4.1. Kernel Production, Infection, Mycotoxin Contamination and Nutrient Composition
Aflatoxins are stressors, exacerbating oxidative damage and disrupting normal cellular functions in developing kernels [
31]. In this study, the AFB1 was significantly higher in inoculated plants than in control plants, and
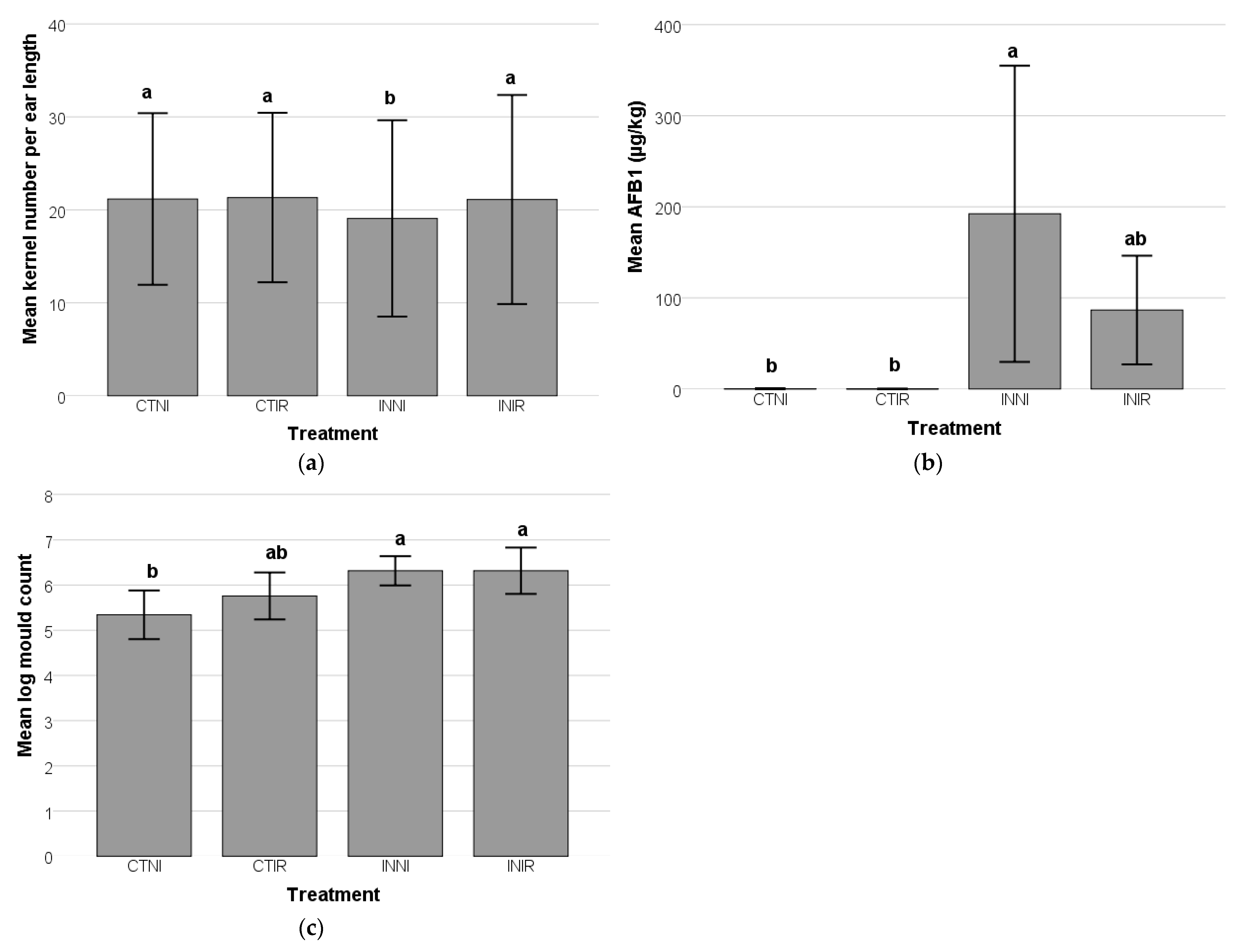
A. flavus inoculation significantly reduced the mean kernel number per ear length compared to the control group (
p < 0.05). This quantitative loss could be because of
A. flavus negatively impacts maize’s reproductive development. This aligns with previous findings that
A. flavus contamination depletes essential nutrients, disrupts metabolic processes, and induces physiological stress through mycotoxin production [
39]. The competition for nutrients between the pathogen and the host plant likely compromises carbohydrate and nitrogen availability for kernel development, leading to reduced reproductive success. One of the primary mechanisms underlying this reduction is the production of aflatoxins, particularly AFB1, which has been shown to impair maize growth and kernel formation [
31]. Beyond nutrient competition and toxin accumulation,
A. flavus infection appears to disrupt hormonal signaling pathways essential for kernel sets. Phytohormones such as auxins and gibberellins regulate reproductive development, and fungal infections have been linked to hormonal imbalances that negatively impact seed formation [
40]. Altered hormone signaling may impair kernel initiation and development, contributing to the decreased kernel number per ear length.
