Optimization of Light Quality for Plant Factory Production of Brassica campestris (Pakchoi)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Growth Conditions and Light Treatments
2.2. Determination of Carbohydrates
2.3. Determination of Nitrate, Free Amino Acids, Soluble Protein, Total Nitrogen, and Protein Nitrogen
2.4. Determination of AsA Content
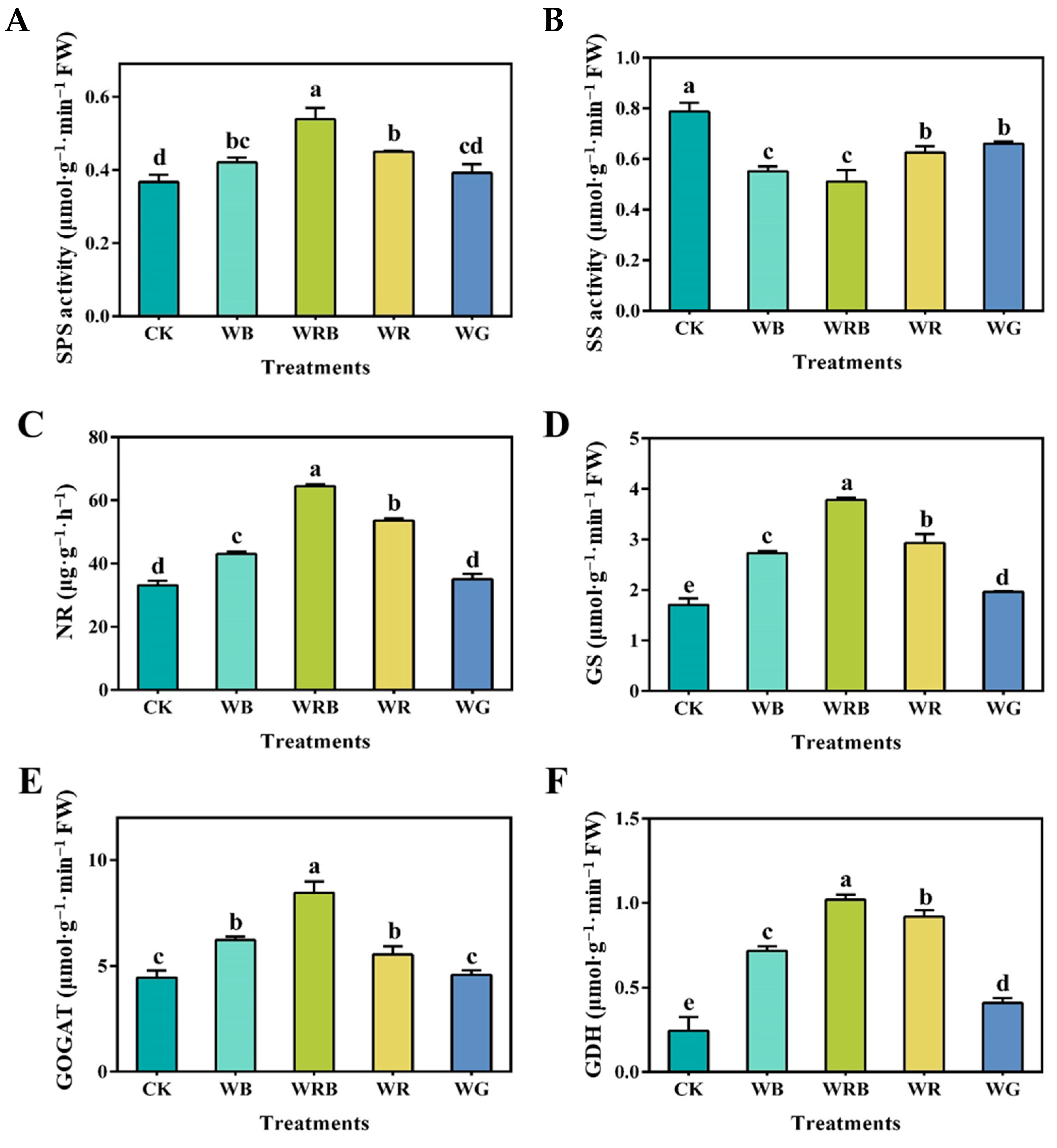
2.5. Determination of Enzyme Activity
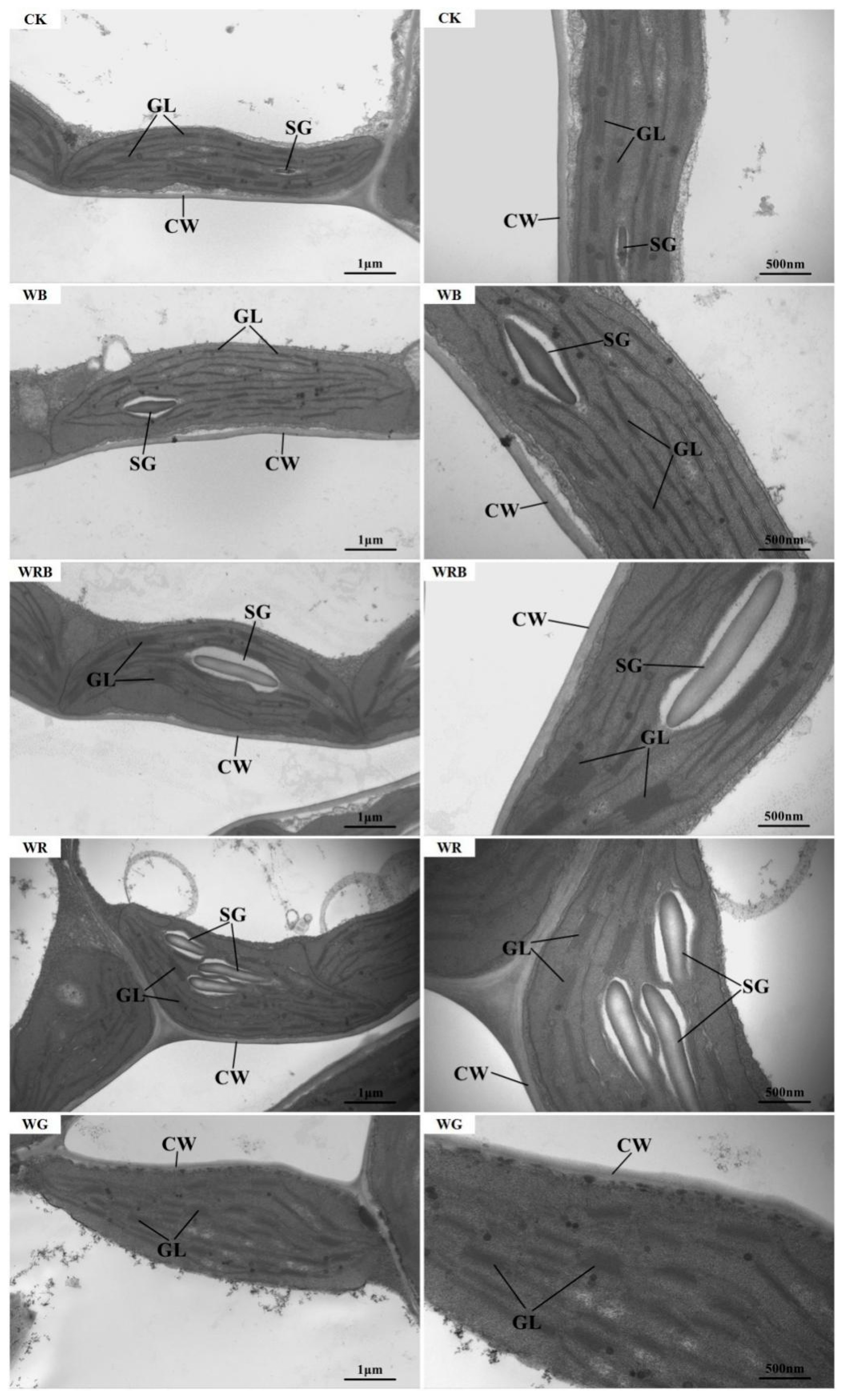
2.6. Observation of Chloroplast Ultrastructure
2.7. Statistical Analysis
3. Results
3.1. Effects of Supplementary Light Quality on the Growth Morphological Indices in Pakchoi
3.2. Effects of Supplementary Light Quality on Chloroplast Ultrastructure in Leaves of Pakchoi
3.3. Effects of Supplementary Light Quality on Quality of Pakchoi
3.4. The Influence of Supplemental Light Quality on the Activities of Key Enzymes
3.5. Quality of Pakchoi Cultivated in Different Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Liu, X.; Li, Y.; Zhu, J.; Li, P. Integrative analysis of transcriptome reveals the possible mechanism of delayed leaf senescence in pak choi (Brassica rapa subsp. chinensis) following melatonin treatment. Food Qual. Saf. 2022, 7, fyac064. [Google Scholar] [CrossRef]
- Fan, X.; Xue, F.; Song, B.; Chen, L. Effects of blue and red light on growth and nitrate metabolism in pakchoi. Open Chem. 2019, 17, 456–464. [Google Scholar] [CrossRef]
- Bai, X.; Jiang, Y.; Miao, H.; Xue, S.; Chen, Z.; Zhou, J. Intensive vegetable production results in high nitrate accumulation in deep soil profiles in China. Environ. Pollut. 2021, 287, 117598. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Kharshiing, E.; Mawphlang, O.; Lama, V.; Bhattacharjee, R.; Sahoo, L. Manipulation of light environment for optimising photoreceptor activity towards enhancing plant traits of agronomic and horticultural importance in crops. J. Hortic. Sci. Biotechnol. 2022, 97, 535–551. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Song, W.; Wang, L.; Guo, W.; Xue, X. Growth and nutritional properties of lettuce affected by different alternating intervals of red and blue LED irradiation. Sci. Hortic. 