Study on the Tissue Heterogeneity and Micromechanical Properties of Maize Kernel
Abstract
1. Introduction
2. Experimental Design
2.1. Experimental Materials
2.2. Experimental Equipment and Methods
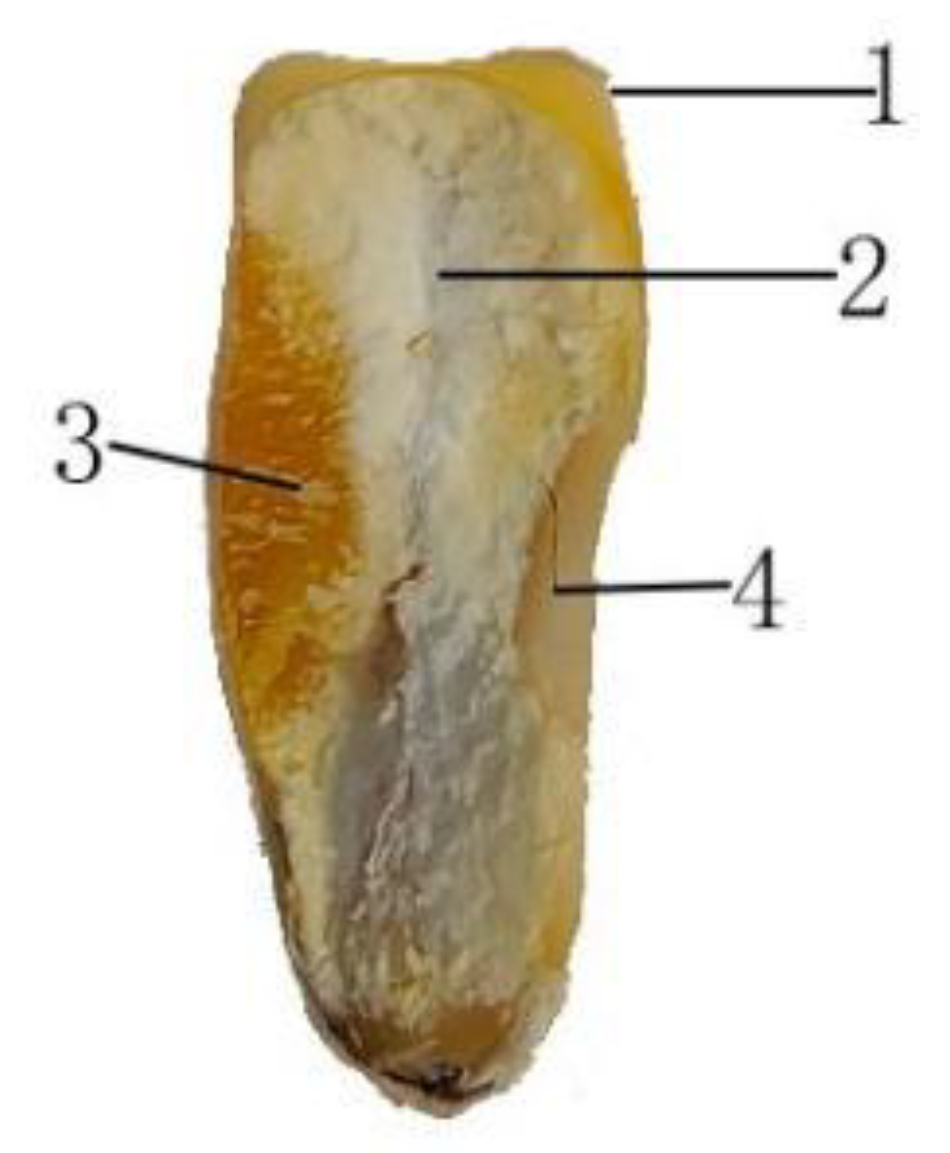
2.2.1. Microstructure Detection
2.2.2. Measurement of Mechanical Properties of Various Tissues in Maize Kernel
- (a)
- Sample preparation: The sample was cut along the short axis of the maize kernel with a blade to expose the internal horny endosperm, farinaceous endosperm, and embryo tissues. The cross-sections were polished using sandpaper of varying coarseness.
- (b)
- Sample placement: To ensure stable loading, the cut surface of the sample was adjusted to be parallel to the working plane of the round probe, and the sample was fixed to the stage using adhesive.
- (c)
- Loading: The round probe was pressed into the maize kernel tissues at a rate of 1 mm/min. The test was terminated when the probe reached a depth of 1.2 mm, and the probe was returned to the initial position.