The significant increase in mold counts and AFB1 levels following
A. flavus inoculation highlighted the pathogen’s aggressive colonization and AFB1 production in maize.
A. flavus is known to be a prolific AFB1 producer, particularly under environmental conditions favorable for fungal growth, such as high humidity and elevated temperatures [
41]. This aligns with previous studies showing that fungal contamination and AFB1 accumulation are closely linked, posing serious threats to maize quality and food safety [
39]. The ability of
A. flavus to deplete essential nutrients from maize kernels contributes to reduced grain development and overall plant health. This nutrient competition further exacerbates the negative impacts of fungal infection, potentially impairing physiological processes critical for yield stability [
39]. Notably, even without visible symptoms, AFB1 contamination can still occur, underscoring the challenge of early detection and management [
42]. The detection of AFB1 in the control group indicates the natural occurrence of
A. flavus in the soil. Several studies have shown that this fungus is commonly present in soil and decomposes plant material and has been linked to the contamination of crops such as maize, cotton, groundnuts, sorghum, and millet [
43,
44]. Soil and plant debris, in particular, act as reservoirs for fungal inoculum, with evidence suggesting that they facilitate the survival and reproduction of
A. flavus. This is attributed to its saprophytic nature, which depends on organic matter for sustenance [
45].
A. flavus inoculation did not significantly correlate to the production of other mycotoxins such as ZEA, FB1 and DON as these mycotoxins are typically associated with other fungal species, particularly from the
Fusarium genus [
46]. However, co-infection scenarios involving both
A. flavus and
Fusarium species can accumulate multiple mycotoxins simultaneously, complicating food safety assessments and management strategies [
46]. Given the potential risks posed by a single mycotoxin, the simultaneous presence of two or more mycotoxins may lead to additive or interactive effects, altering their toxicity in humans and animals in ways that are not yet fully understood [
47].
Recent studies have highlighted the co-occurrence of fumonisins (FBs) and aflatoxins (AFs) in maize-growing regions, where high incidences of human hepatocellular carcinoma (HCC), chronic liver disease, and growth retardation in children have been reported [
48,
49]. The combination of FBs and AFs is particularly concerning due to the well-documented genotoxic effects of AFB1 and the capacity of FB1 to promote regenerative cell proliferation [
50]. Growing awareness of human co-exposure to multiple mycotoxins is particularly evident in cereals such as maize, tree nuts, oilseeds, and legumes. Other co-occurring mycotoxins include ochratoxin A (OTA) and DON in wheat [
51] and AF and DON in cereal-based baby food in Europe [
52] and
Fusarium toxins and OTA in the United States [
53].
The minimal impact of
A. flavus inoculation on starch and protein content suggests that short-term fungal infection does not significantly degrade these macro-nutrients [
54]. This could be attributed to the structural integrity of maize kernels, where starch and protein reserves are compartmentalized within the endosperm and aleurone layer, limiting direct fungal access to enzymatic degradation. However, prolonged fungal colonization or the presence of additional environmental stressors, such as drought or nutrient deficiency, may enhance enzymatic breakdown and compromise kernel composition over time [
55].
Similarly, the stable levels of total polyphenols in inoculated plants indicate that
A. flavus infection did not strongly induce stress-related phenolic metabolism under the experimental conditions tested [
56]. Polyphenols play a crucial role in plant defense, acting as antioxidants and antimicrobial agents; however, their biosynthesis is metabolically energy-intensive. The lack of a significant increase in polyphenol content suggests that the maize plants may have relied on alternative defense mechanisms, such as structural reinforcements, phytohormonal responses, or antimicrobial proteins, rather than investing heavily in phenolic production.
An essential factor influencing these results is the maize hybrid used in this study, SY Orpheus, which exhibits high stress tolerance [
23]. This hybrid’s genetic resilience likely contributed to its ability to maintain nutrient stability under both abiotic and biotic stresses. Its stress-adaptive traits may have facilitated efficient resource allocation, ensuring that kernel composition remained unchanged despite fungal infection and environmental challenges. The hybrid’s ability to sustain physiological homeostasis under varying conditions underscores its suitability for cultivation in regions where drought stress and fungal contamination threaten maize productivity.
While
A. flavus plays a critical role in kernel development and AFB1 contamination, its effects on maize nutritional parameters and secondary metabolites may be less pronounced in the short term. However, given the potential for aflatoxin accumulation and the progressive impact of fungal stress on grain quality, continuous monitoring and management strategies remain essential to ensuring food security and safety in maize production [
40,
57], particularly as climate change alters environmental conditions that influence fungal proliferation and toxin biosynthesis.
The combined effects of
A. flavus inoculation and irrigation significantly influenced maize physiology, particularly in kernel development and AFB1 accumulation. The findings demonstrated that irrigation is protective in mitigating the negative impacts of
A. flavus infection, as evidenced by the comparable kernel numbers in irrigated groups (INIR and CTIR). This suggests that sufficient water availability supports kernel formation even under biotic stress. Conversely, the significant reduction in kernel numbers in the INNI group underscores the compounded effects of fungal infection and water deficiency, likely due to impaired nutrient uptake and increased oxidative stress restricting grain development. These results align with studies indicating that water stress exacerbates fungal proliferation and toxin accumulation in maize [
22].
