Modulating Effects of Grape Pomace on the Intestinal Antioxidative and Inflammatory Status in Fattening Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Analysis of Antioxidant Activity and Polyphenol Content of Experimental Diets
- Assessment of total phenolic content
- Determination of antioxidant activity
- 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) scavenging assay
2.3. Sample Collection and Histological and Immunohistochemical Analyses
2.4. Statistical Analysis
3. Results
3.1. Pig Growth Performance
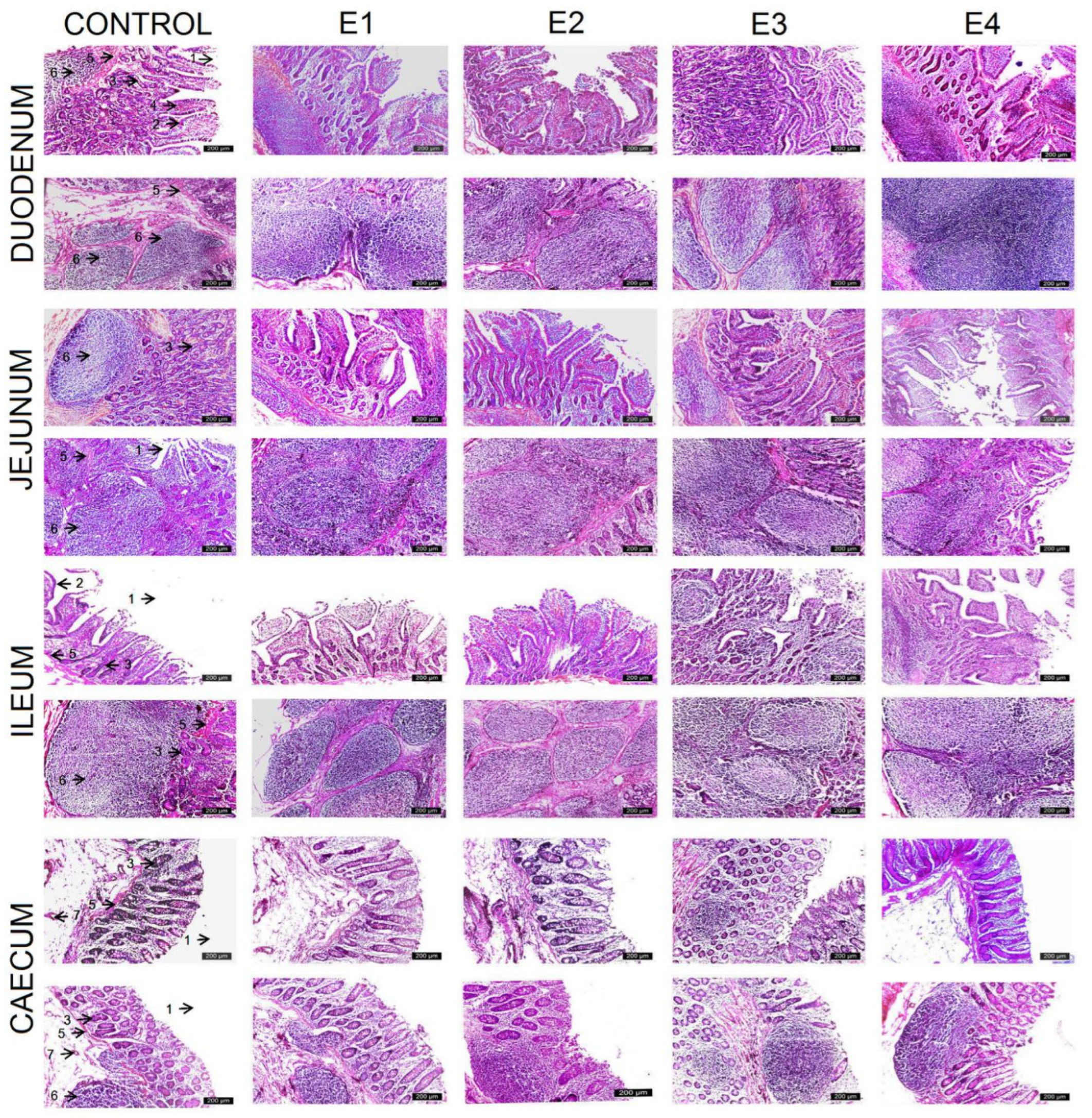
3.2. Histologic Intestinal Analysis
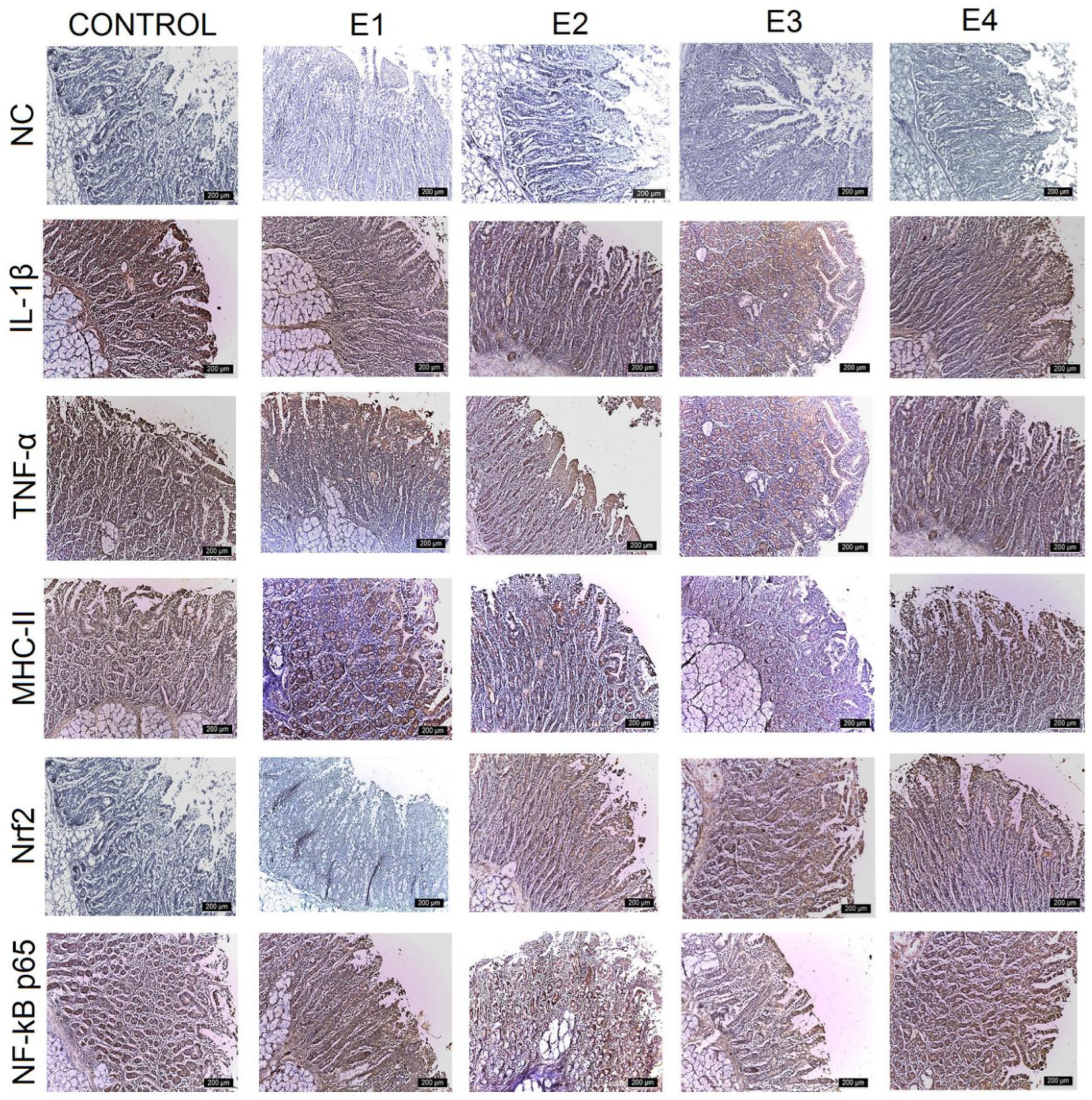
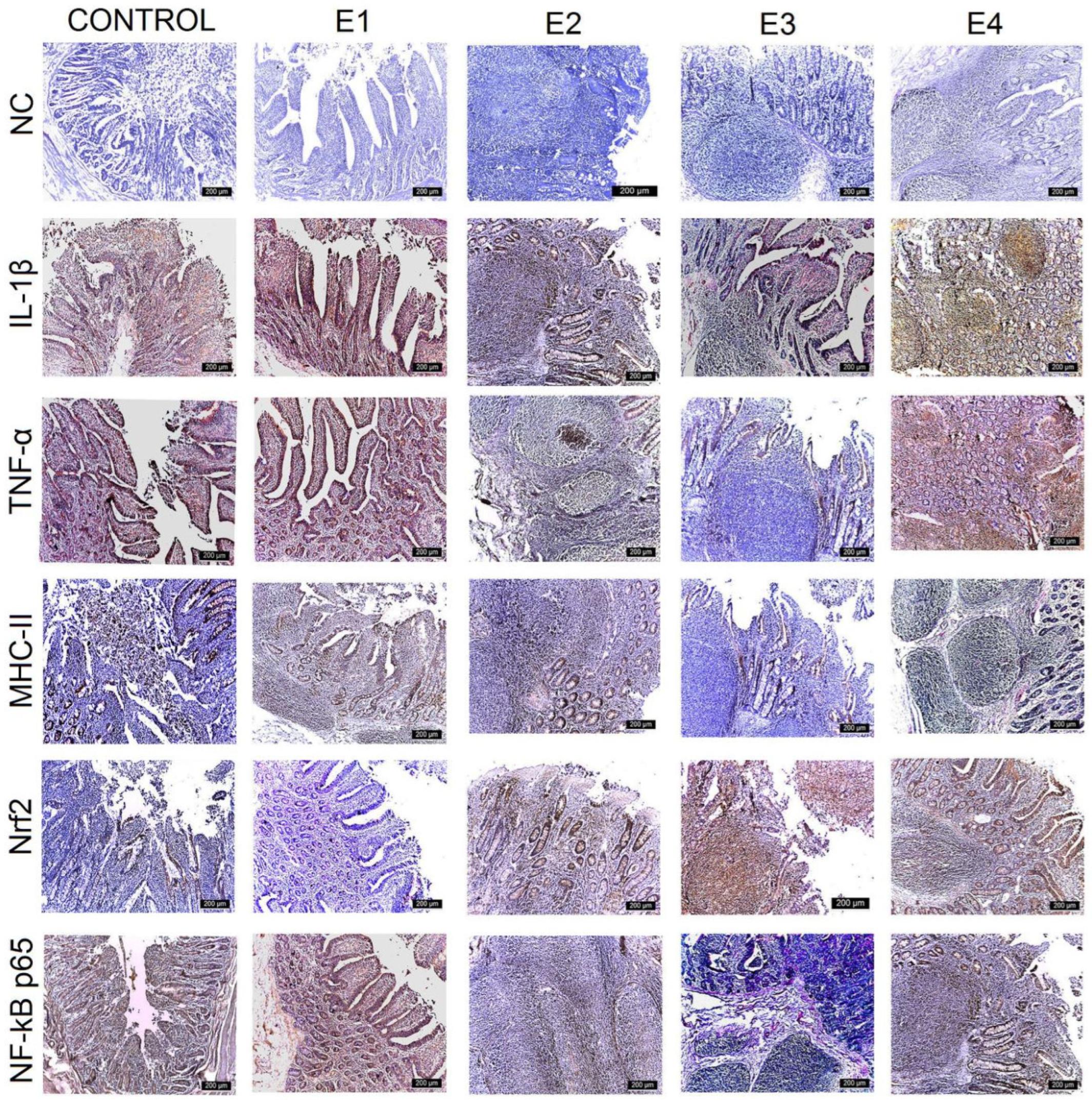
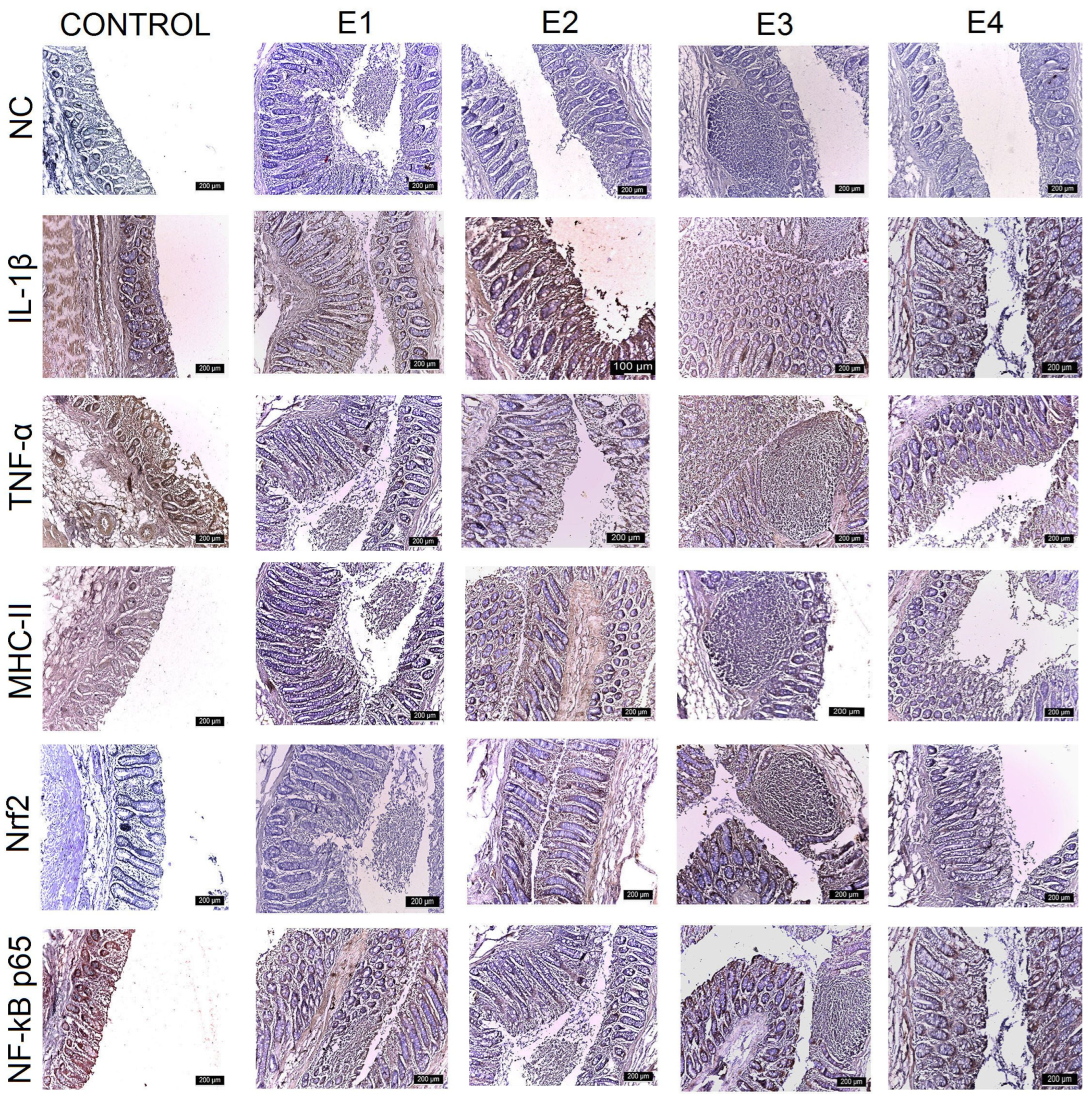
3.3. IHC Intestinal Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Henchion, M.; Moloney, A.P.; Hyland, J.; Zimmermann, J.; McCarthy, S. Review: Trends for meat, milk and egg consumption for the next decades and the role played by livestock systems in the global production of proteins. Animal 2021, 15, 100287. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, M.; Zhu, Q.; Azad, A.K.; Gao, Q.; Kong, X. Dietary betaine addition alters carcass traits, meat quality, and nitrogen metabolism of bama mini-pigs. Front. Nutr. 2021, 8, 728477. [Google Scholar] [CrossRef]
- Erinle, T.J.; Adewole, D.I. Fruit Pomaces—Their nutrient and bioactive components, effects on growth and health of poultry species, and possible optimization techniques. Anim. Nutr. 2022, 9, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.; Neumann, C.; Velten, S.; Liebert, F.; Mörlein, D. Meat quality derived from high inclusion of a micro-alga or insect meal as an alternative protein source in poultry diets: A pilot study. Foods 2018, 7, 34. [Google Scholar] [CrossRef]
- Reckmann, K.; Blank, R.; Traulsen, I.; Krieter, J. Comparative Life Cycle Assessment (LCA) of Pork Using Different Protein Sources in Pig Feed. Arch. Anim. Breed. 2016, 59, 27–36. [Google Scholar] [CrossRef]
- Spigno, G.; Marinoni, L.; Garrido, G.D. State of the art in grape processing by-products. Handb. Grape Process. By-Prod. 2017, 1–27. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.L.; Cabaraux, J.F.; Chentouf, M. Ecological, morpho-agronomical, and bromatological assessment of sorghum ecotypes in Northern Morocco. Sci. Rep. 2023, 13, 15548. [Google Scholar] [CrossRef]
- Jin, L.-Z.; Dersjant-Li, Y.; Giannenas, I. Application of aromatic plants and their extracts in diets of broiler chickens. Feed Addit. 2020, 159–185. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition–a review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent advances in biologically active compounds in herbs and spices: A review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; Intech Open: London, UK, 2020; pp. 1–27. [Google Scholar]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; Morais, S.M.; Lima, A.; Martins, C.G.; Alexandrino, C.D.; Ferreira PA, T.; Rodrigues, A.L.M.; Rodrigues, S.P. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar]
- Taranu, I.; Habeanu, M.; Gras, M.; Pistol, G.; Lefter, N.; Palade, M.; Ropota, M.; Sanda Chedea, V.; Marin, D. Assessment of the effect of grape seed cake inclusion in the diet of healthy fattening-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e30–e42. [Google Scholar]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin b1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon 2019, 162, 24–31. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Rotar, M.C.; Ropota, M.; Taranu, I. Bioactive compounds from dietary whole grape seed meal improved colonic inflammation via inhibition of MAPKs and NF-KB signaling in pigs with DSS induced colitis. J. Funct. Foods 2020, 66, 103708. [Google Scholar] [CrossRef]
