Abstract
Polyphenol-rich plant products are widely used as feed additives for their anti-inflammatory, antioxidant, and antimicrobial properties. The aim of this research is to test the hypothesis that feeding grape pomace (GP) to fattening pigs modulates the intestinal immune and antioxidant response, promotes the morphostructure of the small intestine, and improves growth performance. Fifty Piétrain fattening pigs were randomly divided into five groups, each consisting of 10 pigs. The groups were fed a basal diet with no supplementation (control) or a diet supplemented with 1 gGP/kg (E1), 5 gGP/kg (E2), 10 gGP/kg (E3), or 15 gGP/kg (E4). The doses were selected based on preliminary tests. The pigs were slaughtered after 90 days, and their duodenum, jejunum, ileum, and caecum were sampled. We recommend a dose of 10 gGP/kg because it leads to many beneficial effects, including a significantly increased villous height, intestinal crypt depth, and V/C ratio in the duodenum and jejunum (p < 0.05). It also decreased the expression of pro-inflammatory markers such as IL-1β in the ileum and caecum, TNF-α in multiple intestinal segments, and MHC-II in the duodenum, jejunum, and caecum. Additionally, antioxidant activity was promoted through the increased immunohistochemical expression of Nrf2 and reduced NF-kB p65 expression. Growth performance also improved, with significantly higher ADG (p = 0.01) and ADFI values (p < 0.01) than those in the control group. In conclusion, polyphenol-rich grape pomace can be used as a supplement in fattening pig diets to maintain their health and productive performance.
1. Introduction
The demand for pork is increasing [1], making improvements in pig production efficiency essential [2]. Since feed represents the highest cost in livestock production, identifying cost-effective and sustainable dietary alternatives is crucial. Agricultural by-products present a promising solution, as they are produced in large quantities each year and often discarded or sent to landfills [3]. Numerous studies have shown that reusing these by-products not only reduces costs but also benefits the environment [4,5]. They are plentiful, low-cost, and rich in nutritional components such as fiber, minerals, protein, vitamins, and antioxidants, making them ideal as additives in pig diets due to their bioactive properties [6].
Phenolic compounds possess natural antioxidant properties that help protect biomolecules (such as proteins, lipids, and carbohydrates) from the oxidative damage caused by free radicals [7]. These compounds also exhibit metal chelation behavior, particularly with iron and copper, preventing metal-catalyzed free radical formation. Factors such as the number and position of hydroxyl groups, their relationship to carboxyl groups, and the molecular structure of phenolics influence antioxidant activity [8,9].
Additionally, phenolic compounds can reduce oxidative reactions by decreasing DPPH levels and inhibiting lipid oxidation at concentrations greater than 50%. Their protective role against oxidation is mainly due to their ability to donate electrons or hydrogen, which enables them to act as free radical scavengers and delocalize unpaired electrons within the phenolic ring [10]. In the intestinal mucosa, these compounds help neutralize reactive oxygen species (ROS)—which can damage tissues and impair nutrient absorption—thereby maintaining a healthy intestinal environment [11].
Dietary polyphenols can also enhance gut health and immunity in monogastric animals by stimulating immunoglobulin production and reducing pro-inflammatory cytokine secretion [12]. Furthermore, they can increase the expression of antioxidant enzymes, which may help reduce inflammation [13]. Some flavonoids and terpenoids also exhibit anti-inflammatory properties, with essential oils showing similar effects through interactions with signaling pathways involving cytokines and transcription factors, as well as through influencing pro-inflammatory gene expression [14].
Grape pomace is a good source of valuable nutrients, mainly fibers, and bioactive substances such as phenolic compounds with potential health-promoting effects. GP also provides carbohydrates, lipids, proteins, vitamins, minerals, and a diverse range of polyphenols, including flavonoids (catechin, epicatechin), phenolic acids, stilbenes (resveratrol), and proanthocyanidins [3,15]. The phenolic compounds found in grapes contribute to the flavor, color, astringency, and aroma of wine [16], while also playing a role in neutralizing free radicals and minimizing oxidative reactions [17]. Despite the potential economic and nutritional benefits of using grape pomace in feed, there are some significant drawbacks that need to be carefully managed. Variability in the polyphenol content, palatability issues, and possible adverse effects on digestibility can affect health and performance [18]. To minimize these risks, it is essential to carefully monitor the polyphenol content, improve palatability, and control the amount of fiber.
Research on the influence of grape pomace has been carried out on several farm animal species such as pigs [19,20,21], laying hens [22], broiler chickens [23], rabbits [24], bulls [25], lambs [26], as well as in humans [27]. Recent research in fattening pigs has shown that 6% grape marc can improve meat quality and reduce inflammation and oxidative stress [28]. In addition, at a dose of 25 gGP/kg, grape pomace increases ADFI and ADG and improves the gut microbiota by increasing the population of Campylobacter spp. [29], while 10% dehydrated grape pomace promotes growth performance and meat quality [30]. However, the effects of grape pomace on anti-inflammatory and antioxidant abilities, as well as the optimal dosages to obtain these benefits, have been less explored in the area of livestock nutrition [19].
The long-term aim of this study is to develop dietary strategies that incorporate polyphenol-rich grape by-products into animal feed to enhance animal health. The main objective of this study is to evaluate the effects of the inclusion of grape must in the diet of finishing pigs, focusing on improving performance indicators, promoting health status, and promoting intestinal antioxidant status. The central hypothesis is that grape pomace improves intestinal morphology and structure while modulating the immune response, thereby supporting the oxidative equilibrium of the gastrointestinal tract.
2. Materials and Methods
2.1. Animals and Diets
Fifty healthy Piétrain pigs (25 females and 25 males) with an average body weight of 85.07 ± 0.61 kg were obtained from an intensive rearing farm. They were randomized into five groups of 10 pigs each. The pen for each group provides 1 m2 space for every pig. Initially, the pigs were housed for an adaptation period of seven days, and then the 5 groups were given different diets based on composition as follows: (1) control group, receiving a basal diet; (2) E1 group, fed a basal diet + 1 gGP/kg feed; (3) E2 group, fed a basal diet + 5 gGP/kg feed; (4) E3 group, fed a basal diet + 10 gGP/kg feed; and (5) E4 group, fed a basal diet + 15 gGP/kg feed. The grape pomace (GP) was waste provided by a local wine producer and consisted of grape skins and seeds. Leaves, stems, and other impurities were removed from the GP material, and then it was spread in a thin layer, with constant turning to avoid mold. Drying was performed naturally and evenly in a clean, well-ventilated space at a constant temperature of 20°C. The GP sample was ground using a Grindomix GM 200 mill to a particle size of less than 6 mm and mixed into the basal diet. The diets were formulated in accordance with the National Research Council (NRC) 2012 nutrient recommendations [31] and consisted of completely pelleted feed for fattening pigs. The crude chemical content of the experimental diets was determined using the International Organization for Standardization’s standardized methods (ISO 6496, 2001 [32]; ISO 6498, 2001 [33]; ISO 6492, 2001 [34]; ISO 6865, 2002 [35]; ISO 5983 2005 [36]; ISO 2171, 2010 [37]) (Table 1).

Table 1.
Composition and nutrient content of experimental diets.
Pigs were fed three times daily in a separate pen with dimensions of 2.0 m × 3.0 m, and they had free access to fresh drinking water throughout the study. The room temperature was maintained at 20–22 °C with a relative humidity of 65–75%. A comprehensive approach combining medication, hygiene, biosecurity, and pest control strategies was required to control diseases and pests on the pig farms. To ensure the health and welfare of the pigs, regular veterinary surveillance and good farm management practices were essential (Virkon S, DuPont, Sudbury, UK; Biosan Steridet, Kemper, Milan, Italy). Study pigs were included in the vaccination schedule (CircoMax, Zoetis, Zaventem, Belgium and Gripork, HIPRA, Amer, Spain) and deworming schedule (Toltarox, KRKA, Velja, Croatia; Dectomax, Zoetis, Parsippany, NJ, USA). No medical treatment was administered during the trial period. The duration of the study was 90 days. The animals were taken care of in accordance with Romanian Law 43/2014 on the handling and protection of animals used for experimental purposes and EU Council Directive 98/58/EC on the protection of farm animals.
The body weight of each animal was measured at the beginning and end of the study. The average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated at the end of the study. Feed intake was measured individually by subtracting the amount of feed refused from the amount of feed given, while the ADFI was estimated by dividing the feed intake by the duration.