The elevated AFB1 levels in the INNI group further highlight the increased susceptibility of stressed plants to
A. flavus colonization. The combination of inadequate irrigation and nutrient limitation likely weakened plant defenses, facilitating fungal invasion and AFB1 accumulation. This pattern is consistent with previous research showing that water-stressed maize is more vulnerable to AFB1 contamination, as drought weakens plant immunity and alters metabolic pathways, creating a favorable environment for fungal proliferation [
22]. Notably, while
A. flavus is known to thrive under dry conditions, the high mold counts observed in both the INNI and INIR groups suggest that the fungus is highly adaptable and capable of growing under water-limited and water-sufficient environments. Variable weather conditions during the entire plant-growing period of maize are likely to favor fungi with very different ecological needs and to enhance fungal and mycotoxin co-occurrence [
58,
59]. The physiological responses of the maize plant to these moisture extremes may influence fungal growth and AFB1 biosynthesis in complex ways.
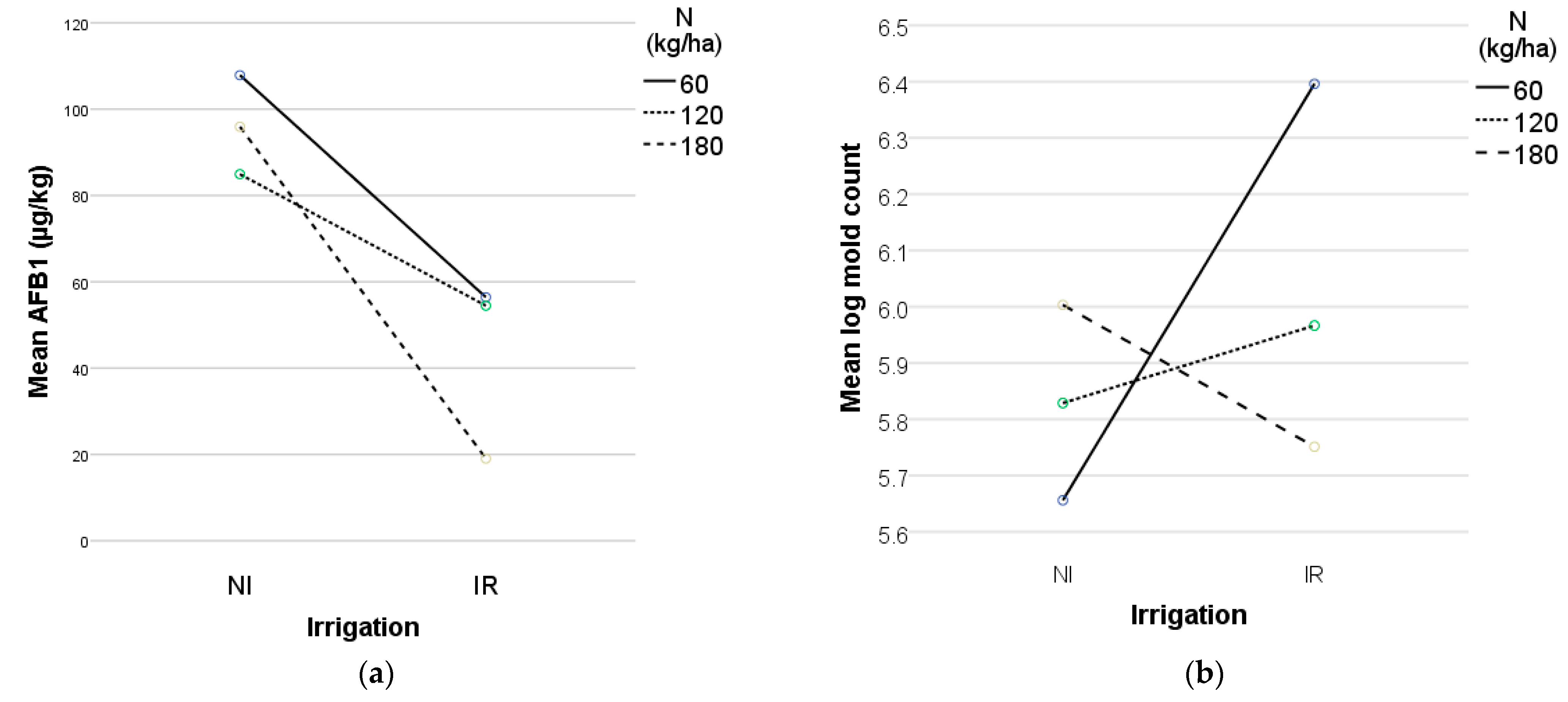
Interestingly, while fungal proliferation was observed under both moisture conditions, the sustained application of irrigation appeared to reduce AFB1 accumulation, supporting previous findings that adequate moisture availability can suppress mycotoxin production [
60]. This suggests that while irrigation promotes kernel development, it may also modulate AFB1 production. Irrigation plays a critical role in mitigating aflatoxin contamination in maize by influencing both plant physiology and fungal development. Well-irrigated maize plants exhibit enhanced physiological defenses, which limit
A. flavus colonization and aflatoxin biosynthesis. Optimal hydration supports cellular function, enhances antioxidant activity, and improves nutrient transport, contributing to plant resilience and reducing fungal infection risks. By maintaining kernel integrity, irrigation minimizes microcracks that could serve as fungal entry points, thereby reducing susceptibility to contamination [
61,
62].
