1. Introduction
The Korean livestock industry faces substantial challenges, including labor shortages driven by an aging workforce, escalating raw material costs, and rising energy prices. To mitigate these challenges, smart-farming technologies have emerged as essential solutions, addressing labor shortages while attracting younger generations to the livestock sector. Smart-farm technology utilizes IoT-based dynamic control algorithms [
1,
2,
3] to enable precise livestock environment management, necessitating comprehensive environmental monitoring systems. Despite advancements in IT and sensor technologies, research on big data-driven decision-support algorithms remains limited, impeding farmers’ ability to optimize productivity [
4,
5,
6]. Among key productivity factors—such as feed intake efficiency, growth rates, and mortality rates—mortality exerts the greatest economic impact on agricultural operations and should be managed through engineering-based approaches.
In pig houses, mortality is predominantly attributed to disease-related factors. While highly contagious infectious diseases such as foot-and-mouth disease are controlled at the national level through preventive measures, chronic wasting syndromes caused by viruses, bacteria, and other environmental factors continue to impose substantial economic burdens on farmers [
7,
8,
9,
10]. In Korea, four major wasting diseases significantly impact commercial pig farms: postweaning multisystemic wasting syndrome (PMWS), porcine respiratory disease complex (PRDC), porcine reproductive and respiratory syndrome (PRRS), and porcine epidemic diarrhea (PED). The complete eradication of these pathogens from farms remains virtually unattainable, as they are persistently transmitted through direct and indirect routes, including human contact, vehicles, equipment, and airborne pathways.
While not all viral exposures result in disease, animals with compromised immunity—often due to environmental stress—are significantly more susceptible [
11,
12]. In particular, suboptimal environmental conditions induce climatic stress, impairing immune function and increasing disease susceptibility [
13]. Consequently, optimizing the livestock environment is essential for minimizing climate-induced stress, maintaining immune resilience, and ultimately reducing disease incidence and mortality rates [
14,
15]. Identifying the critical environmental factors that require management through an engineering-based approach, integrated with mortality rate analysis, is imperative. This comprehensive strategy facilitates the effective identification and mitigation of environmental stressors, thereby enhancing livestock health and reducing disease prevalence.
Inadequate management of temperature, ventilation, and other environmental factors within swine housing can create unfavorable conditions, exposing animals to climate-induced stress that adversely affects their health [
16,
17]. However, previous studies on respiratory diseases and livestock environments have predominantly centered on field experiments investigating viral pathogens, airborne pollutants, and vaccine development for disease prevention [
18,
19,
20,
21,
22,
23,
24]. Further research is required to elucidate the key environmental determinants contributing to the onset and transmission of respiratory diseases.
Respiratory diseases in livestock are multifactorial and influenced by the complex interplay of various stressors, including climate-induced stress resulting from inadequate temperature regulation, humidity fluctuations, airborne dust, and gas accumulation. Additionally, pre-existing health conditions and external environmental factors further exacerbate disease susceptibility [
25,
26,
27,
28,
29,
30]. However, few studies have systematically examined the interconnections among these stressors and their collective impact on disease progression.
Song et al. [
31] investigated the potential airborne transmission of diseases within swine housing using trace gases as experimental indicators, suggesting that airborne dissemination could serve as a significant disease propagation route. Similarly, Seo et al. [
32] conducted field experiments demonstrating that wasting diseases in swine housing can spread through airborne pathways in addition to direct contact transmission.
Future research should prioritize a comprehensive understanding of the interactions between various environmental stressors, respiratory diseases, and livestock mortality. An interdisciplinary approach integrating engineering methodologies with veterinary and environmental sciences is crucial for developing effective disease management strategies to mitigate respiratory disease transmission in livestock.
In this study, a field experiment was conducted to collect monitoring data on the internal livestock environment and investigate the environmental factors contributing to pig mortality. A commercial pig farm facing challenges in environmental control was visited, and the rearing conditions and mortality records were systematically monitored. Through the analysis of extensive environmental data gathered from this livestock facility, the key factors significantly influencing mortality rates were identified.