- Hafeez, A.; Hassni, S.F.; Naz, S.; Alonaizan, R.; Al-Akeel, R.K.; Sifa, D.; Shamsi, S.; Ullah Khan, R. Impact of Grape (Vitis vinifera) Seed extract on egg production traits, nutrients digestability, lipid peroxidation and fertility of golden laying hens (Gallus gallus) during early stage of production. Vet. Q. 2023, 43, 1–7. [Google Scholar] [CrossRef]
- Madkour, M.; Abdel-Fattah, S.A.; Ali, S.I.; Ali, N.G.M.; Shourrap, M.; Hosny, M.; Elolimy, A.A. Impact of in ovo feeding of grape pomace extract on the growth performance, antioxidant status, and immune response of hatched broilers. Poult. Sci. 2024, 103, 103914. [Google Scholar] [CrossRef]
- Derbali, H.; Ben Saïd, S.; Abid, K.; Aroua, M.; Jabri, J.; Dhaouafi, J.; Tissaoui, M.; Malek, A.; Bouzid, K.; Mahouachi, M. Valorization of dehydrated grape pomace waste as a low-cost feed additive to improve reproduction and growth performance of male rabbits. Waste Biomass Valor 2024, 15, 3987–3996. [Google Scholar] [CrossRef]
- Li, Y.; Shi, C.; Deng, J.; Qiu, X.; Zhang, S.; Wang, H.; Qin, X.; He, Y.; Cao, B.; Su, H. Effects of grape pomace on growth performance, nitrogen metabolism, antioxidants, and microbial diversity in Angus Bulls. Antioxidants 2024, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Antunović, Z.; Šalavardić, Ž.K.; Steiner, Z.; Đidara, M.; Drenjančević, M.; Ronta, M.; Pavić, V.; Barron, L.J.; Novoselec, J. Meat quality, metabolic profile and antioxidant status of lambs fed on seedless grape pomace (Vitis vinifera L.). Ann. Anim. Sci. 2023, 23, 809–818. [Google Scholar] [CrossRef]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, D.; Zhao, X.; Xiao, Z.; Sun, J.; Yuan, T.; Wang, Y.; Zuo, X.; Yang, G.; Yu, T. Dietary grape pomace extract supplementation improved meat quality, antioxidant capacity, and immune performance in finishing pigs. Front. Microbiol. 2023, 14, 1116022. [Google Scholar] [CrossRef]
- Avilés Peterson, K.A.; Montalvo Corral, M.; González Ríos, H.; Parra Sánchez, H.; Barrera Silva, M.A.; Pinelli Saavedra, A. Grape pomace on the growth performance and intestinal microbiota of finishing pigs. Biotecnia 2024, 26, 274–282. [Google Scholar] [CrossRef]
- da Silveira Almeida, B.C.; Ludke, M.d.C.M.M.; Bertol, T.M.; Ludke, J.V.; Bernardi, D.M.; Cunha, A., Jr.; Coldebella, A. Growth performance, meat quality, and lipid oxidation in pigs’ fed diets containing grape pomace. Appl. Biosci. 2024, 3, 378–391. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine: Eleventh, Reviseded. Wash. DC: Natl. Acad. Press 2012, 10, 13298. [Google Scholar]
- ISO 6496; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6498; Animal Feeding Stuffs—Preparation of Test Samples. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6492; Animal Feeding Stuffs—Determination of Fat Content. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6865; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. International Organization for Standardization: Geneva, Switzerland, 2002.
- ISO 5983; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 1: Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 2171; Cereals, Pulses and By-Products—Determination of Ash Yield by Incineration. International Organization for Standardization: Geneva, Switzerland, 2010.