2017, 223, 44–52. [Google Scholar] [CrossRef]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Urbonaviciute, A.; Pinho, P.; Samuoliene, G.; Duchovskis, P.; Vitta, P.; Stonkus, A.; Tamulaitis, G.; Zukauskas, A.; Halonen, L. Effect of short-wavelength light on lettuce growth and nutritional quality. Sodininkystė ir daržininkystė 2007, 26, 157–165. [Google Scholar]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2025, 15, 1507628. [Google Scholar] [CrossRef]
- Zhang, R.; He, Q.; Pan, Q.; Feng, Y.; Shi, Y.; Li, G.; Zhang, Y.; Liu, Y.; Khan, A. Blue-green light treatment enhances the quality and nutritional value in postharvest Chinese cabbage (Brassica rapa L. ssp. pekinensis). Food Chem. 2024, 24, 102004. [Google Scholar] [CrossRef]
- Schenkels, L.; Saeys, W.; Lauwers, A.; Proft, M.P.D. Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Sci. Hortic. 2020, 261, 109002. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Pan, Q.; Khan, A.; Bai, X.; Ali, M.; Yang, W.; Zhang, L.; Li, B. The effects of short term blue light treatment on promoting nutrition value in Chinese cabbage. Food Chem. 2023, 412, 135542. [Google Scholar] [CrossRef]
- Izzo, L.G.; Arena, C.; De Micco, V.; Capozzi, F.; Aronne, G. Light quality shapes morpho-functional traits and pigment content of green and red leaf cultivars of Atriplex hortensis. Sci. Hortic. 2019, 246, 942–950. [Google Scholar] [CrossRef]
- Izzo, L.G.; Hay Mele, B.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Commichau, F.; Forchhammer, K.; Stülke, J. Regulatory links between carbon and nitrogen metabolism. Curr. Opin. Microbiol. 2006, 9, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult. 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Ren, M.; Liu, S.; Mao, G.; Tang, C.; Gai, P.; Guo, X.; Zheng, H.; Wang, W.; Tang, Q. Simultaneous Application of Red and Blue Light Regulate Carbon and Nitrogen Metabolism, Induces Antioxidant Defense System and Promote Growth in Rice Seedlings under Low Light Stress. Int. J. Mol. Sci. 2023, 24, 10706. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Shin, K.; Murthy, H.; Heo, J.; Hahn, E.; Paek, K. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Bian, Z.; Cheng, R.; Wang, Y.; Yang, Q.; Lu, C. Effect of green light on nitrate reduction and edible quality of hydroponically grown lettuce (Lactuca sativa L.) under short-term continuous light from red and blue light-emitting diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Maness, N. Extraction and analysis of soluble carbohydrates. In Plant Stress Tolerance; Methods in Molecular Biology; Humana Press: Clifton, NJ, USA, 2010; Volume 639, pp. 341–370. [Google Scholar]
- Wei, J.; Song, K.; Zang, Z.; Yang, H.; Gao, Y.; Zhang, J.; Wang, Z.; Liu, C. Influence of specific tobacco endophytic Bacillus on tobacco leaf quality enhancement during fermentation. Front. Microbiol. 2024, 15, 1468492. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Shi, R.; Liu, W.; Liu, J.; Zeb, A.; Wang, Q.; Wang, J.; Li, J.; Yu, M.; Ali, N.; An, J. Earthworms improve the rhizosphere micro-environment to mitigate the toxicity of microplastics to tomato (Solanum lycopersicum). J. Hazard. Mater. 2024, 472, 134578. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. A natural light/dark cycle regulation of carbon-nitrogen metabolism and gene expression in rice shoots. Front. Plant Sci. 2016, 7, 1318. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, M.; Zhao, H.; Zhang, Y.; Wang, X.; Guo, S.; Zhang, Z.; Liu, T. Deciphering the mechanism of β-aminobutyric acid-induced resistance in wheat to the grain aphid, Sitobion avenae. PLoS ONE 2014, 9, e91768. [Google Scholar] [CrossRef]
- Chen, X.; Xue, X.; Guo, W.; Wang, L.; Qiao, X. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Sohail, H.; Du, H.; Li, J. The impact of short-term nitrogen starvation and replenishment on the nitrate metabolism of hydroponically grown spinach. Sci. Hortic. 2023, 309, 111632. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M. Mechanical flower thinning improves the fruit quality of apples. J. Sci. Food Agric. 2010, 90, 735–741. [Google Scholar] [CrossRef]