2.2. Analysis of Antioxidant Activity and Polyphenol Content of Experimental Diets
- Assessment of total phenolic content
The total phenolic content (TPC) was measured using a previously described method [38] using a SpectroStar Nano Microplate Reader (BMG Labtech, Ortenberg, Germany). The Folin–Ciocalteu method was employed for TPC determination. In short, 50 µL of the sample was combined with 100 µL of Folin–Ciocalteu reagent and shaken well. After 3 min, 75 µL of a 1% sodium carbonate solution was added, and the mixture was incubated in the dark at room temperature for 2 h. A total of 50 µL of the sample was added as a blank to 100 µL of Folin-Ciocalteu reagent without sodium carbonate. Using a 96-well microplate, the absorbance of the blank was measured at 760 nm and the total phenolic content (TPC) was calculated and reported as milligrams of gallic acid equivalents (mg GAE/g) (Table 2).

Table 2.
Antioxidant activity and polyphenol content of experimental diets.
- Determination of antioxidant activity
The antioxidant activity was determined using an in vitro method, specifically radical scavenging (2,2-diphenyl-1-picrylhydrazyl radical) assays, according to previously established methodologies [32].
- 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) scavenging assay
In this process, 50 µL of the sample was added to 150 µL of a 0.004% methanol solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH). After incubating the mixture for 30 min at room temperature in the dark, the absorbance was measured at 517 nm. Blank values were subtracted from both the sample and standard readings. A linear regression curve for the Trolox standards was constructed. The DPPH radical scavenging activity was then expressed as micromoles of Trolox equivalents (µM TRE/g) (Table 2).
2.3. Sample Collection and Histological and Immunohistochemical Analyses
Animals were slaughtered in accordance with EU Council Directive 2010/63/EC at the end of the experiment. Samples were taken from the anterior third of each small intestinal segment (duodenum, jejunum, ileum) and the anterior third of the caecum. The samples were fixed in 10% neutral buffered formalin for a period of 24 h. After one day, the samples were dehydrated in a graded series of ethyl alcohol, cleared in xylene, and embedded in paraffin. The samples were cut into 5 µm sections and subjected to HE staining. Measurements of villi and crypts were in accordance with [39]. For each treatment group, ten intact and well-oriented villi and adjacent crypts were randomly selected to measure the villous height and crypt depth in each segment (duodenum, jejunum, and ileum). In addition, 10 lymphoid follicles from the Peyer’s patches of the jejunum and ileum were selected for measurement, taking into account their length and width. Measurements for the cecum were taken in 10 different areas for each experimental group for the mucosa, submucosa, and muscularis. All measurements were taken using Leica Application Suite software (LAS v4.13), and the villous height-to-crypt depth ratio (VCR) was calculated separately for each treatment group.
Immunohistochemical (IHC) staining was performed using a series of specific antibodies as markers. Four key regions were analyzed (duodenum, jejunum, ileum, and caecum) with inflammation-specific markers (IL-1β, TNF-α, MHC-II, NF-kB p65, and antioxidant Nrf2) (Table 3). Preparations were initially deparaffinized in 3 xylene baths, hydrated in ethyl alcohol, and then microwaved at 95 °C for 10 min in a citrate buffer, pH 6.0, 10× (C9999, Sigma-Aldrich, St. Louis, MO, USA) for antigen retrieval. After this, preparations were allowed to cool for 20 min at room temperature and then rinsed 3 times in PBS (P4417, Sigma-Aldrich, USA) for 5 min each. They were then incubated overnight at 4 °C in a moist room with primary antibodies. The next day, the preparations were washed three times with PBS for 5 min for each bath and then incubated with secondary antibodies. 3,30-diaminobenzidine (DAB, ab64238, Abcam, Cambridge, UK) was added to the sections and they were subsequently counterstained with hematoxylin (Merck KGaA, Darmstadt, Germany). Sections were subsequently examined and photographed using an optical microscope (Leica DM750, Leica Microsystems CMS GmbH, Wetzlar, Germany). Cell counts were determined for intraepithelial and lamina propria leukocytes. Specifically, three different cell counts were performed for each histological section (in three different areas of 0.004 cm2). The immunolocalization of IL-1β, TNF-α, MHC-II, NF-kB p65, and Nrf2 was studied by two scientists (LH and CS) in a blind analysis and visualized photographically using a Leica DM750 (Germany), with a magnification of 400. The amount of positively stained cells and their relationships were expressed by a simple qualitative scoring system, as performed by Zimmermann et al. [40]. The examined slides were scored as ‘+++’ (strong IHC staining), ‘++’ (moderate IHC staining), ‘+’ (weak IHC staining), or ‘−’ (no visible staining).

Table 3.
Information on the antibodies used in the study.
2.4. Statistical Analysis
Data are reported as means ± standard deviation (SD) and were analyzed using SPSS22.0 (SPSS, Chicago, IL, USA). All data were analyzed by a one-way ANOVA followed by a post hoc Tukey test for multiple comparisons. The difference was considered significant when p < 0.05.
3. Results
3.1. Pig Growth Performance
E3 had the highest final body weight, significantly higher than the control (p = 0.006), E1 (p = 0.008), E2 (p = 0.025), and E4 (p = 0.012), but there was no difference between the final weights of control, E1, E2, and E4 (p > 0.05) (Table 4).

Table 4.
Effects of grape pomace on growth performance.
There is a directly proportional increase in daily food intake (ADFI) with dose. E3 had a significantly higher feed intake in comparison to the control and the rest of the experimental groups (p < 0.01). E4 was not significantly different relative to E1 (p = 0.06) and E2 (p = 0.85).
The greatest increase in body weight gain (ADG) was recorded in E3 and was significantly higher than the control (p = 0.01), E1 (p = 0.01), E2 (p = 0.02), and E4 (p = 0.01). There was no significant difference between the control and E1, E2, or E4 (p > 0.05).
There was no significant difference in feed conversion ratio (FCR) between the control group and the rest of the experimental groups (p = 0.57).
3.2. Histologic Intestinal Analysis
Since the duodenum and ileum are the primary locations of nutrient absorption, it is anticipated that histological changes in these areas will be induced by the inclusion of polyphenol-rich plant products in the pig diet. To analyze the potential effects of GP on the absorptive capacity via alterations in villous height and crypt depth, histological sections of these parts of the small intestine were performed (Figure 1). Measurements were also taken for the jejunum and ileum to study the possible reactivity of the lymphoid tissue associated with the intestinal mucosa to polyphenols from grape pomace (Table 5).
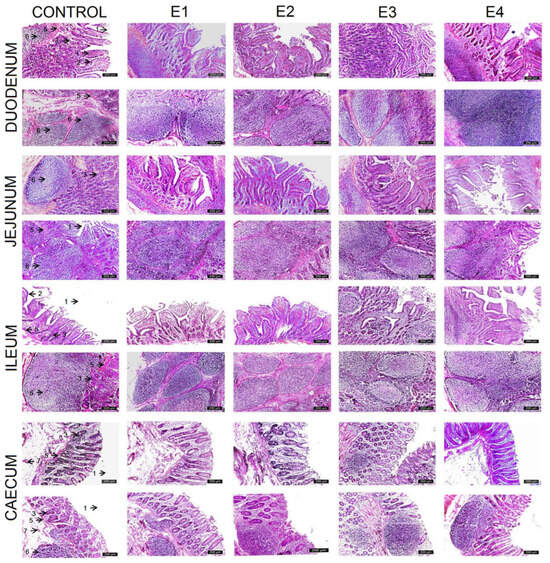
Figure 1.
Reactivity of the intestinal mucosa and MALT following the introduction of GP into pig feed. 1 = intestinal lumen, 2 = columnar epithelium, 3 = intestinal crypt, 4 = intestinal villi, 5 = muscularis mucosae, 6 = lymphoid nodule, 7 = blood vessels. Scale bar is 200 μm. HE stains.

Table 5.
Effects of introducing grape pomace into pig feed on intestinal morphostructure.
Following the introduction of grape pomace (GP) into the pig feed, the height of villi, the depth of intestinal crypts, and the V/C ratio in the duodenum and jejunum of the E3 group (10 gGP/kg) were significantly higher than the control and the rest of the experimental groups (p < 0.05). The duodenum of E3 contained long intestinal villi with a larger diameter, and smooth muscle fibers derived from the muscularis mucosae, blood vessels, and lymphatic vessels belonging to the connective tissue of the lamina propria were observed. The mucosa had numerous lateral folds and the columnar epithelium had a higher integrity rate. No significant differences were found between the duodenal mucosa of E2 (5 gGP/kg) and E4 (15 gGP/kg); between the jejunal mucosa of E1 (1 gGP/kg) and control; and between the ileum mucosa of E2 (5 gGP/kg), E3 (10 gGP/kg), and E4 (15 gGP/kg).
The mucosa of the jejunum and ileum is thicker due to the accumulation of different lymphoplasmacytic populations in the lamina propria. These cells can be found from Lieberkühn’s glands to the tips of the villi. The submucosa is more prominent due to the accumulation of lymphoid follicles, which are large enough to pass through the muscularis mucosae and act as an immune barrier to the intestinal wall. Peyer’s patch sizes, both in the jejunum and ileum, were significantly larger in E3 (10 gGP/kg) compared to the control and the rest of the experimental groups (p < 0.001). Lymph node sizes decreased in the following order: E2 (5 gGP/kg), E4 (15 gGP/kg), E1 (1 gGP/kg), and control.