- Luca, S.V.; Kulinowski, Ł.; Ciobanu, C.; Zengin, G.; Czerwińska, M.E.; Granica, S.; Xiao, J.; Skalicka-Woźniak, K.; Trifan, A. Phytochemical and multi-biological characterization of two Cynara scolymus L. varieties: A glance into their potential large scale cultivation and valorization as bio-functional ingredients. Ind. Crops Prod. 2022, 178, 114623. [Google Scholar] [CrossRef]
- Sehm, J.; Lindermayer, H.; Meyer, H.H.D.; Pfaffl, M.W. The influence of apple- and red-wine pomace rich diet on MRNA expression of inflammatory and apoptotic markers in different piglet organs. Anim. Sci. 2006, 82, 877–887. [Google Scholar] [CrossRef]
- Zimmermann, A.; Camenisch, U.; Rechsteiner, M.P.; Bode-Lesniewska, B.; Rössle, M. Value of immunohistochemistry in the detection of BRAF V600E mutations in fine-needle aspiration biopsies of papillary thyroid carcinoma. Cancer Cytopathol. 2014, 122, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.R.; Davoodi, H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011, 35, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal absorption and antioxidant activity of grape pomace polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef]
- Yan, L.; Kim, I. Effect of dietary grape pomace fermented by saccharomyces boulardii on the growth performance, nutrient digestibility and meat quality in finishing pigs. Asian-Australas. J. Anim. Sci. 2011, 24, 1763–1770. [Google Scholar]
- Trombetta, F.; Fruet, A.; Stefanello, F.; Fonseca, P.; De Souza, A.; Tonetto, C.; Rosado Júnior, A.; Nörnberg, J. Effects of the dietary inclusion of linseed oil and grape pomace on weight gain, carcass characteristics, and meat quality of swine. Int. Food Res. J. 2019, 26, 1741. [Google Scholar]
- Vlaicu, P.A.; Panaite, T.D.; Cornescu, M.G.; Ropota, M.; Olteanu, M.; Drăgotoiu, D. The influence of by-products on the production parameters and nutrient digestibility in fattening pigs diet (60–100 Kg). AgroLife Sci. J. 2019, 8, 261–269. [Google Scholar]
- Rajković, E.; Schwarz, C.; Tischler, D.; Schedle, K.; Reisinger, N.; Emsenhuber, C.; Ocelova, V.; Roth, N.; Frieten, D.; Dusel, G.; et al. Potential of grape extract in comparison with therapeutic dosage of antibiotics in weaning piglets: Effects on performance, digestibility and microbial metabolites of the ileum and colon. Animals 2021, 11, 2771. [Google Scholar] [CrossRef]
- Aguiar, S.C.; Cottica, S.M.; Boeing, J.S.; Samensari, R.B.; Santos, G.T.; Visentainer, J.V.; Zeoula, L.M. Effect of feeding phenolic compounds from propolis extracts to dairy cows on milk production, milk fatty acid composition, and the antioxidant capacity of milk. J Anim. Feed Sci Technol 2014, 193, 148–154. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.L.; Chentouf, M.; Cabaraux, J.F. Characterisation of bitter vetch (Vicia ervilia (L.) Willd) ecotypes: An ancient and promising legume. Exp Agric 2024, 60, 19. [Google Scholar]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.L.; Cabaraux, J.F.; Chentouf, M. Growth performance, carcass characteristics, fatty acid profile, and meat quality of male goat kids supplemented by alternative feed resources: Bitter vetch and sorghum grains. Arch. Anim. Breed. 2024, 67, 481–492. [Google Scholar]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-ΚB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [PubMed]