2. Materials and Methods
2.1. Experimental Design and Site Description
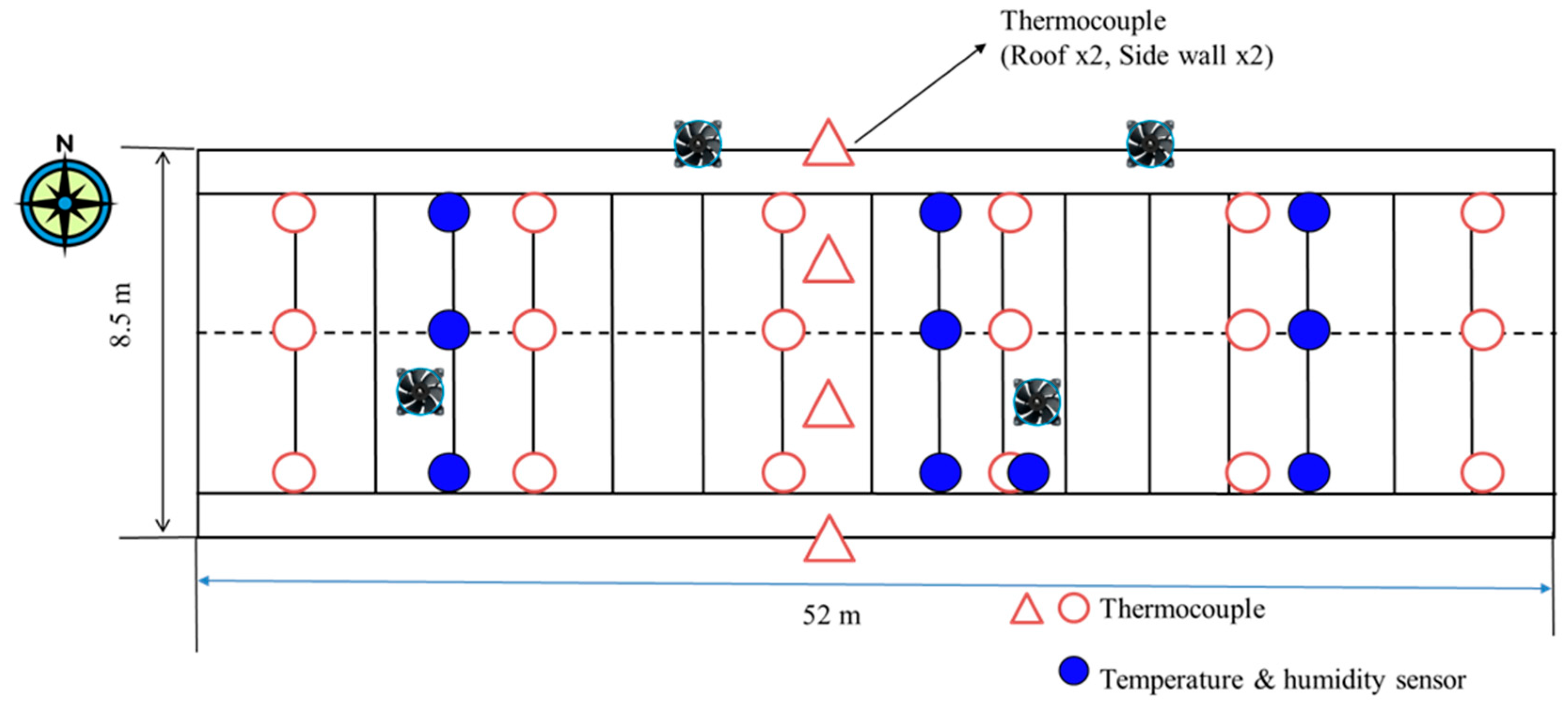
This study was conducted in a commercial pig farm (36°35′ N and 126°32′ E) accommodating approximately 600 growing pigs between 50 and 90 days old. The facility has a length of 52 m, a width of 8.5 m, and height of 3.7 m. The ventilation system includes two 32-inch roof exhaust fans and two 42-inch sidewall exhaust fans, operating sequentially based on internal temperature conditions, while a forced ventilation system is employed, drawing air through a slot-type inlet on the opposite side wall. The experimental pig house utilizes a negatively pressured ventilation system, featuring two 32-inch variable ducts installed on the ceiling. The ventilation rate is regulated based on the set temperature, monitored by a temperature sensor located approximately 2 m above the floor in the center of the facility. When the temperature increases and additional ventilation is required, two 42-inch fans on the north side wall are activated in a sequential manner. The air inlet from the outside utilizes a negatively pressured slot duct located on the same north side wall (
Figure 1).
2.2. Instrumentation and Measurement
To ensure reproducibility, all instruments used in this study and their calibration protocols were carefully documented. For temperature and humidity measurement, T-type thermocouples with an accuracy of ±0.2 °C were employed for continuous monitoring. These thermocouples were strategically installed at two different heights within the facility: 2 m to represent the upper zone and 1 m to reflect the pig zone. Additionally, HOBO data loggers (Onset Corp., Bourne, MA, USA) were used to measure air temperature and relative humidity (
Figure 2). These loggers were placed near the air inlets and outlets to capture variations within the facility. All sensors were calibrated against a certified reference thermometer prior to installation to ensure accuracy.