Histological analysis of the coecum showed that the mucosa of E3 (10 gGP/kg) was significantly larger than that of the control (p = 0.002), E1 (p = 0.005), and E4 (p = 0.002). The composition of Lieberkühn’s glands was the same, but they had a larger diameter in E3; a columnar epithelium with a brush border was observed in both; and there was a predominance of goblet cells to the detriment of absorptive cells. In the submucosa, there were numerous lymphoid nodules that formed Peyer’s patches, which act as a defensive barrier to intestinal immunity. The submucosa of the E3 group was significantly larger than that of the control (p = 0.001) and E1 (p = 0.005), the difference is due to the reactivity of the lymphoid nodules to grape polyphenols. The cecum muscularis layer did not change significantly after the introduction of GP into the pig diets.
3.3. IHC Intestinal Analysis
For the evaluation of the anti-inflammatory potential of grape pomace polyphenols, the following three markers were analyzed simultaneously via IHC: IL-1β, TNF-α, and MHC-II. The results showed that all three markers varied with the amount of GP added to the diet (Table 6). The expressions of these pro-inflammatory markers had the highest IHC intensity in the control, which is a normal response due to simultaneous permanent and physiological dynamic processes at the intestinal level between pro- and anti-inflammatory factors.

Table 6.
Effects of introducing grape pomace into pig feed on intestinal inflammatory markers.
IL-1β expression was strongest (+++) for the control, E1, and E2 throughout the mucosa of the duodenum (Figure 2), jejunum (Figure 3), and caecum, and particularly in the lamina propria cell groups and Lieberkühn’s glands. Major changes in this marker were observed in the ileum (Figure 4), where IL-1β expression was weak (+) in the mucosal cells of the Lieberkühn’s glands and in the epithelium at the surface of the villi in E3 (10 gGP/kg) and E4 (15 gGP/kg). E2 and E1 expression remained moderate (++). Increasing the dose of GP polyphenols gradually reduced pro-inflammatory markers in E3, E4, and E2. The best results were obtained when 10 gGP/kg was added to the pigs’ diets, resulting in weak (+) expression of the IL-1β marker in the ileum and caecum and moderate (++) expression in the duodenum and jejunum (Figure 5).
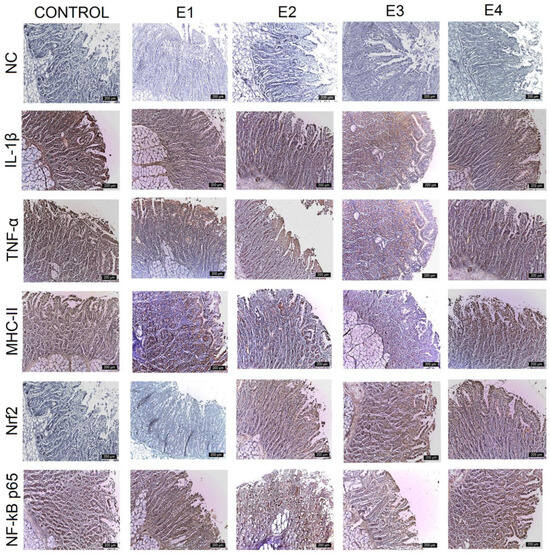
Figure 2.
Immunohistochemical analysis of IL-1β, TNF-α, MHC-II, NF-kB p65, and Nrf2 in the duodenum. The expression of pro-inflammatory markers is evident in the epithelium and lamina propria cells of the duodenum for the control and E1 and progressively decreased for E2, E4, and E3. Nrf2 expression showed a reverse evolution. The most intense labeling is observed for E3, followed by E2 and E4. The expression for E1 and the control decreased to zero. Scale bar is 200 μm.
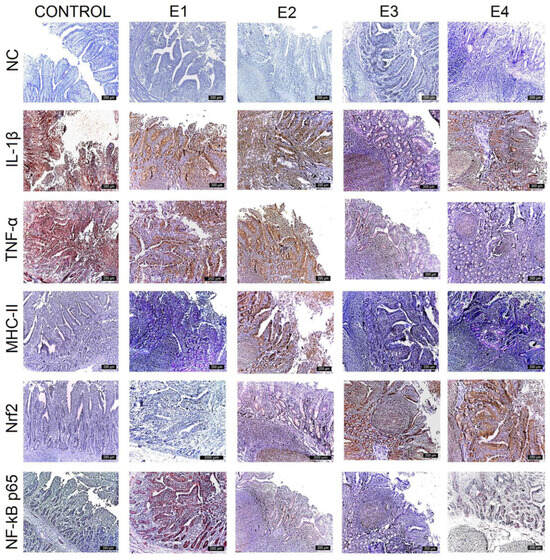
Figure 3.
Immunohistochemical analysis of IL-1β, TNF-α, MHC-II, NF-kB p65, and Nrf2 in the jejunum. The expression of pro-inflammatory markers is evident in the epithelium and lamina propria cells of the jejunum for the control and E1, and progressively decreased for E2, E4, and E3. Nrf2 expression exhibited the reverse evolution. The most intense labeling is observed for E3, followed by E2 and E4. The expression for E1 and the control decreased to zero. Scale bar is 200 μm.
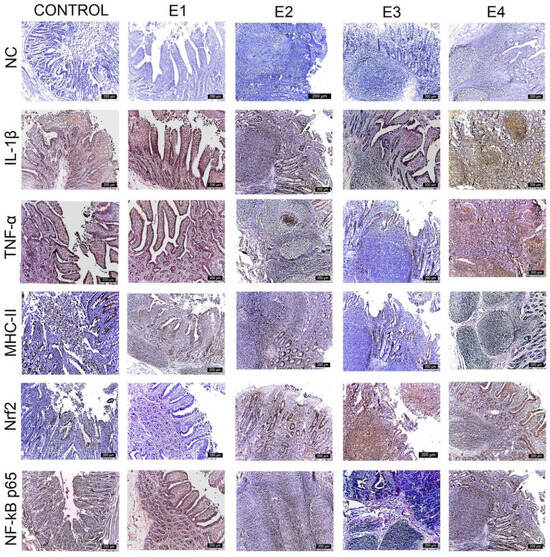
Figure 4.
Immunohistochemical analysis of IL-1β, TNF-α, MHC-II, NF-kB p65, and Nrf2 in the ileum. The expression of pro-inflammatory markers is evident in the epithelium and lamina propria cells of the ileum for the control and E1, and progressively decreased for E2, E4, and E3. Nrf2 expression exhibits the reverse evolution. The most intense labeling is observed for E3, followed by E2 and E4. The expression for E1 and the control decreased to zero. Scale bar is 200 μm.
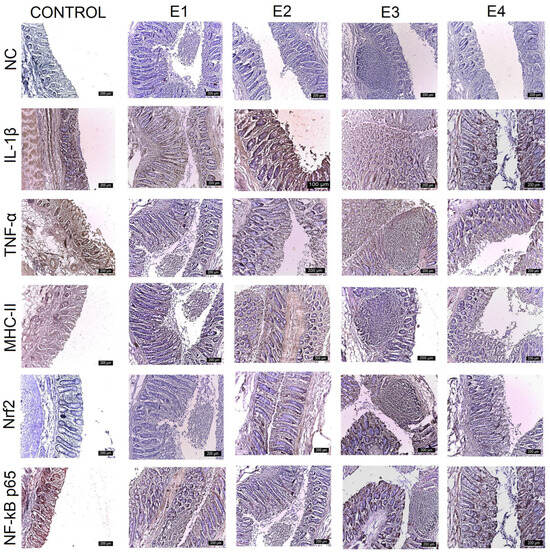
Figure 5.
Immunohistochemical analysis of IL-1β, TNF-α, MHC-II, NF-kB p65, and Nrf2 in the caecum. The expression of pro-inflammatory markers is evident in the epithelium and lamina propria cells of the caecum for the control and E1, and progressively decreased for E2, E4, and E3. Nrf2 expression exhibits the reverse evolution. The most intense labeling is observed for E3, followed by E2 and E4. The expression for E1 and the control decreases to zero. Scale bar is 200 μm.
In the intestine, TNF-α expression was positive in all experimental groups. The strongest expression was observed for the control in the Lieberkühn’s glands and the periglandular space, decreasing in the experimental groups. The strongest expression of TNF-α was observed for the control (+++) in all intestinal segments examined (when the pigs received no polyphenols in their diet). At 1 gGP/kg (E1) and 5 gGP/kg (E2), IHC expression of TNF-α was moderate (++) in the jejunum, ileum, and caecum, and it was weak (+) in all intestinal segments examined for E3 (10 gGP/kg).
