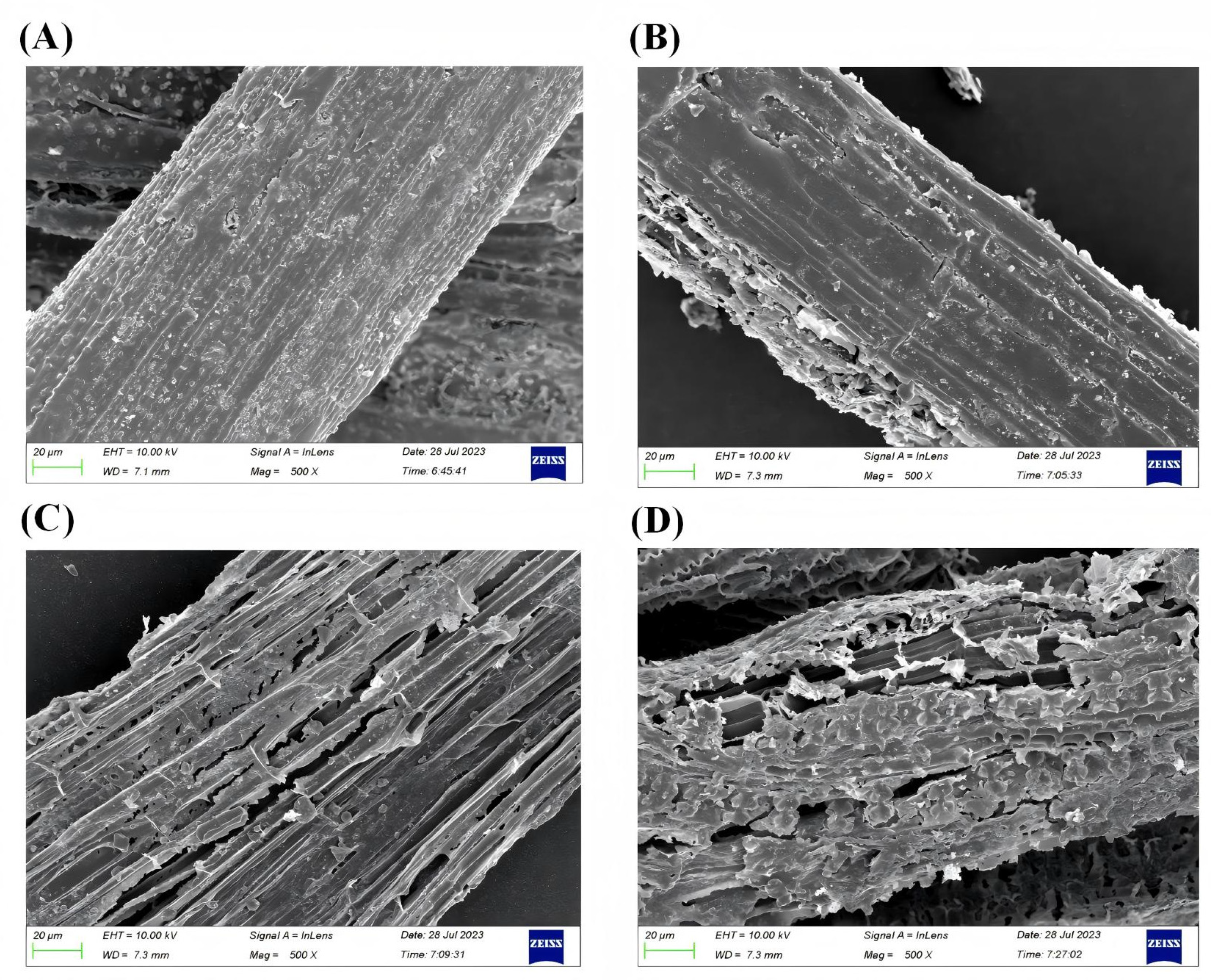
4.1. Effects of Rice Straw Variety on Gas Production and Rumen Fermentation Characteristics
The nutritional value of rice straw significantly influences its in vitro gas production and digestibility. Traditionally regarded as a low-quality feed because of its high fiber and low protein content, the nutritional value of rice straw can vary considerably depending on the rice variety and cultivation practices. In this study, the two tested rice varieties were cultivated under identical conditions, thus isolating the variety as the sole influencing factor. A previous study found that varieties with lower NDF and ADF content typically exhibited higher in vitro gas production and digestibility [
21]. This is because these fibers primarily restrict the accessibility of cellulolytic microbes, thereby affecting the rate and extent of fermentation. Additionally, the crude protein content of rice straw positively correlates with gas production, as it provides essential nitrogen for microbial growth and activity, which in turn enhances the breakdown of fibrous components [
10]. Furthermore, the presence of silica and lignin can hinder the digestion process, resulting in lower gas production and digestibility rates [
22]. In this study, the R1053 group showed higher in vitro gas production and digestibility. This could be because of its numerically higher content of organic matter, crude protein, and WSC, as well as its lower levels of NDF and ADF.
