Investigating the Impact of Sowing Date on Wheat Leaf Morphology Through Image Analysis
Abstract
1. Introduction
2. Materials and Methods
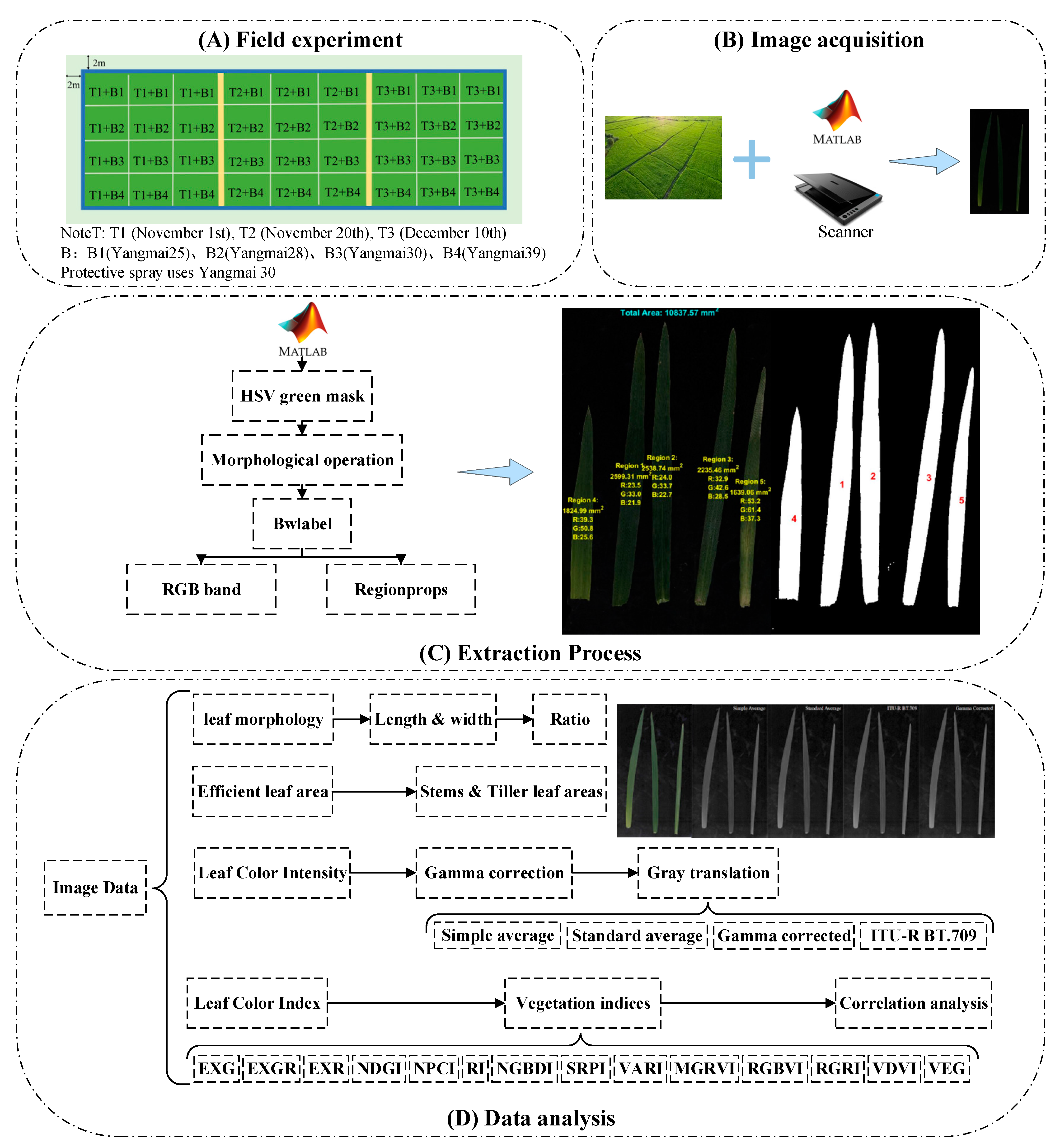
2.1. Experimental Materials and Field Planting
2.2. Data Collection
2.2.1. Field Sampling
2.2.2. Image Acquisition
2.3. Experimental Methods
2.3.1. Image Preprocessing
2.3.2. Data Processing
3. Results
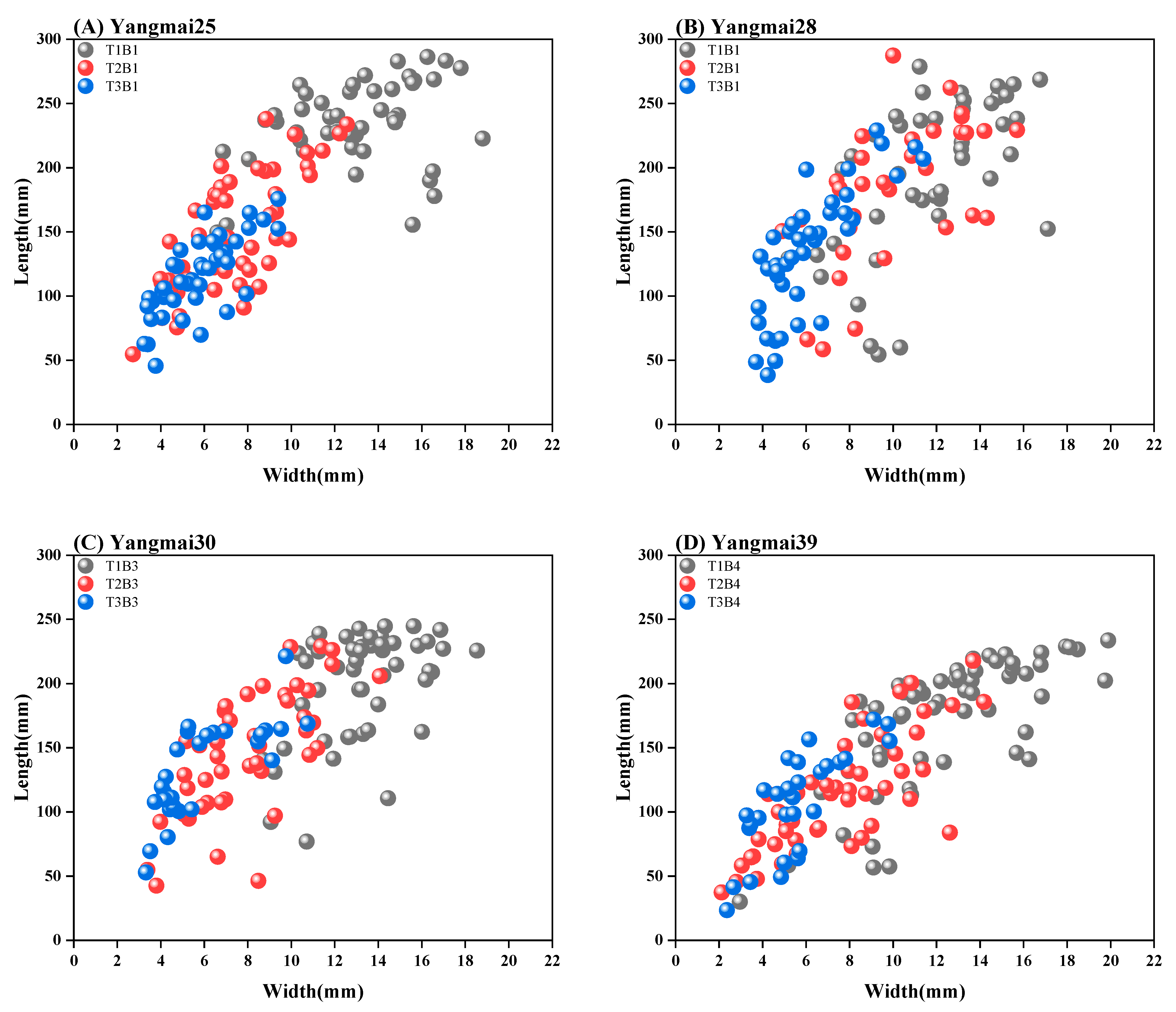
3.1. The Impacts of Sowing Dates on Leaf Morphology
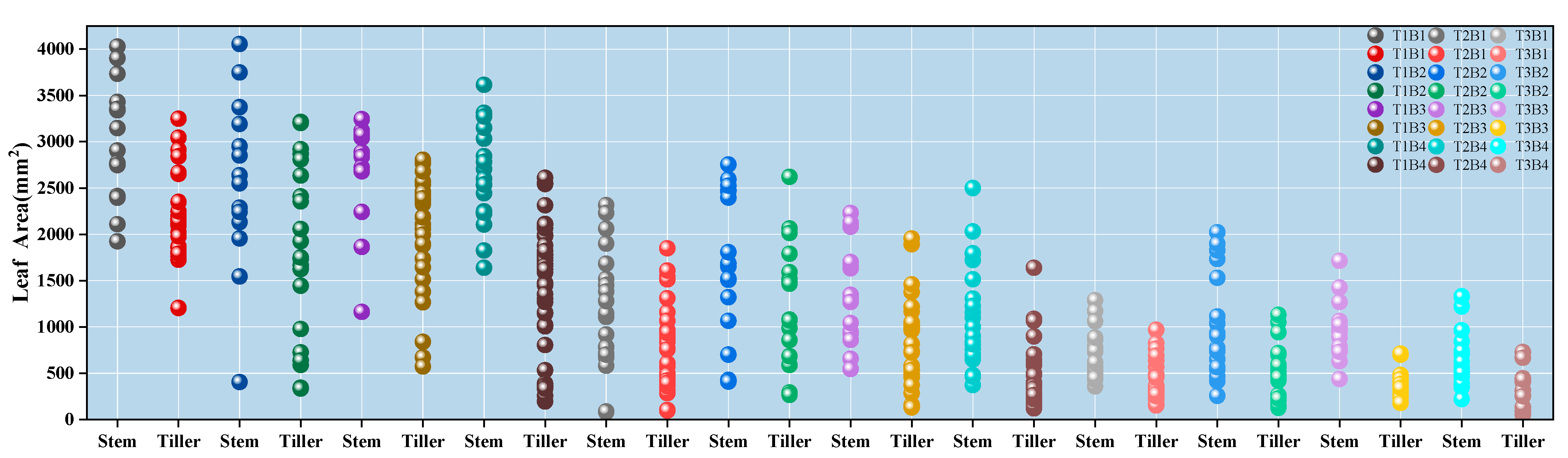
3.2. The Impacts of Different Treatments on the Leaf Area of Winter Wheat
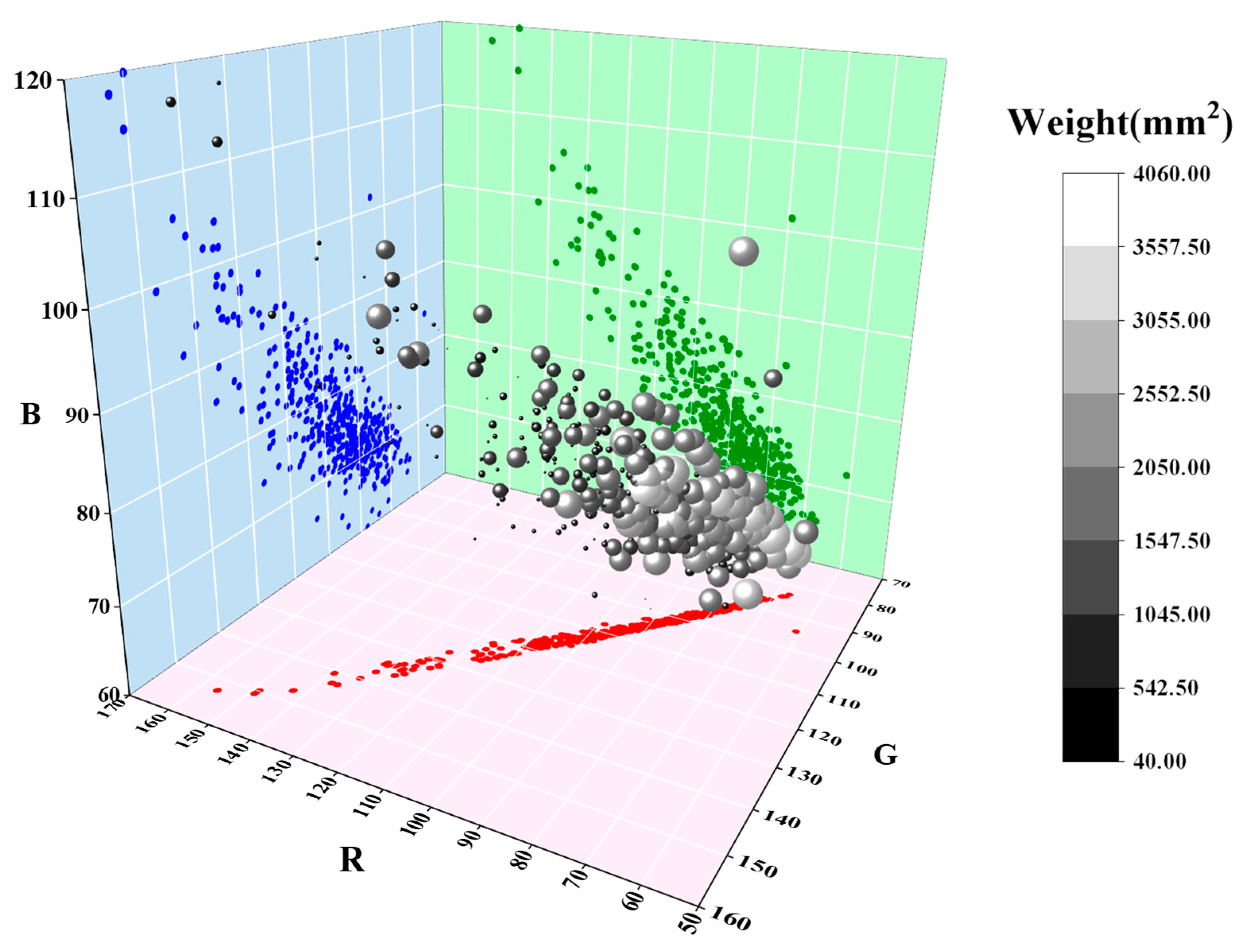
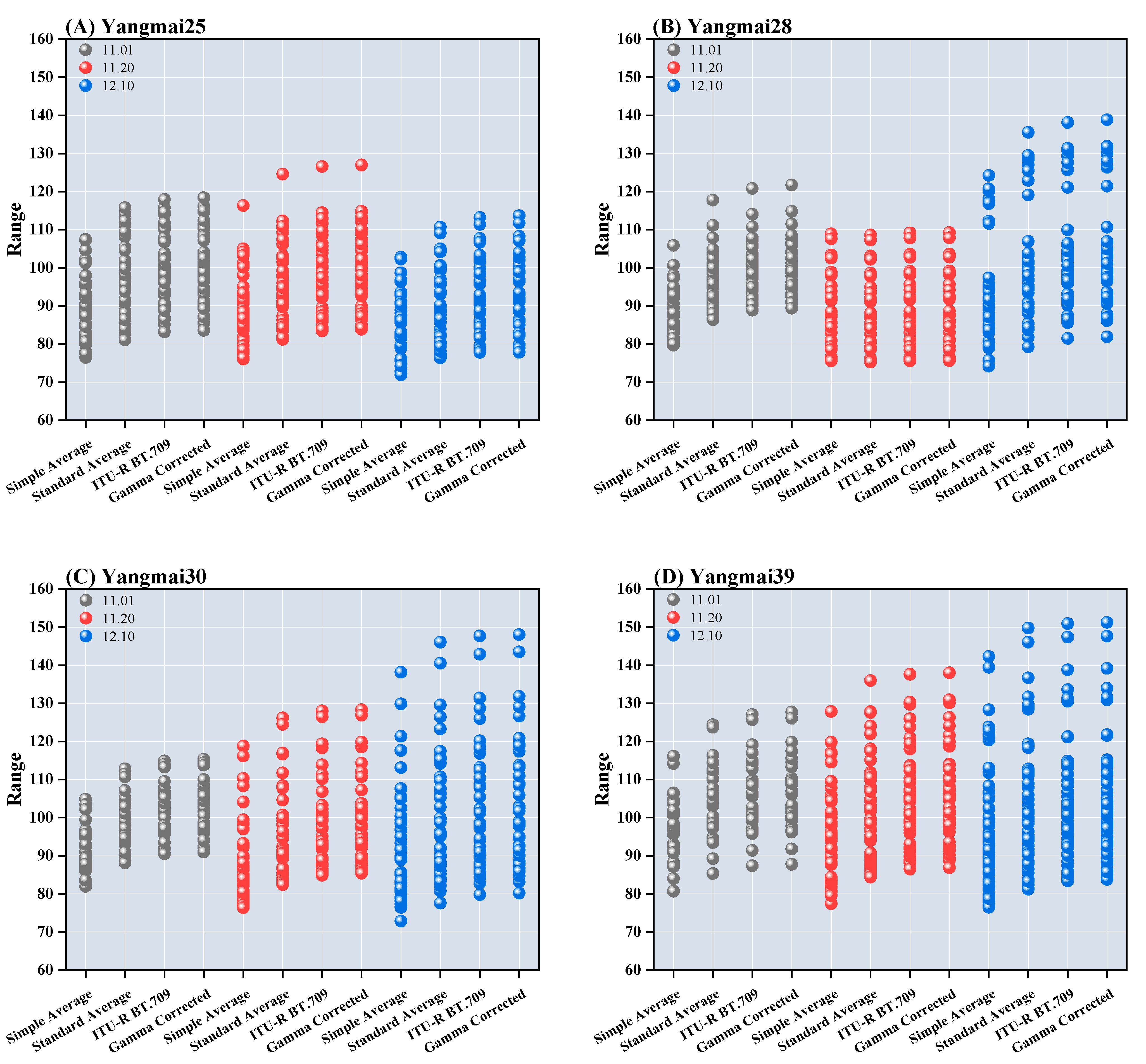
3.3. The Impacts of Sowing Dates on Leaf Color Intensity
3.4. The Impacts of Sowing Dates on the Leaf Color Index
4. Discussion
4.1. Analysis of the Effects and Patterns of Sowing Dates
4.2. Evaluation of the Method
4.3. Application and Extension of the Method
5. Conclusions
- (1)
- The sowing date significantly influences winter wheat leaf morphology. Specifically, as sowing is delayed, the leaf length-to-width ratio decreases gradually, following a convex relationship. This results in a reduction in the leaf length-to-width ratio across varieties, indicating a negative correlation between this ratio and the delayed sowing date.
- (2)
- The leaf area exhibits an exponential decline with delayed sowing. Across all winter wheat varieties, leaf area decreases significantly, with an average reduction of more than 59% for every 20-day delay in sowing. Among the varieties, Yangmai 28 is less sensitive to temperature fluctuations, showing an average reduction of 61.54%, whereas Yangmai 39 is the most affected, with a decline of up to 148.20%. A comprehensive analysis indicates that Yangmai 25 is slightly less affected by sowing date changes compared to Yangmai 30 and Yangmai 39, but is still more sensitive than Yangmai 28.
- (3)
- The leaf color responses to delayed sowing vary among different varieties. The leaf color of Yangmai 25 and Yangmai 39 gradually darkens with later sowing dates, exhibiting an overall positive correlation with sowing delay. In contrast, Yangmai 28 displays a lighter leaf color under late sowing, which deepens significantly under very late sowing, following an opposite trend to that of Yangmai 30. Overall, Yangmai 25, under the optimal sowing date, Yangmai 30, under late sowing, and Yangmai 28, under very late sowing, exhibit darker leaf colors compared to those of other varieties, providing them with a photosynthetic advantage. Conversely, Yangmai 39 maintains a lighter leaf color across all sowing dates.
- (4)
