1. Introduction
Soybeans are a valuable legume crop that is an important source of oil, food, and animal feed. The growing demand for soybeans provides incentives for growers to maximize crop yields, which are directly impacted by nutrient availability. Production of a high-yield crop of soybeans requires up to 380 lbs of nitrogen per acre [
1,
2,
3]. The majority of this nitrogen is supplied by nitrogen-fixation performed by
Bradyrhizobium japonicum in root nodules [
1,
4]. The establishment of soybean nodules requires the presence of symbiotic bacteria, from either seed inoculation or residual
Bradyrhizobium japonicum from soybeans grown in the prior three years, as well as appropriate field conditions. Failure to produce nodules can result from anaerobic soil conditions (.e., flooding [
5]), nematode infection [
6,
7,
8,
9], and excessive nitrate levels [
10,
11,
12]. In these instances, portions of a field experiencing nitrogen deficiency often exhibit chlorosis, while plants in other areas of the field appear healthy.
Soybeans with sufficient nodulation can generally be grown successfully without the application of nitrogen fertilizers, but insufficient nodulation can result in significant yield loss [
13]. Studies have shown that nitrogen fertilization generally results in increased soybean yields [
3,
14,
15], but overapplication of additional nitrogen can result in excessive cost and waste. A previous study showed that applying nitrogen-rich swine manure enhanced soybean yield and plant nitrogen accumulation without negatively impacting the environment, if the application rates did not exceed the plant’s nitrogen uptake capacity [
16]. Interestingly, the authors also used mutant soybeans that could not produce nodules to indicate the role of nitrogen fixation on the dynamics of nitrogen uptake. As expected, they found that soybean plants lacking nodules required an additional application of nitrogen fertilizer to reach maximal crop yields.
We hypothesized that site-specific fertilization of nitrogen-deficient regions of soybean fields could result in yield and profit benefits. This strategy requires timely identification of nitrogen deficiency and information about the amount of nitrogen needed to rescue deficient plants. While there has been a burst of high-throughput phenotyping technology for the analysis of plant health for scientific research [
17], tools for growers to identify and address soybean nitrogen deficiency have been limited. However, the use of aerial photography with unmanned aerial vehicles (drones) is becoming a valuable resource for field scouting and decision making. Previous studies have shown that color classification and edge detection can be used to identify components of plant health [
18]. We predict that digital image analysis could allow soybean farmers to quickly identify and diagnose nitrogen-deficient regions of their fields. Thus, the aim of this project was to begin to develop models for using aerial image analysis to predict when nitrogen deficiency is present and provide information about how much nitrogen needs to be applied to restore yield.
2. Materials and Methods
Materials—Williams 82 [
19] and nodulation-deficient (nod-) Williams 82 seeds derived from a fast neutron mutagenesis population [
20] were obtained from Dr. Minviluz “Bing” Stacy at the University of Missouri. All nitrogen fertilizer treatments were in the form of urea (46.66% nitrogen by weight).
Plot setup—In 2020, plots were located at the Clemson Edisto Research and Education Center in Barnwell County, SC. This growing season was slightly warmer than average (~1 °F), but there were no major disruptions to the normal weather pattern. Williams 82 control and nod- seeds were treated with a standard Bradyrhizobium japonicum inoculant and planted by hand (10 June) with six seeds per foot and 30 in row spacing (~104,500 seeds per acre). Each plot consisted of a block of four 5 ft rows where the two outside rows were untreated Williams 82 (buffer rows) and the two center rows were the treatment. Each treatment was planted in six complete randomized replicates and marked with stakes and flagging tape across the ground to allow for the identification of individual plots via aerial imagery. Nitrogen applications were incorporated into the soil with a rake about 4–6 inches from the plants in each row.
In 2021 and 2022, chlorotic soybean patches were identified in grower’s fields in Barnwell and Allendale Counties, SC. These plots likely represent an array of soybean cultivars, soil quality, and agronomic practices. For each site, four randomized replicates of each treatment were marked and fertilized as described above. Each plot was a single 10 ft long row with 38 in row spacing. The weather in these growing seasons was normal except for above average rain events in August 2021 and June 2022.
Ground measurements—Plant height was measured from the soil to the topmost leaf in five locations within each plot. Normalized Difference Vegetation Index (NDVI) measurements were taken using a handheld Greenseeker™ (NTech Industries Incorporation, Ukiah, CA, USA) held at waist height while walking along the row. Plant tissue analysis was performed by collecting the most recently matured trifoliate from twelve plants in each plot, drying them, then sending them to the Clemson Agricultural Service Lab for nitrogen analysis using established protocols. At maturity (late Oct to early Nov), plants from each plot were bundled and removed from the field, air dried, then hand threshed using an Almaco small bundle thresher. Seed moisture content was determined using a Burrows Model 750 Digital Moisture Computer (Seedburo Equipment Company, Des Plaines, IL, USA) and all calculations for yield comparisons were normalized to 13% moisture content.
