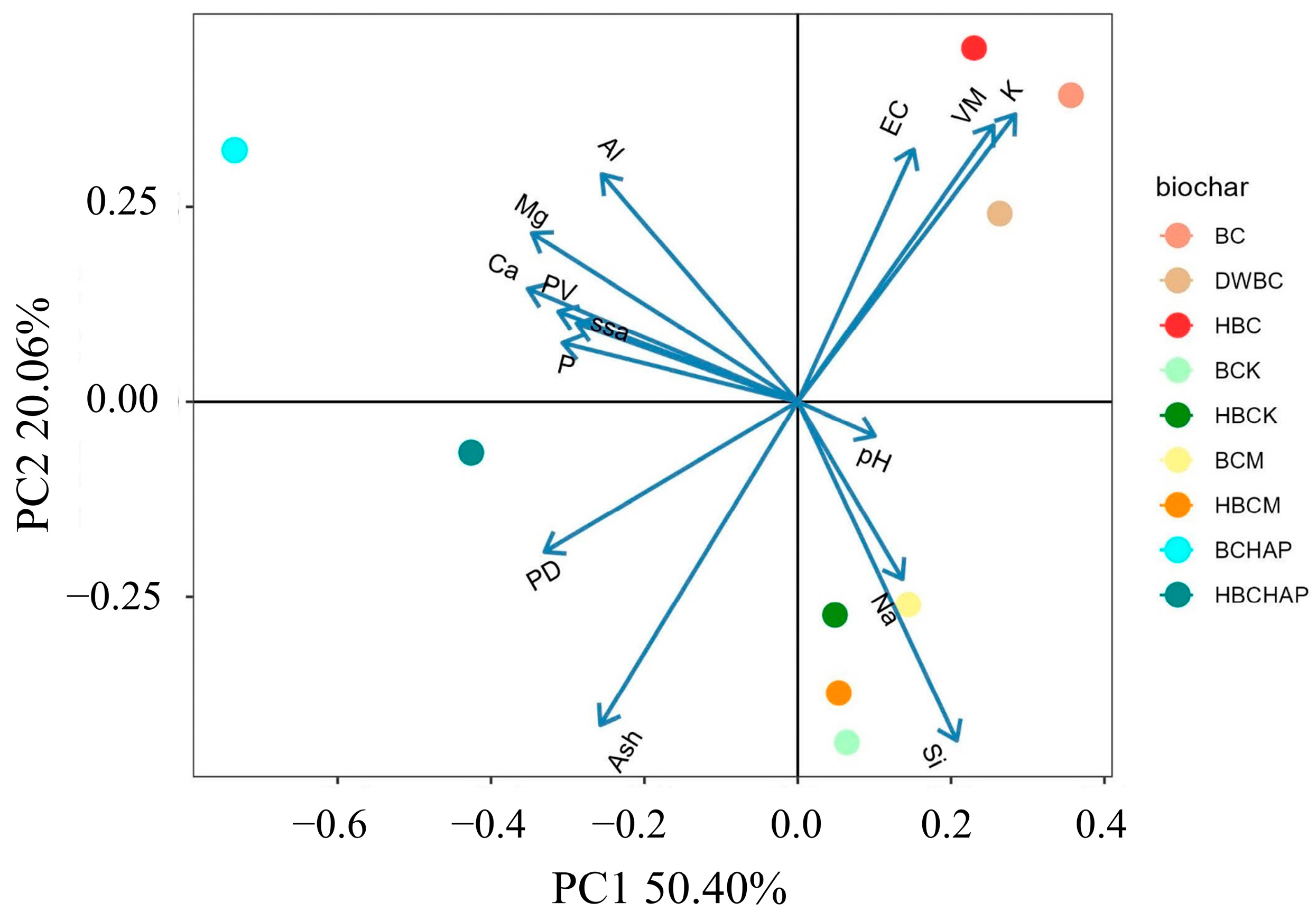
3.5. Principal Component Analysis
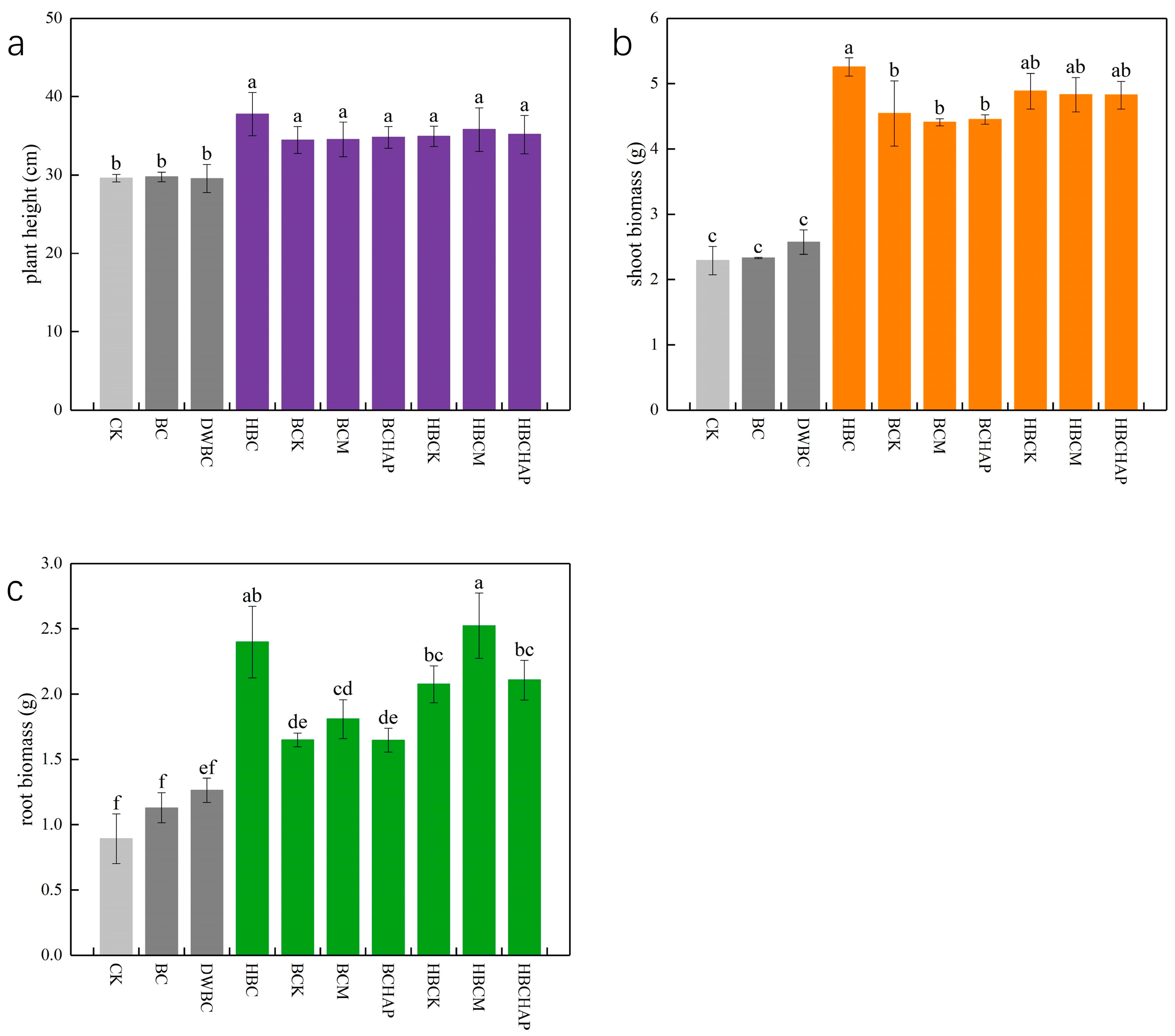
3.6. Enhancement of Plant Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | raw corn straw biochar |
| DWBC | deionized water-washed biochar |
| HBC | H3PO4-modified biochar |
| BCK | kaolinite–biochar composite |
| BCM | montmorillonite–biochar composite |
| BCHAP | hydroxyapatite–biochar composite |
| HBCK | H3PO4–kaolinite–biochar composite |
| HBCM | H3PO4–montmorillonite–biochar composite |
| HBCHAP | H3PO4–hydroxyapatite–biochar composite |
References
- An, Y.; Yang, X.-X.; Zhang, L.; Zhang, J.; Du, B.; Yao, L.; Li, X.-T.; Guo, C. Alfalfa MsCBL4 enhances calcium metabolism but not sodium transport in transgenic tobacco under salt and saline–alkali stress. Plant Cell Rep. 2020, 39, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, Z.; Zhang, Z.; You, L.; Xu, L.; Huang, H.; Wang, X.; Gao, Y.; Cui, X. Treatment of the saline-alkali soil with acidic corn stalk biochar and its effect on the sorghum yield in western Songnen Plain. Sci. Total Environ. 2021, 797, 149190. [Google Scholar] [CrossRef] [PubMed]
- Abulaiti, A.; She, D.; Liu, Z.; Sun, X.; Wang, H. Application of biochar and polyacrylamide to revitalize coastal saline soil quality to improve rice growth. Environ. Sci. Pollut. Res. Int. 2023, 30, 18731–18747. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Zhang, P.; Bing, X.; Jiao, L.; Xiao, H.; Li, B.; Sun, H. Amelioration effects of coastal saline-alkali soil by ball-milled red phosphorus-loaded biochar. Chem. Eng. J. 2022, 431, 133904. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Duan, M.; Yan, R.; Wang, Q.; Zhou, B.; Zhu, H.; Liu, G.; Guo, X.; Zhang, Z. Integrated microbiological and metabolomics analyses to understand the mechanism that allows modified biochar to affect the alkalinity of saline soil and winter wheat growth. Sci. Total Environ. 