In contrast, water stress weakens plant defenses by disrupting metabolic processes and inducing oxidative stress, making maize more vulnerable to
A. flavus infection. Drought conditions impair nutrient uptake and distribution, particularly nitrogen, which is essential for plant defense mechanisms. Adequate water availability counteracts these stress-induced vulnerabilities, preserving kernel structure and sustaining metabolic homeostasis. However, irrigation may also modulate stress-response pathways, potentially altering secondary metabolite production, which influences plant resistance to biotic stress [
37,
63]. Despite this, the net effect of irrigation favors reduced aflatoxin accumulation by alleviating physiological stress that would otherwise promote fungal proliferation.
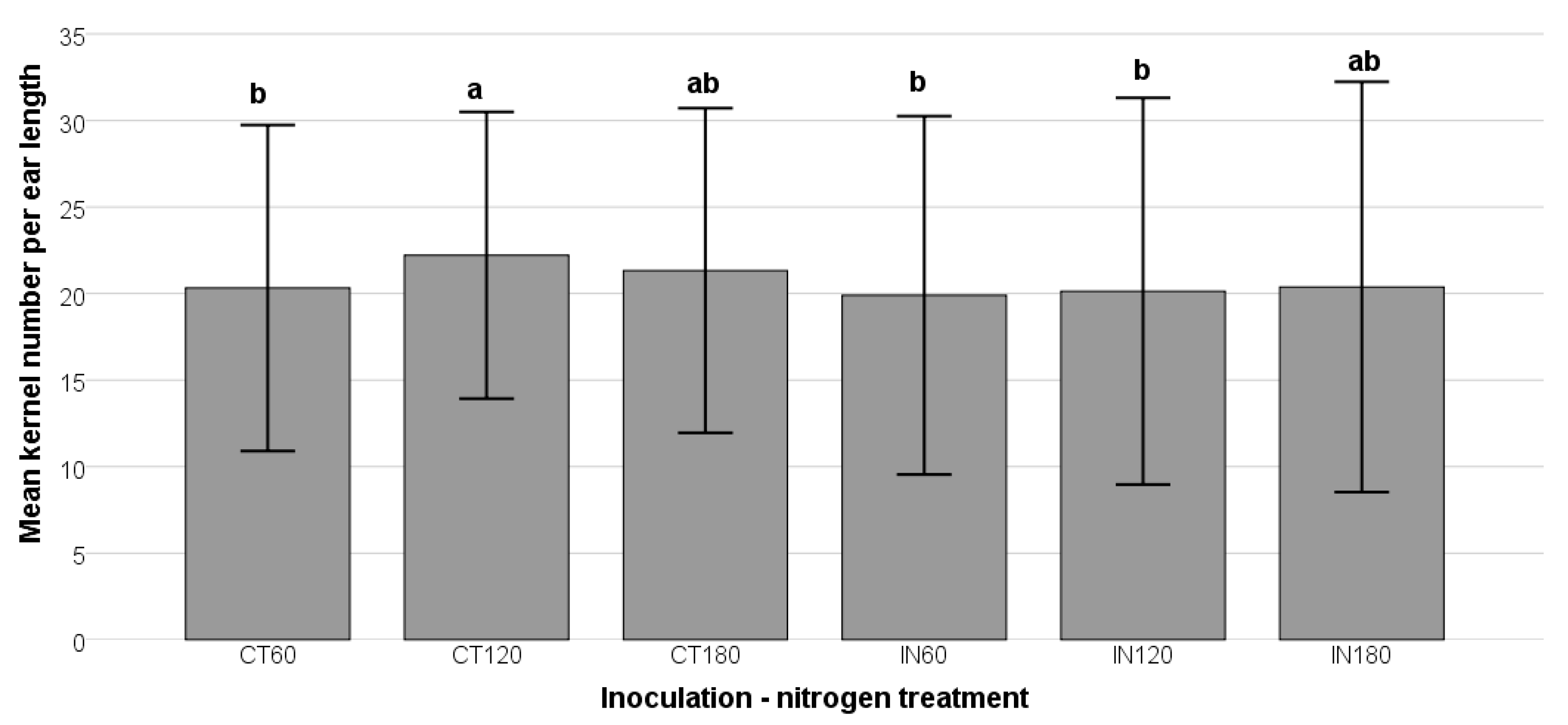
This study demonstrated that nitrogen supplementation is critical in buffering maize kernel development against biotic (
A. flavus infection) and abiotic (nutrient stress) factors. The enhanced kernel numbers in non-inoculated plants at 120 kg/ha nitrogen suggest that nitrogen availability promotes essential physiological processes such as photosynthesis, amino acid synthesis, and stress defense. These findings align with previous studies indicating that nitrogen supports plant growth and resilience, reducing vulnerability to fungal infections and subsequent AFB1 accumulation [
64]. However, in inoculated plants, kernel numbers declined at lower nitrogen levels (IN60 and IN120), likely due to fungal interference with nutrient uptake and energy diversion toward plant defense mechanisms.
Nitrogen application plays a crucial role in reducing AFB1 concentrations and modulating
A. flavus colonization in maize. The decline in AFB1 levels and mold count with increasing nitrogen suggests that nitrogen enhances plant vigor, promoting stronger cellular integrity, antioxidant activity, and efficient metabolic functions, all of which contribute to improved resistance against fungal infection. These findings align with previous studies indicating that nitrogen availability regulates the expression of aflatoxin biosynthetic genes, such as
aflR and
aflD, thereby suppressing toxin production [
65].
