Phytoplasma Infections and Potential Vector Associations in Wheat and Maize in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and DNA Templates
2.2. Insect Collection and DNA Templates
2.3. Phytoplasma Detection and Identification
| Primer | Region Amplified | Sequence 5′-3′ | References |
|---|---|---|---|
| P1 | 16S rDNA | AAGAGTTTGATCCTGGCTCAGGATT | [32] |
| M1R | 16S rDNA | GCCTCAGCGTCAGTAAAGAC | [33] |
| M1 (16R758F) | 16S rDNA | GTCTTTACTGACGCTGAGGC | [33] |
| P7 | 23S rDNA | CGTCCTTCATCGGCTCTT | [34] |
| fU5 | 16S rDNA | CGGCAATGGAGGAAACT | [35] |
| rU3 | 16S rDNA | TTCAGCTACTCTTTGTAACA | |
| R16F2n | 16S rDNA | GAAACGACTGCTAAGACTGG | [36] |
| R16R2 | 16S rDNA | TGACGGGCGGTGTGTACAAACCCCG | |
| fTuf1 | tuf | CACATTGACCACGGTAAAAC | [37] |
| rTuf1 | tuf | CCACCTTCACGAATAGAGAAC | |
| fTuf1n | tuf | CACGTAGACCACGGTAAAAC | [37] |
| rTuf1n | tuf | CCACCTTCACGAATAGAAAAT | modified |
| fTufAY | tuf | GCTAAAAGTAGAGCTTATGA | [37] |
| rTufAY | tuf | CGTTGTCACCTGGCATTACC | |
| RevI-S | ITS | GCAAGTAAGTTAGTTATAATGAAAAAC | This study |
| RevI-R | ITS | AATGCAATTTGCAAGCAAG |
2.4. The Ribosomal RNA Operon-Specific Amplification of rRNA Genes of 16SrI-C Phytoplasma Strains
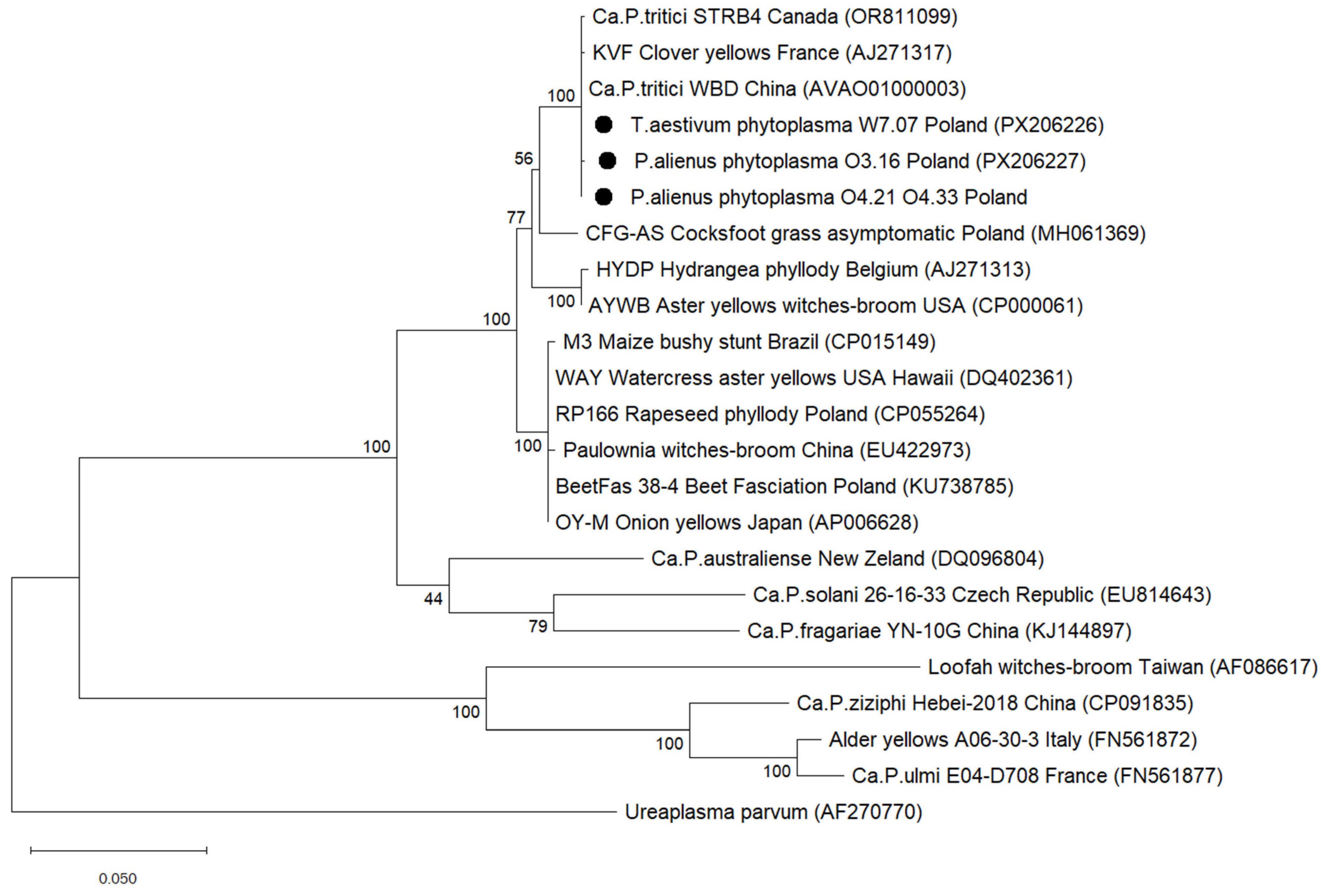
2.5. Phylogenetic Tree Based on the 16S rRNA Gene
2.6. The Tuf Gene Comparative Analysis
2.7. Cereal Viruses Detection in Wheat Using ELISA and PCR
3. Results
3.1. Symptoms Observed in Wheat and Maize
3.2. Phytoplasma Detection and Identification in Plant Samples
3.3. 16S rRNA Gene Phylogeny
3.4. Insect Collection and Species Identification
3.5. Tuf Gene Sequences
3.6. WDV Detection in Wheat Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rao, G.P.; Bertaccini, A.; Fiore, N.; Liefting, L. Phytoplasmas: Plant Pathogenic Bacteria-I: Characterization and Epidemiology of Phytoplasma—Associated Diseases; Springer: Singapore, 2018; ISBN 978-981-13-0119-3. [Google Scholar]
- Huang, W.; MacLean, A.M.; Sugio, A.; Maqbool, A.; Busscher, M.; Cho, S.-T.; Kamoun, S.; Kuo, C.-H.; Immink, R.G.; Hogenhout, S.A. Parasitic Modulation of Host Development by Ubiquitin-Independent Protein Degradation. Cell 2021, 184, 5201–5214.e12. [Google Scholar] [CrossRef]
- Alma, A.; Lessio, F.; Nickel, H. Insects as Phytoplasma Vectors: Ecological and Epidemiological Aspects. In Phytoplasmas: Plant Pathogenic Bacteria—II: Transmission and Management of Phytoplasma—Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 1–25. ISBN 978-981-13-2832-9. [Google Scholar]