VFA, including acetate, propionate, and butyrate, are indispensable to ruminant animals, supplying over 70% of their energy needs [
23]. The production of VFA within the rumen is influenced by the feed’s composition, the microbial community, and ambient conditions [
23]. An elevated total VFA production, as well as increased concentrations of individual VFA, was observed in the R1053 group. This can be attributed to the numerically higher levels of WSC and crude protein in R1053, which are more susceptible to fermentation. Theoretical insights suggest that these components are crucial for enhancing the fermentation process [
23]. As a result, the numerically higher contents of these fermentable substrates in the R1053 group likely contributes to its superior VFA production. Fermenting structural carbohydrates generally results in higher acetate proportion than fermenting starch [
23]. This difference in fermentation outcomes may account for the elevated proportion of acetate observed in the Z17 group, which could be because of its numerically higher contents of NDF and ADF.
4.2. Effects of Rice Straw Variety on Rumen Bacterial Diversity and Community Composition
Rumen microbial alpha diversity is vital for the health and productivity of ruminants. Jia et al. [
24] reported that a high alpha diversity signified a complex microbial community, which boosted the host’s resilience to environmental stress and enhanced nutrient utilization efficiency. This study found that the R1053 group exhibited greater richness and evenness than the Z17 group, suggesting that it is more conducive to the stability and resilience of the rumen microbial community. This increased diversity, in turn, promotes nutrient digestion, as indicated by higher gas production and in vitro dry matter digestibility. Verrucomicrobiota and Chloroflexi possess the ability to break down complex carbohydrates, including cellulose, hemicellulose, and polysaccharides, thereby generating short-chain fatty acids that serve as a crucial energy source for ruminants [
25]. In the context of this study, it was observed that the relative abundances of these two phyla were higher in the Z17 group than in the R1053 group. This difference is likely attributed to the higher fiber content in the Z17 group. Similarly, the genera
Saccharofermentans,
Probable genus 10, and
Lachnospiraceae AC2044 group also play significant roles in the process of fiber degradation [
26]. Therefore, it is not surprising to observe their higher relative abundances in the Z17 group. Patescibacteria are a group of uncultured bacteria that have lost the genes necessary for key metabolic pathways, including the synthesis of amino acids, nucleotides, fatty acids, and cofactors. Despite these losses, they have evolved pilus-like structures that enable them to adhere to other microorganisms. These structures are hypothesized to function as tunnels, facilitating the exchange of metabolites [