- The sowing date significantly impacts winter wheat leaf color indices. As sowing is delayed, EXG and EXGR indices decline, reflecting a reduction in greenness, while the EXR index increases, indicating red pigment accumulation. The declines in the SRPI and VARI indices suggest a deterioration in leaf health. Correlation analysis reveals notable differences among various leaf color indices. The correlation between NDGI and EXGR is 0.906, indicating strong consistency in representing leaf health and color. Similarly, EXG and EXR exhibit a correlation of 0.848, suggesting high consistency in extracting leaf color variation features. However, such high correlations may indicate multicollinearity issues, necessitating the selection of indices with lower correlations. For example, RI, which displays correlations of −0.166 with EXG and −0.17 with EXGR, was selected for independent red-edge analysis. Additionally, RGRI shows a correlation of 0.0338 with SRPI and 0.702 with EXR, indicating that RGRI independently captures different leaf characteristics. Meanwhile, RGBVI exhibits a correlation of 0.569 with EXG and 0.811 with EXGR, suggesting that RGBVI can complement feature extraction from other dimensions.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Minoli, S.; Jägermeyr, J.; Asseng, S.; Urfels, A.; Müller, C. Global crop yields can be lifted by timely adaptation of growing periods to climate change. Nat. Commun. 2022, 13, 7079. [Google Scholar] [CrossRef]
- Hussain, I.; Ijaz, M.; Ul-Allah, S.; Sattar, A.; Sher, A.; Nawaz, A.; Ghaffar, A.; Rahman, M.H.U.; Ahmad, S.; Rasheed, I.; et al. Optimum zinc fertilization and sowing date improved growth, yield components, and grain Zn contents of bread wheat under different tillage systems. J. Soil Sci. Plant Nutr. 2023, 23, 2344–2353. [Google Scholar] [CrossRef]
- Subedi, K.; Ma, B.; Xue, A. Planting date and nitrogen effects on grain yield and protein content of spring wheat. Crop Sci. 2007, 47, 36–44. [Google Scholar] [CrossRef]
- Kosina, R. Morphometry of lodicules in the genus Triticum L. Genet. Resour. Crop Evol. 2011, 58, 1129–1142. [Google Scholar] [CrossRef]
- Fernandez-Gallego, J.A.; Kefauver, S.C.; Vatter, T.; Gutiérrez, N.A.; Nieto-Taladriz, M.T.; Araus, J.L. Low-cost assessment of grain yield in durum wheat using RGB images. Eur. J. Agron. 2019, 105, 146–156. [Google Scholar] [CrossRef]
- Zhou, B.W.; Elazab, A.; Bort, J.; Vergara, O.; Serret, M.D.; Araus, J.L. Low-cost assessment of wheat resistance to yellow rust through conventional RGB images. Comput. Electron. Agric. 2015, 116, 20–29. [Google Scholar] [CrossRef]