2023, 866, 161330. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Zheng, H.; Li, M.; Liu, Y.; Wang, X.; Peng, Y.; Luo, X.; Li, F.; Li, X.; et al. Biochar as a sustainable tool for improving the health of salt-affected soils. Soil Environ. Health 2023, 1, 100033. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Kumar, M.; Rao, C.S.; Nataraj, K.C.; Singh, G.; Vinu, A.; Bhowmik, A.; Sharma, H.; et al. Biochar modulating soil biological health: A review. Sci. Total Environ. 2024, 914, 169585. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zheng, L.; Zhao, J.; Ma, J.; Li, X. Biochar Application Maintains Photosynthesis of Cabbage by Regulating Stomatal Parameters in Salt-Stressed Soil. Sustainability 2023, 15, 4206. [Google Scholar] [CrossRef]
- Shahzad, A.S.; Younis, U.; Naz, N.; Danish, S.; Syed, A.; Elgorban, A.M.; Eswaramoorthy, R.; Huang, S.; Battaglia, M.L. Acidified biochar improves lead tolerance and enhances morphological and biochemical attributes of mint in saline soil. Sci. Rep. 2023, 13, 8720. [Google Scholar] [CrossRef]
- Kazemi, R.; Ronaghi, A.; Yasrebi, J.; Ghasemi-Fasaei, R.; Zarei, M. Effect of shrimp waste–derived biochar and arbuscular mycorrhizal fungus on yield, antioxidant enzymes, and chemical composition of corn under salinity stress. J. Soil Sci. Plant Nutr. 2019, 19, 758–770. [Google Scholar]
- Xu, W.; Wang, G.; Deng, F.; Zou, X.; Ruan, H.; Chen, H.Y.H. Responses of soil microbial biomass, diversity and metabolic activity to biochar applications in managed poplar plantations on reclaimed coastal saline soil. Soil Use Manag. 2018, 34, 597–605. [Google Scholar]
- Bao, D.; Li, Z.; Tang, R.; Wan, C.; Zhang, C.; Tan, X.; Liu, X. Metal-modified sludge-based biochar enhance catalytic capacity: Characteristics and mechanism. J. Environ. Manag. 2021, 284, 112113. [Google Scholar] [CrossRef]