Aerial imagery—Aerial images were captured using a DJI Phantom 3 UAV (SZ DJI Technology Co., Ltd., Shenzhen, China) equipped with a standard 2.7 K RGB camera. Geo-referenced images coupled with spatial definitions of plot layout allowed for analysis by plot. Images were analyzed using Clemson’s Batch Load Image Processor software (version 1.1), which discriminates between plant and soil pixels to calculate canopy fraction and outputs summaries of pixel color space values [
21].
3. Results
3.1. Creating Nitrogen Deficiency
Detection of nitrogen deficiency in soybeans by aerial photography requires ground truth data from plants where the only difference is the amount of nitrogen present. We predicted that the sandy soils present in the coastal plain region of South Carolina combined with a nodulation-deficient soybean mutant [
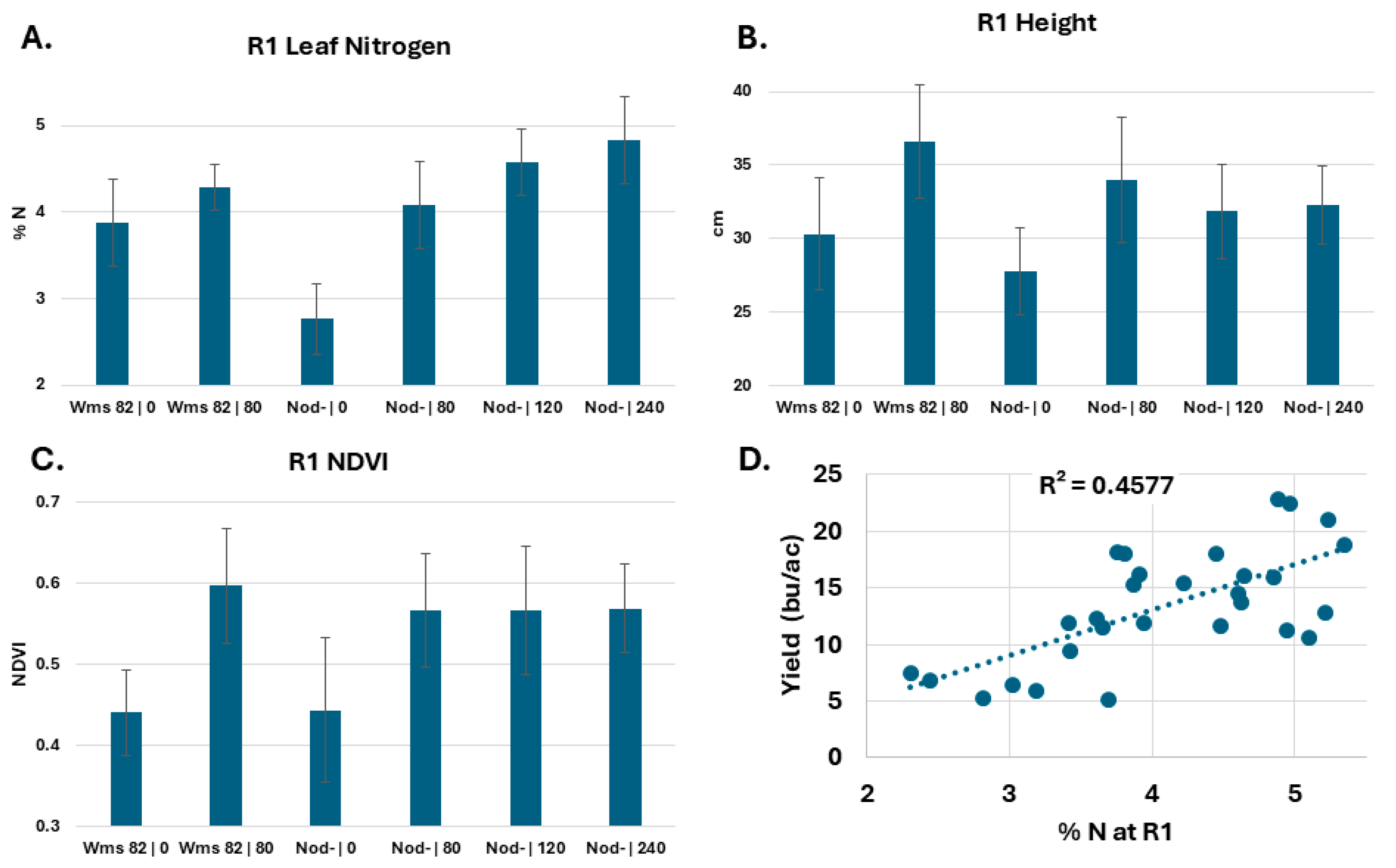
20] would produce the needed nitrogen deficiencies. In 2020, we observed that the application of differing amounts of nitrogen (0, 80, 160, and 240 pounds per acre in the form of urea) at planting produced a gradient of leaf nitrogen levels in nodulation-deficient soybeans at the R1 stage (
Figure 1A). nod- plants with no nitrogen treatment had about 3% leaf nitrogen, while fertilization at planting with 80 lbs or more nitrogen raised the leaf nitrogen content of nod- plants to the normal range of 4–5%. In contrast to the change observed for nod- plants, the application of 80 lbs per acre of nitrogen to Wiliams 82 plants did not result in a significant increase in leaf nitrogen levels.