27]. The higher relative abundance of Patescibacteria in the R1053 group suggests a potentially greater metabolic activity and an enhanced capacity for microbial cooperation to maintain the stability and functional diversity of the rumen community, which could be indirectly inferred from the higher alpha diversity in this group.
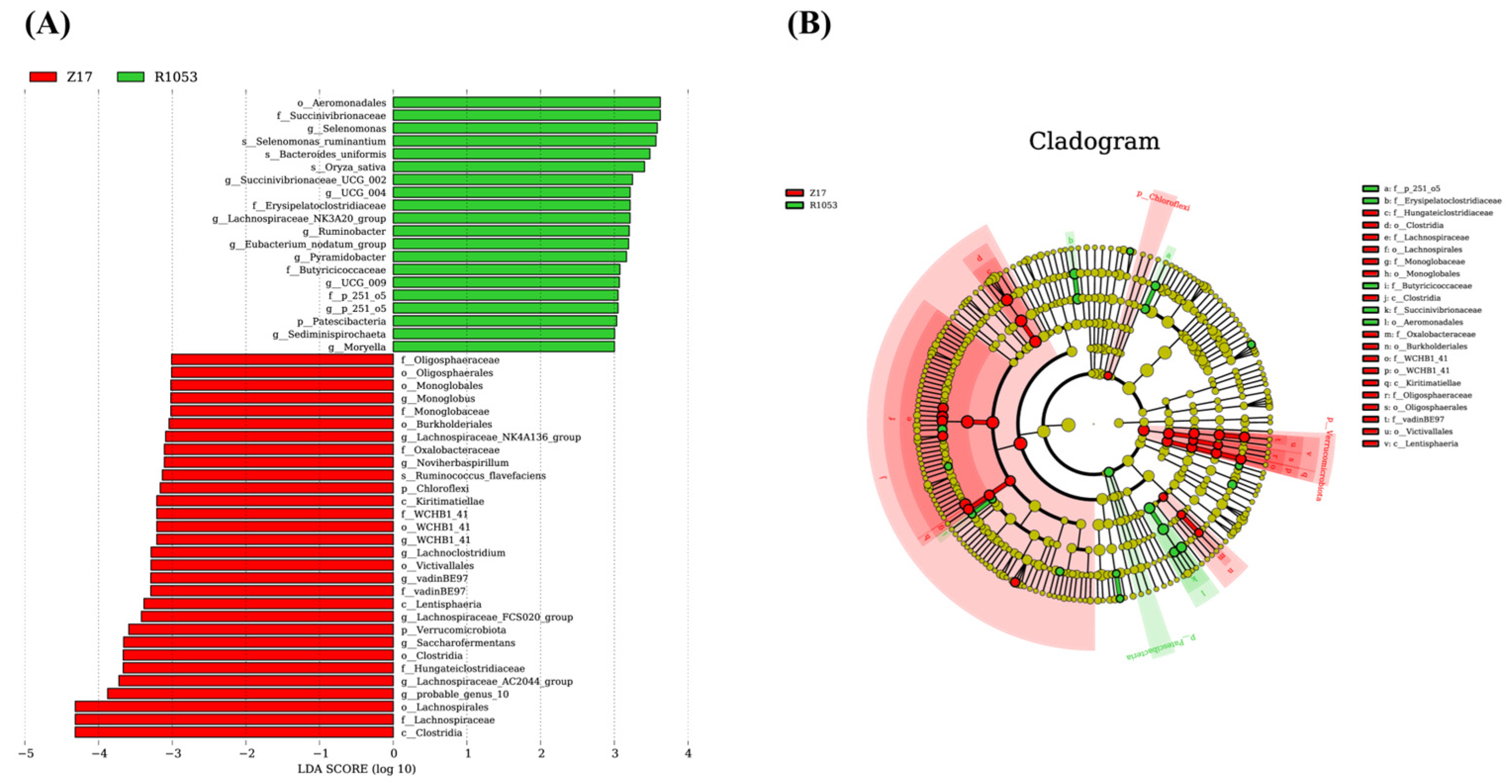
LEfSe analysis provides a more comprehensive perspective on the differential microbes at different taxonomic levels between the Z17 and R1053 groups.
Ruminococcus flavefaciens is a significant cellulose-degrading bacterium identified within the rumen of ruminants, possessing the capability to break down cellulose via a sophisticated enzyme system [
28]. This bacterium also collaborates with other rumen microorganisms, such as Clostridia and Hungateiclostridiaceae, in the process of cellulose degradation. Together, these microorganisms employ distinct enzyme systems and metabolic pathways to effectively degrade and ferment cellulose. This study revealed that the relative abundances of these microorganisms were notably higher in the Z17 group, thereby further substantiating their role in cellulose degradation. Members of the order Monoglobales are equipped with pectin-specific glycoside hydrolase domains and cell wall-anchored S-layer homology motifs. These features empower them to efficiently degrade a wide range of pectins, rhamnogalacturonan-I, and galactans, thereby producing polysaccharide degradation products [
29]. Members of the Oligosphaeraceae and Burkholderiales, as well as its affiliated Oxalobacteraceae and
Noviherbaspirillum, are primarily involved in the fermentation of glucose, fructose, and sucrose, a process that results in the production of acetate, which serves as a crucial energy source for ruminant animals [
30]. Kiritimatiellae, WCHB1_41, and VadinBE97 exhibit strict anaerobic and fermentative metabolic pathways, utilizing carbohydrates as their primary substrate. These bacteria derive energy through fermentation in oxygen-deprived environments, thereby playing a crucial role in intricate energy networks. For instance, during cold seasons when feed is scarce, they can modulate their nutritional requirements to acclimate to harsh conditions [
31]. Lentisphaera and Victivallis are capable of utilizing a diverse array of carbohydrates as carbon sources, generating acetate via fermentation. In the present study, the elevated relative abundances of the aforementioned microorganisms in the Z17 group are mirrored by a correspondingly higher proportion of acetate within the same group.
Aeromonadales, including Succinivibrionaceae and
Succinivibrionaceae UCG 002, exhibit a diverse array of enzymatic activities, including those of proteases, amylases, and lipases [
32]. Their primary metabolic products consist of short-chain fatty acids, including acetic acid, propionic acid, butyric acid, and succinic acid. Given the elevated levels of crude protein, ether extract, and WSC in the R1053 group, it is expected that these bacteria would have a higher relative abundance in this group.