- Bhandari, G.; Gangola, S.; Dhasmana, A.; Rajput, V.; Gupta, S.; Malik, S.; Slama, P. Nano-biochar: Recent progress, challenges, and opportunities for sustainable environmental remediation. Front. Microbiol. 2023, 14, 1214870. [Google Scholar] [CrossRef]
- Song, J.; Zhang, S.; Li, G.; Du, Q.; Yang, F. Preparation of montmorillonite modified biochar with various temperatures and their mechanism for Zn ion removal. J. Hazard. Mater. 2020, 391, 121692. [Google Scholar] [CrossRef]
- Viglašová, E.; Galamboš, M.; Danková, Z.; Krivosudský, L.; Lengauer, C.L.; Hood-Nowotny, R.; Soja, G.; Rompel, A.; Matík, M.; Briančin, J. Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Fang, J.; Zhang, M.; Chen, H.; Zhou, Y.; Creamer, A.E.; Sun, Y.; Yang, L. Characterization and environmental applications of clay–biochar composites. Chem. Eng. J. 2014, 242, 136–143. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, N.; Feng, C.; Wang, C.; Huang, R.; Tao, Q.; Tang, X.; Wu, Y.; Luo, Y.; Li, Q.; et al. Pore size and organic carbon of biochar limit the carbon sequestration potential of Bacillus cereus SR. Ecotoxicol. Environ. Saf. 2024, 274, 116229. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Bordoloi, S.; Ng, C.W.W.; Sarmah, A.K. Influence of pore fluid salinity on shrinkage and water retention characteristics of biochar amended kaolin for landfill liner application. Sci. Total Environ. 2022, 838, 156493. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Guan, J.; Tang, W.; Chen, J. Mechanistic insight into adsorptive removal of ionic NOR and nonionic DEP organic contaminates by clay-biochar composites. Environ. Pollut. 2022, 310, 119881. [Google Scholar] [CrossRef]
- Cui, S.; Kong, F.; Li, Y.; Jiang, Z.; Xi, M. Effect of mineral loaded biochar on the leaching performances of nitrate and phosphate in two contrasting soils from the coastal estuary area. Sci. Total Environ. 2021, 779, 146346. [Google Scholar] [CrossRef]