- Xu, K.; Li, H.M.; Cao, W.X.; Zhu, Y.; Chen, R.J.; Ni, J. Recognition of weeds in wheat fields based on the fusion of RGB images and depth images. IEEE Access 2020, 8, 110362–110370. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, H.; Lv, X.; Yang, L.; Shang, J.; Sun, Q.; Zheng, X.; Zhou, C.; Zhao, B.; Wu, J. Applying convolutional neural networks for detecting wheat stripe rust transmission centers under complex field conditions using RGB-based high spatial resolution images from UAVs. Comput. Electron. Agric. 2022, 200, 107211. [Google Scholar] [CrossRef]
- Zang, H.; Peng, Y.; Zhou, M.; Li, G.; Zheng, G.; Shen, H. Automatic detection and counting of wheat spike based on DMseg-Count. Sci. Rep. 2024, 14, 29676. [Google Scholar] [CrossRef]
- Sales, C.R.; Molero, G.; Evans, J.R.; Taylor, S.H.; Joynson, R.; Furbank, R.T.; Hall, A.; Carmo-Silva, E. Phenotypic variation in photosynthetic traits in wheat grown under field versus glasshouse conditions. J. Exp. Bot. 2022, 73, 3221–3237. [Google Scholar] [CrossRef]
- Carmo-Silva, E.; Andralojc, P.J.; Scales, J.C.; Driever, S.M.; Mead, A.; Lawson, T.; Raines, C.A.; Parry, M.A. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 2017, 68, 3473–3486. [Google Scholar] [CrossRef]
- Niu, Z.; Liang, N.; He, Y.Y.; Xu, C.J.; Sun, S.S.; Zhou, Z.J.; Qiu, Z.J. A novel method for wheat spike phenotyping based on instance segmentation and classification. Appl. Sci. 2024, 14, 6031. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Yang, G.J.; Liang, D.; Hu, J.; Yang, H.; Yue, J.B.; Yan, R.R.; Han, L.; Huang, L.S.; Xu, L.J. Recognizing black point in wheat kernels and determining its extent using multidimensional feature extraction and a naive Bayes classifier. Comput. Electron. Agric. 2021, 180, 105919. [Google Scholar] [CrossRef]
- Tian, B.J.; Zhu, J.C.; Liu, X.W.; Huang, S.B.; Pu, W. Interacting leaf dynamics and environment to optimize maize sowing date in North China Plain. J. Integr. Agric. 2020, 19, 1227–1240. [Google Scholar] [CrossRef]
- Zhao, W.X.; Zhao, H.Y.; Wang, H.S.; He, Y. Research progress on the relationship between leaf senescence and quality, yield and stress resistance in horticultural plants. Front. Plant Sci. 2022, 13, 1044500. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.F.; Shang, J.L. Estimating effective leaf area index of winter wheat using simulated observation on unmanned aerial vehicle-based point cloud data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 2874–2887. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy leaf area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Sun, S.Q.; Huang, Y.J.; Inoue, K.; Hara, K. Order space-based morphology for color image processing. J. Imaging 2023, 9, 139. [Google Scholar] [CrossRef]