- Qian, C.; Chen, Q.; Jiang, L.; Yang, X.; Rao, S.; Zhang, W.; Xu, F. Physiological Mechanism of Exogenous Selenium in Alleviating Mercury Stress on Pakchoi (Brassica campestris L.). Phyton-Int. J. Exp. Bot. 2024, 93, 951–962. [Google Scholar] [CrossRef]
- Lauria, G.; Lo Piccolo, E.; Ceccanti, C.; Guidi, L.; Bernardi, R.; Araniti, F.; Cotrozzi, L.; Pellegrini, E.; Moriconi, M.; Giordani, T.; et al. Supplemental red LED light promotes plant productivity, “photomodulates” fruit quality and increases Botrytis cinerea tolerance in strawberry. Postharvest Biol. Technol. 2023, 198, 112253. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 447–461. [Google Scholar] [CrossRef]
- Son, K.; Jeon, Y.; Oh, M. Application of supplementary white and pulsed light-emitting diodes to lettuce grown in a plant factory with artificial lighting. Hortic. Environ. Biotechnol. 2016, 57, 560–572. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Chen, B.; Wan, Z.; Li, S.; Wang, G.; Song, H.; Wang, X. Effects of nitrate supply on plant growth, nitrate accumulation, metabolic nitrate concentration and nitrate reductase activity in three leafy vegetables. Plant Sci. 2004, 167, 635–643. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W. Substituting green light for partial red light promoted the growth and quality, and regulated the nitrogen metabolism of Medicago sativa grown under red-blue LEDs. Environ. Exp. Bot. 2024, 220, 105623. [Google Scholar] [CrossRef]
- Sun, N.; Wei, M.; Li, Y.; Wang, X.; Yang, F.; Shi, Q. Effects of light quality on carbon and nitrogen metabolism and enzyme activities in tomato seedlings. Acta Hortic. Sin. 2016, 43, 80–88. [Google Scholar]
- Li, J.; Liu, Y.; Wang, J.; Liu, M.; Li, Y.; Zheng, J. Effects of Different LED Spectra on the Antioxidant Capacity and Nitrogen Metabolism of Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). Plants 2024, 13, 2958. [Google Scholar] [CrossRef]