The MHC-II marker was intensely labeled (+++) in all intestinal segments examined for the control and in the jejunum, ileum, and caecum for 1 gGP/kg (E1). Positive lamina propria cell populations and mucosal cells in Lieberkühn’s glands were labeled intensely. Moderate (++) MHC-II staining was observed in the duodenum for E1 and E2, in the jejunum for E2 and E4, and in the ileum for E2, E3, and E4. In the cecum, MHC-II expression was moderate (++) for E2 and E4. The weakest (+) MHC-II IHC labeling was observed in the duodenum, jejunum, and caecum at 10 gGP/kg.
To evaluate the antioxidant potential of the GP polyphenols, the following two markers were analyzed simultaneously via IHC: Nrf2 and NF-kB p65. The results show that both NF-kB p65 and Nrf2 vary with the amount of GP added to the diet. The expression of Nrf2 is intense (+++) in the lamina propria and intraepithelial leukocytes of the duodenum, jejunum, ileum, and caecum for E3 and gradually decreases for E4, E2, E1, and the control. At the same time, the expression of NF-kB p65 is weakest for E3 (+) and gradually increases for the other experimental batches in the order of E4, E2, E1, and control. In other words, it can be concluded that an increase in Nrf2 expression leads to a simultaneous decrease in NF-kB p65 expression in the intestine at 10 gGP/kg, which is the recommended dose of GP as an additive in pig feed.
4. Discussion
Natural plant products and their derived metabolites are alternative feed additives that are commonly utilized in animal husbandry [41]. The effect of incorporating grape by-products into pig diets on growth performance depends on the growth stage of the animals and the by-product dose used. In general, lower by-product doses are not considered to have a significant effect on growth performance, although this varies with concentration and animal age. For example, pigs supplemented with 9% GP showed an increase in ADG (average daily gain) in all trials [42], while those supplemented with 5% GP showed no change [43]. Piglets fed 3% GP performed better only during the growing period, probably due to higher digestibility [44]. It must be noted that pre-treatment of the by-products (e.g., fermentation) may influence the results as fermentation may increase the phenolic contents in the product. Studies have also shown that doses of 9% GP administered over 30 days can increase ADG without affecting the feed intake or FCR [42]. However, some studies reported that grape by-products had no significant effects on these parameters at 3.5% and 7% GP [45], 1% GS [46], or 0,015% by-product [47]. Despite the variability in the results, the increase in ADG observed in [41] suggests that grape pomace (9%) has a promising effect on the growth performance of piglets (20 days old). In this experiment, the best results were obtained when 10 gGP/kg was fed to finishing pigs, as the final weight, daily feed intake, and daily weight gain were significantly higher in E3 than in the control group (p < 0.05). Although grape pomace can be a useful source of antioxidants and fiber for pigs, we chose the doses with caution, choosing to administer grape pomace in a moderate amount over a long period of time (3 months) to avoid toxicity and nutritional imbalances. Moreover, the pigs in our experiment were healthy and no inflammatory processes were induced at the digestive level, supporting our aim of demonstrating that grape pomace promotes pig production and pig digestive health. The lack of additional benefits at 15 gGP/kg may suggest a saturation point in the pigs’ abilities to utilize polyphenols effectively. Future studies should explore whether exceeding 10 gGP/kg results in metabolic inefficiencies or an altered gut microbiota composition. Beneficial effects of polyphenols have also been observed in other species, such as ruminants, using other natural product sources. Phenolic compounds from propolis extracts (3.81, 3.27, 1.93 mg phenolic compounds/kg dry matter (DM) ingested) had effects on milk production by modifying the fatty acid composition and antioxidant capacity of milk in dairy cows [48]. Bitter vetch and sorghum grains are also rich sources of antioxidants, vitamins and minerals [49]. With a total polyphenol content of 3.86 g/kg DM−1 of bitter vetch and a total polyphenol content of 12.44 g/kg DM−1 of sorghum grains, used as alternative feed sources in the diet of male goat kids, promoted meat quality by improving the fatty acid profile and carcass characteristics [50].
The state of health is influenced by the morphostructure of the intestine. Long villi mean a larger intestinal surface area, leading to greater nutrient absorption. Our results showed that the addition of 10 gGP/kg significantly increased the V/C ratio and the height of the duodenal and ileal villi in the duodenum and jejunum in E3 in comparison with the control and the rest of the experimental groups. Similar results were obtained by Gessner et al. [51], who administered 10 g GSGME (grape seed and grape marc meal extract) to 6-week-old piglets, leading to an improvement in the intestinal morphostructure by increasing the V/C ratio. Wang et al. [52] reported that the villous height increased following the introduction of 50 g GP into the diet of 28-day-old piglets. The intestinal morphostructure was also improved by the administration of 250 g/kg of proanthocyanin extract to weaning piglets [53]. In contrast, Fiesel et al. [54] and Taranu et al. [20] reported that polyphenols derived from grape by-products had no effect on the intestinal morphostructure in 5-week-old piglets and growing-finishing pigs, respectively.
There are numerous studies investigating the effect of grape compounds on intestinal inflammation, but most of them focus on the action of a single compound, such as resveratrol; proanthocyanidins; or on a small group of compounds such as flavonols. In this context, less is known about the effects of the whole range of grape compounds and their possible combinatorial activity. The anti-inflammatory effects resulting from the synergistic interaction between the bioactive compounds in GP are also supported by the results of our experiment, which indicate the combined action of these substances in reducing intestinal inflammation.
Similar to this study, Pistol et al. [21] found that 8% grape seed meal in the diet (weaned piglets with induced DSS) caused a reduction in pro-inflammatory cytokine genes in the intestines of pigs. In the same line, Fiesel et al. [54] demonstrated that feeding weaned piglets with 1% polyphenol-rich grape marc and grape seed meal extract causes down-regulation of the pro-inflammatory cytokines IL-8, TNF-α, IL-1β, and ICAM1 in the mucosa of different segments of the intestine (duodenum, ileum, and colon). Gessner et al. [55] reported that supplementation with polyphenol-rich grape seed and grape meal extract in the diet reduced the expression of IL-1b, IL-8, MCP-1, and CXCL1 genes in Caco-2 intestinal cells, while Taranu et al. [19] demonstrated that feeding pigs with 5% polyphenol-rich grape seed cake causes down-regulation of gene expression and protein concentrations in the pro-inflammatory cytokines IL-8, IL-1β, IFN-γ, and TNF-α. Our results are the closest to those of Taranu et al. [19] because, in both our and their experiments, the pigs were in the growing-finishing period and were also healthy animals.
In this study, dietary polyphenols increased the number of leukocytes in the lamina propria and intraepithelial zone, indicating massive leukocyte migration in the experimental groups. During inflammation of the gut, the recruitment of white blood cells to the site of inflammation involves complex interactions with other immune cells and the release of chemical mediators necessary for the inflammatory response, which contribute to mucosal healing [56]. Previous studies have shown that leukocytes can directly influence disease pathology when they are excessively recruited and activated, releasing toxic substances and causing morphological changes in intestinal villi and crypts, as well as extensive mucosal damage. In this way, leukocytes can act paradoxically, contributing to homeostasis by eliminating pathogens, but also contributing to harmful inflammatory processes by exacerbating inflammation through the release of pro-inflammatory mediators. Our results demonstrate that GP did not alter the intestinal structure, but rather promoted the intestinal villous height and V/C ratio and decreased the IHC expression of pro-inflammatory markers (MHC-II, TNF-α, IL-1α); thus, the presence of a high number of leukocytes in the lamina propria suggests that polyphenols from GP, such as those from olives [57], will not promote inflammation in adult pigs. This effect and its relevance need to be further investigated, as we know that a basal level of inflammation is necessary in the body, as inflammation is involved in various physiological functions. The increased presence of leukocytes in the lamina propria suggests enhanced immune surveillance. However, whether this reflects a beneficial immune modulation or a compensatory response to dietary polyphenols requires further investigation.
Cytokines such as TNF-α and IL-1β are produced by the intestinal epithelium and may be involved in the initial recruitment of immune cells to the intestinal mucosa and are greatly increased in reaction to infection by microbes [58,59]. In this study, TNF-α was highly expressed in the control, despite the fact that all animals had no clinical signs of disease prior to grape pomace administration. Low levels of TNF-α have previously been observed in clinically healthy pigs [60,61]. The production of pro-inflammatory cytokines is almost immediately followed by the production of anti-inflammatory cytokines [62]; thus, the inflammatory process is self-limiting. TNF-α is a powerful cytokine that is produced by many different cell subsets, including macrophages and lymphocytes, in response to inflammation. TNF-α is not only a marker of inflammation, but also a factor involved in regulating the regeneration of the intestinal epithelium, being produced by fibroblasts and keratinocytes [63]. A caspase cascade can be triggered by the exposure of cells to TNF-α, resulting in apoptosis. TNF-α activates two key transcription factors, NF-κB and AP-1, which promote the expression of genes involved in both acute and chronic inflammatory responses [64,65]. These signaling pathways play a crucial role in the regulation of inflammation and cell death [66].