- Wang, R.; Yu, H.; Fang, H.; Jin, Y.; Zhao, Y.; Shen, J.; Zhou, C.; Li, R.; Wang, J.; Fu, Y. Effects of dietary grape pomace on the intestinal microbiota and growth performance of weaned piglets. Arch. Anim. Nutr. 2020, 74, 296–308. [Google Scholar]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016, 7, 80313. [Google Scholar]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef]
- Gessner, D.; Ringseis, R.; Siebers, M.; Keller, J.; Kloster, J.; Wen, G.; Eder, K. Inhibition of the pro-inflammatory NF-κB pathway by a grape seed and grape marc meal extract in intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1074–1083. [Google Scholar]
- Fournier, B.; Parkos, C. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef]
- Varricchio, E.; Coccia, E.; Orso, G.; Lombardi, V.; Imperatore, R.; Vito, P.; Paolucci, M. Influence of polyphenols from olive mill wastewater on the gastrointestinal tract, alveolar macrophages and blood leukocytes of pigs. Ital. J. Anim. Sci. 2019, 18, 574–586. [Google Scholar]
- Jung, H.C.; Eckmann, L.; Yang, S.; Panja, A.; Fierer, J.; Morzycka-Wroblewska, E.; Kagnoff, M. A Distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 1995, 95, 55–65. [Google Scholar]
- Lallès, J.-P.; Boudry, G.; Favier, C.; Le Floc’h, N.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef]
- Zhu, Y.; Österlundh, I.; Hultén, F.; Magnusson, U. Tumor Necrosis Factor-α, Interleukin-6, serum amyloid A, haptoglobin, and cortisol concentrations in sows following intramammary inoculation of Escherichia coli. Am. J. Vet. Res. 2004, 65, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Davis, B.; Skjolaas, K.; Burkey, T.; Dritz, S.; Johnson, B.; Minton, J. Effects of feeding Salmonella Enterica Serovar Typhimurium or Serovar Choleraesuis on growth performance and circulating Insulin-like Growth Factor-I, Tumor Necrosis Factor-α, and Interleukin-1β in weaned pigs. J. Anim. Sci. 2007, 85, 1161–1167. [Google Scholar] [CrossRef]
- Philpott, M.; Ferguson, L.R. Immunonutrition and cancer. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2004, 551, 29–42. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear Factor-ΚB—A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef]
- Chang, H.Y.; Yang, X. Proteases for cell suicide: Functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 2000, 64, 821–846. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-ΚB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Hayden, M.; West, A.; Ghosh, S. NF-ΚB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar]
- Cho, S.-J.; Jung, U.J.; Park, H.-J.; Kim, H.-J.; Park, Y.B.; Kim, S.R.; Choi, M.-S. Combined ethanol extract of grape pomace and omija fruit ameliorates adipogenesis, hepatic steatosis, and inflammation in diet-induced obese mice. Evid.-Based Complement. Altern. Med. 2013, 2013, 212139. [Google Scholar]
- Chuang, C.-C.; Bumrungpert, A.; Kennedy, A.; Overman, A.; West, T.; Dawson, B.; McIntosh, M.K. Grape powder extract attenuates Tumor Necrosis Factor α-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J. Nutr. Biochem. 2011, 22, 89–94. [Google Scholar]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Giguère, A.; Lessard, M. Dietary supplementation with different forms of flax in late gestation and lactation: Effects on sow and litter performances, endocrinology, and immune response. J. Anim. Sci. 2010, 88, 225–237. [Google Scholar]