- Phillips, K.; Tarragó-Trani, M.; Gebhardt, S.; Exler, J.; Patterson, K.; Haytowitz, D.; Holden, J. Stability of Vitamin C in frozen raw fruit and vegetable homogenates. J. Food Compos. Anal. 2010, 23, 253–259. [Google Scholar] [CrossRef]
- Mastrangelo, D.; Massai, L.; Fioritoni, G.; Lo Coco, F.; Noguera, N.; Testa, U. High Doses of Vitamin C and Leukemia: In Vitro Update. In Myeloid Leukemia; Lasfer, A., Ed.; InTech: London, UK, 2018. [Google Scholar][Green Version]
- Ntagkas, N.; Woltering, E.; Marcelis, L. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Massot, C.; Genard, M.; Stevens, R.; Gautier, H. Fluctuations in sugar content are not determinant in explaining variations in vitamin C in tomato fruit. Plant Physiol. Biochem. 2010, 48, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Marcelis, L.; Nicole, C.; Woltering, E. High light intensity applied shortly before harvest improves lettuce nutritional quality and extends the shelf life. Front. Plant Sci. 2021, 12, 615355. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Z.; Liu, W.; Bian, Z.; Lu, W.; Zhou, B.; Wang, S.; Li, Q.; Yang, Q. High-Proportion Blue Light Irradiation at the End-of-Production Stage Promotes the Biosynthesis and Recycling of Ascorbate in Lettuce. Int. J. Mol. Sci. 2023, 24, 16524. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Setiawan, C.K.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Maezawa, S.; Sato, H.; Kanemitsu, N.; Kato, M. Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in postharvest broccoli. Postharvest Biol. Technol. 2014, 94, 97–103. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Xin, Y.; Mei, Z.; Gao, A.; Liu, W.; Yu, L.; Chen, X.; Chen, Z.; Wang, N. Analyses of the photosynthetic characteristics, chloroplast ultrastructure, and transcriptome of apple (Malus domestica) grown under red and blue lights. BMC Plant Biol. 2021, 21, 483. [Google Scholar] [CrossRef]