The mammalian NF-κB family comprises five proteins—p65 (RelA), RelB, c-Rel, p105/p50 (NF-κB1), and p100/p52 (NF-κB2)—of which the p65:p50 heterodimer is the most prevalent and relevant in inflammatory processes [67]. Under resting cell conditions, this heterodimer is retained in the cytoplasm by attachment to the inhibitory protein IκB. Following an inflammatory stimulus, IκB kinaseB (IκKB) is activated, leading to the phosphorylation of the IκB-a protein [68]. Following degradation of pIκB-a, p65:p50 heterodimers translocate to the cell nucleus, where they attach to κB sequences in the promoter or enhancer regions of many pro-inflammatory genes, thereby stimulating their expression (TNF-α, IL-1α) [64,68].
Polyphenols in green tea reduce the release of cytokines that promote inflammation (TNF-alpha, IL-1 beta) and block the TLR4 signaling pathway [69]. Downregulating NF-κB could be a mechanism through which grapes’ bioactive compounds exert their action in the liver [70]. In addition, other studies have shown that the whole cascade of inflammation, involving MAPK in the cytoplasm and NF-κB in the nucleus, is inhibited by the inhibition mechanism of grape compounds [71,72]. The same results were obtained in growing-finishing pigs with grape seed meal (5%), which reduced the expression of NF-κB and its target genes (pro-inflammatory cytokines, COX2, iNOS, and eNOS) [19], as well as in weaning piglets. In the latter, NF-kB gene expression was attenuated in the intestine by the addition of 8% grape seed meal to their diet [21]. Our experiment demonstrated that 10 gGP/kg reduced IHC expression of NF-kB in the duodenum, jejunum, and cecum. A reduction in NF-kB levels under the influence of GSGME was also demonstrated in vitro [55]. This may have synergistic effects with other phytochemicals in grapeseed such as PUFA, which are known to reduce inflammation by affecting the phosphorylation state of nuclear receptors [19,73,74,75].
Dendritic cells (DCs) play a key role in the activation of T cell-dependent immune responses and are the most potent antigen-presenting cells (APCs) localized in tissues such as the intestinal mucosa [76]. DCs take up antigens, convert these into immunogenic peptides and present them to the major histocompatibility complex (MHC) class II to trigger an immediate immune response. Furthermore, it has been suggested that DCs play an essential role in the development of immunological tolerance [77].
Recently, dietary factors have been shown to contribute to the prevention and treatment of immunological diseases. Akiyama et al. [78] showed that apple polyphenol extract inhibits food allergies in mouse models by inhibiting antigen presentation by APCs and reducing the expression of MHC-II and costimulatory molecules. Similar results were obtained by us: bioactive compounds from GP downregulated the IHC expression of MHC-II throughout the study, and weak MHC-II expression was observed in E3, who received 10 gGP/kg in their diet. Other polyphenols such as quercetin, curcumin, silibinin, and apigenin inhibit the maturation of murine DCs and reduce MHC expression, antigen uptake, and the secretion of pro-inflammatory interleukins (IL-12, IL-2, IL-1, IL-6) [79,80,81,82].
Grape polyphenols improve the antioxidant status and reduce ROS levels in pigs through systemic anti-inflammatory effects [83,84]. They also stimulate the activity of endogenous antioxidant enzymes such as CAT, GPx, and SOD by activating Nrf2, a transcription factor that regulates cellular antioxidant defense [85,86]. The Nrf2 pathway is an important target for antioxidant research and is promising for the treatment and prevention of diseases associated with oxidative stress [87]. Nrf2 regulates the cellular redox balance and is bound to Keap1 in the cytoplasm under normal conditions, helping to maintain basal levels of antioxidant enzymes. Under conditions of oxidative stress, Keap1 is altered and Nrf2 is activated and moves to the cell nucleus, stimulating the upregulation of antioxidant enzymes to protect cells from free radicals [88]. Several studies have demonstrated polyphenols’ ability to induce Nrf2 activation, which in turn increases the expression of a number of antioxidative and cell-protective genes in the small intestine [85,89]. The activation of Nrf2 in response to other dietary components has also been demonstrated. Dihydromyricetin (0.03–0.05%) increased Nrf2 nuclear protein and phospho-ERK (p-ERK) expression in the jejunum of growing-finishing pigs via the ERK/Nrf2/HO-1 pathway. ERK2 of the MAPK subfamily may enhance the nuclear translocation of Nrf2 and induce antioxidant gene transcription [90].
Flavonoids are generally not well absorbed across the intestinal mucosa [91], and the gut is the primary location of antioxidative defense [92]. When Nrf2 is inactivated or missing, downstream antioxidant enzymes are decreased and cells become vulnerable to oxidative stress toxicity, which can lead to cell malfunctions or apoptosis. Studies suggest that polyphenols from various waste products may have an inhibitory effect on antioxidant enzymes [93], which appears to be dose-dependent. Thus, high doses of green tea polyphenols decrease the level of antioxidant enzymes, whereas low and medium doses have a stimulating effect [94]. This was confirmed in our experiment. The IHC expression of Nrf2 was highest at the dose of 10 g GP/kg in the diet (E3), while 1 gGP/kg (E1) had similar results to the control group and 5 gGP/kg (E2) and 15 gGP/kg (E4) led to moderate and low IHC expressions, respectively. Contrary to our results, previous studies presented different results: Zhang et al. [95] showed that grape seed extract (2 g/kg) reduced plasma lipid peroxidation in piglets, but did not affect the total antioxidant capacity. Gessner et al. [51] observed the grape seed and GP had no significant effects on lipid peroxidation or antioxidant capacity. Similarly, Taranu et al. [19] found that the liver antioxidant capacity of pigs fed grape seed (5%) was not significantly different when compared with the control group. The anti-inflammatory and antioxidant effects of GP observed in our study can be attributed to the fact that the pigs were in the fattening phase, as obesity is known to be associated with inflammatory factors. The observation of anti-inflammatory and antioxidative effects not only in the duodenal and jejunal regions, but also in the ileal and cecal regions, suggests that the active constituents of polyphenolic-rich plant products are not fully absorbed or broken down in the anterior portion of the intestine, and remain available and active, at least in part, in the posterior part of the intestine. The present study supports the concept that polyphenols could represent a useful dietary approach to inhibit inflammation and promote antioxidant capacity in the porcine gut, regardless of the exact mode of action of NF-κB inhibition and Nrf2 activation.
GP is a significant source of dietary fiber, which is composed of cellulose, hemicellulose, pectin, and lignin. Fiber is the predominant component of grape pomace (29–58% dry weight), alongside residual polyphenols and other bioactive compounds [96,97]. The insoluble fraction, which includes cellulose and lignin, is present in abundance, while the soluble fiber fraction (pectins, oligosaccharides) is present in lower amounts. The fiber composition is related to the grape variety, vinification technique, and processing conditions. For instance, red wine pomace has more lignin and structural polysaccharides as opposed to white wine pomace that retains more soluble fiber due to different fermentation processes [98].
The fiber in GP is referred to as antioxidant dietary fiber (ADF) due to its capacity to adsorb phenolic compounds, which seems to affect gut health differently from just its physical presence [97]. Complex carbohydrates such as hemicelluloses and pectins play a role in determining the functional properties of GP, such as its fermentability in the gut and its possible use as a prebiotic [99].
The fiber fraction of GP has a vital role in modifying intestinal morphology, especially in pigs, which are sensitive to dietary fiber. The effects of the fiber from GP on morphological features such as the villus height, crypt depth, V/C, mucosal thickness, and Peyer’s patch size depend on physicochemical properties such as solubility, fermentability, and particle size [100]. It has been determined that insoluble fiber, especially from GP, has a tendency to increase the height of the villi and the depth of the crypts in the small intestine, which increases the surface area for absorption [101]. In piglets, diets containing insoluble fiber from GP have been found to result in longer villi and deeper crypts, especially in the jejunum and ileum, which is indicative of an increased epithelial turnover and adaptation of the gut to fiber-containing diets [42].
Nevertheless, the crypt depth is not always a good marker of gut health. A higher crypt depth means a higher cell turnover, which is advantageous in conjunction with longer villi if the V/C ratio is also increased, but it may also indicate increased epithelial stress if villi growth does not occur concurrently [102]. Some research shows that high levels of fiber from the diet (especially lignin) can lead to a decrease in the V/C ratio without the corresponding villus lengthening in weaned piglets through crypt hyperplasia [103].