- Bertaccini, A. Phytoplasmas: Diversity, Taxonomy, and Epidemiology. Front. Biosci. 2007, 12, 673–689. [Google Scholar] [CrossRef]
- Wei, W.; Lee, I.M.; Davis, R.E.; Suo, X.; Zhao, Y. Automated RFLP Pattern Comparison and Similarity Coefficient Calculation for Rapid Delineation of New and Distinct Phytoplasma 16Sr Subgroup Lineages. Int. J. Syst. Evol. Microbiol. 2008, 58, 2368–2377. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, Y. Phytoplasma Taxonomy: Nomenclature, Classification, and Identification. Biology 2022, 11, 1119. [Google Scholar] [CrossRef]
- Jung, H.Y.; Miyata, S.I.; Oshima, K.; Kakizawa, S.; Nishigawa, H.; Wei, W.; Suzuki, S.; Ugaki, M.; Hibi, T.; Namba, S. First Complete Nucleotide Sequence and Heterologous Gene Organization of the Two rRNA Operons in the Phytoplasma Genome. DNA Cell Biol. 2003, 22, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zwolińska, A.; Borodynko-Filas, N. Intra and Extragenomic Variation between 16S rRNA Genes Found in 16SrI-B-related Phytopathogenic Phytoplasma Strains. Ann. Appl. Biol. 2021, 179, 368–381. [Google Scholar] [CrossRef]
- Katanić, Z.; Krstin, L.; Jezić, M.; Zebec, M.; Ćurković-Perica, M. Molecular Characterization of Elm Yellows Phytoplasmas in Croatia and Their Impact on Ulmus spp. Plant Pathol. 2016, 65, 1430–1440. [Google Scholar] [CrossRef]
- Makarova, O.; Contaldo, N.; Paltrinieri, S.; Kawube, G.; Bertaccini, A.; Nicolaisen, M. DNA Barcoding for Identification of “Candidatus Phytoplasmas” Using a Fragment of the Elongation Factor Tu Gene. PLoS ONE 2012, 7, e52092. [Google Scholar] [CrossRef]
- Martini, M.; Lee, I.M.; Bottner, K.D.; Zhao, Y.; Botti, S.; Bertaccini, A.; Harrison, N.A.; Carraro, L.; Marcone, C.; Khan, A.J.; et al. Ribosomal Protein Gene-Based Phylogeny for Finer Differentiation and Classification of Phytoplasmas. Int. J. Syst. Evol. Microbiol. 2007, 57, 2037–2051. [Google Scholar] [CrossRef]
- Wu, Y.F.; Hao, X.G.; Li, Z.N.; Gu, P.W.; An, F.Q.; Xiang, J.Y.; Wang, H.N.; Luo, Z.P.; Liu, J.J.; Xiang, Y. Identification of the Phytoplasma Associated with Wheat Blue Dwarf Disease in China. Plant Dis. 2010, 94, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-T.; Kung, H.-J.; Huang, W.; Hogenhout, S.A.; Kuo, C.-H. Species Boundaries and Molecular Markers for the Classification of 16SrI Phytoplasmas Inferred by Genome Analysis. Front. Microbiol. 2020, 11, 1531. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, W.; Davis, R.E.; Lee, M.; Bottner-Parker, K.D. The Agent Associated with Blue Dwarf Disease in Wheat Represents a New Phytoplasma Taxon,‘Candidatus Phytoplasma Tritici. Int. J. Syst. Evol. Microbiol. 2021, 71, 004604. [Google Scholar] [CrossRef]