- Barbedo, J.G.A. A novel algorithm for semi-automatic segmentation of plant leaf disease symptoms using digital image processing. Trop. Plant Pathol. 2016, 41, 210–224. [Google Scholar] [CrossRef]
- Li, X.S.; Liu, M.L.; Ling, Q. Pixel-wise gamma correction mapping for low-light image enhancement. IEEE Trans. Circuits Syst. Video Technol. 2023, 34, 681–694. [Google Scholar] [CrossRef]
- Cao, G.; Huang, L.H.; Tian, H.W.; Huang, X.L.; Wang, Y.B.; Zhi, R.C. Contrast enhancement of brightness-distorted images by improved adaptive gamma correction. Comput. Electr. Eng. 2018, 66, 569–582. [Google Scholar]
- Khudhair, Z.N.; Khdiar, A.N.; El Abbadi, N.K.; Mohamed, F.; Saba, T.; Alamri, F.S.; Rehman, A. Color to grayscale image conversion based on singular value decomposition. IEEE Access 2023, 11, 54629–54638. [Google Scholar]
- Sowmya, V.; Govind, D.; Soman, K.P. Significance of incorporating chrominance information for effective color-to-grayscale image conversion. Signal Image Video Process. 2017, 11, 129–136. [Google Scholar]
- Alrubaie, S.a.H.; Hameed, A.H. Dynamic weights equations for converting grayscale image to RGB image. J. Univ. Babylon Pure Appl. Sci. 2018, 26, 122–129. [Google Scholar]
- Lee, M.K.; Golzarian, M.R.; Kim, I. A new color index for vegetation segmentation and classification. Precis. Agric. 2021, 22, 179–204. [Google Scholar]
- Wang, J.; Chen, C.; Wang, J.; Yao, Z.; Wang, Y.; Zhao, Y.; Sun, Y.; Wu, F.; Han, D.; Yang, G. NDVI Estimation Throughout the Whole Growth Period of Multi-Crops Using RGB Images and Deep Learning. Agronomy 2024, 15, 63. [Google Scholar] [CrossRef]
- Štroner, M.; Urban, R.; Suk, T. Filtering green vegetation out from colored point clouds of Rocky terrains based on various vegetation indices: Comparison of simple statistical methods, support vector machine, and neural network. Remote Sens. 2023, 15, 3254. [Google Scholar] [CrossRef]
- Meyer, G.E.; Neto, J.C. Verification of color vegetation indices for automated crop imaging applications. Comput. Electron. Agric. 2008, 63, 282–293. [Google Scholar]
- Clay, D.E.; Kim, K.I.; Chang, J.; Clay, S.A.; Dalsted, K. Characterizing water and nitrogen stress in corn using remote sensing. Agron. J. 2006, 98, 579–587. [Google Scholar] [CrossRef]
- Xie, Q.Y.; Dash, J.D.; Huang, W.J.; Peng, D.L.; Qin, Q.M.; Mortimer, H.; Casa, R.; Pignatti, S.; Laneve, G.; Pascucci, S. Vegetation indices combining the red and red-edge spectral information for leaf area index retrieval. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 1482–1493. [Google Scholar]