Fiber, especially insoluble fiber, increases mucosal thickness through mechanical irritation and epithelial cell regeneration [104]. An increased mucosal thickness is protective against pathogens; a well-developed epithelial barrier does not permit microbe penetration or the development of systemic inflammation.
The integrity of tight junctions is also controlled by GP fiber through the regulation of mucin production and goblet cell activity. It has been established that the pectins and hemicelluloses present in GP enhance the secretion of mucins, which enrich the intestinal mucus barrier and enhance the protective mechanisms of the epithelium [105].
Peyer’s patches are dynamically regulated by fiber. Insoluble fiber from GP can act as an adjuvant to increase the contact of antigens with the immune system and induce more effective mucosal immunity. Studies in pigs have revealed that fiber-containing diets stimulate the development of Peyer’s patches and immune cells, which may help to enhance the immune response of the gut [106]. However, overstimulation of the immune system by fiber, especially the lignin fraction, may theoretically lead to chronic inflammation if not well managed.
There is a high degree of morphological changes in the gut of pigs upon feeding with GP fiber, but the same can be observed in other monogastric animals. Lignan-rich fiber from GP has been found to increase the crypt depth and cause villus erosion in rabbits, which is consistent with more marked mucosal irritation [104]. In poultry, the use of GP fiber has been reported to improve gut integrity by increasing the production of short-chain fatty acids in the cecum, which in turn increases the villus height and strengthens the intestinal barrier [103]. In ruminants, however, the effects of GP fiber are directed towards the modulation of rumen fermentation rather than the morphology of the intestine. Nonetheless, studies have shown that GP inclusion alters the microbiota, which affects gut health [107].
In the context of the present study, since the crude fiber content of the experimental diets was only moderately higher than that of the basal diet, the beneficial effects of grape by-products are considered to be mainly due to polyphenols, which have significant bioactivities. Although these by-products can be useful in animal production, their potential has not been fully exploited. The optimal dose for animals is difficult to determine due to the variation in the polyphenol composition of these by-products. More research is needed to determine the optimal dose and bioavailability of grape polyphenols, rather than the by-products themselves, and their effect on animal health. The interaction of polyphenols with the gut microbiota also plays a key role in maintaining gut health by influencing nutrient absorption and reducing the translocation of pro-inflammatory and oxidative stimuli. Plant polyphenols have been shown to reduce local and systemic inflammation and improve animal performance, especially during the fattening period in monogastric species, and also in challenged or stressed animals. These effects are due to reduced inflammation and oxidative processes that normally reduce feed intake and alter metabolism. However, research should also address the potential adverse effects of polyphenols, such as interactions with essential minerals or inhibition of digestive enzymes. The antimicrobial benefits of grape products are already being studied, but further research is needed to better understand their effects on gut microflora and animal performance. In conclusion, polyphenols from grape by-products have significant potential to improve animal health, but further research is needed to optimize their use and understand their full impact on animal nutrition and health.
5. Conclusions
In conclusion, the present study shows that the oral administration of a GP rich in polyphenols and fiber promotes growth performance, as demonstrated by significantly higher values for ADG (p = 0.01) and ADFI (p < 0.01) at 10 gGP/kg than those in the control group; however, it does not influence the FCR (p = 0.57). In the duodenum, grape pomace promotes the intestinal morphostructure by increasing the villus height, intestinal crypt depth, and V/C ratio, decreasing the expression of pro-inflammatory markers (TNF-α, MCH-II, and NF-kB p65), and promoting antioxidant activity by increasing the immunohistochemical expression of Nrf2 at a dose of 10 gGP/kg. In the jejunum, GP promotes the intestinal morphostructure and the structure of Peyer’s patches, decreases the expression of pro-inflammatory markers TNF-α, MCH-II, and NF-kB p65, and increases the expression of Nrf2 at a dose of 10 gGP/kg. In the ileum, GP increases the villus height, improves the structure of Peyer’s patches, decreases the expression of pro-inflammatory markers IL-1β, TNF-α, and MCH-II, and promotes antioxidant activity at a dose of 10 gGP/kg. In the caecum, GP increases the width of the mucosa and submucosa, decreases the expression of pro-inflammatory markers IL-1β, TNF-α, MCH-II, and NF-kB p65, and leads to a high immunohistochemical expression of Nrf2 at a dose of 10 gGP/kg. Taken together, these results indicate that polyphenol-rich plant extracts, such as GP, may be suitable feed supplements for pigs. We recommend a dose of 10 gGP/kg over a 3-month period for healthy growing-finishing pigs to promote both performance and animal health.
Author Contributions
Conceptualization, L.H., A.C.P., G.S. and C.S.; methodology, L.H., B.G.Ș., A.T., G.P., G.S. and C.S.; validation, G.S. and C.S.; formal analysis, L.H., B.G.Ș., A.T., G.P., G.S. and C.S.; investigation, A.C.P., L.H., B.G.Ș., A.T., G.P., G.S. and C.S.; writing—original draft preparation, L.H., G.S. and C.S.; writing—review and editing, L.H., C.S. and G.S; supervision, C.S and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was carried out with the consent of the Ethics Commission of the Faculty of Veterinary Medicine within the “Ion Ionescu de la Brad” University of Life Sciences in Iași, in accordance with the Research Law no. 206/27.05.2004 on good practices in scientific research, technological development, and innovation, as well as European Legislation.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Henchion, M.; Moloney, A.P.; Hyland, J.; Zimmermann, J.; McCarthy, S. Review: Trends for meat, milk and egg consumption for the next decades and the role played by livestock systems in the global production of proteins. Animal 2021, 15, 100287. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, M.; Zhu, Q.; Azad, A.K.; Gao, Q.; Kong, X. Dietary betaine addition alters carcass traits, meat quality, and nitrogen metabolism of bama mini-pigs. Front. Nutr. 2021, 8, 728477. [Google Scholar] [CrossRef]
- Erinle, T.J.; Adewole, D.I. Fruit Pomaces—Their nutrient and bioactive components, effects on growth and health of poultry species, and possible optimization techniques. Anim. Nutr. 2022, 9, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.; Neumann, C.; Velten, S.; Liebert, F.; Mörlein, D. Meat quality derived from high inclusion of a micro-alga or insect meal as an alternative protein source in poultry diets: A pilot study. Foods 2018, 7, 34. [Google Scholar] [CrossRef]
- Reckmann, K.; Blank, R.; Traulsen, I.; Krieter, J. Comparative Life Cycle Assessment (LCA) of Pork Using Different Protein Sources in Pig Feed. Arch. Anim. Breed. 2016, 59, 27–36. [Google Scholar] [CrossRef]
- Spigno, G.; Marinoni, L.; Garrido, G.D. State of the art in grape processing by-products. Handb. Grape Process. By-Prod. 2017, 1–27. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.L.; Cabaraux, J.F.; Chentouf, M. Ecological, morpho-agronomical, and bromatological assessment of sorghum ecotypes in Northern Morocco. Sci. Rep. 2023, 13, 15548. [Google Scholar] [CrossRef]
- Jin, L.-Z.; Dersjant-Li, Y.; Giannenas, I. Application of aromatic plants and their extracts in diets of broiler chickens. Feed Addit. 2020, 159–185. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition–a review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent advances in biologically active compounds in herbs and spices: A review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; Intech Open: London, UK, 2020; pp. 1–27. [Google Scholar]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; Morais, S.M.; Lima, A.; Martins, C.G.; Alexandrino, C.D.; Ferreira PA, T.; Rodrigues, A.L.M.; Rodrigues, S.P. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar]
- Taranu, I.; Habeanu, M.; Gras, M.; Pistol, G.; Lefter, N.; Palade, M.; Ropota, M.; Sanda Chedea, V.; Marin, D. Assessment of the effect of grape seed cake inclusion in the diet of healthy fattening-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e30–e42. [Google Scholar]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin b1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon 2019, 162, 24–31. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Rotar, M.C.; Ropota, M.; Taranu, I. Bioactive compounds from dietary whole grape seed meal improved colonic inflammation via inhibition of MAPKs and NF-KB signaling in pigs with DSS induced colitis. J. Funct. Foods 2020, 66, 103708. [Google Scholar] [CrossRef]
- Hafeez, A.; Hassni, S.F.; Naz, S.; Alonaizan, R.; Al-Akeel, R.K.; Sifa, D.; Shamsi, S.; Ullah Khan, R. Impact of Grape (Vitis vinifera) Seed extract on egg production traits, nutrients digestability, lipid peroxidation and fertility of golden laying hens (Gallus gallus) during early stage of production. Vet. Q. 2023, 43, 1–7. [Google Scholar] [CrossRef]
- Madkour, M.; Abdel-Fattah, S.A.; Ali, S.I.; Ali, N.G.M.; Shourrap, M.; Hosny, M.; Elolimy, A.A. Impact of in ovo feeding of grape pomace extract on the growth performance, antioxidant status, and immune response of hatched broilers. Poult. Sci. 2024, 103, 103914. [Google Scholar] [CrossRef]
- Derbali, H.; Ben Saïd, S.; Abid, K.; Aroua, M.; Jabri, J.; Dhaouafi, J.; Tissaoui, M.; Malek, A.; Bouzid, K.; Mahouachi, M. Valorization of dehydrated grape pomace waste as a low-cost feed additive to improve reproduction and growth performance of male rabbits. Waste Biomass Valor 2024, 15, 3987–3996. [Google Scholar] [CrossRef]
- Li, Y.; Shi, C.; Deng, J.; Qiu, X.; Zhang, S.; Wang, H.; Qin, X.; He, Y.; Cao, B.; Su, H. Effects of grape pomace on growth performance, nitrogen metabolism, antioxidants, and microbial diversity in Angus Bulls. Antioxidants 2024, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Antunović, Z.; Šalavardić, Ž.K.; Steiner, Z.; Đidara, M.; Drenjančević, M.; Ronta, M.; Pavić, V.; Barron, L.J.; Novoselec, J. Meat quality, metabolic profile and antioxidant status of lambs fed on seedless grape pomace (Vitis vinifera L.). Ann. Anim. Sci. 2023, 23, 809–818. [Google Scholar] [CrossRef]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, D.; Zhao, X.; Xiao, Z.; Sun, J.; Yuan, T.; Wang, Y.; Zuo, X.; Yang, G.; Yu, T. Dietary grape pomace extract supplementation improved meat quality, antioxidant capacity, and immune performance in finishing pigs. Front. Microbiol. 2023, 14, 1116022. [Google Scholar] [CrossRef]
- Avilés Peterson, K.A.; Montalvo Corral, M.; González Ríos, H.; Parra Sánchez, H.; Barrera Silva, M.A.; Pinelli Saavedra, A. Grape pomace on the growth performance and intestinal microbiota of finishing pigs. Biotecnia 2024, 26, 274–282. [Google Scholar] [CrossRef]
- da Silveira Almeida, B.C.; Ludke, M.d.C.M.M.; Bertol, T.M.; Ludke, J.V.; Bernardi, D.M.; Cunha, A., Jr.; Coldebella, A. Growth performance, meat quality, and lipid oxidation in pigs’ fed diets containing grape pomace. Appl. Biosci. 2024, 3, 378–391. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine: Eleventh, Reviseded. Wash. DC: Natl. Acad. Press 2012, 10, 13298. [Google Scholar]
- ISO 6496; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6498; Animal Feeding Stuffs—Preparation of Test Samples. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6492; Animal Feeding Stuffs—Determination of Fat Content. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6865; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. International Organization for Standardization: Geneva, Switzerland, 2002.