Air quality was monitored using a PAC III gas detector (Dräger, Lübeck, Germany), capable of detecting ammonia concentrations within the range of 0 to 200 ppm with an accuracy of ±5%. Dust concentrations, including PM10 and PM2.5 levels, were measured using a portable aerosol spectrometer (Model 1.108, Grimm Aerosol Technik GmbH & Co. KG, Ainring, Germany). Both instruments were calibrated using certified gas and particulate standards to maintain the reliability of the data.
Ventilation rates were evaluated using a thermal anemometer (Testo 425, Germany) with a velocity range of 0 to 20 m/s and an accuracy of ±0.03 m/s. Airflow measurements were conducted at multiple points along the ventilation outlets to calculate the air exchange rate (AER), providing an accurate assessment of the ventilation system’s performance.
External weather conditions were continuously monitored using a weather station (Vantage Pro2, Davis Instruments, Hayward, CA, USA) installed on the roof of the pig house. The station recorded key environmental parameters, including ambient temperature, relative humidity, wind speed, solar radiation, and precipitation, at 10 min intervals. These external data were critical for understanding the influence of external climatic conditions on the internal environment of the pig house.
2.3. Feed and Management Practices
All pigs were fed a standardized commercial feed formulated for growing pigs (13.5% crude protein, 3.2% crude fat). Feed and water were provided ad libitum using automatic feeders and drinkers. Management practices followed routine protocols, including weekly cleaning of pens, routine vaccination schedules, and deworming treatments. Mortality and health status were closely monitored by the farm staff and veterinarians.
During the experimental period, environmental variables were systematically controlled to ensure the reliability of the study. Ventilation rates were carefully managed using a six-stage system that adjusted airflow based on internal temperature readings. These rates ranged from a minimum air exchange rate (AER, h−1) of 18 to a maximum of 44. On-site measurements indicated that the actual AER was 30 h−1, which accounted for 67% of the system’s design capacity. Daily verification of ventilation adjustments was performed to maintain consistency. Temperature control was another critical aspect of the experiment. External temperatures during the study period ranged from 18.1 °C to 28.7 °C. Diurnal temperature variations were closely monitored and analyzed to assess their potential impact on pig mortality, particularly in areas where environmental instability might exacerbate health risks. To minimize confounding factors, pigs were distributed evenly across pens based on their age (ranging from 70 to 110 days) and weight. This approach ensured uniformity in the experimental population and reduced variability caused by individual differences. These measures provided a controlled environment to accurately evaluate the relationship between environmental conditions and pig mortality.
2.4. Data Analysis
Monitoring and data analysis were conducted to establish environmental criteria aimed at reducing the mortality rate associated with pig wasting diseases. The study focused on monitoring mortality caused by PRRS (porcine reproductive and respiratory syndrome) and PCV2 (porcine circovirus type 2), which are representative of pig wasting diseases. Environmental factors such as temperature, humidity, dust levels, and gas concentrations were measured to identify potential contributors to these health issues.
To determine the causes of pig deaths, autopsies were performed by local veterinarians. Viral infections were analyzed using RT-PCR (real-time polymerase chain reaction), a technique that quantifies viral DNA and RNA to detect the presence of pathogens. This method was used to test for nine viruses commonly found in domestic pig farms in Korea, including PRRS, PCV-2, M. pneumoniae, and Salmonella.
Additionally, the specific locations and dates of pig deaths within each pigpen were meticulously recorded. These mortality data were integrated with the collected environmental measurements to analyze key factors contributing to climate stress within the pig house. By combining these data sources, the study provided insights into the environmental conditions that exacerbate pig mortality, particularly in relation to viral infections and environmental stressors. To quantify the relationship between microclimate variability and pig mortality, Pearson correlation analysis was conducted to examine the association between diurnal temperature deviation and mortality rate. Additionally, a linear regression analysis was performed to assess the statistical significance of temperature fluctuations on mortality. The statistical significance of the results was determined using a p-value threshold of 0.05. All data were analyzed using SPSS (version 25.0) to control for confounding factors.
3. Results
3.1. Pig Mortality
After changing the ventilation structure of the experimental pig house before the experiment, there was a significant increase in mortality. The mortality rate reached alarming levels, exceeding 40%, which was largely attributed to respiratory diseases, estimated by coughing and shivering of pigs. The grower pigs appeared to be in good health prior to their transfer into the experimental pig house. However, their health gradually deteriorated after the transfer, resulting in a rapid increase in the mortality rate within a span of approximately two weeks.
Figure 3 provides an overview of the pig mortality rate during the experimental period. The number of pigs in the experimental pig house accounted for around 20% of the total animal number in the pig farm, yet approximately 68.9% of the total mortality occurred within this specific facility. This indicates a significant issue with the breeding environment, including the number of pigs that died after being moved to the experimental pig house. We aimed to demonstrate the relationship between environmental influences and mortality during periods 1 and 2 in
Figure 3, where the mortality rate intensively increased, by distinguishing each period into two-week intervals.