- Gamarra, D.G.; Villar, C.M.; Suarez, G.T.; Esteban, W.D.I.; Contaldo, N.; Lozano, E.C.C.; Bertaccini, A. Diverse Phytoplasmas Associated with Maize Bushy Stunt Disease in Peru. Eur. J. Plant Pathol. 2022, 163, 223–235. [Google Scholar] [CrossRef]
- Jović, J.; Cvrković, T.; Mitrović, M.; Krnjajić, S.; Petrović, A.; Redinbaugh, M.G.; Pratt, R.C.; Hogenhout, S.A.; Toševski, I. Stolbur Phytoplasma Transmission to Maize by Reptalus Panzeri and the Disease Cycle of Maize Redness in Serbia. Phytopathology 2009, 99, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Orlovskis, Z.; Canale, M.C.; Haryono, M.; Lopes, J.R.S.; Kuo, C.H.; Hogenhout, S.A. A Few Sequence Polymorphisms among Isolates of Maize Bushy Stunt Phytoplasma Associate with Organ Proliferation Symptoms of Infected Maize Plants. Ann. Bot. 2017, 119, 869–884. [Google Scholar] [CrossRef]
- Ramos, A.; Esteves, M.B.; Cortés, M.T.B.; Lopes, J.R.S. Maize Bushy Stunt Phytoplasma Favors Its Spread by Changing Host Preference of the Insect Vector. Insects 2020, 11, 600. [Google Scholar] [CrossRef]
- Kosovac, A.; Rekanović, E.; Ćurčić, Ž.; Stepanović, J.; Duduk, B.; Kosovac, A.; Rekanović, E.; Ćurčić, Ž.; Stepanović, J.; Duduk, B. Plants under Siege: Investigating the Relevance of ‘Ca. P. Solani’ Cixiid Vectors through a Multi-Test Study. Plants 2023, 12, 4157. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, C.R.; Atkinson, L.M.; Samac, D.A.; Larsen, J.E.; Motteberg, C.D.; Abrahamson, M.D.; Glogoza, P.; MacRae, I.V. Region and Field Level Distributions of Aster Yellows Phytoplasma in Small Grain Crops. Plant Dis. 2008, 92, 623–630. [Google Scholar] [CrossRef]
- Pfrieme, A.-K.; Will, T.; Pillen, K.; Stahl, A. The Past, Present, and Future of Wheat Dwarf Virus Management—A Review. Plants 2023, 12, 3633. [Google Scholar] [CrossRef] [PubMed]
- Parizipour, M.G.; Behjatnia, S.; Afsharifar, A.; Izadpanah, K. Natural Hosts and Efficiency of Leafhopper Vector in Transmission of Wheat Dwarf Virus. J. Plant Pathol. 2016, 98, 483–492. [Google Scholar]
- Wei, S.; Liu, L.; Chen, G.; Yang, H.; Huang, L.; Gong, G.; Luo, P.; Zhang, M. Molecular Evolution and Phylogeographic Analysis of Wheat Dwarf Virus. Front. Microbiol. 2024, 15, 1314526. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Isidoro, N.; Riolo, P. Natural Phytoplasma Infection of Four Phloem-Feeding Auchenorrhyncha Across Vineyard Agroecosystems in Central-Eastern Italy. J. Econ. Entomol. 2013, 106, 604–613. [Google Scholar] [CrossRef]