2.2.3. Maize Kernel Compression Test
2.2.4. Maize Kernel Tomographic Scanning
3. Results and Discussion
3.1. Microstructure of Maize Kernels
3.2. Mechanical Properties of Maize Kernel Tissues
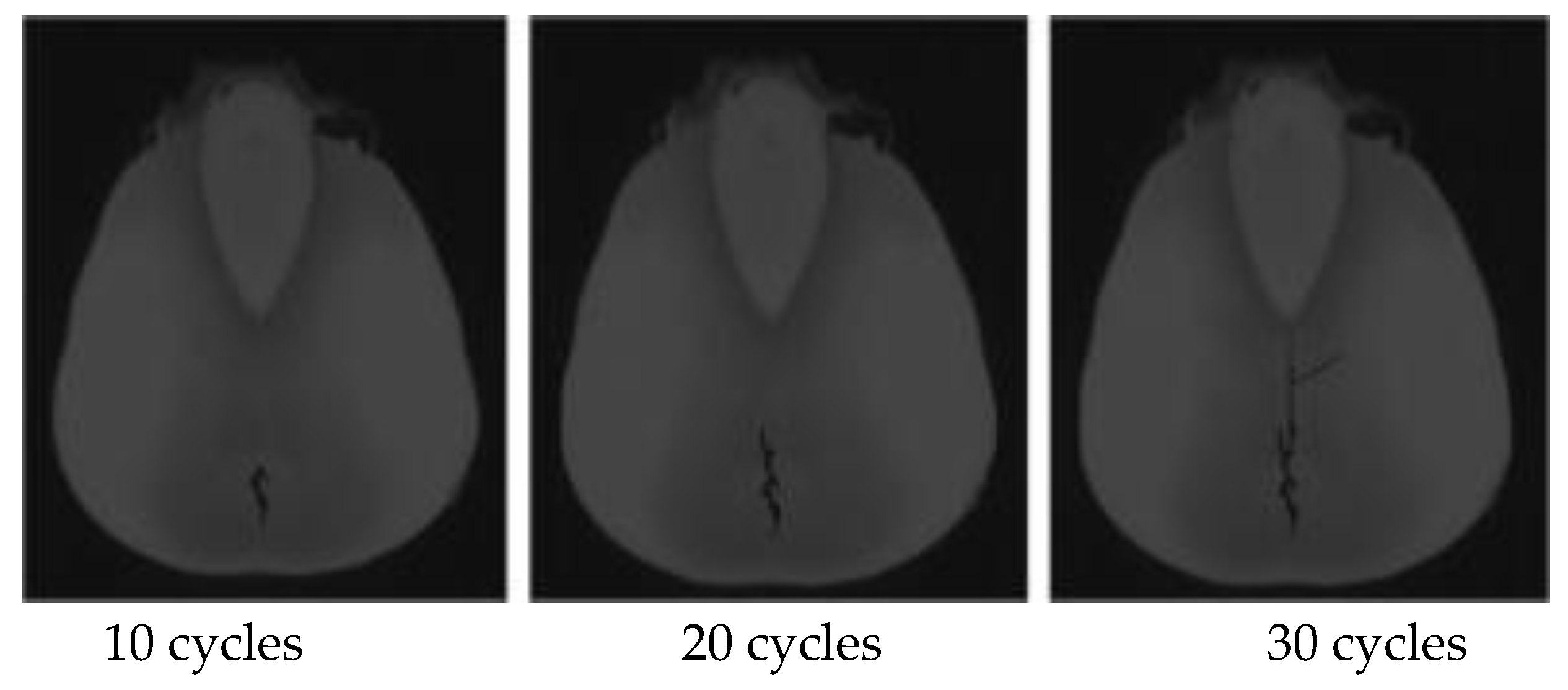
3.3. Analysis of Maize Kernel Damage and Fracture Performance
4. Finite Element Simulation and Analysis
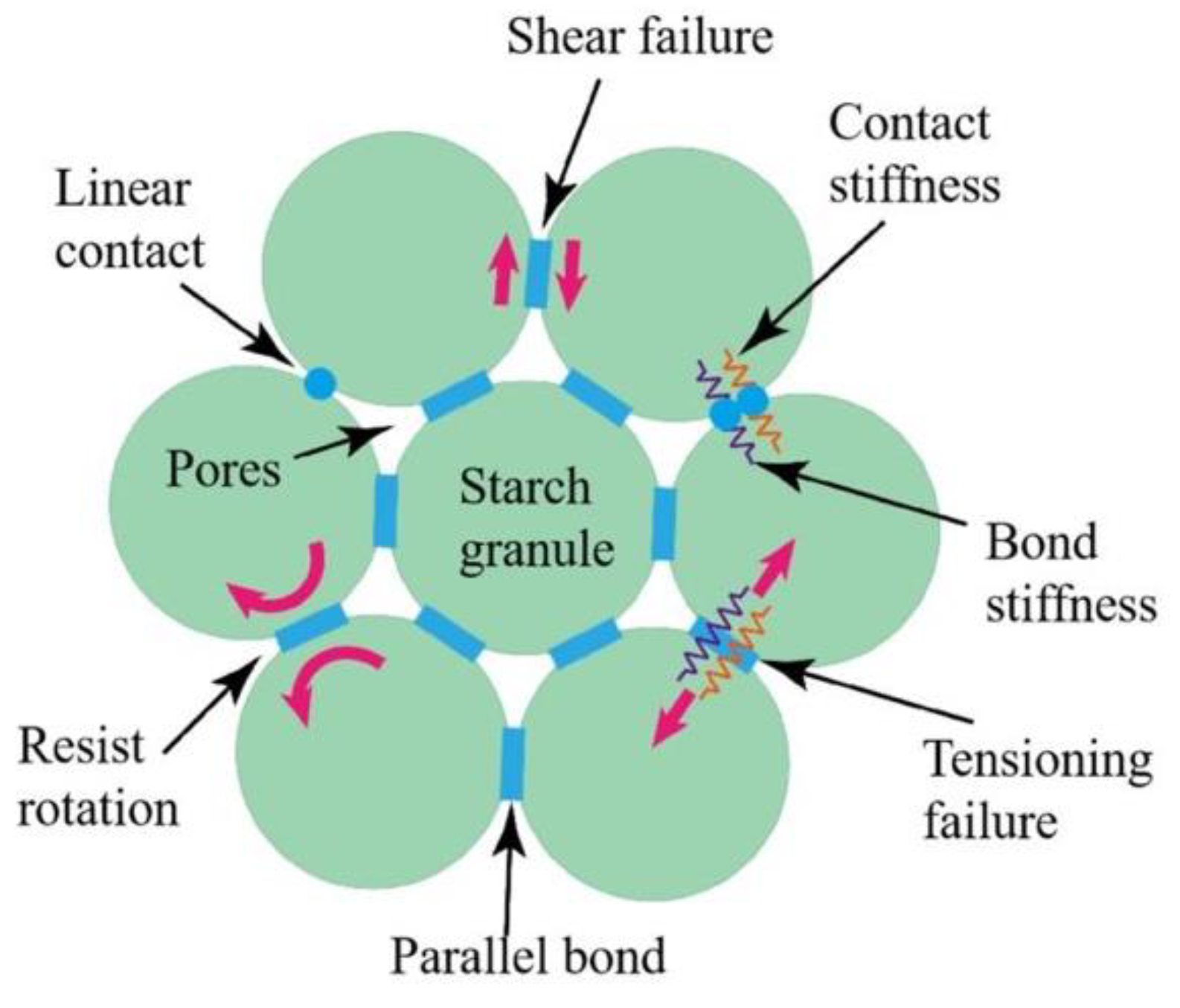
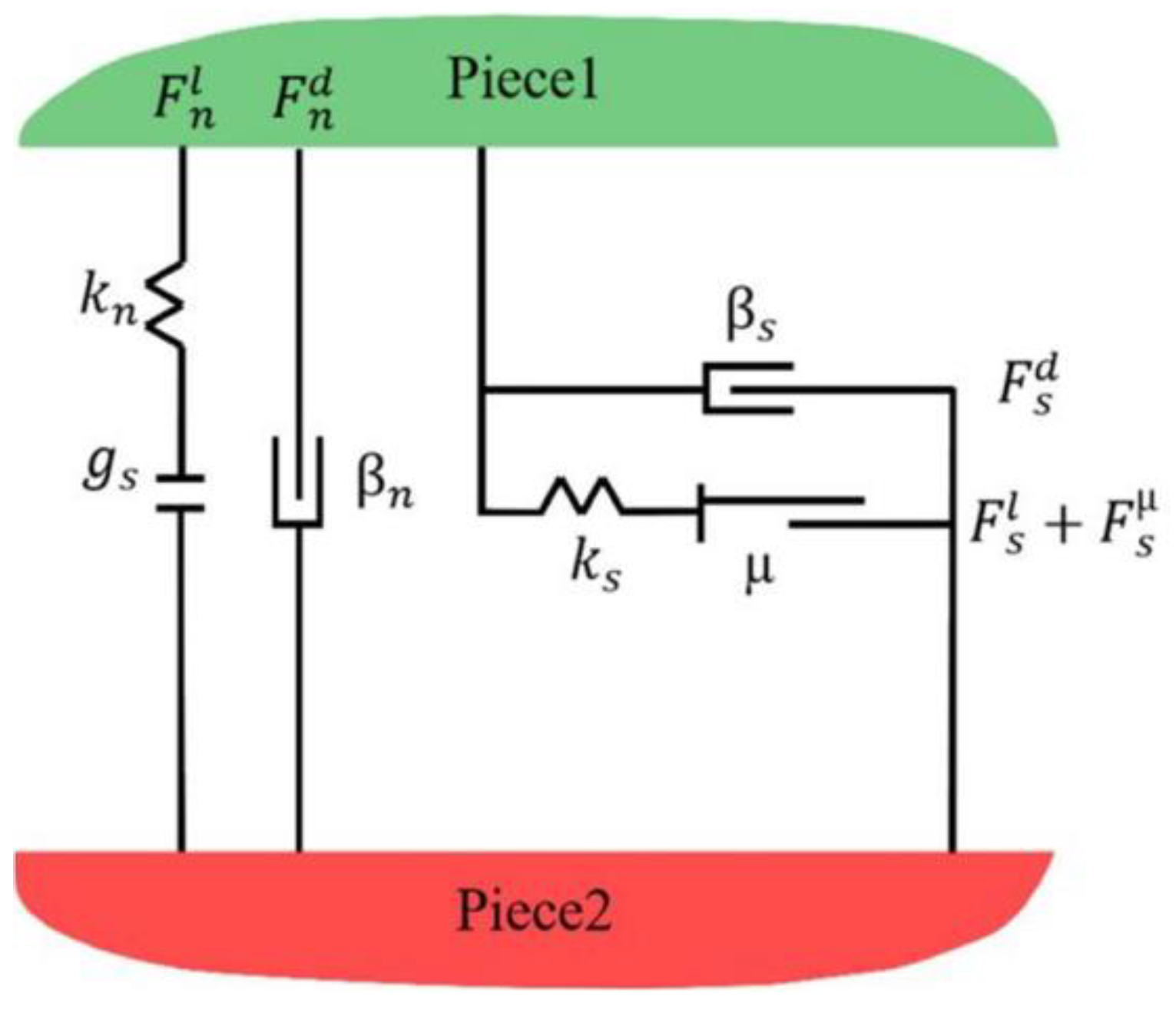
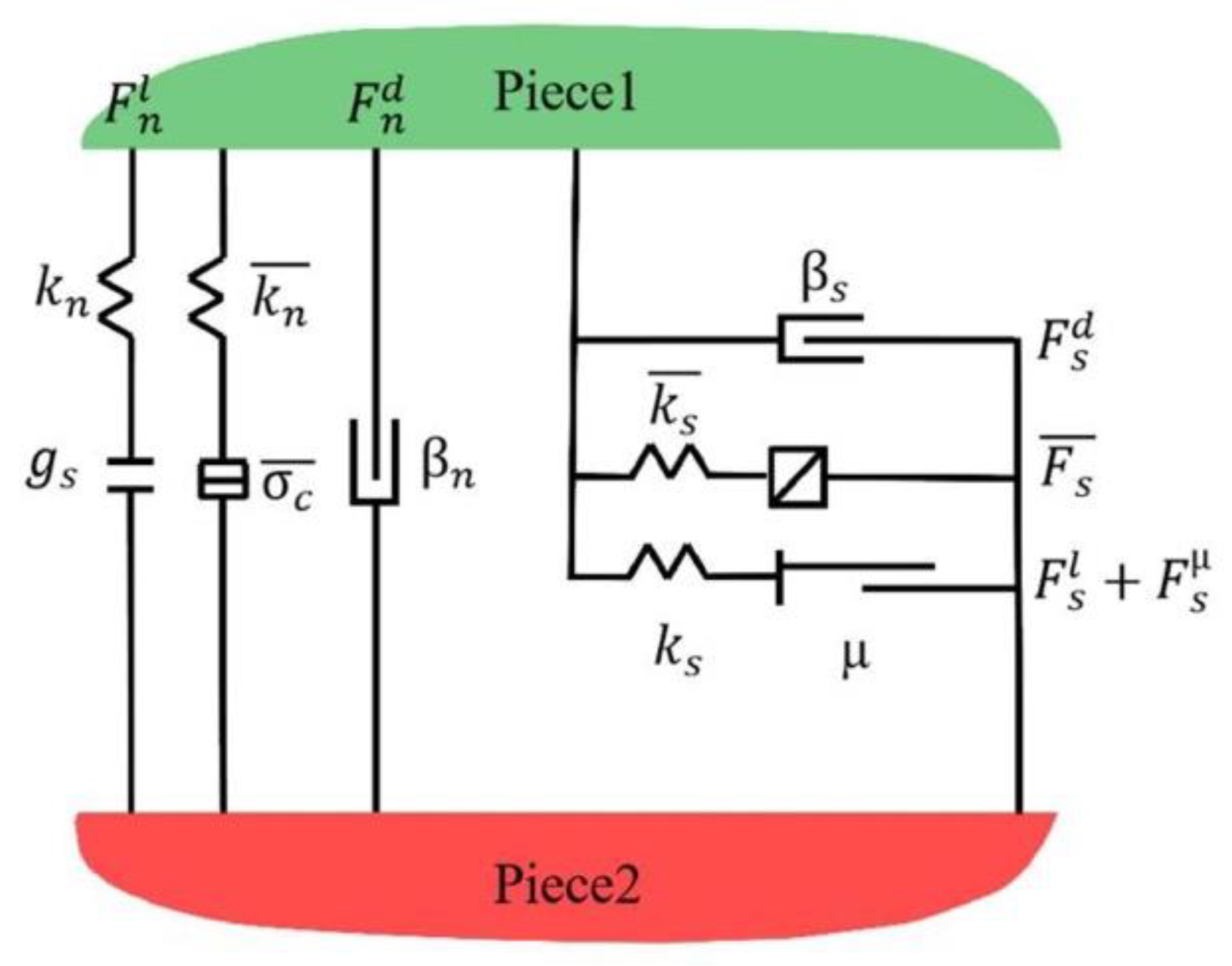
4.1. Contact Model Setup
4.2. Structural Parameter Setup
4.2.1. Porosity Statistics
4.2.2. Parameter Characterization
4.3. Establishment of the Particle Flow Model
4.4. Simulation Results and Analysis
4.4.1. Force and Deformation During the Loading Process
4.4.2. Speed Field Analysis During the Loading Process
5. Conclusions
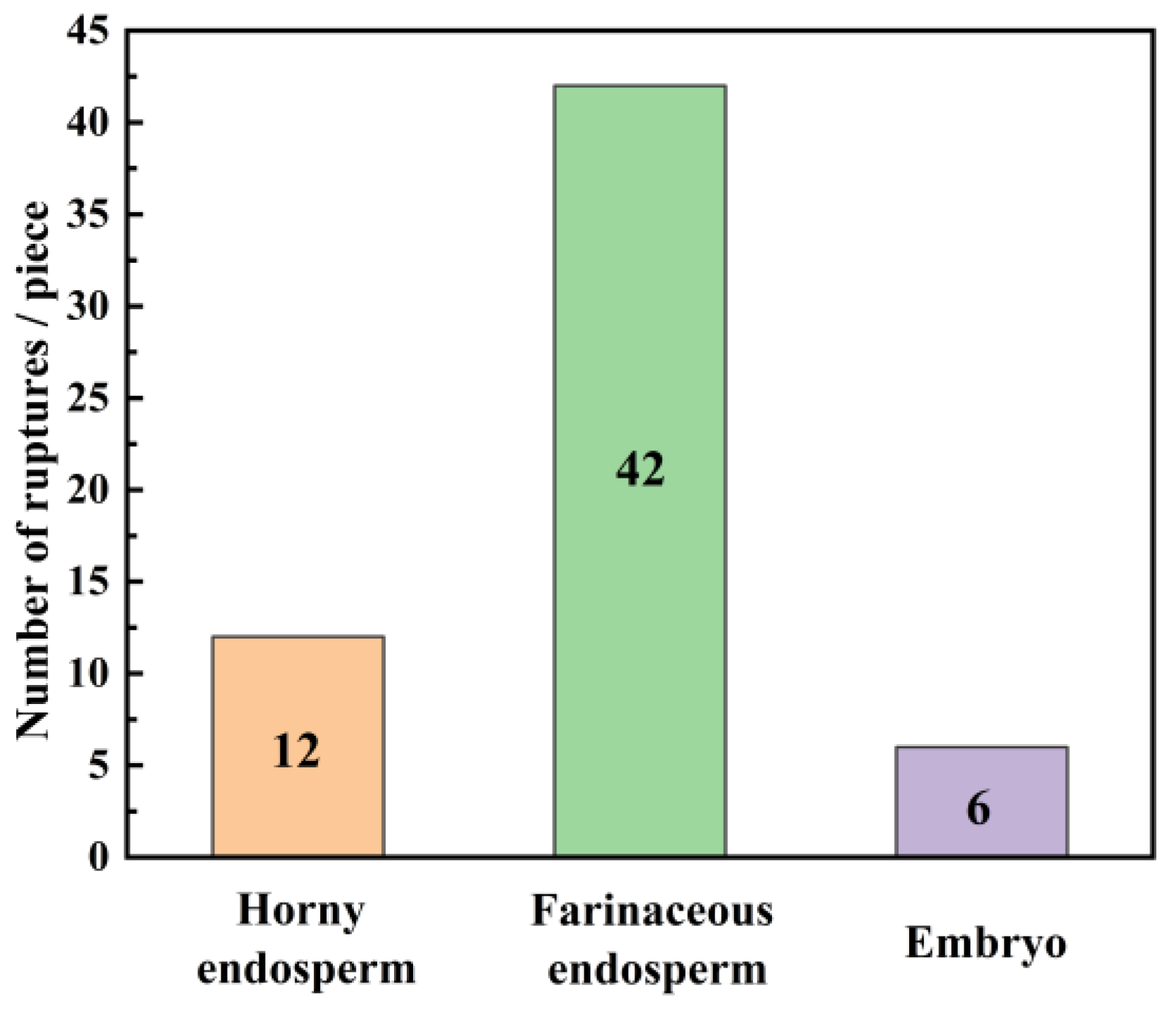
- There is a significant difference in the mechanical properties of different tissues in maize kernels. The starch particles in the horny endosperm are of a regular polyhedral shape, tightly arranged, resulting in higher maximum rupture force (128 N) and elasticity modulus (353 MPa). Therefore, the horny endosperm exhibits good structural stability and bearing capacity at the macroscopic level. Starch particles in the powdery endosperm are spheroid, arranged loosely and irregularly with more gaps, which makes the maximum rupture force (38 N) and elastic modulus (136 MPa) of the powdery endosperm relatively small. The powdery endosperm is more prone to deformation and rupture. Under compression loading, the initial damage in maize kernels mainly occurs in the farinaceous endosperm.