Interestingly, while nitrogen fertilization reduced AFB1 biosynthesis, it had a relatively minor effect on total mold counts. The presence of high fungal loads in inoculated maize suggests that A. flavus successfully colonized the tissues but did not produce significant toxins under high-nitrogen conditions. This indicates that nitrogen does not prevent fungal establishment but rather alters host–pathogen interactions in a way that limits aflatoxin accumulation. By enhancing plant immune responses, nitrogen may stimulate the production of antifungal metabolites or structural defenses that inhibit fungal metabolism and secondary metabolite synthesis.
Moreover, the stability of FB1 concentrations across treatments revealed FB1 independence from
A. flavus presence, which fungus primarily produces AFB1 but not fumonisins [
66]. The absence of DON in inoculation treatments (IN120 and IN180) may indicate a shift in fungal metabolic priorities or reduced fungal activity due to resource exhaustion or competitive microbial interactions. Additionally, the marginally higher polyphenol levels in inoculated treatments suggest an induced biotic stress response as polyphenols are known to contribute to plant protection under pathogen attack.
Overall, these findings highlight the multifaceted role of nitrogen in modulating A. flavus infection and mycotoxin production. Although nitrogen generally supports fungal colonization, its influence on A. flavus metabolism appears to suppress AFB1 biosynthesis. The lower mold counts in high-nitrogen treatments suggest that excess nitrogen may inhibit fungal proliferation or alter fungal pathogenicity. These results underscore the importance of nitrogen management in agricultural practices to mitigate AFB1 contamination and optimize maize yield. Future studies should explore the molecular mechanisms underlying nitrogen-induced changes in fungal metabolism and plant defense and optimal nitrogen application strategies for balancing crop productivity and pathogen resistance.
The findings suggest that adequate irrigation and 180 kg/ha nitrogen were the most effective strategies for managing
A. flavus growth and AFB1 biosynthesis in maize (
Figure 3) while maintaining high kernel production. Inoculated treatments at 180 kg/ha nitrogen exhibited significantly lower AFB1 levels and mold counts, indicating nitrogen’s potential role in suppressing
A. flavus colonization and toxin production. Nitrogen played a crucial role in enhancing plant defenses, influencing leaf wetness, shaping microbial competition, and interacting with irrigation to support plant health. By improving metabolic functions, nitrogen promoted the synthesis of defense-related proteins, amino acids, and secondary metabolites, which are crucial for pathogen resistance. Studies indicate that nitrogen-treated plants exhibit elevated concentrations of pathogenesis-related proteins and essential metabolites, strengthening their defense mechanisms against fungal infections [
67]. Thus, nitrogen acted not only as a growth nutrient but also as a key regulator of plant immunity.
Nitrogen also influenced leaf morphology, altering leaf wetness duration and moisture retention. Increased nitrogen levels contribute to denser foliage, which can trap more moisture, potentially affecting fungal colonization by modifying the micro-climate around leaf surfaces [
68]. Additionally, nitrogen availability may have altered soil microbial competition, favoring beneficial microbes that suppress the growth of pathogenic fungi. Nitrogen supplementation has been reported to enhance microbial biomass and functional gene diversity, improving nutrient cycling and bolstering plant health [
69,
70]. However, excessive nitrogen can disrupt microbial balance, lower soil pH, and inadvertently promote pathogenic dominance [
71,
72]. Combined, irrigation enhanced nitrogen uptake, ensuring the maize plants received adequate nutrients for optimal growth and defense responses towards
A. flavus proliferation and AFB1 accumulation.
The positive correlation between AFB1 and mold count (r = 0.479,
p < 0.01) reinforces the well-established link between
A. flavus proliferation and mycotoxin production. This correlation suggests that environmental conditions favoring fungal growth also promote AFB1 biosynthesis, aligning with previous studies indicating that high mold counts are often accompanied by elevated AFB1 contamination [
73,
74]. Given that
A. flavus thrives under stress conditions such as drought, high temperature, and nutrient imbalance, effective environmental management strategies are critical to mitigating fungal colonization and subsequent mycotoxin production. Nazari et al. (2019) [
75] also reported a positive correlation between mycotoxins and fungal DNA in the wheat kernel, but only for
Fusarium sporotrichioides and
Fusarium poae.
The significant negative correlation between starch and protein content (r = −0.664,
p < 0.01) highlights a metabolic trade-off in maize, wherein carbohydrate storage increases at the expense of protein synthesis. This phenomenon is consistent with plant metabolic resource allocation, as carbohydrate reserves are often prioritized under certain environmental or physiological conditions, potentially reducing nitrogen assimilation and protein biosynthesis [
41,
76]. This trade-off is particularly relevant under stress conditions, where plants must balance growth, defense, and storage processes to optimize survival and productivity.
The positive correlation between starch content and mold count (r = 0.313,
p > 0.05) suggests that starch may be a readily available carbon source for fungal growth, potentially fueling mycotoxin biosynthesis. While this correlation was not statistically significant in the present study, previous research indicates that fungal pathogens exploit plant carbohydrate reserves for their metabolic needs, increasing mold proliferation and toxin accumulation [
74,
77]. This relationship implies that maize genotypes with higher starch reserves may be more susceptible to fungal colonization, necessitating targeted breeding strategies to balance yield and disease resistance. In addition, the ability of maize plants to maintain relatively stable starch and protein levels under certain conditions suggests that short-term fungal stress may not drastically alter nutrient composition; however, prolonged exposure could lead to more pronounced metabolic shifts [
78].