- Hur, S.J.; Kang, S.H.; Jung, H.S.; Kim, S.C.; Jeon, H.S.; Kim, I.H.; Lee, J.D. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr. Res. 2012, 32, 801–816. [Google Scholar]
- Zhan, Z.; Huang, F.; Luo, J.; Dai, J.; Yan, X.; Peng, J. Duration of feeding linseed diet influences expression of inflammation-related genes and growth performance of growing-finishing barrows. J. Anim. Sci. 2009, 87, 603–611. [Google Scholar] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar]
- Shortman, K.; Heath, W.R. Immunity or tolerance? that is the question for dendritic cells. Nat. Immunol. 2001, 2, 988–989. [Google Scholar]
- Akiyama, H.; Sato, Y.; Watanabe, T.; Nagaoka, M.H.; Yoshioka, Y.; Shoji, T.; Kanda, T.; Yamada, K.; Totsuka, M.; Teshima, R. Dietary unripe apple polyphenol inhibits the development of food allergies in murine models. FEBS Lett. 2005, 579, 4485–4491. [Google Scholar]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [PubMed]
- Huang, R.-Y.; Yu, Y.-L.; Cheng, W.-C.; OuYang, C.-N.; Fu, E.; Chu, C.-L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [PubMed]
- Lee, J.S.; Kim, S.G.; Kim, H.K.; Lee, T.; Jeong, Y.; Lee, C.; Yoon, M.; Na, Y.J.; Suh, D.; Park, N.C. Silibinin Polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells. J. Cell. Physiol. 2007, 210, 385–397. [Google Scholar] [PubMed]
- Yoon, M.-S.; Lee, J.S.; Choi, B.-M.; Jeong, Y.-I.; Lee, C.-M.; Park, J.-H.; Moon, Y.; Sung, S.-C.; Lee, S.K.; Chang, Y.H. Apigenin inhibits immunostimulatory function of dendritic cells: Implication of immunotherapeutic adjuvant. Mol. Pharmacol. 2006, 70, 1033–1044. [Google Scholar]
- Hou, X.; Zhang, J.; Ahmad, H.; Zhang, H.; Xu, Z.; Wang, T. Evaluation of antioxidant activities of ampelopsin and its protective effect in lipopolysaccharide-induced oxidative stress piglets. PLoS ONE 2014, 9, e108314. [Google Scholar]
- Wang, M.; Suo, X.; Gu, J.; Zhang, W.; Fang, Q.; Wang, X. Influence of grape seed proanthocyanidin extract in broiler chickens: Effect on chicken coccidiosis and antioxidant Status. Poult. Sci. 2008, 87, 2273–2280. [Google Scholar]
- Cheng, Y.-T.; Wu, C.-H.; Ho, C.-Y.; Yen, G.-C. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J. Nutr. Biochem. 2013, 24, 475–483. [Google Scholar]
- Hao, R.; Li, Q.; Zhao, J.; Li, H.; Wang, W.; Gao, J. Effects of Grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest. Sci. 2015, 178, 237–242. [Google Scholar]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-ΚB are coordinated effectors of the rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar]
- Scapagnini, G.; Sonya, V.; Nader, A.G.; Calogero, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [PubMed]
- Wei, C.; Chen, X.; Chen, D.; Yu, B.; Zheng, P.; He, J.; Chen, H.; Yan, H.; Luo, Y.; Huang, Z. Dihydromyricetin enhances intestinal antioxidant capacity of growing-finishing pigs by activating ERK/Nrf2/HO-1 signaling pathway. Antioxidants 2022, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar]
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000, 33, 819–830. [Google Scholar]
- Bobek, P.; Ozdín, L.; Hromadova, M. The effect of dried tomato, grape and apple pomace on the cholesterol metabolism and antioxidative enzymatic system in rats with hypercholesterolemia. Food/Nahrung 1998, 42, 317–320. [Google Scholar] [PubMed]
- Inoue, H.; Maeda-Yamamoto, M.; Nesumi, A.; Tanaka, T.; Murakami, A. Low and medium but not high doses of green tea polyphenols ameliorated dextran sodium sulfate-induced hepatotoxicity and nephrotoxicity. Biosci. Biotechnol. Biochem. 2013, 77, 1223–1228. [Google Scholar]