- Walls, J., III; Rajotte, E.; Rosa, C. The Past, Present, and Future of Barley Yellow Dwarf Management. Agriculture 2019, 9, 23. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Hendrickson, R.C. Changes to Virus Taxonomy and the ICTV Statutes Ratified by the International Committee on Taxonomy of Viruses (2023). Arch. Virol. 2023, 168, 175. [Google Scholar] [CrossRef]
- Trzmiel, K. Identification of Barley Yellow Dwarf Viruses in Poland. J. Plant Pathol. 2017, 99, 493–497. [Google Scholar]
- Trzmiel, K.; Hasiów-Jaroszewska, B. Molecular Characteristics of Barley Yellow Dwarf Virus—PAS—The Main Causal Agent of Barley Yellow Dwarf Disease in Poland. Plants 2023, 12, 3488. [Google Scholar] [CrossRef]
- Biedermann, R.; Niedringhaus, R. Die Zikaden Deutschlands: Bestimmungstafeln Für Alle Arten; Wabv-Fründ: Scheeßel, Germany, 2004; ISBN 3-939202-00-2. [Google Scholar]
- Zhao, Y.; Wei, W.; Lee, I.-M.; Shao, J.; Suo, X.; Davis, R.E. Construction of an Interactive Online Phytoplasma Classification Tool, iPhyClassifier, and Its Application in Analysis of the Peach X-Disease Phytoplasma Group (16SrIII). Int. J. Syst. Evol. Microbiol. 2009, 59, 2582–2593. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Gundersen-Rindal, D.E.; Davis, R.E.; Bartoszyk, I.M. Revised Classification Scheme of Phytoplasmas Based on RFLP Analyses of 16S rRNA and Ribosomal Protein Gene Sequences. Int. J. Syst. Bacteriol. 1998, 48, 1153–1169. [Google Scholar] [CrossRef]
- Deng, S.J.; Hiruki, C. Amplification of 16S Ribosomal-RNA Genes from Culturable and Nonculturable Mollicutes. J. Microbiol. Methods 1991, 14, 53–61. [Google Scholar] [CrossRef]
- Gibb, K.S.; Padovan, A.C.; Mogen, B.D. Studies on Sweet-Potato Little-Leaf Phytoplasma Detected in Sweet-Potato and Other Plant-Species Growing in Northern Australia. Phytopathology 1995, 85, 169–174. [Google Scholar] [CrossRef]
- Schneider, B.; Seemüller, E.; Smart, C.D.; Kirkpatrick, B.C. Phylogenic Classification of Plant Pathogenic Mycoplasmalike Organisms or Phytoplasmas. In Molecular and Diagnostic Procedures in Mycoplasmology; Academic Press: San Diego, CA, USA, 1995; pp. 369–380. [Google Scholar]
- Lorenz, K.; Schneider, B.; Ahrens, U.; Seemüller, E. Detection of the Apple Proliferation and Pear Decline Phytoplasmas by PCR Amplification of Ribosomal and Nonribosomal DNA. Phytopathology 1995, 85, 771–776. [Google Scholar] [CrossRef]