- By further particle flow simulation of the loading process of powdered endosperm, it is concluded that the damage evolution of the powdered endosperm during the whole loading process is divided into four stages, firstly, cracks start to sprout from the inner endosperm, and then gradually expand, and the powdered endosperm starts to rupture when the maximum force reaches 803 N. The process of damage accumulation of the powdered endosperm during the simulation is consistent with the process of change in maize after loading by test. And the direction of crack development is also consistent.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yadava, P.; Abhishek, A.; Singh, R.; Singh, I.; Kaul, T.; Pattanayak, A.; Agrawal, P.K. Advances in Maize Transformation Technologies and Development of Transgenic Maize. Front. Plant Sci. 2017, 7, 1949. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Xu, S.; Du, Z.; Ma, Y.; Ma, F.; Liu, J. Low-Damage Corn Threshing Technology and Corn Threshing Devices: A Review of Recent Developments. Agriculture 2023, 13, 1006. [Google Scholar] [CrossRef]
- Shi, M.L.; Zhu, G.Q.; Chen, G.Y.; Xue, L.; Hu, J.R.; Yin, Z.T.; Chen, G.Q. Medicinal value of maize and its industrialization development and application. Chin. Food Nutr. 2004, 12, 23–25. [Google Scholar]
- Ariyanti, D.; Rimantho, D.; Leonardus, M.; Ardyani, T.; Fiviyanti, S.; Sarwana, W.; Hanifah, Y.; Agustian, E.; Meliana, Y.; Susparini, N.T.; et al. Valorization of corn cob waste for furfural production. A circular economy approach. Biomass Bioenergy 2025, 194, 0961–9534. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Li, X.; Yu, J.; Cai, M.; Jin, M. Integrated bioethanol production from mixtures of corn and corn stove. Bioresour. Technol. 2018, 258, 18–25. [Google Scholar] [CrossRef]
- Cao, H.; Yan, L.; Song, X.; Liu, L.; Xu, S.; Hu, S.; Zhang, S. Determining the nutritional values of new corn varieties on pigs and broilers. Anim. Nutr. Metab. 2024, 11, 1358773. [Google Scholar] [CrossRef]
- Cui, Z.K.; Zhang, H.; Zhou, J.; Di, Z.F.; Wang, X.J. Design and experiment of intelligent corn grain harvester. J. Chin. Agric. Mech. 2019, 40, 26–30. [Google Scholar]
- Ke, Y.; Chunxia, J.; Wei, Z.; Enke, L.; Guangqian, Z.; Dongmei, Z. Study on the Effects of Different Treatment on Mechanical Harvest Broken Rate of Maize Grains. Crops 2024, 40, 138–143. [Google Scholar]
- Wang, F.; Alimu, M.; Zhang, J.; Li, Q.; Xu, L. Design and Experiment of Pre-screening Cleaning Device for Combined Screen Surface of Corn Grain Harvester. Trans. Chin. Soc. Agric. Mach. 2024, 55, 135–147+166. [Google Scholar]
- Cui, T.; Fan, C.L.; Zhang, D.X.; Yang, L.; Li, Y.B.; Zhao, H.H. Research Progress of Maize Mechanized Harvesting Technology. Trans. Chin. Soc. Agric. Mach. 2019, 50, 1–13. [Google Scholar]
- Geng, D.; Wang, Q.; Li, H.; He, Q.; Yue, D.; Ma, J.; Wang, Y.; Xu, H. Online detection technology for broken corn kernels based on deep learning. Trans. Chin. Soc. Agric. Eng. 2023, 39, 270–278. [Google Scholar]
- Li, X.; Ma, F.; Gao, L. Drop impact test of corn seed. Trans. Chin. Soc. Agric. Eng. 2009, 25, 113–116. [Google Scholar]