To investigate the causes of death in the experimental pig house, virus examinations were conducted on the carcasses and feces of pigs (
Table 1). It was found that pigs between 8 and 14 weeks of age, being raised in the experimental pig house, were infected with PRRS and PCV-2 virus. Additionally,
M. hyopneumoniae,
P. multocida,
A. pleuropneumoniae,
H. parasuis, and
Salmonella viruses were also detected. Through virus detection and autopsies, the direct causes of swine death were identified as complications such as pneumonia caused by infectious diseases. In general, the experimental farms showed the spread of PCV-2 infections, which are responsible for PMWS. Moreover, there was a high risk of infection with Glässer’s disease and salmonellosis. The main pathways for swine mortality can be estimated as follows: (1) Pigs infected with PRRS and PCV-2 viruses experienced weakened immune systems, allowing PCV-2 to proliferate and trigger PMWS. (2) Diseased pigs with severely compromised immune systems were prone to secondary bacterial infections and complex infections. (3) There was an increased risk of mortality from PRDC (porcine respiratory disease complex), characterized by symptoms such as severe coughing. These factors contribute to the understanding of the primary causes of swine mortality within the experimental pig house (
Figure 4).
Viruses can spread through various routes within this farm. The majority of livestock in the pig house are susceptible to viral infections, as they can be transmitted through air droplets from infected animals by feces, saliva, and coughing. However, not all animals manifest the diseases, as individual immunity levels vary. Despite having the same diet and receiving the same vaccination regimen, differences in immunity are attributed to environmental variations in each region. To address this issue, it is crucial to maintain high levels of immunity by reducing stress. This can be achieved through improving the breeding environment, implementing vaccination protocols, and utilizing antibiotics that enhance immunity.
3.2. Internal Environmental Conditions
To investigate the internal environmental conditions, periodic visits and monitoring of the experimental pig house were conducted. A poor breeding environment can be seen where pigs were coughing frequently and severely and often remained lying down without movement (
Figure 4). Additionally, a common clinical symptom of PMWS was detected by the severely exposed spines of pigs. These observations provided indications that the pigs were subjected to a suboptimal breeding environment. To support these speculations, various environmental factors were measured, and the locations of deceased pigs were recorded for each room.
The average concentration of particulate matter measured in the experimental pig house was observed to be TSP 240 μg/m3, PM-10 106 μg/m3, and PM-2.5 45 μg/m3 under stable conditions. The occupational safety standard for respirable dust particles smaller than 4 μm is 160 μg/m3, indicating that the particulate matter concentration in the experimental pig house was not high. This is believed to be a result of effective ventilation in sufficient amounts to reduce the particulate matter. Results from the size-specific analysis using the aerosol spectrometer showed that particle concentrations around the 10 μm size were relatively high. This is influenced by the dust from the feed supplied on site. The suspended dust inside the experimental pig house serves as a major carrier for viruses and germs causing respiratory diseases.
The average concentration of ammonia measured from a height of 1 to 1.2 m above the floor was 7.0 ppm in the western space, where ventilation systems were densely installed, while in the eastern space, with relatively fewer ventilation systems, it was 10.7 ppm. Due to issues with the rearing environment and cleaning state, ammonia appears to be generated from the pig waste accumulated on the floor and in the slurry pit, with the concentration at the floor level averaging 28.6 ppm. While it is difficult to assert that the concentration of ammonia is at a level that could lead to mortality, it was acting as a significant stress factor for the pigs.
Despite the poor environmental conditions in the experimental pig house during the experimental period, the concentrations of ammonia and particulate matter were not severe, thanks to adequate ventilation. The ventilation system was adjustable across six stages. Considering the lower outside temperature during the onset of the experiment, which took place in a transitional season, the ventilation rate was maintained at 18 AER (air exchange rate, h−1) and was set to increase to a maximum of 43.8 AER (h−1) depending on external temperatures. However, direct monitoring of the ventilation rate on site showed an actual rate of 29.4 AER (h−1), which is 67% of the designed capacity, indicating its efficiency.