- Gundersen, D.E.; Lee, I.-M. Ultrasensitive Detection of Phytoplasmas by Nested-PCR Assays Using Two Universal Primer Pairs. Phytopathol. Mediterr. 1996, 35, 114–151. [Google Scholar]
- Schneider, B.; Gibb, K.S.; Seemuller, E. Sequence and RFLP Analysis of the Elongation Factor Tu Gene Used in Differentiation and Classification of Phytoplasmas. Microbiology 1997, 143, 3381–3389. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. Clustal-W—Improving the Sensitivity of Progressive Multiple Sequence Alignment Through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A. Characteristics of the Microplate Method of Enzyme-Linked Immunosorbent Assay for the Detection of Plant Viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Trzmiel, K. Occurrence of Wheat Dwarf Virus and Barley Yellow Dwarf Virus Species in Poland in the Spring of 2019. J. Plant Prot. Res. 2020, 60, 345–350. [Google Scholar]
- Trzmiel, K.; Klejdysz, T. Detection of Barley-and Wheat-Specific Forms of Wheat Dwarf Virus in Their Vector Psammotettix Alienus by Duplex PCR Assay. J. Plant Prot. Res. 2018, 58, 54–57. [Google Scholar] [CrossRef]
- Zwolińska, A.; Krawczyk, K.; Borodynko-Filas, N.; Pospieszny, H. Non-Crop Sources of Rapeseed Phyllody Phytoplasma (“Candidatus Phytoplasma Asteris”: 16SrI-B and 16SrI-(B/L)L), and Closely Related Strains. Crop Prot. 2019, 119, 59–68. [Google Scholar] [CrossRef]
- Llácer, G.; Medina, V.; Archelós, D. A Survey of Potential Vectors of Apricot Chlorotic Leaf Roll. Agronomie 1988, 8, 79–83. [Google Scholar] [CrossRef]
- Salehi, M.; Siampour, M.; Esmailzadeh Hosseini, S.; Bertaccini, A. Characterization and Vector Identification of Phytoplasmas Associated with Cucumber and Squash Phyllody in Iran. Bull. Insectology 2015, 68, 311–319. [Google Scholar]
- Wilson, M.; Stewart, A.; Biedermann, R.; Nickel, H.; Niedringhaus, R. The Planthoppers and Leafhoppers of Britain and Ireland Identification Keys to All Families and Genera and All British and Irish Species Not Recorded from Germany; Wabv-Fründ: Scheeßel, Germany, 2015; ISBN 9783939202066. [Google Scholar]
- Galvão, S.R.; Sabato, E.O.; Bedendo, I.P. Occurrence and Distribution of Single or Mixed Infection of Phytoplasma and Spiroplasma Causing Corn Stunting in Brazil. Trop. Plant Pathol. 2021, 46, 152–155. [Google Scholar] [CrossRef]
- Perez-Lopez, E.; Olivier, C.Y.; Luna-Rodriguez, M.; Rodriguez, Y.; Iglesias, L.G.; Castro-Luna, A.; Adame-Garcia, J.; Dumonceaux, T.J. Maize Bushy Stunt Phytoplasma Affects Native Corn at High Elevations in Southeast Mexico. Eur. J. Plant Pathol. 2016, 145, 963–971. [Google Scholar] [CrossRef]