- Pan, X.; Shi, M.; Chen, X.; Kuang, S.; Ullah, H.; Lu, H.; Riaz, L. An investigation into biochar, acid-modified biochar, and wood vinegar on the remediation of saline−alkali soil and the growth of strawberries. Front. Environ. Sci. 2022, 10, 1057384. [Google Scholar] [CrossRef]
- Ji, L.; Tian, C.; Kuramae, E.E. Phosphorus-mediated succession of microbial nitrogen, carbon, and sulfur functions in rice-driven saline-alkali soil remediation. Soil Biol. Biochem. 2023, 184, 109125. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, M.; Li, Q.; Lu, S.; Zhou, D.; Feng, L.; Hou, Z.; Yu, J. Comparative analysis of the properties of biochars produced from different pecan feedstocks and pyrolysis temperatures. Ind. Crops Prod. 2023, 197, 116638. [Google Scholar] [CrossRef]
- Harvey, O.R.; Kuo, L.J.; Zimmerman, A.R.; Louchouarn, P.; Amonette, J.E.; Herbert, B.E. An index-based approach to assessing recalcitrance and soil carbon sequestration potential of engineered black carbons (biochars). Env. Sci. Technol. 2012, 46, 1415–1421. [Google Scholar] [CrossRef]
- Zhao, F.; Mu, B.; Zhang, T.; Dong, C.; Zhu, Y.; Zong, L.; Wang, A. Synthesis of biochar/clay mineral nanocomposites using oil shale semi-coke waste for removal of organic pollutants. Biochar 2023, 5, 7. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O.; Sohi, S.P.; Brownsort, P.; Cross, A. The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 2012, 5, 122–131. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Abduljabbar, A.; Ok, Y.S.; Al-Wabel, M.I. Date palm waste-derived biochar composites with silica and zeolite: Synthesis, characterization and implication for carbon stability and recalcitrant potential. Environ. Geochem. Health 2017, 41, 1687–1704. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xu, X.; Wei, L.; Fan, L.; Huang, H.; Li, J.; Lu, Q.; Li, J.; Zhou, W. Biochar stability assessment by incubation and modelling: Methods, drawbacks and recommendations. Sci. Total Environ. 2019, 664, 11–23. [Google Scholar] [CrossRef]