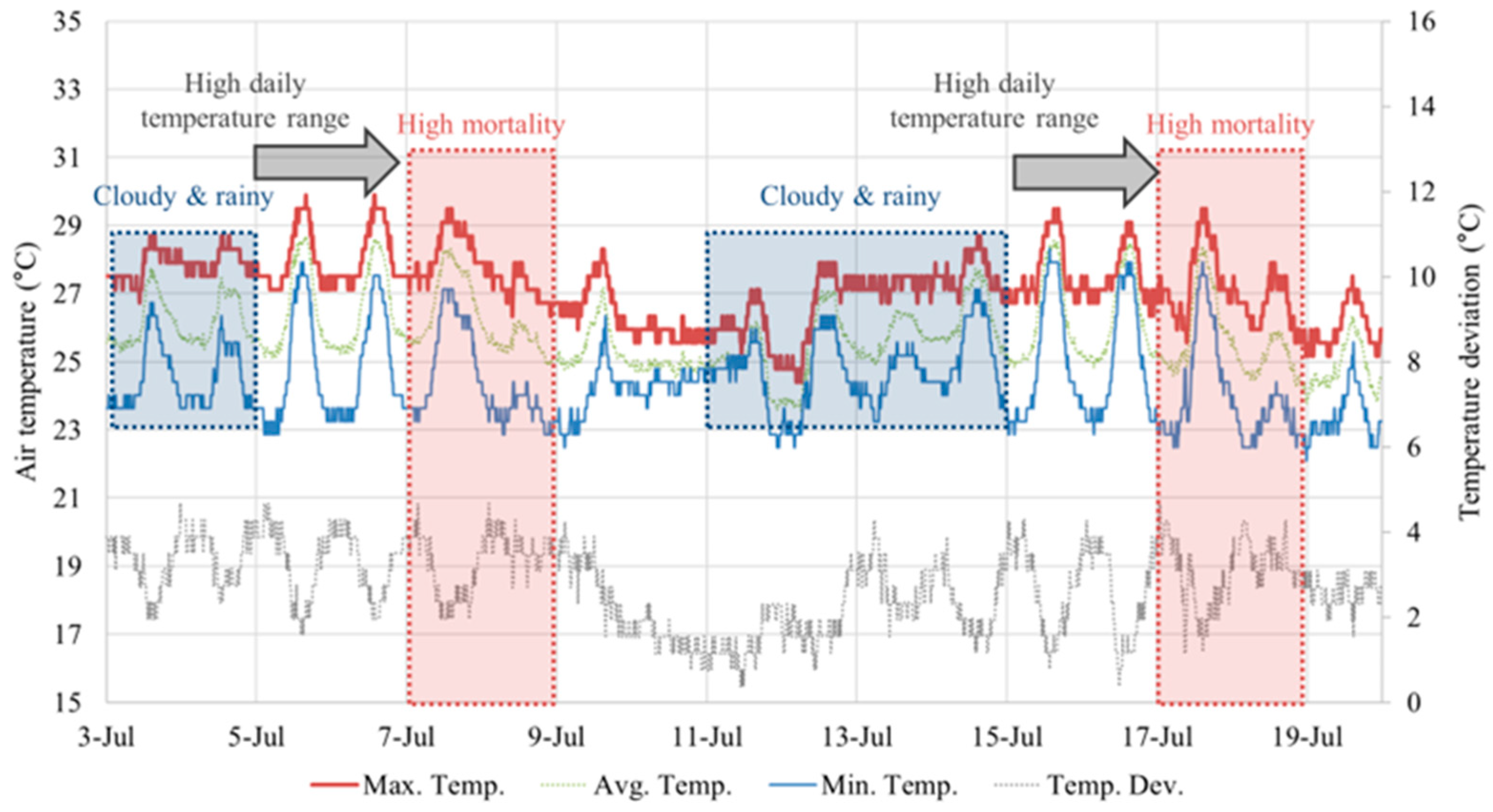
The influx of air due to ventilation is impossible to observe visually on site. Therefore, considering the temperature difference between the interior and exterior, multiple thermocouples were installed to indirectly analyze the air flow pattern by thermal environment. While the average temperature change throughout the entire pig house is important, the local uniformity and the stability of the internal temperature against external temperature changes are also crucial. To analyze this, 40 thermocouples installed lengthwise were used to monitor the temperature in real time. The maximum, minimum, and average temperatures, and temperature variations, within the pig pens were analyzed, as shown in
Figure 5. Additionally, to understand the patterns and periods when most mortality occurred, spatial temperature contours were studied. The analysis of continuous temperature monitoring revealed that the mortality rate spiked during the changing seasons and the rainy season, when there is a high diurnal temperature range. Despite consistent high levels of ammonia and dust concentration during each period, the mortality rate varied across specific periods. This led to the hypothesis that rapid temperature changes were the primary cause of environmental stress. Cold air, particularly during the night and rainy period, can significantly impact pig immunity and increase the risk of pneumonia with severe coughing. However, it was very challenging to derive a statistical correlation between the mortality rate and metrics like average daily temperature or temperature deviation. While there is a direct association between the environment and the mortality rate due to disease, quantifying it is difficult. This is because of the latency of viruses, and the time it takes for disease to manifest and lead to mortality varies depending on the individual characteristics of each pig.