- Jović, J.; Cvrković, T.; Mitrović, M.; Petrović, A.; Krstić, O.; Krnjajić, S.; Toševski, I. Multigene Sequence Data and Genetic Diversity among ‘Candidatus Phytoplasma Ulmi’ Strains Infecting Ulmus spp. in Serbia. Plant Pathol. 2011, 60, 356–368. [Google Scholar] [CrossRef]
- Malembic-Maher, S.; Desqué, D.; Khalil, D.; Salar, P.; Bergey, B.; Danet, J.-L.; Duret, S.; Dubrana-Ourabah, M.-P.; Beven, L.; Ember, I.; et al. When a Palearctic Bacterium Meets a Nearctic Insect Vector: Genetic and Ecological Insights into the Emergence of the Grapevine Flavescence Dorée Epidemics in Europe. PLoS Pathog. 2020, 16, e1007967. [Google Scholar] [CrossRef]
- Fos, A.; Danet, J.L.; Zreik, L.; Garnier, M.; Bove, J.M. Use of a Monoclonal-Antibody to Detect the Stolbur Mycoplasma-like Organism in Plants and Insects and to Identify a Vector in France. Plant Dis. 1992, 76, 1092–1096. [Google Scholar] [CrossRef]
- Maczey, N.; Masters, G.J.; Hollier, J.A.; Mortimer, S.R.; Brown, V.K. Community Associations of Chalk Grassland Leafhoppers (Hemiptera: Auchenorrhyncha): Conclusions for Habitat Conservation. J. Insect Conserv. 2005, 9, 281–298. [Google Scholar] [CrossRef]
- Marion-Poll, F.; Giustina, W.D.; Mauchamp, B. Changes of Electric Patterns Related to Feeding in a Mesophyll Feeding Leafhopper. Entomol. Exp. Appl. 1987, 43, 115–124. [Google Scholar] [CrossRef]
- Batlle, A.; Martínez, M.A.; Laviña, A. Occurrence, Distribution and Epidemiology of Grapevine Yellows in Spain. Eur. J. Plant Pathol. 2000, 106, 811–816. [Google Scholar] [CrossRef]


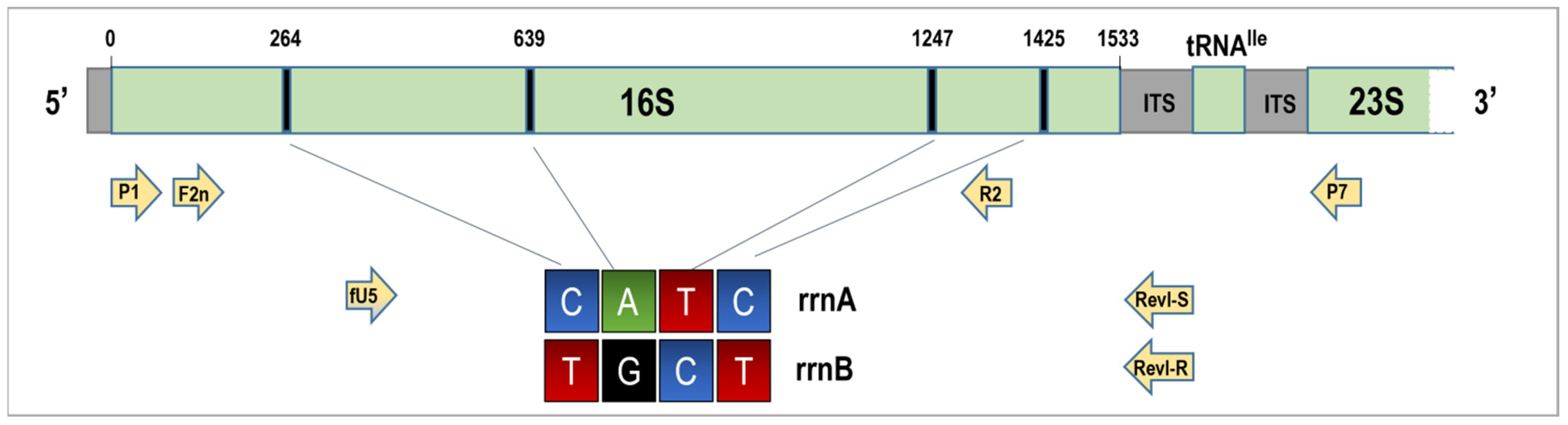



| Crop | Field (Batch) | Number of Samples | Collection Date | Growth Stage (BBCH Scale) | Latitude | Longitude |
|---|---|---|---|---|---|---|
| Wheat | 1 (W1) | 50 | 3 May 2019 | 39 | 50°58′34″ N | 16°42′24″ E |
| 2 (W2) | 50 | 4 May 2019 | 39 | 51°12′26″ N | 17°08′42″ E | |
| 3 (W7) | 50 | 10 November 2019 | 24 | 50°58′17″ N | 16°42′46″ E | |
| 4 (W8) | 50 | 11 November 2019 | 25 | 51°12′53″ N | 17°08′26″ E | |
| Maize | 1 (W3) | 50 | 10 June 2019 | 17 | 50°58′40″ N | 16°42′48″ E |
| 2 (W4) | 50 | 11 June 2019 | 18 | 51°12′52″ N | 17°08′22″ E | |
| 1 (W5) | 50 | 29 July 2019 | 39 | 50°58′40″ N | 16°42′48″ E | |
| 2 (W6) | 50 | 29 July 2019 | 39 | 51°12′52″ N | 17°08′22″ E |
| Crop | Field (Batch) | Number of Insects | Collection Date | Growth Stage (BBCH Scale) | Latitude | Longitude |