- Liu, G.; Pan, X.; Ma, X.; Xin, S.; Xin, Y. Effects of feedstock and inherent mineral components on oxidation resistance of biochars. Sci. Total Environ. 2020, 726, 138672. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, R.; Donne, S.W.; Beyad, Y.; Liu, X.; Duan, X.; Yang, T.; Su, P.; Sun, H. Co-pyrolysis of wood chips and bentonite/kaolin: Influence of temperatures and minerals on characteristics and carbon sequestration potential of biochar. Sci. Total Environ. 2022, 838, 156081. [Google Scholar] [CrossRef]
- Ba, Y.; Liu, J.; Han, J.; Zhang, X. Application of Vis-NIR spectroscopy for determination the content of organic matter in saline-alkali soils. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117863. [Google Scholar] [CrossRef]
- Ganapati, R.K.; Naveed, S.A.; Zafar, S.; Wang, W.; Xu, J. Saline-Alkali Tolerance in Rice: Physiological Response, Molecular Mechanism, and QTL Identification and Application to Breeding. Rice Sci. 2022, 29, 412–434. [Google Scholar] [CrossRef]
- Pan, Y.; She, D.; Shi, Z.; Cao, T.; Xia, Y.; Shan, J. Salinity and high pH reduce denitrification rates by inhibiting denitrifying gene abundance in a saline-alkali soil. Sci. Rep. 2023, 13, 2155. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Ma, R.; Chen, M.; Wei, W.; Ding, X. Carbon sequestration under different organic amendments in saline-alkaline soils. Catena 2021, 196, 104882. [Google Scholar] [CrossRef]
- Yang, D.; Tang, L.; Cui, Y.; Chen, J.; Liu, L.; Guo, C. Saline-alkali stress reduces soil bacterial community diversity and soil enzyme activities. Ecotoxicology 2022, 31, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, C.; Wang, Y.; He, L.; Lu, H.; Yang, S. Vermiculite modification increases carbon retention and stability of rice straw biochar at different carbonization temperatures. J. Clean. Prod. 2020, 254, 120111. [Google Scholar] [CrossRef]