- Di, Q.; Li, J.; Du, Y.; Wei, M.; Shi, Q.; Li, Y.; Yang, F. Combination of Red and Blue Lights Improved the Growth and Development of Eggplant (Solanum melongena L.) Seedlings by Regulating Photosynthesis. J. Plant Growth Regul. 2021, 40, 1477–1492. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, T.; Huang, K.; Liu, Y.; Liu, M.; Wang, J. Effect of LED Spectrum on the Quality and Nitrogen Metabolism of Lettuce Under Recycled Hydroponics. Front. Plant Sci. 2021, 12, 678197. [Google Scholar] [CrossRef]
- Yang, X.; Hu, J.; Wang, Z.; Huang, T.; Xiang, Y.; Zhang, L.; Peng, J.; Tomás-Barberán, F.; Yang, Q. Pre-harvest Nitrogen Limitation and Continuous Lighting Improve the Quality and Flavor of Lettuce (Lactuca sativa L.) under Hydroponic Conditions in Greenhouse. J. Agric. Food Chem. 2023, 71, 710–720. [Google Scholar] [CrossRef] [PubMed]
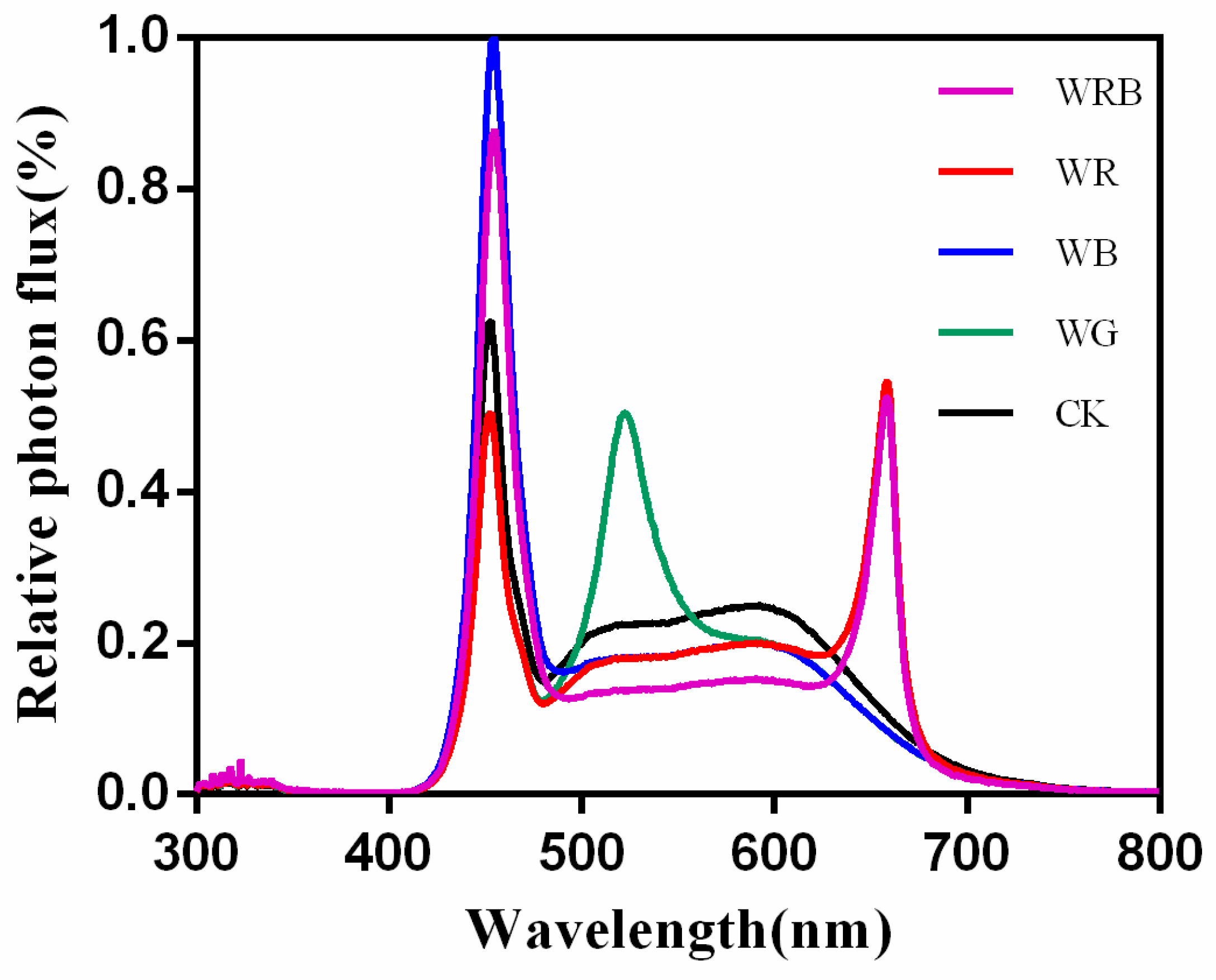
| Treatments | PPFD of W (µmol·m−2·s−1) | PPFD of B (µmol·m−2·s−1) | PPFD of R (µmol·m−2·s−1) | PPFD of G (µmol·m−2·s−1) | Total PPFD (µmol·m−2·s−1) |
|---|---|---|---|---|---|
| CK | 250 | \ | \ | \ | 250 |
| WB | 200 | 50 | \ | \ | 250 |
| WRB | 150 | 50 | 50 | \ | 250 |
| WR | 200 | \ | 50 | \ | 250 |
| WG | 200 | \ | \ | 50 | 250 |
| Treatments | Plant Height (cm) | Leaf Area (cm2) | Shoot Fresh Weight (g) | Shoot Dry Weight (g) |
|---|---|---|---|---|
| CK | 10.80 ± 0.54 b | 236.07 ± 34.91 c | 8.62 ± 1.37 c | 0.606 ± 0.08 c |
| WB | 10.10 ± 0.26 c | 216.91 ± 19.60 c | 7.98 ± 0.93 c | 0.558 ± 0.08 c |
| WRB | 12.25 ± 0.44 a | 333.99 ± 28.06 a | 13.84 ± 1.03 a | 0.898 ± 0.07 a |
| WR | 11.17 ± 0.66 b | 294.97 ± 28.44 b | 11.62 ± 1.43 b | 0.762 ± 0.08 b |
| WG | 10.88 ± 0.26 b | 245.49 ± 14.59 c | 9.19 ± 0.72 c | 0.632 ± 0.06 c |
| Treatments | Number of Thylakoid Layers | Thylakoid Layer Width (nm) | Length of Thylakoid Layers (nm) | Thylakoid Size (nm2) |
|---|---|---|---|---|
| CK | 4.17 ± 1.6 bc | 71.54 ± 12.07 bc | 309.46 ± 30.92 c | 4533.4 ± 845.8 a |
| WB | 3 ± 0.63 c | 59.42 ± 4.29 c | 450.92 ± 40.62 ab | 3116.2 ± 497.7 b |
| WRB | 6.5 ± 2.26 a | 134.61 ± 60.46 a | 504.43 ± 61.79 a | 3802 ± 487.5 a |
| WR | 5.5 ± 1.52 ab | 106.17 ± 28.69 ab | 427.85 ± 19.06 b | 3497.6 ± 498.1 b |
| WG | \ | \ | \ | 4511.6 ± 469.3 ab |
| Compound | CK | WB | WRB | WR | WG |
|---|---|---|---|---|---|