- Li, X.; Gao, L.; Ma, F. Experimental study on threshing characteristics of maize seeds. J. Agric. Mech. Res. 2007, 156–158. [Google Scholar]
- Li, X.; Gao, L.; Ma, F. Experimental study on impact damage of maize seed. J. Shenyang Agric. Univ. 2007, 38, 5. [Google Scholar]
- Cai, C.J.; Chen, Z.; Han, Z.D.; Liu, G.M.; Zhang, Z.L.; Hao, J.F. Study on Relationship of Biomechanical Characteristics of Corn Seed and Threshing Performance. J. Agric. Mech. Res. 2017, 39, 192–196. [Google Scholar]
- Liu, J.; Liu, Q.; Chen, X.; Deng, Z.; Yuan, J.; Kong, F. Diversity and influencing factors of kernel breakage tolerance of different maize cultivars. J. China Agric. Univ. 2021, 26, 207–220. [Google Scholar]
- Zhang, Y.; Gao, L.; Liu, H.; Li, X. Experimental Study on Corn Kernel Shear Crash. J. Agric. Mech. Res. 2007, 136–138. [Google Scholar]
- Wang, B.; Gao, M.; Geng, D.; Zhou, S. Study on damage mechanism and crack growth of the corn grain. J. Fail. Anal. Prev. 2022, 22, 1526–1534. [Google Scholar]
- Wang, G. Finite Element Analysis of compression mechanical properties of Corn grains. Chin. J. Agric. Mech. 2016, 37, 117–120. [Google Scholar]
- Tao, T.; Wei, X. Finite element analysis of corn seed under impact load. J. Agric. Mech. Res. 2019, 41, 35–39. [Google Scholar]
- Tang, H.; Zhu, G.; Sun, Z.; Xu, C.; Wang, J. Impact damage evolution rules of maize kernel based on FEM. Biosyst. Eng. 2024, 247, 162–174. [Google Scholar] [CrossRef]
- Gao, P. Study on Mechanical Properties of Corn Grains and Their Relationship with Grain Disintegration in Storage. Master’s Thesis, Henan University of Technology, Zhengzhou, China, 2024. [Google Scholar]