In addition to measuring leaf nitrogen levels at R1, we also measured height and NDVI by hand. As expected, we observed that the addition of nitrogen to nodulation-deficient plants resulted in taller (
Figure 1B) and greener and/or larger canopy plants (higher NDVI values,
Figure 1C). Williams 82 plants were also taller and greener from the nitrogen treatment, indicating that the additional available nitrogen stimulated growth. As Williams 82 is categorized as Maturity group III, it had a relatively short growing season in SC, resulting in relatively low yields. With no additional nitrogen application, we observed increased yield from plants with higher R1 leaf nitrogen levels (
Figure 1D). Together, these results indicate that nitrogen deficiency can be detected at or before flowering and that nitrogen-deficient plants have a significantly lower yield.
3.2. Detecting Nitrogen Deficiency with Aerial Imagery
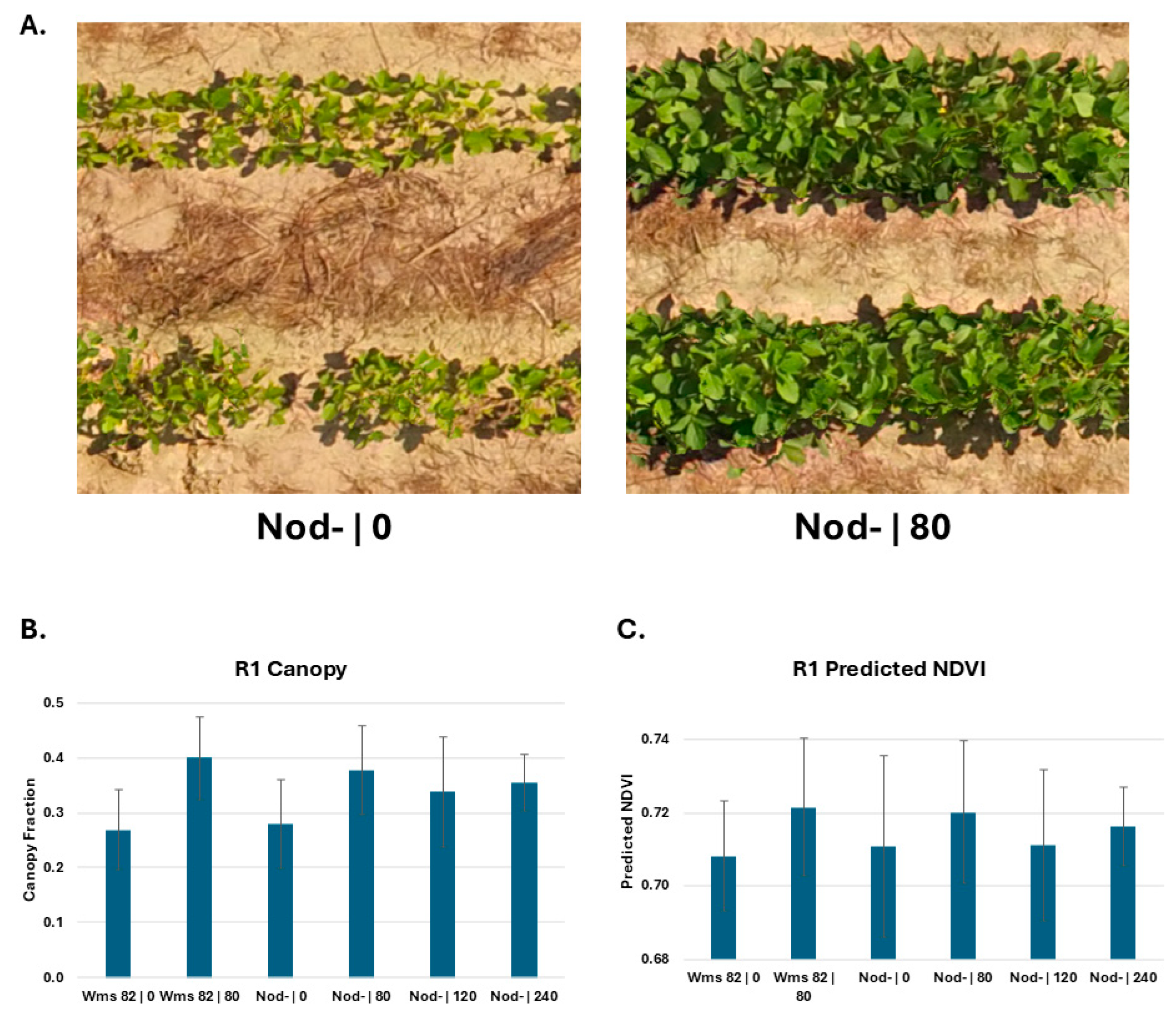
Aerial images were captured at the R1 stage, including both a single, coarser ground sampling distance (i.e., fewer pixels per plot) image of the field and a mosaic of images captured from lower altitudes. Visual inspection of these images shows the obvious phenotypic differences between nitrogen-deficient and nitrogen-sufficient plants (
Figure 2A). We used these images to calculate the canopy fraction based on the percentage of an image representing leaves and soil (
Figure 2B). These results were comparable to ground-based measurements, with increased nitrogen fertilization corresponding to larger plants. The average predicted NDVI (
Figure 2C) calculated from these aerial images showed a similar pattern to the ground NDVI measurements, but with a larger degree of error. This suggests that aerial image analysis can be used as a proxy for ground measurements, simplifying the process of identifying nitrogen-deficient patches.
3.3. Rescuing Nitrogen-Deficient Plants
At the R1 stage, rescue applications of fertilizer (80, 160, and 240 pounds per acre in the form of urea) were applied to a subset of plants with low nitrogen content (nod-). Within two weeks, these nitrogen treatments appeared to green up the nitrogen-deficient plants (
Figure 3A). NDVI measurement showed that the lowest (80 lbs/acre) rescue application of nitrogen produced the strongest increase in NDVI in the nod- plants (