4.2. Physiological Changes in the Maize Plant
The unexpected increase in chlorophyll content following
A. flavus inoculation suggests a stress-induced compensatory response aimed at maintaining photosynthetic efficiency and efficacy under pathogen pressure (
Table 5). Biotic stress can trigger complex physiological adjustments, including enhanced chlorophyll biosynthesis and delayed degradation, as part of the plant’s defense strategy [
79,
80]. One possible mechanism is the activation of stress-signaling pathways, such as those mediated by salicylic acid (SA) and jasmonic acid (JA), which regulate chlorophyll metabolism in response to pathogen attack [
81,
82]. SA, in particular, has been linked to chlorophyll retention by modulating antioxidant enzyme activity and reducing chlorophyll degradation under stress conditions. Additionally,
A. flavus infection may induce reactive oxygen species (ROS) production, which, at moderate levels, acts as a signaling molecule to enhance plant defense and metabolic adjustments, including chlorophyll stabilization [
82]. Prolonged
A. flavus infection can lead to significant physiological changes in maize plants, particularly through the disruption of photosynthesis and chloroplast function. One of the earliest signs of
A. flavus infection is chlorosis, which is characterized by the yellowing of the leaves. This phenomenon is primarily attributed to a decrease in chlorophyll synthesis, which is essential for photosynthetic activity. Several interrelated factors contribute to this reduction in chlorophyll content. First,
A. flavus competes for key nutrients within the plant, such as magnesium and iron, both of which are crucial for chlorophyll biosynthesis. The depletion of these nutrients is integral to the chlorophyll molecule’s structural integrity [
83], and results in reduced chlorophyll production and, consequently, diminished photosynthetic capacity [
84]. In addition to nutrient competition, fungal infection induces oxidative stress in maize plants. The accumulation of ROS as a result of fungal colonization can damage cellular components, including chloroplast membranes, further impairing chlorophyll synthesis and functionality [
85]. Excessive ROS also disrupts the balance of enzymatic activities necessary for chlorophyll biosynthesis, inhibiting the action of chlorophyll-synthesizing enzymes [
86]. This oxidative damage diminishes chlorophyll content and directly impacts the plant’s photosynthetic efficiency.
The relationship between chlorophyll content and the plant’s water and nutrient status is also critical, as both can be negatively affected by
A. flavus infection [
87]. When the plant’s physiological balance is disturbed—whether through drought stress or nutrient deficiencies—chlorophyll concentrations typically decline. This suggests that
A. flavus, by disrupting the plant’s overall health and nutrient acquisition, can lead to substantial reductions in chlorophyll content.
Furthermore, the activation of defense mechanisms in maize in response to
A. flavus infection adds complexity to the situation. While the plant activates defense pathways to combat the pathogen, these responses can divert energy and resources away from essential physiological functions, including chlorophyll production [
88]. Additionally, the altered hormonal balance during infection, including increased levels of abscisic acid (ABA), can lead to enhanced stomatal closure, thereby reducing photosynthetic rates and further diminishing chlorophyll content [
89].
Metabolic alterations resulting from
A. flavus infection also influence gene expression related to chlorophyll biosynthesis. Studies have shown that different maize varieties exhibit distinct patterns of gene expression related to chlorophyll biosynthesis when infected by
A. flavus [
90,
91,
92]. These variations highlight the genetic basis of chlorophyll regulation, suggesting that breeding programs aimed at improving chlorophyll retention could contribute significantly to developing maize cultivars with enhanced resistance to fungal pathogens [
93].
However, spectral changes, particularly the REP shift toward shorter wavelengths in inoculated plants, indicate physiological disruptions. This shift is often associated with chlorophyll degradation, increased stress-related pigments (such as total polyphenols), or structural modifications in leaf tissue [
94]. While increased chlorophyll content may reflect an initial defense response, the REP shift suggests that fungal stress eventually compromises photosynthetic integrity, potentially altering leaf optical properties and signaling pathways.
The decline in the FD725/FD702 ratio, an index of photosynthetic efficiency and pigment composition, in inoculated plants further confirms disruptions in photosystem II (PSII) function. Aflatoxins, particularly AFB1, induce oxidative stress, disrupt membrane stability, and impair chloroplast function, altering chlorophyll fluorescence properties [
95,
96]. This oxidative burden may initially stimulate chlorophyll synthesis as a short-term response while concurrently reducing PSII efficiency, affecting overall photosynthetic performance.
Furthermore, the observed increase in total polyphenol content in inoculated plants highlights the role of oxidative stress responses in fungal defense mechanisms. Polyphenols are crucial in mitigating oxidative damage and enhancing plant resilience against pathogens, consistent with the literature on plant stress adaptation [
80]. However, their accumulation and changes in spectral indices suggest an underlying metabolic cost associated with fungal resistance.