- Zhang, H.J.; Jiang, X.R.; Mantovani, G.; Lumbreras, A.E.V.; Comi, M.; Alborali, G.; Savoini, G.; Dell’Orto, V.; Bontempo, V. Modulation of Plasma antioxidant activity in weaned piglets by plant polyphenols. Ital. J. Anim. Sci. 2014, 13, 3242. [Google Scholar]
- Prata, C.; Zalambani, C.; Rossi, F.; Rossello, S.; Cerchiara, T. Nutrients and Nutraceuticals from Vitis vinifera L. Pomace: Biological Activities, Valorization, and Potential Applications. Nutrients 2025, 17, 583. [Google Scholar] [CrossRef]
- González-Barrio, R.; Gasch-Tolrá, J.; Tomás-Barberán, F.A.; Selma, M.V. Grape Pomace as a Cardiometabolic Health-Promoting Ingredient: Activity in the Intestinal Environment. Antioxidants 2023, 12, 979. [Google Scholar] [CrossRef]
- Guaita, M.; Motta, S.; Messina, S.; Casini, F.; Bosso, A. Polyphenolic Profile and Antioxidant Activity of Green Extracts from Grape Pomace Skins and Seeds of Italian Cultivars. Foods 2023, 12, 3880. [Google Scholar] [CrossRef]
- Blasi, F.; Trovarelli, V.; Mangiapelo, L.; Ianni, F. Grape Pomace for Feed Enrichment to Improve the Quality of Animal-Based Foods. Foods 2024, 13, 3541. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Bulgaru, V.C.; Taranu, I. Grape By-Products and Their Efficiency in Alleviating the Intestinal Disorders in Post-Weaning Piglets. Arch. Zootech. 2023, 26, 56–77. [Google Scholar]
- Schedle, K.; Pfaffl, M.W.; Plitzner, C. Effect of Insoluble Fiber on Intestinal Morphology and mRNA Expression Pattern of Inflammatory, Cell Cycle and Growth Marker Genes in a Piglet Model. Arch. Anim. Nutr. 2008, 62, 427–438. [Google Scholar] [PubMed]
- Modina, S.C.; Polito, U.; Rossi, R.; Corino, C. Nutritional Regulation of Gut Barrier Integrity in Weaning Piglets. Animals 2019, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M. Grape By-Products as Feedstuff for Pig and Poultry Production. Animals 2022, 12, 2239. [Google Scholar] [CrossRef]
- Chiou, P.W.S.; Bi, Y.; Chang, L. Effect of Different Components of Dietary Fiber on the Intestinal Morphology of Domestic Rabbits. Comp. Biochem. Physiol. Part A Physiol. 1994, 109, 637–647. [Google Scholar]
- Patra, A.K.; Amasheh, S. Modulation of Gastrointestinal Barrier and Nutrient Transport Function in Farm Animals by Natural Plant Bioactive Compounds—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3237–3268. [Google Scholar] [CrossRef]
- Saliu, E.M.; Martínez-Vallespín, B. Dietary Fiber and Its Role in Performance, Welfare, and Health of Pigs. Anim. Health Res. Rev. 2022, 23, 165–193. [Google Scholar]
- Asadnezhad, B.; Pirmohammadi, R.; Alijoo, Y. The Effects of Dietary Supplementation with Red Grape Pomace Treated with Ozone Gas on Ruminal Fermentation Activities, Nutrient Digestibility, and Lactational Performance. J. Agric. Food Res. 2024, 18, 100489. [Google Scholar]

| Ingredient (%) | Basal diet | E1 | E2 | E3 | E4 |
|---|---|---|---|---|---|
| Corn | 46.84 ± 2.29 a | 46.00 ± 2.96 ab | 44.98 ± 2.33 ab | 41.92 ± 3.22 bc | 39.98 ± 2.31 c |
| Wheat | 19.56 ± 2.58 a | 19.44 ± 2.02 a | 16.50 ± 1.91 ab | 15.00 ± 2.07 bc | 12.00 ± 2.08 c |
| Barley | 15.00 ± 1.79 | 15.00 ± 1.80 | 15.00 ± 1.84 | 15.00 ± 1.85 | 15.00 ± 1.26 |
| Soybean meal | 8.40 ± 1.91 | 8.90 ± 1.95 | 9.40 ± 2.90 | 10.04 ± 2.36 | 10.00 ± 3.46 |
| Sunflower meal | 6.00 ± 2.01 | 5.50 ± 2.01 | 5.00 ± 1.98 | 4.00 ± 2.00 | 4.00 ± 2.29 |