- Li, F.; Cao, X.; Zhao, L.; Wang, J.; Ding, Z. Effects of mineral additives on biochar formation: Carbon retention, stability, and properties. Environ. Sci. Technol. 2014, 48, 11211–11217. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The Interfacial Behavior between Biochar and Soil Minerals and Its Effect on Biochar Stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef]
- Zama, E.F.; Reid, B.J.; Sun, G.-X.; Yuan, H.-Y.; Li, X.-M.; Zhu, Y.-G. Silicon (Si) biochar for the mitigation of arsenic (As) bioaccumulation in spinach (Spinacia oleracean) and improvement in the plant growth. J. Clean. Prod. 2018, 189, 386–395. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Butt, M.; Irfan, M.; Rizwan, M.S.; Ali, H.; Cheema, M.A. Interactive Effect of Biochar and Silicon on Improving Morpho-Physiological and Biochemical Attributes of Maize by Reducing Drought Hazards. J. Soil Sci. Plant Nutr. 2020, 20, 1819–1826. [Google Scholar] [CrossRef]
- Ahmad, M.; Rafique, M.I.; Akanji, M.A.; Al-Wabel, M.I.; Al-Swadi, H.A.; Al-Farraj, A.S.F. Silica modified biochar mitigates the adverse effects of salt and drought stress and improves safflower (Carthamus tinctorius L.) growth. J. Soils Sediments 2022, 23, 172–192. [Google Scholar] [CrossRef]
- Bouras, H.; Bouaziz, A.; Choukr-Allah, R.; Hirich, A.; Devkota, K.P.; Bouazzama, B. Phosphorus Fertilization Enhances Productivity of Forage Corn (Zea mays L.) Irrigated with Saline Water. Plants 2021, 10, 2608. [Google Scholar] [CrossRef]
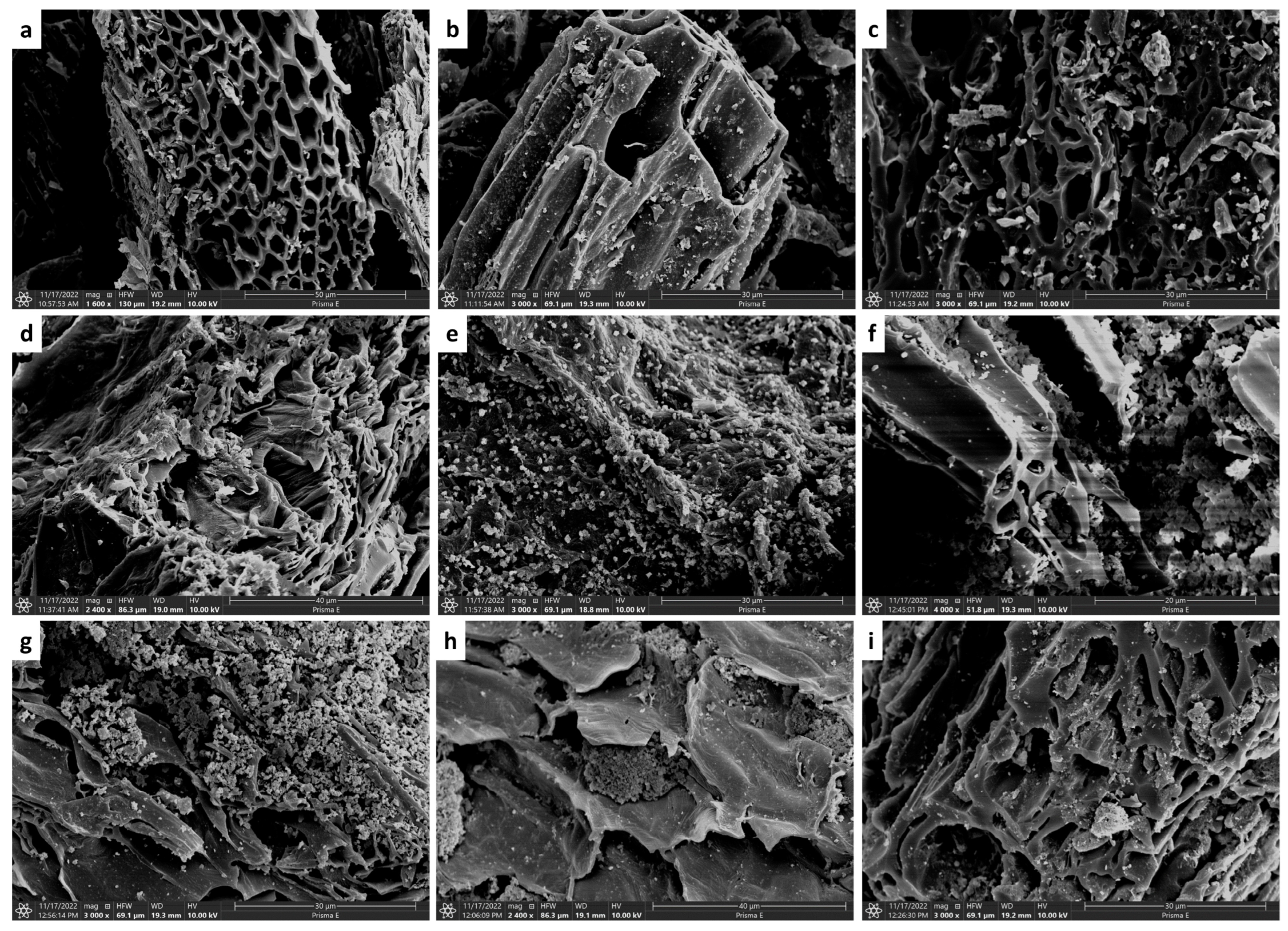
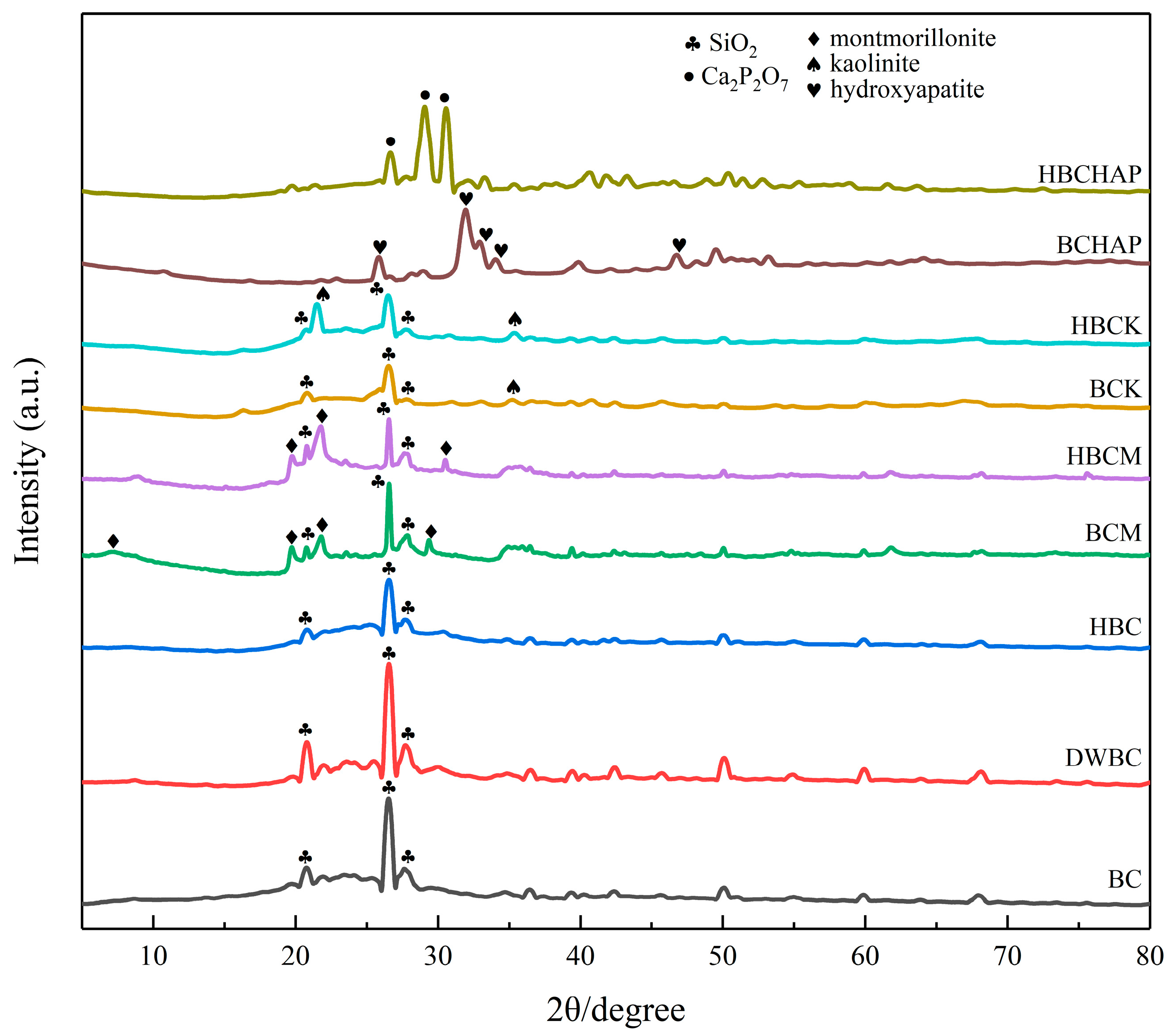
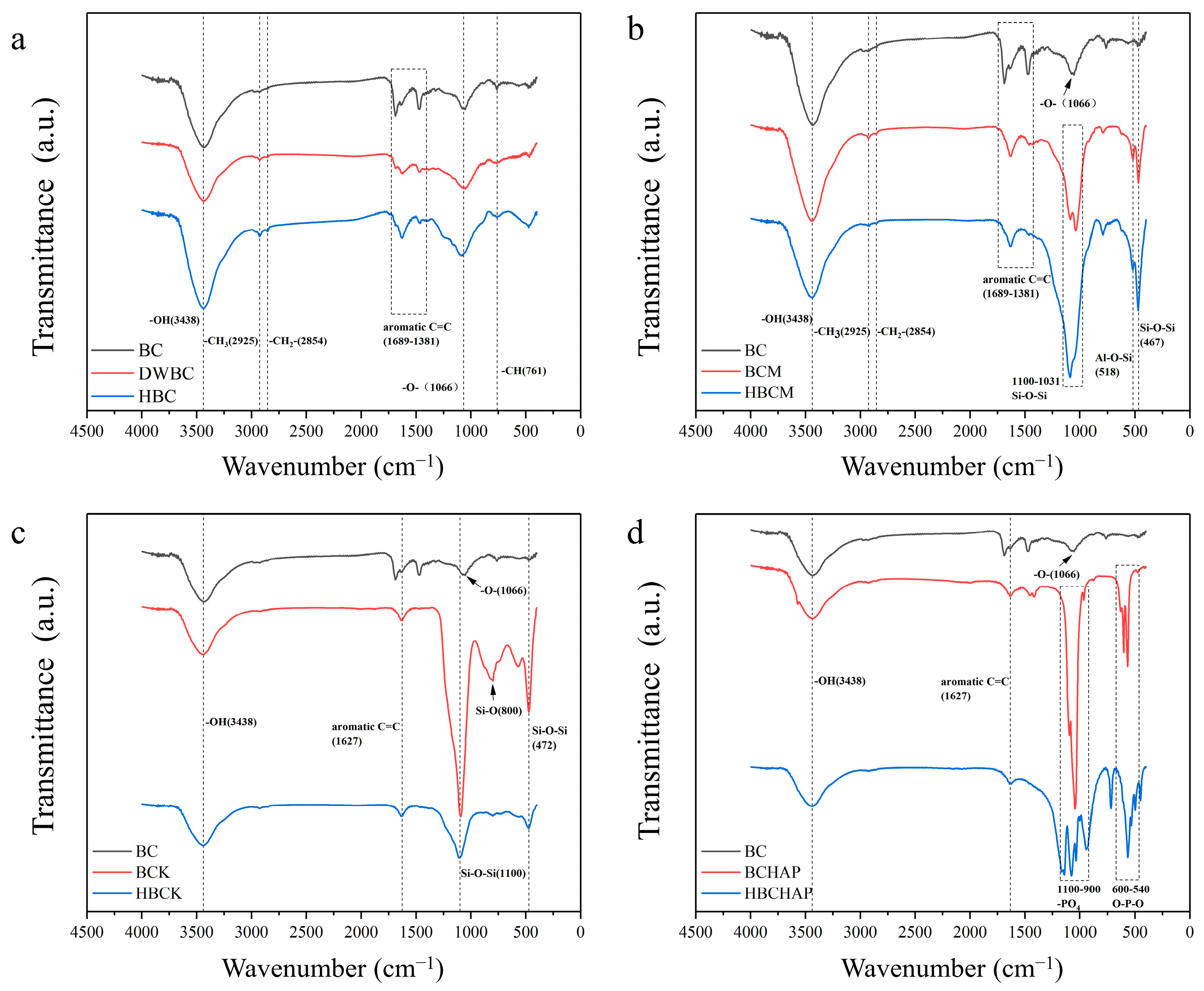
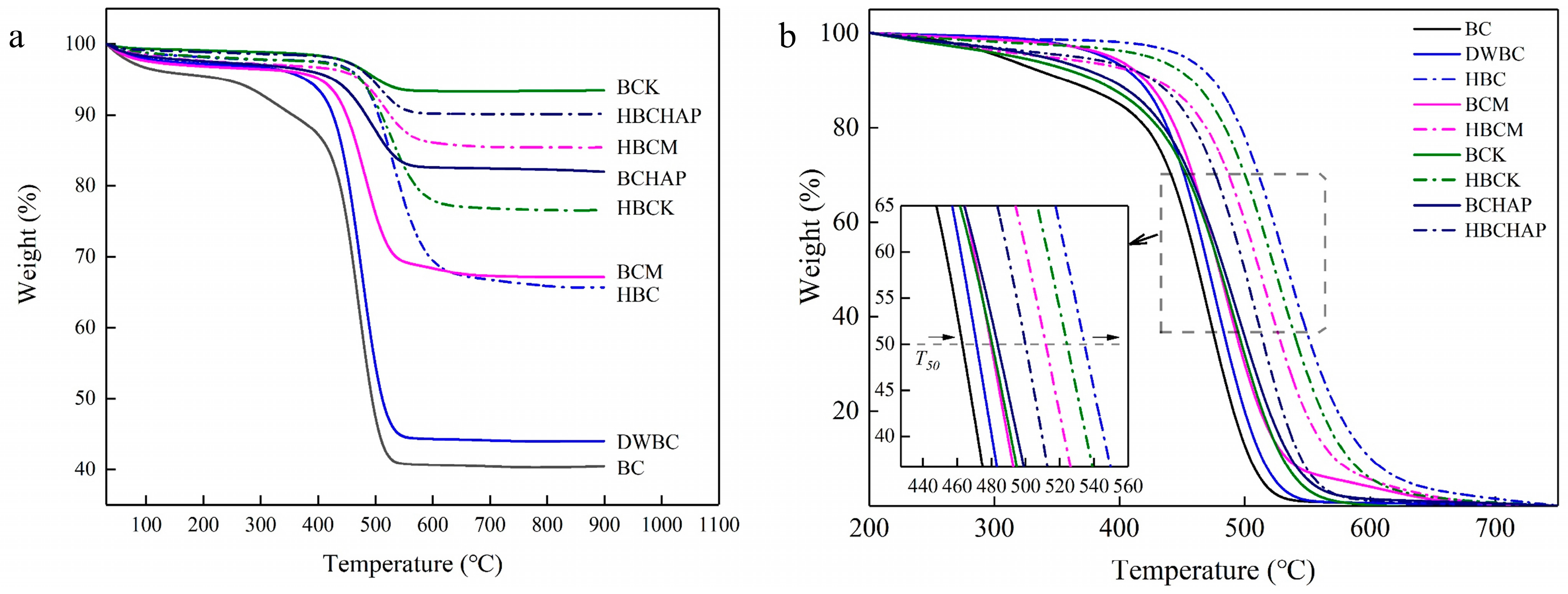