3.3. Cause Analysis of Mortality
The relationship between mortality and environmental conditions was analyzed using daily records of pig deaths, including their specific time and location within the facility. Direct statistical analysis of the correlation between environmental factors and mortality was challenging due to the influence of confounding factors such as disease prevalence, individual immunity variations, and management practices. However, the analysis of environmental conditions suggested that temperature fluctuations, rather than air quality parameters such as dust and ammonia concentration, were likely to be a significant contributing factor. Although ventilation appeared to be functioning adequately, the spatial distribution of mortality events indicated that variations in airflow and temperature fluctuations may have exacerbated localized environmental stress.
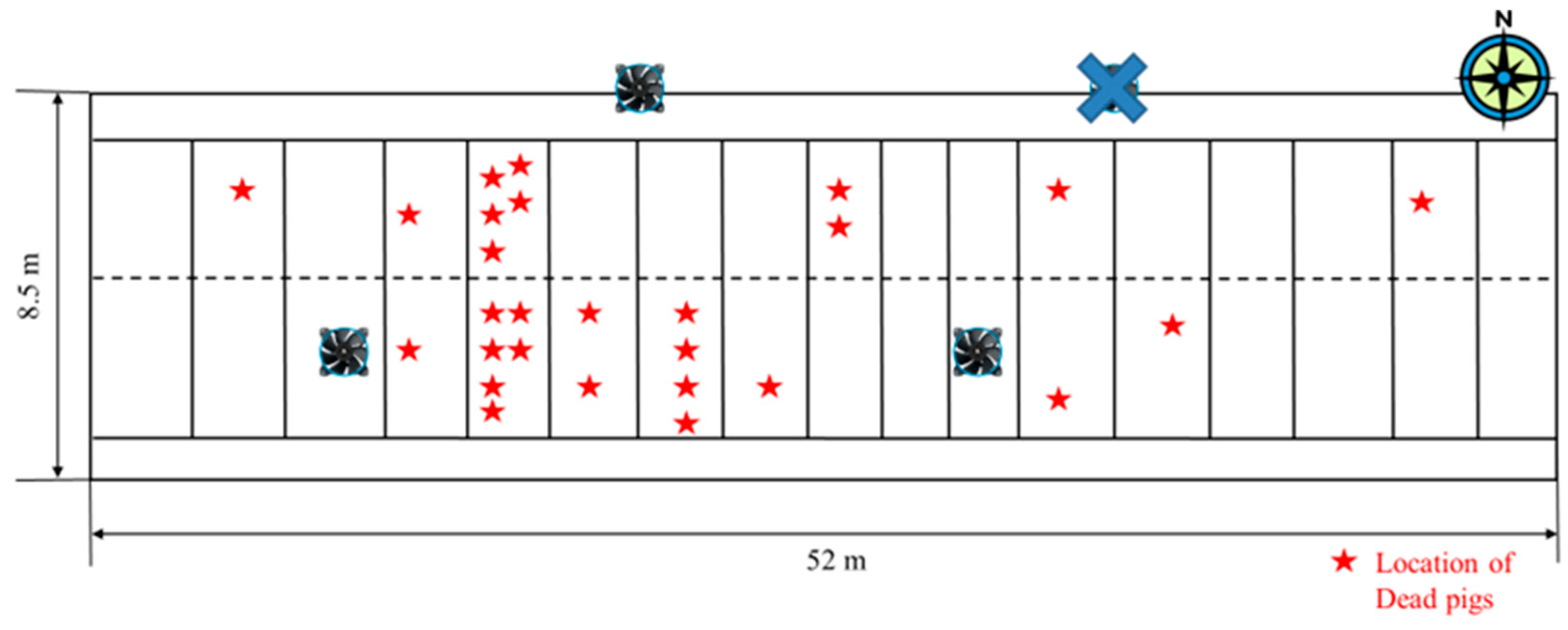
During Period 1, which occurred during the transitional season with significant diurnal temperature variation, only three of the four ventilation fans were operational. Over a span of ten days, mortality events were concentrated in areas where ventilation was most active (
Figure 6). While increased ventilation effectively reduced harmful gases such as ammonia and dust, it also led to greater exposure to external temperature fluctuations. On 20 May, at 2 p.m., temperature variations within the facility were minimal (less than 2 °C), indicating stable conditions. However, by 2 a.m. on 21 May, the diurnal temperature range at certain locations had increased to 15 °C, particularly near the ventilation inlets. Such drastic temperature changes within a short time frame are a well-documented source of thermal stress, weakening pig immunity and increasing their susceptibility to respiratory diseases (
Figure 7 and
Figure 8). The mortality rate spiked in areas experiencing the highest temperature fluctuations, reinforcing the hypothesis that unstable thermal conditions contributed to increased pig deaths.