Selenomonas and
Selenomonas ruminantium are crucial for gluconeogenesis and the production of propionate in ruminants, being particularly abundant in the rumen of high-performing animals [
33]. This study observed higher relative abundances of these bacteria in the R1053 group, which correlates with increased in vitro dry matter digestibility and elevated propionate levels.
Ruminobacter is extensively prevalent in the rumen of cattle and sheep, playing a pivotal role in the breakdown of dietary starch. Research has indicated that
Ruminobacter,
Selenomonas, and
Succinivibrio not only synergistically interact but also exhibit a significant positive correlation with feed efficiency [
30]. During the degradation of
β-glucan,
Bacteroides uniformis generates metabolites like glucose and nicotinamide. These metabolites serve as substrates for other beneficial bacteria, such as
Lactobacillus johnsonii, thereby fostering their growth [
34]. This synergistic interaction contributes to the maintenance of the rumen microbial community’s equilibrium and augments overall digestive efficiency. Erysipelatoclostridiaceae exhibits a positive correlation with the concentration of ammonia nitrogen in the rumen. Meanwhile,
Pyramidobacter aids in the digestion of cellulose by leveraging enzymes indirectly [
35]. These two bacterial groups significantly enhance the efficiency of nitrogen utilization and protein degradation in the rumen, which was verified by the numerically higher abundance of the metabolic pathway of metabolism of other amino acids. Butyricicoccaceae and
Eubacterium nodatum group supply an energy source for rumen epithelial cells through the production of butyrate, while also strengthening the tight junctions of the intestinal epithelium and reducing inflammatory states [
36]. This, in turn, improves hindgut function and boosts the immune capabilities of animals. This study observed that the relative abundance of Butyricicoccaceae was notably higher in the R1053 group, aligning with the elevated levels and proportions of butyrate in this group. Furthermore,
Sediminispirochaeta,
UCG 004, and
Moryella were identified as being positively correlated with feed efficiency [
37]. These findings from rumen microbiota abundance imply that hybrid rice straw may have a higher feed value.
4.3. Effect of Rice Straw Variety on Rumen Metabolites
Estradiol and fipronil-desulfinyl interact with specific receptors on rumen microbial cells, thereby disrupting endocrine and amino acid metabolic pathways. Consequently, this interaction results in cellular macromolecule damage, impairs neural signal transmission, and induces disordered energy metabolism, ultimately leading to a suppression of rumen metabolic activity [
38]. Previous research demonstrated that eicosapentaenoic acid markedly diminishes the levels of acetate and ammonia nitrogen in the rumen, simultaneously reducing the diversity of the rumen microbiota [
39]. Additionally, abietic acid shows a synergistic interaction with eicosapentaenoic acid within the rumen, which is involved in terpenoid metabolism. The metabolites previously mentioned are notably elevated in the Z17 group, suggesting that Z17 is unfavorable for the fermentative metabolic processes of rumen microorganisms. This unfavorable condition is reflected in a decline in the production of VFA and a decrease in microbial diversity.
Aerobactin is an iron chelator that forms iron–aerobactin complexes, thereby facilitating more efficient iron acquisition by bacteria from the rumen environment, as seen from its metabolic pathway of microbial metabolism in diverse environments. This process not only promotes bacterial growth and reproduction but also helps maintain the stability of the microbial community [
40]. Stanozolol, a steroid hormone, significantly enhances protein synthesis and is utilized to improve feed utilization efficiency and growth in ruminants [
41]. Manumycin A, known for its anti-tumor, anti-inflammatory, and immune-regulatory activities, shares similarities with saccharocin and canthiumine in its ability to inhibit the growth of harmful bacteria [
42]. By increasing the populations of cellulolytic and lactic acid bacteria in the rumen, it boosts the degradation efficiency of cellulose and starch, thereby promoting rumen fermentation and increasing the production of VFA. In the R1053 group, these metabolites were found to be significantly upregulated. When considering the rumen fermentation characteristics and microbial diversity within this group, it becomes evident that the straw of hybrid rice can enhance rumen fermentation, implying that feeding this type of straw could lead to a greater energy input in the rumen.
It is crucial to note that the current findings are based solely on rumen fluid collected from dairy cows during their dry period. To enhance the applicability of hybrid rice straw as a feedstock for ruminants, future studies might consider including a broader spectrum of animal species, such as beef cattle, buffaloes, sheep, and goats. Additionally, incorporating rumen fluid samples from a greater number of donors across various physiological stages would be beneficial for the precision feeding of different varieties of rice straw.