| Ash [%] | Volatile Matter [%] | T50 | R50 | pH | EC [μs/cm] | Specific Surface Area [m2/g] | Pore Volume [cm3/g] | Pore Diameter [nm] | |
|---|---|---|---|---|---|---|---|---|---|
| BC | 34.89 ± 2.49 f | 14.07 ± 0.07 a | 462.93 | 52.25 | 10.17 ± 0.24 a | 849.00 ± 31.04 b | 1.26 | 0.0028 | 80.19 |
| DWBC | 38.69 ± 2.65 f | 8.99 ± 0.38 b | 471.26 | 53.19 | 9.74 ± 0.08 b | 789.50 ± 19.19 b | 8.91 | 0.0115 | 50.47 |
| HBC | 46.76 ± 3.44 e | 9.18 ± 1.12 b | 534.43 | 60.32 | 2.32 ± 0.01 h | 2700.00 ± 89.81 a | 1.22 | 0.0035 | 122.01 |
| BCK | 88.34 ± 3.90 ab | 5.15 ± 1.33 c | 480.35 | 54.22 | 6.55 ± 0.01 d | 185.85 ± 8.53 e | 11.13 | 0.0531 | 186.26 |
| BCM | 66.70 ± 0.19 d | 8.29 ± 0.45 b | 479.72 | 54.14 | 10.18 ± 0.01 a | 837.00 ± 14.70 b | 16.85 | 0.0568 | 132.90 |
| BCHAP | 83.29 ± 0.52 bc | 4.86 ± 0.18 cd | 483.53 | 54.57 | 6.98 ± 0.04 c | 341.50 ± 9.39 d | 46.27 | 0.3239 | 277.27 |
| HBCK | 78.77 ± 0.70 c | 4.40 ± 0.33 cd | 524.12 | 59.16 | 2.91 ± 0.00 g | 638.00 ± 2.45 c | 4.37 | 0.0136 | 121.91 |
| HBCM | 82.51 ± 2.91 bc | 5.08 ± 0.46 cd | 511.71 | 57.76 | 5.41 ± 0.01 f | 401.00 ± 16.33 d | 5.95 | 0.0316 | 207.66 |
| HBCHAP | 89.58 ± 0.59 a | 3.36 ± 0.87 d | 499.85 | 56.42 | 6.13 ± 0.05 e | 209.50 ± 2.86 e | 6.54 | 0.0420 | 252.93 |
| P [g/kg] | Si [g/kg] | K [g/kg] | Ca [g/kg] | Mg [g/kg] | Na [g/kg] | Al [g/kg] | |
|---|---|---|---|---|---|---|---|
| BC | 2.47 ± 0.12 e | 75.25 ± 6.69 c | 16.00 ± 0.89 a | 6.18 ± 0.80 c | 0.60 ± 0.25 c | 4.92 ± 1.42 c | 2.26 ± 0.43 bc |
| DWBC | 2.42 ± 0.11 e | 83.82 ± 6.33 c | 15.71 ± 0.55 a | 5.70 ± 1.17 cd | 0.59 ± 0.21 c | 5.02 ± 1.21 c | 2.55 ± 0.48 b |
| HBC | 58.64 ± 5.10 c | 75.19 ± 6.45 c | 13.95 ± 0.71 b | 4.73 ± 0.83 cd | 0.49 ± 0.25 c | 4.32 ± 1.11 cd | 2.43 ± 0.69 bc |
| BCK | 2.19 ± 0.11 e | 164.66 ± 2.71 a | 6.37 ± 0.58 de | 2.50 ± 0.30 cd | 0.18 ± 0.06 c | 2.77 ± 0.20 cde | 1.26 ± 0.22 d |