|---|---|---|---|---|---|---|
| Maize | 1 (O1) | 155 | 2 August 2018 | 51 | 50°59′31″ N | 16°41′23″ E |
| 2 (O2) | 120 | 3 August 2018 | 51 | 51°12′48″ N | 17°08′51″ E | |
| Wheat | 1 (W1, O3) | 63 | 3 May 2019 | 39 | 50°58′34″ N | 16°42′24″ E |
| 2 (W2, O4) | 70 | 4 May 2019 | 39 | 51°12′26″ N | 17°08′42″ E |
| Crop | Date | Sample | Insect | Phytoplasma | |
|---|---|---|---|---|---|
| 1 | Maize | 1 August 2018 | O1.204 | Z. scutellaris | 16SrV-C |
| 2 | Maize | 1 August 2018 | O1.242 | Z. scutellaris | 16SrV-C |
| 3 | Maize | 1 August 2018 | O2.260 | Z. scutellaris | 16SrV-C |
| 4 | Maize | 1 August 2018 | O2.284 | P. alienus | 16SrI-C |
| 5 | Wheat | 3 May 2019 | O3.04 | P. alienus | 16SrI-C |
| 6 | Wheat | 3 May 2019 | O3.06 | P. alienus | 16SrI-C |
| 7 | Wheat | 3 May 2019 | O3.08 | P. alienus | 16SrI-C |
| 8 | Wheat | 3 May 2019 | O3.09 | P. alienus | 16SrI-C |
| 9 | Wheat | 3 May 2019 | O3.10 | P. alienus | 16SrI-C |
| 10 | Wheat | 3 May 2019 | O3.16 | P. alienus | 16SrI-C |
| 11 | Wheat | 3 May 2019 | O3.38 | P. alienus | 16SrI-C |
| 12 | Wheat | 4 May 2019 | O4.21 | P. alienus | 16SrI-C |
| 13 | Wheat | 4 May 2019 | O4.31 | P. alienus | 16SrI-B |
| 14 | Wheat | 4 May 2019 | O4.33 | P. alienus | 16SrI-C |
| Collection Date | Symptomatic | Asymptomatic | WDV Detection |
|---|---|---|---|
| May 2019 | W1.22 | yes | |
| W1.36 | yes | ||
| W1.47 | yes | ||
| November 2019 | W7.07 | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwolińska, A.; Jurga-Zotow, M.; Trzmiel, K.; Klejdysz, T.; Hasiów-Jaroszewska, B. Phytoplasma Infections and Potential Vector Associations in Wheat and Maize in Poland. Agriculture 2025, 15, 2571. https://doi.org/10.3390/agriculture15242571
Zwolińska A, Jurga-Zotow M, Trzmiel K, Klejdysz T, Hasiów-Jaroszewska B. Phytoplasma Infections and Potential Vector Associations in Wheat and Maize in Poland. Agriculture. 2025; 15(24):2571. https://doi.org/10.3390/agriculture15242571
Chicago/Turabian StyleZwolińska, Agnieszka, Marta Jurga-Zotow, Katarzyna Trzmiel, Tomasz Klejdysz, and Beata Hasiów-Jaroszewska. 2025. "Phytoplasma Infections and Potential Vector Associations in Wheat and Maize in Poland" Agriculture 15, no. 24: 2571. https://doi.org/10.3390/agriculture15242571
APA StyleZwolińska, A., Jurga-Zotow, M., Trzmiel, K., Klejdysz, T., & Hasiów-Jaroszewska, B. (2025). Phytoplasma Infections and Potential Vector Associations in Wheat and Maize in Poland. Agriculture, 15(24), 2571. https://doi.org/10.3390/agriculture15242571