- Van Beek, J.; Tits, L.; Somers, B.; Deckers, T.; Janssens, P.; Coppin, P. Viewing geometry sensitivity of commonly used vegetation indices towards the estimation of biophysical variables in orchards. J. Imaging 2016, 2, 15. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar]
- Ruwanpathirana, P.; Sakai, K.; Jayasinghe, G.; Nakandakari, T.; Yuge, K.; Wijekoon, W.; Priyankara, A.; Samaraweera, M.; Madushanka, P. Evaluation of Sugarcane Crop Growth Monitoring Using Vegetation Indices Derived from RGB-Based UAV Images and Machine Learning Models. Agronomy 2024, 14, 2059. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Wang, J.; Zhao, Y.; Wang, H.; Zhang, W.; Yao, Z.; Liu, S.; Zhong, X.; Sun, C. Research on the estimation of wheat AGB at the entire growth stage based on improved convolutional features. J. Integr. Agric. 2024, 24, 1403–1423. [Google Scholar]
- Zhang, L.Y.; Niu, Y.X.; Zhang, H.H.; Han, W.T.; Li, G.; Tang, J.D.; Peng, X.S. Maize canopy temperature extracted from UAV thermal and RGB imagery and its application in water stress monitoring. Front. Plant Sci. 2019, 10, 1270. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Y.; Sun, Y.; Wang, J.; Yao, Z.; Chen, C.; Zhong, X.; Liu, S.; Sun, C.; Li, T. High-throughput identification of fusarium head blight resistance in wheat varieties using field robot-assisted imaging and deep learning techniques. J. Clean. Prod. 2024, 480, 144024. [Google Scholar]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar]
- Cao, Z.Y.; Chen, Z.H.; Tang, B.; Zeng, Q.; Guo, H.L.; Huang, W.H.; Luo, Y.; Shen, S.; Zhou, S.L. The effects of sowing date on maize: Phenology, morphology, and yield formation in a hot subtropical monsoon region. Field Crops Res. 2024, 309, 109309. [Google Scholar] [CrossRef]
- Mishiba, K. Fast guided median filter. IEEE Trans. Image Process. 2023, 32, 737–749. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, Z.D.; Liu, W.B.; Zeng, N.Y.; Lauria, S.; Prieto, C.; Sikström, F.; Yu, H.; Liu, X.H. A novel depth-connected region-based convolutional neural network for small defect detection in additive manufacturing. Cogn. Comput. 2025, 17, 36. [Google Scholar]
- Friedman, J.M.; Hunt Jr, E.R.; Mutters, R.G. Assessment of leaf color chart observations for estimating maize chlorophyll content by analysis of digital photographs. Agron. J. 2016, 108, 822–829. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, P.; Singh, J.P. A unified lightweight CNN-based model for disease detection and identification in corn, rice, and wheat. IETE J. Res. 2024, 70, 2481–2492. [Google Scholar] [CrossRef]