| Total sugar/mg·g−1 (DW) | 91.43 ± 1.85 c | 100.33 ± 4.25 b | 110.05 ± 2.46 a | 99.17 ± 3.81 b | 95.53 ± 2.35 bc |
| Soluble sugar/mg·g−1 (DW) | 51.81 ± 1.49 c | 55.00 ± 0.34 a | 54.33 ± 0.82 ab | 54.51 ± 1.10 ab | 53.25 ± 0.60 bc |
| Sucrose/mg·g−1 (DW) | 9.58 ± 0.91 c | 9.35 ± 0.17 c | 11.00 ± 0.54 b | 13.40 ± 0.23 a | 11.28 ± 1.10 b |
| Starch/mg·g−1 (DW) | 95.43 ± 1.13 b | 93.80 ± 4.38 b | 106.63 ± 0.42 a | 93.06 ± 0.56 b | 83.79 ± 1.31 c |
| Free amino acids/mg·g−1 (FW) | 0.168 ± 0.01 bc | 0.178 ± 0.01 b | 0.195 ± 0.01 a | 0.178 ± 0.01 b | 0.16 ± 0.01 c |
| Soluble protein/mg·g−1 (FW) | 4.57 ± 0.24 d | 5.38 ± 0.23 b | 6 ± 0.11 a | 5.51 ± 0.17 b | 4.87 ± 0.26 c |
| Nitrate/mg·g−1 (FW) | 0.292 ± 0.01 a | 0.157 ± 0.01 c | 0.106 ± 0.01 d | 0.112 ± 0.01 d | 0.175 ± 0.01 b |
| Total nitrogen/mg·g−1 (DW) | 25.26 ± 0.58 ab | 25.87 ± 1.37 ab | 27.61 ± 1.70 a | 24.03 ± 1.80 b | 18.67 ± 1.03 c |
| Protein nitrogen/mg·g−1 (DW) | 16.92 ± 0.69 d | 20.89 ± 0.07 b | 21.88 ± 0.39 a | 20.84 ± 0.06 b | 19.93 ± 0.43 c |
| AsA/mg·g−1 (FW) | 2.32 ± 0.02 c | 2.3 ± 0.02 c | 2.43 ± 0.01 a | 2.38 ± 0.02 b | 2.36 ± 0.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhou, K.; Hu, J.; Zhang, X.; Li, Q. Optimization of Light Quality for Plant Factory Production of Brassica campestris (Pakchoi). Agriculture 2025, 15, 347. https://doi.org/10.3390/agriculture15030347
Zhou C, Zhou K, Hu J, Zhang X, Li Q. Optimization of Light Quality for Plant Factory Production of Brassica campestris (Pakchoi). Agriculture. 2025; 15(3):347. https://doi.org/10.3390/agriculture15030347
Chicago/Turabian StyleZhou, Chengbo, Kangwen Zhou, Jiangtao Hu, Xu Zhang, and Qingming Li. 2025. "Optimization of Light Quality for Plant Factory Production of Brassica campestris (Pakchoi)" Agriculture 15, no. 3: 347. https://doi.org/10.3390/agriculture15030347
APA StyleZhou, C., Zhou, K., Hu, J., Zhang, X., & Li, Q. (2025). Optimization of Light Quality for Plant Factory Production of Brassica campestris (Pakchoi). Agriculture, 15(3), 347. https://doi.org/10.3390/agriculture15030347