- ISO 5983; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 1: Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 2171; Cereals, Pulses and By-Products—Determination of Ash Yield by Incineration. International Organization for Standardization: Geneva, Switzerland, 2010.
- Luca, S.V.; Kulinowski, Ł.; Ciobanu, C.; Zengin, G.; Czerwińska, M.E.; Granica, S.; Xiao, J.; Skalicka-Woźniak, K.; Trifan, A. Phytochemical and multi-biological characterization of two Cynara scolymus L. varieties: A glance into their potential large scale cultivation and valorization as bio-functional ingredients. Ind. Crops Prod. 2022, 178, 114623. [Google Scholar] [CrossRef]
- Sehm, J.; Lindermayer, H.; Meyer, H.H.D.; Pfaffl, M.W. The influence of apple- and red-wine pomace rich diet on MRNA expression of inflammatory and apoptotic markers in different piglet organs. Anim. Sci. 2006, 82, 877–887. [Google Scholar] [CrossRef]
- Zimmermann, A.; Camenisch, U.; Rechsteiner, M.P.; Bode-Lesniewska, B.; Rössle, M. Value of immunohistochemistry in the detection of BRAF V600E mutations in fine-needle aspiration biopsies of papillary thyroid carcinoma. Cancer Cytopathol. 2014, 122, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.R.; Davoodi, H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011, 35, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal absorption and antioxidant activity of grape pomace polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef]
- Yan, L.; Kim, I. Effect of dietary grape pomace fermented by saccharomyces boulardii on the growth performance, nutrient digestibility and meat quality in finishing pigs. Asian-Australas. J. Anim. Sci. 2011, 24, 1763–1770. [Google Scholar]
- Trombetta, F.; Fruet, A.; Stefanello, F.; Fonseca, P.; De Souza, A.; Tonetto, C.; Rosado Júnior, A.; Nörnberg, J. Effects of the dietary inclusion of linseed oil and grape pomace on weight gain, carcass characteristics, and meat quality of swine. Int. Food Res. J. 2019, 26, 1741. [Google Scholar]
- Vlaicu, P.A.; Panaite, T.D.; Cornescu, M.G.; Ropota, M.; Olteanu, M.; Drăgotoiu, D. The influence of by-products on the production parameters and nutrient digestibility in fattening pigs diet (60–100 Kg). AgroLife Sci. J. 2019, 8, 261–269. [Google Scholar]
- Rajković, E.; Schwarz, C.; Tischler, D.; Schedle, K.; Reisinger, N.; Emsenhuber, C.; Ocelova, V.; Roth, N.; Frieten, D.; Dusel, G.; et al. Potential of grape extract in comparison with therapeutic dosage of antibiotics in weaning piglets: Effects on performance, digestibility and microbial metabolites of the ileum and colon. Animals 2021, 11, 2771. [Google Scholar] [CrossRef]
- Aguiar, S.C.; Cottica, S.M.; Boeing, J.S.; Samensari, R.B.; Santos, G.T.; Visentainer, J.V.; Zeoula, L.M. Effect of feeding phenolic compounds from propolis extracts to dairy cows on milk production, milk fatty acid composition, and the antioxidant capacity of milk. J Anim. Feed Sci Technol 2014, 193, 148–154. [Google Scholar] [CrossRef]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.L.; Chentouf, M.; Cabaraux, J.F. Characterisation of bitter vetch (Vicia ervilia (L.) Willd) ecotypes: An ancient and promising legume. Exp Agric 2024, 60, 19. [Google Scholar]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Louvieaux, J.; Hornick, J.L.; Cabaraux, J.F.; Chentouf, M. Growth performance, carcass characteristics, fatty acid profile, and meat quality of male goat kids supplemented by alternative feed resources: Bitter vetch and sorghum grains. Arch. Anim. Breed. 2024, 67, 481–492. [Google Scholar]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-ΚB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [PubMed]
- Wang, R.; Yu, H.; Fang, H.; Jin, Y.; Zhao, Y.; Shen, J.; Zhou, C.; Li, R.; Wang, J.; Fu, Y. Effects of dietary grape pomace on the intestinal microbiota and growth performance of weaned piglets. Arch. Anim. Nutr. 2020, 74, 296–308. [Google Scholar]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016, 7, 80313. [Google Scholar]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef]
- Gessner, D.; Ringseis, R.; Siebers, M.; Keller, J.; Kloster, J.; Wen, G.; Eder, K. Inhibition of the pro-inflammatory NF-κB pathway by a grape seed and grape marc meal extract in intestinal epithelial cells. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1074–1083. [Google Scholar]
- Fournier, B.; Parkos, C. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef]
- Varricchio, E.; Coccia, E.; Orso, G.; Lombardi, V.; Imperatore, R.; Vito, P.; Paolucci, M. Influence of polyphenols from olive mill wastewater on the gastrointestinal tract, alveolar macrophages and blood leukocytes of pigs. Ital. J. Anim. Sci. 2019, 18, 574–586. [Google Scholar]
- Jung, H.C.; Eckmann, L.; Yang, S.; Panja, A.; Fierer, J.; Morzycka-Wroblewska, E.; Kagnoff, M. A Distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 1995, 95, 55–65. [Google Scholar]
- Lallès, J.-P.; Boudry, G.; Favier, C.; Le Floc’h, N.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef]
- Zhu, Y.; Österlundh, I.; Hultén, F.; Magnusson, U. Tumor Necrosis Factor-α, Interleukin-6, serum amyloid A, haptoglobin, and cortisol concentrations in sows following intramammary inoculation of Escherichia coli. Am. J. Vet. Res. 2004, 65, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Davis, B.; Skjolaas, K.; Burkey, T.; Dritz, S.; Johnson, B.; Minton, J. Effects of feeding Salmonella Enterica Serovar Typhimurium or Serovar Choleraesuis on growth performance and circulating Insulin-like Growth Factor-I, Tumor Necrosis Factor-α, and Interleukin-1β in weaned pigs. J. Anim. Sci. 2007, 85, 1161–1167. [Google Scholar] [CrossRef]
- Philpott, M.; Ferguson, L.R. Immunonutrition and cancer. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2004, 551, 29–42. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear Factor-ΚB—A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef]
- Chang, H.Y.; Yang, X. Proteases for cell suicide: Functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 2000, 64, 821–846. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-ΚB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Hayden, M.; West, A.; Ghosh, S. NF-ΚB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar]
- Cho, S.-J.; Jung, U.J.; Park, H.-J.; Kim, H.-J.; Park, Y.B.; Kim, S.R.; Choi, M.-S. Combined ethanol extract of grape pomace and omija fruit ameliorates adipogenesis, hepatic steatosis, and inflammation in diet-induced obese mice. Evid.-Based Complement. Altern. Med. 2013, 2013, 212139. [Google Scholar]