As ventilation was concentrated in specific areas, the resulting uneven temperature distribution further intensified environmental stress. During periods of low outside temperatures, maintaining stable internal conditions became increasingly difficult, as areas with high ventilation rates experienced a rapid drop in temperature. This issue was most pronounced during transitional seasons when ventilation rates fluctuated significantly, as opposed to winter, when ventilation remained at a minimum level. Ineffective temperature regulation under such conditions can ultimately lead to increased mortality due to immune suppression and respiratory complications.
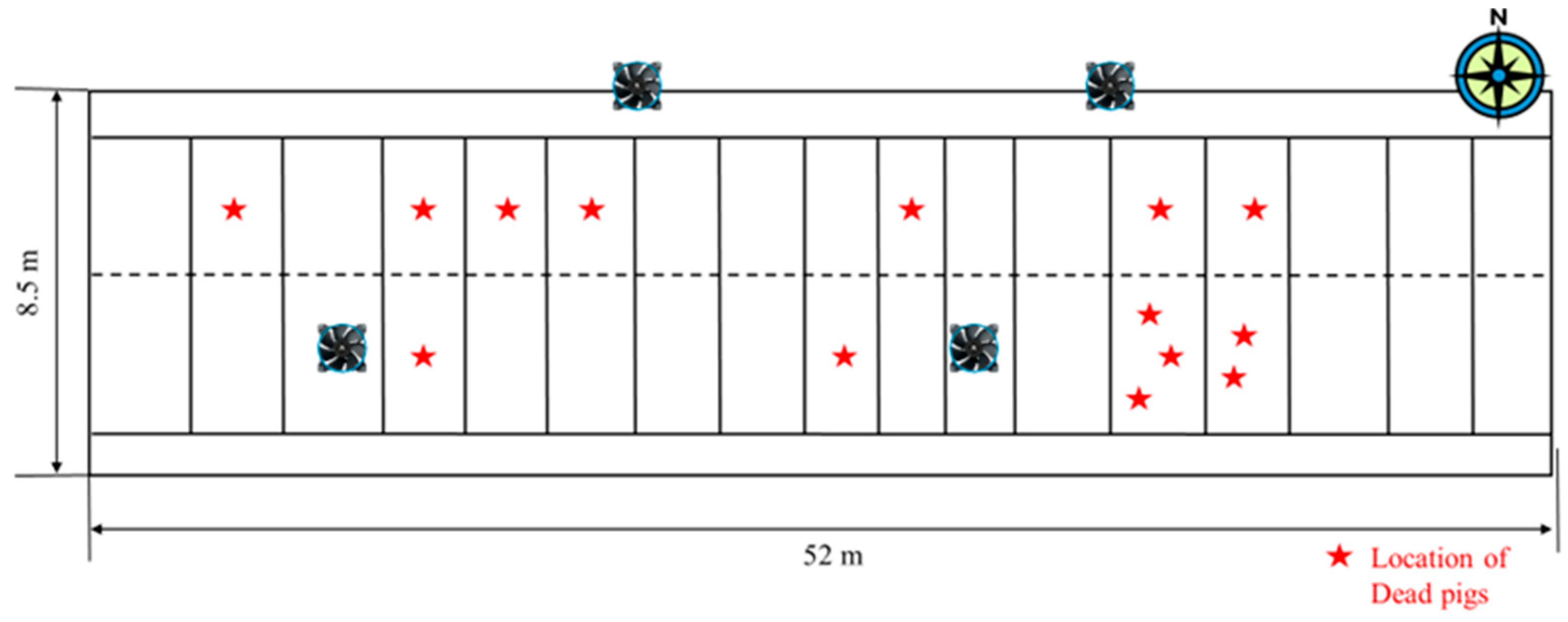
A similar trend was observed during Period 2, which corresponded to a phase of maximum ventilation. Unlike Period 1, mortality events during this phase were more evenly distributed throughout the facility (
Figure 9). Further analysis of mortality timing revealed a pattern: pig deaths were highest two to three days after experiencing large diurnal temperature variations, particularly following multiple consecutive days of overcast or rainy weather, which resulted in a decrease in ambient temperature. For example, on 6 July at 2 p.m., temperature readings across different areas of the facility were relatively uniform, with a mean temperature of 28.1 °C and a spatial variation of 2.8 °C. However, by 2 a.m. on 7 July, the temperature dropped sharply in some regions, with the coldest areas reaching 21.8 °C, while other sections remained at 26 °C. This resulted in a high diurnal temperature fluctuation of 8.7 °C, aligning with a peak in mortality events observed the following day (
Figure 10). This trend suggests that prolonged exposure to unstable thermal conditions was a key factor in pig deaths.