- Mahony, N.O.; Campbell, S.; Carvalho, A.; Harapanahalli, S.; Velasco-Hernández, G.A.; Krpalkova, L.; Riordan, D.; Walsh, J. Deep Learning vs. Traditional Computer Vision. arXiv 2019, arXiv:1910.13796. [Google Scholar]
- Münke, F.R.; Schützke, J.; Berens, F.; Reischl, M. A review of adaptable conventional image processing pipelines and deep learning on limited datasets. Mach. Vision Appl. 2024, 35, 17. [Google Scholar] [CrossRef]
- Hamuda, E.; Mc Ginley, B.; Glavin, M.; Jones, E. Automatic crop detection under field conditions using the HSV colour space and morphological operations. Comput. Electron. Agric. 2017, 133, 97–107. [Google Scholar] [CrossRef]
- Thenappan, S.; Arun, C.A. Wheat leaf diseases classification and severity analysis using HT-CNN and Hex-D-VCC-based boundary tracing mechanism. Environ. Monit. Assess. 2023, 195, 20. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Song, X.; Yang, G.; Du, X.; Mei, X.; Yang, X. Remote sensing monitoring of rice and wheat canopy nitrogen: A review. Remote Sens. Environ. 2022, 14, 5712. [Google Scholar] [CrossRef]
- Shafian, S.; Rajan, N.; Schnell, R.; Bagavathiannan, M.; Valasek, J.; Shi, Y.; Olsenholler, J. Unmanned aerial systems-based remote sensing for monitoring sorghum growth and development. PLoS ONE 2018, 13, e0196605. [Google Scholar] [CrossRef]
- Ni, Q.; Zuo, Y.; Zhi, Z.; Shi, Y.; Liu, G.; Ou, Q. Diagnosis of corn leaf diseases by FTIR spectroscopy combined with machine learning. Vibr. Spectrosc. 2024, 135, 103744. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, L.; Wang, J.; Luo, J.; Du, S.; Huang, W. Advances in remote sensing monitoring of crop diseases and insect pests. Trans. Chin. Soc. Agric. Eng. 2012, 28, 1–11. [Google Scholar]



| Traits | Yangmai 25 | Yangmai 28 | Yangmai 30 | Yangmai 39 |
|---|---|---|---|---|
| Growth Duration (days) | 202 | 196 | 196 | 200.2 |
| Seedling Characteristics | Semi-prostrate seedling, strong tillering | Upright seedling, weaker tillering | Upright seedling, strong tillering | Upright seedling, relatively strong tillering |
| Leaf Characteristics | Leaves erect, uniform spike layer | Wide and long leaves, uniform spike layer | Dark green leaves, flag leaf flat | Wide and long leaves, yellow-green color |
| Protein Content (%) | 13.56 | 12.53 | 12.8 | 13.7 |
| Wet Gluten Content (%) | 28.5 | 24.8 | 21 | 29.4 |
| No. | Grayscale Transformation | Calculation Formulas | References |
|---|---|---|---|
| 1 | Simple Average | [22] | |
| 2 | Standard Average | [23] | |
| 3 | ITU-R BT.709 | [23] | |
| 4 | Gamma Corrected | [24] |
| No. | Color Indices | Calculation Formulas | References |
|---|---|---|---|
| 1 | EXG | − | [25] |