- Qiao, M.; Xia, G.; Cui, T.; Xu, Y.; Gao, X.; Su, Y.; Li, Y.; Fan, H. Effect of moisture, protein, starch, soluble sugar contents and microstructure on mechanical properties of maize kernels. Food Chem. 2022, 379, 13214. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Finner, M.F.; Rohatgi, P.K.; Buelow, F.H.; Schaller, M. Structure and mechanical properties of corn kernels: A hybrid composite material. Mater. Sci. 1991, 26, 274–284. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J. Mechanical properties of maize kernel horny endosperm, floury endosperm and germ. Int. J. Food Prop. 2019, 22, 863–877. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, H. Starch Granule Size, Pasting Properties and Textural Properties of Horny Endosperm and Silty Endosperm in Maize. J. Chin. Cereals Oils Assoc. 2014, 29, 27–32. [Google Scholar]
- Yin, X.; Ming, B.; Hou, J.; Ming, B.; Zhang, Y.; Guo, X.; Gao, S.; Wang, K.; Hou, P.; Li, S. Effects of various grain positions of ear on the internal Structural parameters of maize grain using X-ray μCT. Trans. Chin. Soc. Agric. Eng. 2021, 37, 8–14. [Google Scholar]
- Xu, A. Development and Characteristics of Corn Keratin and Silty Endosperm Starch. Master’s Thesis, Yangzhou University, Yangzhou, China, 2020. [Google Scholar]








| Number | Maximum Rupture Force/N | Rupture Deformation/mm | Modulus of Elasticity/MPa |
|---|---|---|---|
| 1 | 121.37 | 0.883 | 345.91 |
| 2 | 123.69 | 0.889 | 346.93 |
| 3 | 135.12 | 0.884 | 349.79 |
| 4 | 131.95 | 0.894 | 350.45 |