To further validate these observations, a statistical analysis was conducted to assess the relationship between diurnal temperature deviation and pig mortality. The Pearson correlation coefficient (r) between temperature deviation and the mortality rate was −0.66, indicating a moderate negative correlation. This suggests that greater temperature fluctuations were associated with an increase in mortality. A regression analysis further quantified this relationship, with a p-value of 0.029, confirming that the correlation between temperature instability and the mortality rate was statistically significant at a 95% confidence level. These findings reinforce the importance of maintaining stable environmental conditions in pig housing to mitigate mortality risks.
4. Discussion
The impact of the pig-raising environment on productivity is well documented. However, previous studies have primarily investigated this issue from either an engineering-based perspective, focusing on environmental control systems, or a veterinary perspective, emphasizing disease management and immune responses. A comprehensive understanding of how environmental factors influence disease-related mortality remains a critical research gap that necessitates further investigation.
Several studies have highlighted the significant impact of environmental fluctuations on pig health and mortality. For instance, it has been reported that heat stress in swine results in reduced feed intake and growth rates, adversely affecting productivity [
33]. Furthermore, thermal stress negatively influences reproductive performance, with consequences extending to offspring, affecting thermoregulation and metabolic processes. These findings align with our study, where mortality rates increased by 40% during periods of high diurnal temperature variations, reaching a maximum fluctuation of 11.4 °C.
Additionally, studies on ventilation efficiency and air quality have indicated that inadequate ventilation leads to elevated ammonia concentrations and increased incidence of respiratory diseases. Ammonia concentrations exceeding 20 ppm have been associated with up to a 12% reduction in pig growth rates, while concentrations surpassing 50 ppm have been linked to heightened susceptibility to respiratory infections [
34]. Our findings corroborate these results, as we observed that regions with poor ventilation exhibited average ammonia concentrations of 10.7 ppm, with levels reaching 28.6 ppm near the floor, coinciding with increased mortality rates. These results underscore the critical role of ventilation management in mitigating ammonia accumulation and ensuring a stable rearing environment.
However, discrepancies exist. Studies conducted in tropical climates have identified high humidity, rather than temperature fluctuations, as the primary factor influencing increased pig mortality [
35]. In contrast, our study primarily focused on the effects of temperature variations. This suggests that future research should explore the combined impact of temperature and humidity on pig mortality, particularly across different climate zones.
Our findings provide practical implications for optimizing livestock management strategies. Given the significant effects of temperature instability and ventilation inefficiency, our study highlights the need for dynamic ventilation control systems that adapt to real-time environmental fluctuations. Moreover, integrating engineering-based monitoring solutions with veterinary disease management strategies is essential for improving overall pig health and productivity.
By comparing our results with previous studies, we have contributed to a broader understanding of how environmental stressors influence pig mortality. These findings reinforce the importance of maintaining thermal uniformity and stable air circulation to mitigate disease risks, further emphasizing the need for future research on adaptive climate control strategies for smart livestock farming.
5. Conclusions
This study demonstrates that the breeding environment significantly influences pig mortality, particularly through climate-induced stress and its impact on immune function. Our field experiments revealed a strong correlation between temperature instability and increased mortality rates, particularly in areas experiencing high diurnal temperature variations exceeding 10 °C, with mortality rates increasing by up to 40%. Furthermore, regions with poor ventilation exhibited ammonia concentrations averaging 10.7 ppm, peaking at 28.6 ppm near the floor, reinforcing the role of environmental stressors in exacerbating health risks.
The relationship between temperature fluctuations and swine mortality was particularly evident during cold periods following cloudy or rainy weather. Our data indicate that diurnal temperature variations exceeding 6 °C resulted in a sharp rise in mortality, particularly when cold air infiltration led to localized temperature drops as low as 21.8 °C in certain areas of the pig house. In contrast, stable temperature conditions (variations < 2 °C) were associated with significantly lower mortality rates. This underscores the necessity of maintaining temperature suitability, stability, and uniformity to mitigate climate stress and enhance livestock welfare.
However, our study also identified challenges in quantitatively assessing local ventilation efficiency through field experiments alone. To address this limitation, computational fluid dynamics (CFD) simulations should be incorporated to supplement field-based data. A combination of long-term monitoring data and CFD simulations would enable a more precise evaluation of airflow dynamics and ventilation effectiveness in commercial pig houses, allowing for a more proactive approach to managing environmental stressors.
The application of smart-farm technologies leveraging IoT and artificial intelligence (AI) presents a promising solution for optimizing livestock environmental control. A real-time monitoring system integrating temperature, humidity, and gas sensors with AI-driven decision-making could dynamically adjust ventilation rates to maintain optimal environmental conditions. Additionally, machine learning models trained on historical climate and mortality data could predict high-risk periods, enabling proactive interventions before stress-induced mortality spikes occur. These advancements are crucial for enhancing precision livestock farming, reducing climate stress-related mortality, and improving overall farm profitability in modern pig production systems.