| 2 | EXGR | [26] | |
| 3 | EXR | − | [27] |
| 4 | NDGI | − | [28] |
| 5 | NPCI | − | [29] |
| 6 | RI | − | [30] |
| 7 | NGBDI | − | [28] |
| 8 | SRPI | [31] | |
| 9 | VARI | − − | [32] |
| 10 | MGRVI | (G2 − R2)/(G2 + R2) | [33] |
| 11 | RGBVI | (G2 − R B)/(G2 + R B) | [34] |
| 12 | RGRI | [35] | |
| 13 | VDVI | [36] | |
| 14 | VEG | [37] |
| Sowing Date | Yangmai 25 | Yangmai 28 | Yangmai 30 | Yangmai 39 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Length | Width | Ratio | Length | Width | Ratio | Length | Width | Ratio | Length | Width | Ratio | |
| T1 (11.01) | 233.88 | 12.91 | 18.12 | 194.96 | 11.66 | 16.73 | 199.40 | 13.31 | 14.98 | 167.89 | 11.96 | 14.04 |
| T2 (11.20) | 149.53 | 7.47 | 20.02 | 179.58 | 10.02 | 17.91 | 147.29 | 8.11 | 18.17 | 115.23 | 7.76 | 14.84 |
| T3 (12.10) | 115.51 | 5.70 | 20.26 | 133.15 | 6.12 | 21.74 | 133.91 | 6.16 | 21.72 | 105.62 | 5.57 | 18.98 |
| Cultivars | T1 (11.01) | T2 (11.20) | T3 (12.10) | |||
|---|---|---|---|---|---|---|
| Effective Leaf Area | RGR | RGR | Effective Leaf Area | RGR | Effective Leaf Area | |
| Yangmai 25 | 19,392.31 | 96.65% | 274.12% | 9861.39 | 90.25% | 5183.51 |
| Yangmai 28 | 15,830.38 | 63.89% | 160.91% | 9659.13 | 59.20% | 6067.41 |
| Yangmai 30 | 18,368.42 | 106.42% | 351.51% | 8898.72 | 118.74% | 4068.24 |
| Yangmai 39 | 20,474.29 | 169.77% | 535.63% | 7589.48 | 135.62% | 3221.12 |
| Treatments | Bands of the Original Images | Bands After Gamma Correction | ||||
|---|---|---|---|---|---|---|
| Red | Green | Blue | Red | Green | Blue | |
| T1B1 | 26.47 | 36.73 | 19.99 | 82.09 | 96.73 | 71.38 |
| T1B2 | 31.80 | 42.16 | 23.42 | 89.98 | 103.67 | 77.24 |
| T1B3 | 32.60 | 42.80 | 22.63 | 91.03 | 104.38 | 75.94 |
| T1B4 | 33.35 | 43.15 | 25.91 | 92.14 | 104.85 | 81.27 |
| T2B1 | 29.82 | 40.35 | 20.92 | 87.16 | 101.41 | 73.01 |
| T2B2 | 32.79 | 43.58 | 22.53 | 91.35 | 105.37 | 75.76 |
| T2B3 | 28.99 | 39.26 | 20.04 | 85.87 | 99.98 | 71.46 |
| T2B4 | 33.37 | 43.74 | 23.64 | 92.04 | 105.47 | 77.61 |
| T3B1 | 30.91 | 41.25 | 21.56 | 88.73 | 102.53 | 74.14 |
| T3B2 | 30.26 | 41.01 | 19.78 | 87.84 | 102.25 | 71.01 |
| T3B3 | 32.50 | 43.48 | 21.57 | 90.96 | 105.25 | 74.14 |
| T3B4 | 35.46 | 45.91 | 24.36 | 94.91 | 108.07 | 78.79 |
| Treatments | Grayscale Transformation | |||
|---|---|---|---|---|
| Simple Average | Standard Average | ITU-R BT.709 | Gamma Corrected | |
| T1B1 | 83.40 | 89.46 | 91.78 | 92.22 |
| T1B2 | 90.30 | 96.56 | 98.85 | 99.25 |
| T1B3 | 90.45 | 97.15 | 99.49 | 99.92 |
| T1B4 | 92.75 | 98.36 | 100.45 | 100.78 |
| T2B1 | 87.19 | 93.91 | 96.33 | 96.79 |
| T2B2 | 90.83 | 97.80 | 100.25 | 100.72 |
| T2B3 | 85.77 | 92.51 | 94.92 | 95.40 |