| Limestone | 2.00 ± 0.91 | 1.90 ± 0.65 | 1.80 ± 0.73 | 1.70 ± 0.84 | 1.60 ± 0.97 |
| Lysine | 0.30 ± 0.11 | 0.29 ± 0.11 | 0.28 ± 0.11 | 0.27 ± 0.14 | 0.26 ± 0.12 |
| Methionine | 0.02 ± 0.01 b | 0.04 ± 0.01 a | 0.06 ± 0.01 a | 0.06 ± 0.01 a | |
| Salt | 0.40 ± 0.01 | 0.40 ± 0.01 | 0.40 ± 0.01 | 0.40 ± 0.01 | 0.40 ± 0.01 |
| Choline premix | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 |
| Monocalcium phosphate | 0.40 ± 0.18 | 0.45 ± 0.21 | 0.50 ± 0.23 | 0.55 ± 0.21 | 0.60 ± 0.18 |
| Vitamin Mineral Premix * | 1.00 ± 0.15 | 1.00 ± 0.15 | 1.00 ± 0.15 | 1.00 ± 0.15 | 1.00 ± 0.15 |
| Grape pomace | 0.10 ± 0.01 d | 0.50 ± 0.01 c | 1.00 ± 0.01 b | 1.50 ± 0.01 a | |
| Nutrient content | |||||
| Crude protein (%) | 15.30 ± 2.03 | 15.18 ± 1.87 | 15.06 ± 2.55 | 14.98 ± 2.19 | 14.86 ± 2.18 |
| Metabolizable energy (Kcal/kg) | 2.90 ± 0.02 | 2.8984 ± 2.02 | 2.8968 ± 0.02 | 2.8952 ± 0.02 | 2.8936 ± 0.02 |
| Fat (%) | 3.90 ± 3.32 | 4.07 ± 3.34 | 4.24 ± 3.31 | 4.41 ± 3.35 | 4.58 ± 3.33 |
| Crude fiber (%) | 3.90 ± 3.50 | 4.03 ± 3.20 | 4.17 ± 3.30 | 4.30 ± 3.88 | 4.44 ± 3.94 |
| Lysine (%) | 0.83 ± 0.78 | 0.83 ± 0.75 | 0.83 ± 0.76 | 0.83 ± 0.76 | 0.83 ± 0.77 |
| Digestible Lysine (%) | 0.740 ± 0.70 | 0.745 ± 0.72 | 0.750 ± 0.71 | 0.755 ± 0.73 | 0.76 ± 0.72 |
| Met+ Cys (%) | 0.53 ± 0.38 | 0.53 ± 0.37 | 0.53 ± 0.38 | 0.53 ± 0.37 | 0.53 ± 0.53 |
| Calcium (%) | 0.63 ± 0.19 | 0.60 ± 0.28 | 0.57 ± 0.25 | 0.54 ± 0.26 | 0.51 ± 0.23 |
| Phosphorus (%) | 0.24 ± 0.20 | 0.23 ± 0.21 | 0.22 ± 0.21 | 0.21 ± 0.19 | 0.20 ± 0.15 |
| Item | Basal Diet | E1 | E2 | E3 | E4 |
|---|---|---|---|---|---|
| TPC (mg GAE/g) | 38.38 ± 0.03 e | 43.79 ± 0.04 d | 65.45 ± 0.03 c | 92.53 ± 0.02 b | 119.6 ± 0.03 a |
| DPPH (µM TRE/g) | 20.61 ± 0.03 e | 32.71 ± 0.03 d | 81.11 ± 0.03 c | 141.61 ± 0.03 b | 202.11 ± 0.03 a |
| Name | Cat. No. | Dilution | Host | Manufacturer |
|---|---|---|---|---|
| Primary antibodies | ||||
| IL-1β | 16806-1-AP | 1:100 | Rabbit | Proteintech, York, UK |
| TNF-α | AF690 | 1:200 | Goat | Thermo Fisher, Waltham, MA, USA |
| MHC-II | MA1-19143 | 1:200 | Mouse | Thermo Fisher, Waltham, MA, USA |
| NF-kB p65 | PA5-16545 | 1:100 | Rabbit | Thermo Fisher, Waltham, MA, USA |
| Nrf2 | ABIN676673 | 1:200 | Rabbit | Limerick, MA, USA |
| Secondary antibodies | ||||
| Goat Anti-Rabbit | ab205718 | 1:200 | Abcam, Cambridge, UK | |
| Control | E1 | E2 | E3 | E4 | |
|---|---|---|---|---|---|
| Initial weight (kg) | 85.09 ± 2.31 | 85.06 ± 1.89 | 85.08 ± 1.90 | 85.05 ± 2.26 | 85.07 ± 2.18 |
| Final weight (kg) | 148.34 ± 1.99 b | 148.39 ± 2.08 b | 148.95 ± 1.88 b | 150.97 ± 1.82 a | 148.80 ± 1,67 b |
| ADFI (g) | 268.79 ± 1.92 d | 274.33 ± 1.10 c | 275.67 ± 0.97 b | 282 ± 3.26 a | 275.56 ± 1.57 bc |
| ADG (g) | 702.82 ± 22.32 b | 703.69 ± 23.01 b | 709.72 ± 21.49 b | 732.54 ± 20.04 a | 708.15 ± 19.03 b |
| FCR (g/kg) | 0.383 ± 0.01 | 0.390 ± 0.01 | 0.389 ± 0.01 | 0.385 ± 0.01 | 0.389 ± 0.01 |
| Control | E1 | E2 | E3 | E4 | |
|---|---|---|---|---|---|
| Duodenum | |||||
| Villus height (μm) | 202.23 ± 23.13 d | 225.48 ± 22.19 c | 293.61 ± 16.29 b | 356.60 ± 21.25 a | 299.31 ± 16.09 b |
| Crypt depth (μm) | 425.44 ± 21.80 b | 426.95 ± 22.00 b | 435.15 ± 20.64 ab | 457.36 ± 29.83 a | 438.68 ± 21.09 ab |
| V/C | 0.474 ± 0.04 d | 0.529 ± 0.05 c | 0.675 ± 0.04 bc | 0.783 ± 0.08 a | 0.683 ± 0.03 b |
| Jejunum | |||||
| Villus height (μm) | 146.47 ± 25.12 d | 149.77 ± 25.58 d | 233.48 ± 21.91 c | 325.38 ± 23.42 a | 270.00 ± 11.56 b |