Water availability is crucial in shaping maize physiological responses, particularly regarding chlorophyll retention and photosynthetic efficiency. The significantly higher SPAD values observed in NI plants (
p < 0.05;
Table 5) suggest an adaptive response to water stress conditions. This aligns with previous research indicating that plants often maintain or even increase chlorophyll under water stress as a survival strategy to sustain photosynthetic activity despite limited water availability [
97]. This response is likely mediated by abscisic acid signaling, which promotes stomatal closure, enhances water-use efficiency, and reduces transpiration-related water loss [
98]. In contrast, the lower SPAD values in IR plants may reflect a dilution effect due to higher leaf water content, which reduces chlorophyll concentration per unit area without necessarily impacting total chlorophyll levels. This observation is consistent with the literature, which indicates that increased leaf hydration can lead to lower SPAD readings despite improved photosynthetic performance [
99].
The stability of REP values between IR and NI treatments suggests that irrigation had minimal effects on leaf structural integrity or chlorophyll degradation. This suggests that plants may employ compensatory physiological mechanisms to maintain reproductive efficiency (REP) despite variations in water availability. Previous studies have shown that while irrigation influences chlorophyll content, its impact on leaf structural properties is often less pronounced [
99]. The significantly higher FD725/FD702 ratio in irrigated plants suggests enhanced electron transport efficiency, likely due to reduced photoinhibition stress under optimal water conditions [
100]. In contrast, the lower FD725/FD702 ratio in NI plants indicates a decline in PSII quantum yield. This suggests that plants under water stress relied more on non-photochemical quenching mechanisms to dissipate excess light energy. This is a well-documented adaptation strategy, where plants shift from photochemical energy conversion to thermal dissipation to mitigate oxidative damage [
101]. These findings highlight the contrasting physiological adjustments between irrigated and non-irrigated maize plants. While irrigation enhances photosynthetic efficiency and reduces photoinhibition stress, water stress conditions trigger adaptive responses such as increased chlorophyll retention and altered energy dissipation mechanisms.
Leaf position relative to the maize cob has a significant influence on chlorophyll content, spectral characteristics, and photosynthetic efficiency (
Table 5). The significantly higher SPAD values observed in the cob-adjacent leaf (
p < 0.01) suggest its critical role in supplying photosynthates to developing kernels, which aligns with previous studies highlighting the functional specialization of different leaf positions in maize [
102]. This enhanced chlorophyll retention likely ensures sustained photosynthetic activity to support grain filling.
In contrast, the upper leaf exhibited lower SPAD values, which may be attributed to greater exposure to light intensity and potential photoinhibition. Increased light exposure can lead to slight reductions in chlorophyll content as a protective mechanism against excessive excitation energy [
103]. The significantly higher REP value in the cob-adjacent leaf (
p < 0.05) further confirms its superior chlorophyll concentration and overall leaf health. REP shifts toward longer wavelengths are typically associated with higher chlorophyll content and better physiological status, reinforcing the importance of this leaf in maintaining maize productivity under field conditions.
The FD725/FD702 ratio, an indicator of photochemical energy conversion efficiency, was also significantly higher in the cob-adjacent leaf (
p < 0.05). This suggests that the leaf next to the cob operates under more favorable physiological conditions, experiencing reduced stress impact and maintaining efficient photosynthetic electron transport. The lower FD725/FD702 ratio in the upper leaf implies greater exposure to environmental stressors, such as fluctuating temperatures and excess light, which may induce non-photochemical quenching mechanisms to dissipate excess absorbed energy and prevent photodamage [
103].
The findings indicate that increasing nitrogen application significantly influenced chlorophyll content (SPAD values), red edge position (REP), and the FD725/702 ratio in maize plants (
Table 5). Nitrogen availability is fundamental in regulating chlorophyll biosynthesis, photosynthetic efficiency, and stress resilience in maize. The significantly higher SPAD values at 180 kg/ha nitrogen application confirm improved leaf nitrogen status and enhanced chlorophyll content, aligning with previous research indicating that nitrogen is a key component of chlorophyll molecules [
104]. Higher nitrogen levels promote increased carbon fixation, ensuring optimal photosynthetic activity. The observed REP shifts toward longer wavelengths at higher nitrogen levels further support the relationship between nitrogen availability and leaf health. Increased nitrogen enhances chlorophyll retention, delays senescence, and strengthens leaf structure, improving spectral properties [
105]. This finding aligns with studies demonstrating that nitrogen fertilization enhances chlorophyll fluorescence parameters, indicating better light absorption and photosynthetic efficiency in maize [
106]. The FD725/FD702 ratio, a photochemical energy conversion efficiency index, was significantly higher in maize plants receiving 180 kg/ha nitrogen. This suggests improved electron transport efficiency and reduced reliance on non-photochemical quenching mechanisms. Conversely, the lower FD725/FD702 ratio at 60 kg/ha suggests nitrogen limitation, which may have reduced PSII efficiency and increased stress-related photoprotective responses [
107]. Nitrogen deficiency has been shown to trigger premature chlorophyll degradation and impair photosynthetic performance, increasing plant susceptibility to oxidative stress and environmental fluctuations [
108].
Beyond its role in chlorophyll synthesis, nitrogen availability influences antioxidant production and secondary metabolite biosynthesis, which can enhance plant resilience against oxidative stress caused by mycotoxins. Given the interplay between nitrogen status and stress responses, optimizing nitrogen fertilization strategies could mitigate stress-induced physiological disruptions in maize while maximizing photosynthetic performance and yield potential.