| T2B4 | 91.71 | 98.28 | 100.61 | 101.02 |
| T3B1 | 88.47 | 95.17 | 97.54 | 97.99 |
| T3B2 | 87.03 | 94.38 | 96.93 | 97.46 |
| T3B3 | 90.11 | 97.43 | 99.96 | 100.47 |
| T3B4 | 93.93 | 100.80 | 103.16 | 103.59 |
| Indices | Calculation Formulas | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | |||||||||
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| EXG | 27.01 | 29.96 | 30.03 | 29.11 | 31.85 | 31.98 | 30.36 | 29.49 | 32.89 | 27.04 | 30.47 | 32.01 |
| EXGR | 9.94 | 9.14 | 8.32 | 8.01 | 8.48 | 9.39 | 7.36 | 8.95 | 8.97 | 6.26 | 7.38 | 6.72 |
| EXR | 17.06 | 20.83 | 21.71 | 21.10 | 23.37 | 22.59 | 23.00 | 20.55 | 23.93 | 20.78 | 23.08 | 25.29 |
| NDGI | 0.16 | 0.15 | 0.14 | 0.14 | 0.14 | 0.15 | 0.14 | 0.15 | 0.14 | 0.13 | 0.13 | 0.13 |
| NPCI | 0.14 | 0.18 | 0.18 | 0.15 | 0.19 | 0.21 | 0.18 | 0.18 | 0.20 | 0.13 | 0.17 | 0.19 |
| RI | −0.16 | −0.15 | −0.14 | −0.14 | −0.14 | −0.15 | −0.14 | −0.15 | −0.14 | −0.13 | −0.13 | −0.18 |
| NGBDI | 0.30 | 0.32 | 0.31 | 0.29 | 0.32 | 0.35 | 0.31 | 0.32 | 0.34 | 0.25 | 0.30 | 0.31 |
| SRPI | 0.76 | 0.70 | 0.70 | 0.74 | 0.69 | 0.65 | 0.69 | 0.69 | 0.66 | 0.78 | 0.71 | 0.69 |
| VARI | 0.24 | 0.21 | 0.20 | 0.21 | 0.20 | 0.21 | 0.19 | 0.21 | 0.20 | 0.19 | 0.19 | 0.18 |
| MGRVI | 0.32 | 0.29 | 0.28 | 0.27 | 0.28 | 0.29 | 0.27 | 0.29 | 0.28 | 0.25 | 0.26 | 0.25 |
| RGBVI | 0.44 | 0.45 | 0.44 | 0.41 | 0.44 | 0.48 | 0.43 | 0.45 | 0.46 | 0.37 | 0.42 | 0.42 |
| RGRI | 0.72 | 0.74 | 0.75 | 0.75 | 0.75 | 0.74 | 0.76 | 0.74 | 0.75 | 0.77 | 0.76 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, J.; Wang, J.; Wang, Z.; Zhao, L.; Yan, Y.; Li, J.; Xu, H.; Sun, C.; Liu, T. Investigating the Impact of Sowing Date on Wheat Leaf Morphology Through Image Analysis. Agriculture 2025, 15, 770. https://doi.org/10.3390/agriculture15070770
Chen J, Wang J, Wang J, Wang Z, Zhao L, Yan Y, Li J, Xu H, Sun C, Liu T. Investigating the Impact of Sowing Date on Wheat Leaf Morphology Through Image Analysis. Agriculture. 2025; 15(7):770. https://doi.org/10.3390/agriculture15070770
Chicago/Turabian StyleChen, Junfan, Jianliang Wang, Jiacheng Wang, Zhian Wang, Lihan Zhao, Yaohua Yan, Jiayue Li, Hanzeyu Xu, Chengming Sun, and Tao Liu. 2025. "Investigating the Impact of Sowing Date on Wheat Leaf Morphology Through Image Analysis" Agriculture 15, no. 7: 770. https://doi.org/10.3390/agriculture15070770
APA StyleChen, J., Wang, J., Wang, J., Wang, Z., Zhao, L., Yan, Y., Li, J., Xu, H., Sun, C., & Liu, T. (2025). Investigating the Impact of Sowing Date on Wheat Leaf Morphology Through Image Analysis. Agriculture, 15(7), 770. https://doi.org/10.3390/agriculture15070770