| Crypt depth (μm) | 380.04 ± 24.11 c | 382.82 ± 25.80 c | 400.94 ± 21.84 bc | 445.40 ± 26.97 a | 430.89 ± 19.79 a |
| V/C | 0.384 ± 0.05 c | 0.392± 0.065 c | 0.584 ±0.064 b | 0.731 ± 0.046 a | 0.627 ± 0.033 b |
| Ileum | |||||
| Villus height (μm) | 125.22 ± 19.18 b | 128.04 ± 19.95 b | 140.28 ± 18.95 ab | 156.45 ± 19.22 a | 150.04 ± 14.45 a |
| Crypt depth (μm) | 393.44 ± 26.68 | 394.83 ± 26.03 | 411.25 ± 34.27 | 420.62 ± 29.14 | 412.11 ± 34.30 |
| V/C | 0.318 ± 0.04 b | 0.324 ± 0.04 bc | 0.341 ± 0.05 abc | 0.371 ± 0.03 a | 0.364 ± 0.03 a |
| Size of jejunal Peyer’s patches | |||||
| Length (μm) | 300 ± 32.10 e | 407.24 ± 18.67 d | 655.09 ± 19.83 b | 793.71 ± 29.28 a | 620.95 ± 21.68 c |
| Width (μm) | 245 ± 29.78 d | 250.37 ± 16.78 d | 383.36 ± 19.57 b | 461.25 ± 27.26 a | 360.63 ± 17.46 c |
| Size of ileal Peyer’s patches | |||||
| Length (μm) | 213.07 ± 20.38 e | 323.70 ± 44.13 d | 655.09 ± 19.83 b | 954.31 ± 32.17 a | 520.88 ± 20.53 c |
| Width (μm) | 156 ±19.81 d | 245 ± 29.78 d | 360.63 ± 17.46 b | 487.36 ± 28.56 a | 355.63 ± 14.22 c |
| Caecum | |||||
| Mucosa (μm) | 362.20 ± 3.52 b | 364.10 ± 2.23 b | 364.70 ± 1.76 ab | 367.10 ± 1.91 a | 364.20 ± 1.68 b |
| Submucosa (μm) | 159.60 ± 2.22 b | 160.30 ± 3.09 b | 161.90 ± 3.24 ab | 165.30 ± 3.74 a | 162.50 ± 2.22 ab |
| Tunica muscularis (μm) | 363.90 ± 7.78 | 364.10 ± 14.35 | 368.50 ± 11.32 | 370.40 ± 18.06 | 365.70 ± 10.20 |
| Control | E1 | E2 | E3 | E4 | |
|---|---|---|---|---|---|
| IL-1β | |||||
| Duodenum | +++ | +++ | +++ | ++ | ++ |
| Jejunum | +++ | +++ | +++ | ++ | +++ |
| Ileum | +++ | ++ | ++ | + | + |
| Caecum | +++ | +++ | ++ | + | ++ |
| TNF-α | |||||
| Duodenum | +++ | ++ | ++ | + | + |
| Jejunum | +++ | ++ | ++ | + | ++ |
| Ileum | +++ | ++ | ++ | + | ++ |
| Caecum | +++ | ++ | ++ | + | ++ |
| MHC-II | |||||
| Duodenum | +++ | ++ | ++ | + | + |
| Jejunum | +++ | +++ | ++ | + | ++ |
| Ileum | +++ | +++ | ++ | ++ | ++ |
| Caecum | +++ | +++ | ++ | + | ++ |
| Nrf2 | |||||
| Duodenum | + | + | ++ | +++ | ++ |
| Jejunum | + | + | ++ | +++ | ++ |
| Ileum | ++ | ++ | ++ | +++ | ++ |
| Caecum | + | + | ++ | +++ | ++ |
| NF-kB p65 | |||||
| Duodenum | +++ | ++ | ++ | + | + |
| Jejunum | +++ | +++ | ++ | + | ++ |
| Ileum | +++ | ++ | ++ | ++ | ++ |
| Caecum | +++ | +++ | ++ | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horodincu, L.; Proca, A.C.; Șlencu, B.G.; Trifan, A.; Pavel, G.; Solcan, G.; Solcan, C. Modulating Effects of Grape Pomace on the Intestinal Antioxidative and Inflammatory Status in Fattening Pigs. Agriculture 2025, 15, 740. https://doi.org/10.3390/agriculture15070740
Horodincu L, Proca AC, Șlencu BG, Trifan A, Pavel G, Solcan G, Solcan C. Modulating Effects of Grape Pomace on the Intestinal Antioxidative and Inflammatory Status in Fattening Pigs. Agriculture. 2025; 15(7):740. https://doi.org/10.3390/agriculture15070740
Chicago/Turabian StyleHorodincu, Loredana, Andrei Claudiu Proca, Bogdan Gabriel Șlencu, Adriana Trifan, Geta Pavel, Gheorghe Solcan, and Carmen Solcan. 2025. "Modulating Effects of Grape Pomace on the Intestinal Antioxidative and Inflammatory Status in Fattening Pigs" Agriculture 15, no. 7: 740. https://doi.org/10.3390/agriculture15070740
APA StyleHorodincu, L., Proca, A. C., Șlencu, B. G., Trifan, A., Pavel, G., Solcan, G., & Solcan, C. (2025). Modulating Effects of Grape Pomace on the Intestinal Antioxidative and Inflammatory Status in Fattening Pigs. Agriculture, 15(7), 740. https://doi.org/10.3390/agriculture15070740