- Chuang, C.-C.; Bumrungpert, A.; Kennedy, A.; Overman, A.; West, T.; Dawson, B.; McIntosh, M.K. Grape powder extract attenuates Tumor Necrosis Factor α-mediated inflammation and insulin resistance in primary cultures of human adipocytes. J. Nutr. Biochem. 2011, 22, 89–94. [Google Scholar]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Giguère, A.; Lessard, M. Dietary supplementation with different forms of flax in late gestation and lactation: Effects on sow and litter performances, endocrinology, and immune response. J. Anim. Sci. 2010, 88, 225–237. [Google Scholar]
- Hur, S.J.; Kang, S.H.; Jung, H.S.; Kim, S.C.; Jeon, H.S.; Kim, I.H.; Lee, J.D. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr. Res. 2012, 32, 801–816. [Google Scholar]
- Zhan, Z.; Huang, F.; Luo, J.; Dai, J.; Yan, X.; Peng, J. Duration of feeding linseed diet influences expression of inflammation-related genes and growth performance of growing-finishing barrows. J. Anim. Sci. 2009, 87, 603–611. [Google Scholar] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar]
- Shortman, K.; Heath, W.R. Immunity or tolerance? that is the question for dendritic cells. Nat. Immunol. 2001, 2, 988–989. [Google Scholar]
- Akiyama, H.; Sato, Y.; Watanabe, T.; Nagaoka, M.H.; Yoshioka, Y.; Shoji, T.; Kanda, T.; Yamada, K.; Totsuka, M.; Teshima, R. Dietary unripe apple polyphenol inhibits the development of food allergies in murine models. FEBS Lett. 2005, 579, 4485–4491. [Google Scholar]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [PubMed]
- Huang, R.-Y.; Yu, Y.-L.; Cheng, W.-C.; OuYang, C.-N.; Fu, E.; Chu, C.-L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [PubMed]
- Lee, J.S.; Kim, S.G.; Kim, H.K.; Lee, T.; Jeong, Y.; Lee, C.; Yoon, M.; Na, Y.J.; Suh, D.; Park, N.C. Silibinin Polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells. J. Cell. Physiol. 2007, 210, 385–397. [Google Scholar] [PubMed]
- Yoon, M.-S.; Lee, J.S.; Choi, B.-M.; Jeong, Y.-I.; Lee, C.-M.; Park, J.-H.; Moon, Y.; Sung, S.-C.; Lee, S.K.; Chang, Y.H. Apigenin inhibits immunostimulatory function of dendritic cells: Implication of immunotherapeutic adjuvant. Mol. Pharmacol. 2006, 70, 1033–1044. [Google Scholar]
- Hou, X.; Zhang, J.; Ahmad, H.; Zhang, H.; Xu, Z.; Wang, T. Evaluation of antioxidant activities of ampelopsin and its protective effect in lipopolysaccharide-induced oxidative stress piglets. PLoS ONE 2014, 9, e108314. [Google Scholar]
- Wang, M.; Suo, X.; Gu, J.; Zhang, W.; Fang, Q.; Wang, X. Influence of grape seed proanthocyanidin extract in broiler chickens: Effect on chicken coccidiosis and antioxidant Status. Poult. Sci. 2008, 87, 2273–2280. [Google Scholar]
- Cheng, Y.-T.; Wu, C.-H.; Ho, C.-Y.; Yen, G.-C. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J. Nutr. Biochem. 2013, 24, 475–483. [Google Scholar]
- Hao, R.; Li, Q.; Zhao, J.; Li, H.; Wang, W.; Gao, J. Effects of Grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest. Sci. 2015, 178, 237–242. [Google Scholar]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-ΚB are coordinated effectors of the rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar]
- Scapagnini, G.; Sonya, V.; Nader, A.G.; Calogero, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [PubMed]
- Wei, C.; Chen, X.; Chen, D.; Yu, B.; Zheng, P.; He, J.; Chen, H.; Yan, H.; Luo, Y.; Huang, Z. Dihydromyricetin enhances intestinal antioxidant capacity of growing-finishing pigs by activating ERK/Nrf2/HO-1 signaling pathway. Antioxidants 2022, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar]
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000, 33, 819–830. [Google Scholar]
- Bobek, P.; Ozdín, L.; Hromadova, M. The effect of dried tomato, grape and apple pomace on the cholesterol metabolism and antioxidative enzymatic system in rats with hypercholesterolemia. Food/Nahrung 1998, 42, 317–320. [Google Scholar] [PubMed]
- Inoue, H.; Maeda-Yamamoto, M.; Nesumi, A.; Tanaka, T.; Murakami, A. Low and medium but not high doses of green tea polyphenols ameliorated dextran sodium sulfate-induced hepatotoxicity and nephrotoxicity. Biosci. Biotechnol. Biochem. 2013, 77, 1223–1228. [Google Scholar]
- Zhang, H.J.; Jiang, X.R.; Mantovani, G.; Lumbreras, A.E.V.; Comi, M.; Alborali, G.; Savoini, G.; Dell’Orto, V.; Bontempo, V. Modulation of Plasma antioxidant activity in weaned piglets by plant polyphenols. Ital. J. Anim. Sci. 2014, 13, 3242. [Google Scholar]
- Prata, C.; Zalambani, C.; Rossi, F.; Rossello, S.; Cerchiara, T. Nutrients and Nutraceuticals from Vitis vinifera L. Pomace: Biological Activities, Valorization, and Potential Applications. Nutrients 2025, 17, 583. [Google Scholar] [CrossRef]
- González-Barrio, R.; Gasch-Tolrá, J.; Tomás-Barberán, F.A.; Selma, M.V. Grape Pomace as a Cardiometabolic Health-Promoting Ingredient: Activity in the Intestinal Environment. Antioxidants 2023, 12, 979. [Google Scholar] [CrossRef]
- Guaita, M.; Motta, S.; Messina, S.; Casini, F.; Bosso, A. Polyphenolic Profile and Antioxidant Activity of Green Extracts from Grape Pomace Skins and Seeds of Italian Cultivars. Foods 2023, 12, 3880. [Google Scholar] [CrossRef]
- Blasi, F.; Trovarelli, V.; Mangiapelo, L.; Ianni, F. Grape Pomace for Feed Enrichment to Improve the Quality of Animal-Based Foods. Foods 2024, 13, 3541. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Bulgaru, V.C.; Taranu, I. Grape By-Products and Their Efficiency in Alleviating the Intestinal Disorders in Post-Weaning Piglets. Arch. Zootech. 2023, 26, 56–77. [Google Scholar]
- Schedle, K.; Pfaffl, M.W.; Plitzner, C. Effect of Insoluble Fiber on Intestinal Morphology and mRNA Expression Pattern of Inflammatory, Cell Cycle and Growth Marker Genes in a Piglet Model. Arch. Anim. Nutr. 2008, 62, 427–438. [Google Scholar] [PubMed]
- Modina, S.C.; Polito, U.; Rossi, R.; Corino, C. Nutritional Regulation of Gut Barrier Integrity in Weaning Piglets. Animals 2019, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M. Grape By-Products as Feedstuff for Pig and Poultry Production. Animals 2022, 12, 2239. [Google Scholar] [CrossRef]
- Chiou, P.W.S.; Bi, Y.; Chang, L. Effect of Different Components of Dietary Fiber on the Intestinal Morphology of Domestic Rabbits. Comp. Biochem. Physiol. Part A Physiol. 1994, 109, 637–647. [Google Scholar]
- Patra, A.K.; Amasheh, S. Modulation of Gastrointestinal Barrier and Nutrient Transport Function in Farm Animals by Natural Plant Bioactive Compounds—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3237–3268. [Google Scholar] [CrossRef]
- Saliu, E.M.; Martínez-Vallespín, B. Dietary Fiber and Its Role in Performance, Welfare, and Health of Pigs. Anim. Health Res. Rev. 2022, 23, 165–193. [Google Scholar]
- Asadnezhad, B.; Pirmohammadi, R.; Alijoo, Y. The Effects of Dietary Supplementation with Red Grape Pomace Treated with Ozone Gas on Ruminal Fermentation Activities, Nutrient Digestibility, and Lactational Performance. J. Agric. Food Res. 2024, 18, 100489. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).