| 5 | 129.08 | 0.888 | 360.17 |
| 6 | 127.24 | 0.895 | 363.45 |
| 7 | 125.44 | 0.899 | 366.37 |
| 8 | 121.39 | 0.893 | 358.12 |
| 9 | 133.76 | 0.910 | 360.43 |
| 10 | 123.36 | 0.895 | 359.48 |
| Mean | 127.24 | 0.893 | 356.11 |
| SD | 5.0525 | 0.0078 | 7.2263 |
| Upper confidence limits of 95% | 123.6259 | 0.8874 | 350.9409 |
| Lower confidence limits of 95% | 130.8541 | 0.8986 | 361.2790 |
| Number | Maximum Rupture Force/N | Rupture Deformation/mm | Modulus of Elasticity/MPa |
|---|---|---|---|
| 1 | 30.34 | 0.825 | 115.36 |
| 2 | 31.82 | 0.827 | 116.47 |
| 3 | 32.84 | 0.829 | 116.52 |
| 4 | 32.91 | 0.835 | 115.29 |
| 5 | 34.56 | 0.837 | 116.92 |
| 6 | 36.45 | 0.840 | 117.66 |
| 7 | 36.12 | 0.845 | 116.67 |
| 8 | 33.31 | 0.843 | 123.8 |
| 9 | 30.68 | 0.846 | 121.31 |
| 10 | 30.67 | 0.823 | 122.7 |
| Mean | 32.97 | 0.835 | 118.27 |
| SD | 2.1977 | 0.0085 | 3.1243 |
| Upper confidence limits of 95% | 31.3979 | 0.8289 | 116.0351 |
| Lower confidence limits of 95% | 34.5421 | 0.8411 | 120.5049 |
| Number | Maximum Rupture force/N | Rupture Deformation/mm | Modulus of Elasticity/MPa |
|---|---|---|---|
| 1 | 17.93 | 0.892 | 30.16 |
| 2 | 17.91 | 0.889 | 29.27 |
| 3 | 17.89 | 0.917 | 29.97 |
| 4 | 19.59 | 0.912 | 30.19 |
| 5 | 20.36 | 0.926 | 31.45 |
| 6 | 21.65 | 0.963 | 34.65 |
| 7 | 19.36 | 0.814 | 34.37 |
| 8 | 18.49 | 0.896 | 35.43 |
| 9 | 21.38 | 0.923 | 36.02 |
| 10 | 20.84 | 0.938 | 30.99 |
| Mean | 19.54 | 0.907 | 32.25 |
| SD | 1.46473 | 0.0396 | 2.5711 |
| Upper confidence limits of 95% | 18.4923 | 0.8786 | 30.4108 |