| BCM | 1.38 ± 0.08 e | 171.27 ± 7.99 a | 8.30 ± 0.40 c | 3.57 ± 0.98 cd | 0.33 ± 0.31 c | 17.96 ± 1.23 a | 2.59 ± 0.13 b |
| BCHAP | 105.25 ± 1.16 b | 21.21 ± 0.87 d | 4.78 ± 0.26 f | 134.40 ± 3.23 a | 4.09 ± 0.09 a | 1.46 ± 0.20 e | 4.43 ± 0.60 a |
| HBCK | 48.10 ± 1.83 d | 133.47 ± 3.94 b | 5.66 ± 0.23 ef | 2.35 ± 0.44 d | 0.60 ± 0.64 c | 2.43 ± 0.14 de | 1.56 ± 0.14 cd |
| HBCM | 53.84 ± 1.89 c | 165.57 ± 3.40 a | 7.11 ± 0.34 cd | 3.51 ± 1.12 cd | 0.30 ± 0.27 c | 14.39 ± 1.09 b | 2.62 ± 0.26 b |
| HBCHAP | 142.51 ± 1.80 a | 15.31 ± 0.70 d | 3.09 ± 0.27 g | 120.45 ± 2.05 b | 2.28 ± 0.21 b | 1.23 ± 0.14 e | 2.71 ± 0.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Liu, Z.; Yu, J.; Teng, X.; Liu, L.; Jia, M.; Xue, J. Assessment of the Characters of a Novel Phosphoric Acid and Mineral-Comodified Biochar Composite and Its Potential Application in Saline–Alkali Soil Improvement. Agriculture 2025, 15, 785. https://doi.org/10.3390/agriculture15070785
Dai H, Liu Z, Yu J, Teng X, Liu L, Jia M, Xue J. Assessment of the Characters of a Novel Phosphoric Acid and Mineral-Comodified Biochar Composite and Its Potential Application in Saline–Alkali Soil Improvement. Agriculture. 2025; 15(7):785. https://doi.org/10.3390/agriculture15070785
Chicago/Turabian StyleDai, Hao, Zhuangzhuang Liu, Jinping Yu, Xiaoming Teng, Lei Liu, Mingyun Jia, and Jianhui Xue. 2025. "Assessment of the Characters of a Novel Phosphoric Acid and Mineral-Comodified Biochar Composite and Its Potential Application in Saline–Alkali Soil Improvement" Agriculture 15, no. 7: 785. https://doi.org/10.3390/agriculture15070785
APA StyleDai, H., Liu, Z., Yu, J., Teng, X., Liu, L., Jia, M., & Xue, J. (2025). Assessment of the Characters of a Novel Phosphoric Acid and Mineral-Comodified Biochar Composite and Its Potential Application in Saline–Alkali Soil Improvement. Agriculture, 15(7), 785. https://doi.org/10.3390/agriculture15070785