In this study, the highest chlorophyll content (SPAD value) was recorded in maize plants that were inoculated, non-irrigated, received 180 kg/ha of nitrogen and were measured at the cob-adjacent leaf position. In contrast, the highest REP value and FD725/FD702 ratio were observed in maize plants that were inoculated, irrigated, received 180 kg/ha of nitrogen, and were measured at the cob-adjacent leaf position. These findings suggest that chlorophyll synthesis and retention are strongly influenced by nitrogen availability and leaf position, with the cob-adjacent leaf likely benefiting from greater nutrient allocation. The interaction between inoculation and irrigation appears to differentially affect spectral properties, with irrigation potentially mitigating stress effects and promoting structural and biochemical changes that enhance REP and FD725/FD702 ratios.
4.3. Micro-Climatic Changes in the Maize Field
The increased T
max in irrigated fields, likely due to enhanced canopy transpiration and greater soil moisture, created a micro-climate conducive to fungal proliferation (
Table 6). This was reflected in slightly higher mold counts, though the difference was not statistically significant (
Table S3). While higher T
max could have favored fungal growth, increased soil moisture likely mitigated extreme temperature fluctuations, preventing excessive proliferation. The lower T
min in irrigated fields, attributed to evaporative cooling at night, may have further influenced pathogen dynamics by slowing fungal growth and prolonging leaf wetness duration. The longer transition time observed in irrigated fields supports this hypothesis, as extended leaf wetness is often associated with increased fungal activity.
Despite these micro-climatic conditions, significantly lower AFB1 concentrations in irrigated maize suggest a protective role of irrigation against mycotoxin accumulation. Reduced plant stress and enhanced physiological defenses likely suppressed AFB1 biosynthesis. Although relative humidity was higher in irrigated fields, mold counts remained statistically similar between treatments, indicating that humidity alone did not markedly influence fungal colonization. Overall, irrigated maize exhibited improved physiological resilience, likely due to reduced oxidative stress and enhanced nutrient uptake, leading to better kernel quality and lower AFB1 contamination. Conversely, non-irrigated plants experienced greater water stress, which may have weakened defense mechanisms, making them more susceptible to fungal colonization and toxin biosynthesis. Optimizing irrigation schedules to balance soil moisture while avoiding excessive leaf wetness could further mitigate fungal proliferation and AFB1 accumulation. Additionally, integrating resistant maize varieties with irrigation management may offer a sustainable approach to reducing A. flavus colonization and mycotoxin contamination.
The stable average temperature between the upper and lower canopy suggests a relatively uniform thermal environment. However, significantly higher T
max in the lower canopy of irrigated maize, particularly around noon, may have resulted from greater soil exposure to radiation due to south–north row alignment (
Table 6). Factors such as leaf angle and the height of the highest leaf area density relative to temperature probes could have influenced airflow restriction and increased heat retention at lower levels. In contrast, significantly lower T
min in the upper canopy suggests greater exposure of upper leaves to night-time radiative cooling, which may impact metabolic processes and pathogen development. The lower canopy retained more moisture due to reduced air circulation and increased transpiration from densely packed leaves, as reflected in higher RH
min values. Increased evapotranspiration in the lower 0.3 m of the canopy also contributed to localized humidity, potentially favoring fungal colonization.
Longer LW
wet in the upper canopy suggests higher atmospheric moisture deposition, likely from dew formation, while extended transition times in the lower canopy indicate prolonged leaf wetness, increasing fungal infection risk. The stability of T
avg across nitrogen treatments suggests that nitrogen fertilization did not significantly alter the overall thermal environment (
Table 6). However, reduced T
max at 120 kg/ha may be due to enhanced canopy development, providing greater shading and reducing heat accumulation. The increase in T
min at 120 kg/ha, likely due to better canopy insulation, suggests a micro-climate less prone to temperature extremes. Higher relative humidity (RH) at 60 kg/ha may be attributed to denser canopy structure, which limited airflow and increased moisture retention.
Nitrogen levels also influenced leaf wetness duration. At 60 kg/ha, longer LWwet suggests reduced transpiration, prolonging surface moisture retention. Conversely, the faster transition time and longer LWdry at 120 kg/ha indicate enhanced plant vigor and transpiration, promoting quicker drying and reducing fungal risk. The micro-climatic changes observed align with fungal contamination and mycotoxin accumulation trends. Prolonged leaf wetness and higher Tmax at 60 kg/ha likely created favorable conditions for fungal growth and AFB1 production. In contrast, improved plant health and faster moisture dissipation at 120–180 kg/ha may have reduced fungal proliferation and toxin accumulation, reinforcing the role of adequate nitrogen levels in mitigating mycotoxin contamination.
The correlations observed (
Table 7) support these findings. The positive relationships between temperature variables (T
avg, T
max, and T
min) suggest a consistent thermal trend across day and night. The inverse correlation between temperature and relative humidity reflects the expected reduction in atmospheric moisture at higher temperatures. Strong correlations between LW
wet and humidity parameters indicate that increased atmospheric moisture prolongs leaf wetness, favoring fungal activity. Conversely, the negative correlation between LW
wet and T
avg suggests that higher temperatures accelerate leaf drying, potentially limiting fungal proliferation. Additionally, the positive correlation between transition time and RH
avg suggests that higher humidity levels delay leaf drying, prolonging conditions conducive to fungal growth. Finally, the negative correlation between LW
dry and relative humidity supports the idea that high humidity levels sustain moisture retention on leaf surfaces, reducing dry periods and potentially increasing fungal pathogen risk.