| Lower confidence limits of 95% | 20.5877 | 0.9354 | 34.0892 |
| Part | Pore Number | Pore Area/μm2 | Total Area/μm2 | Porosity/% |
|---|---|---|---|---|
| Powdery endosperm | 981 | 176,342.2 | 3,093,656.3 | 5.70 |
| Group | Particle Size (mm) | Proportions (%) |
|---|---|---|
| 1 | 2~4 | 2.3 |
| 2 | 4~8 | 26.7 |
| 3 | 8~12 | 31.7 |
| 4 | 12~16 | 31.1 |
| 5 | 16~18 | 5.8 |
| 6 | 18~22 | 2.4 |
| Parameter | Value |
|---|---|
| Rmax/μm | 2 |
| Rmin/μm | 22 |
| ρ/kg·m−3 | 1300 |
| porosity/% | 5.71 |
| kn/N·m−1 | 0.85 × 108 |
| kratio | 1.5 |
| fric | 0.41 |
| pb_coh/MPa | 0.65 |
| pb_ten/MPa | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Bi, J.; Xu, P.; Li, R.; Zhang, G.; Geng, D.; Lan, Y.; Wang, B. Study on the Tissue Heterogeneity and Micromechanical Properties of Maize Kernel. Agriculture 2025, 15, 636. https://doi.org/10.3390/agriculture15060636
Shi Z, Bi J, Xu P, Li R, Zhang G, Geng D, Lan Y, Wang B. Study on the Tissue Heterogeneity and Micromechanical Properties of Maize Kernel. Agriculture. 2025; 15(6):636. https://doi.org/10.3390/agriculture15060636
Chicago/Turabian StyleShi, Zhou, Jingcun Bi, Peng Xu, Rui Li, Guohai Zhang, Duanyang Geng, Yubin Lan, and Bolong Wang. 2025. "Study on the Tissue Heterogeneity and Micromechanical Properties of Maize Kernel" Agriculture 15, no. 6: 636. https://doi.org/10.3390/agriculture15060636
APA StyleShi, Z., Bi, J., Xu, P., Li, R., Zhang, G., Geng, D., Lan, Y., & Wang, B. (2025). Study on the Tissue Heterogeneity and Micromechanical Properties of Maize Kernel. Agriculture, 15(6), 636. https://doi.org/10.3390/agriculture15060636