Figure 3B). Measurements of mature plant heights showed that rescue nitrogen applications generally increased plant growth, although the 240 lbs/acre rescue application was not an improvement over lower application rates (
Figure 3C). These results indicate that higher nitrogen application rates may result in stress or shock to plants.
Yield analysis of Williams 82 and nod- plants treated with a rescue nitrogen application at R1 are shown in
Figure 3D. This indicates that the application of 80 lbs or more nitrogen per acre to nod- plants restored the yield to levels observed for unfertilized nodulating Williams 82. This significant yield increase suggests that poorly nodulated soybeans can be rescued if action is taken by the time the plants flower.
3.4. Field Application
In 2021 and 2022, large patches of short chlorotic plants were identified in soybean fields in South Carolina (
Table 1). These patches were generally located in low lying or very sandy regions that likely had insufficient nodulation. In addition to these chlorotic plants, nearby plots of healthy plants were identified for each experimental location. Baseline measurements of leaf nitrogen, NDVI, height, and soil fertility were taken. Initial leaf nitrogen levels of these plots showed that chlorotic plots had relatively low levels of leaf nitrogen (3.04% on average), while adjacent healthy plants showed normal levels of leaf nitrogen (4.58% on average) (
Table 1). These results confirmed that at least one of the causes of chlorosis was low nitrogen levels, but do not rule out other nutrient deficiencies or diseases.
At the R1 stage, each chlorotic patch was divided into four replicates of 10 ft row plots, which were randomly assigned into four nitrogen treatments (0, low = 40, med = 80, and high = 120 pounds of nitrogen per acre). These treatments consisted of side dressing with the appropriate amount of urea in a shallow trench about 6 inches from the plants and covering the fertilizer with a thin layer of soil to prevent it from drifting into other treatments. The health of the plots was reevaluated one month after nitrogen application, including leaf tissue analysis, height, and NDVI. We found that rescue nitrogen application increased the leaf nitrogen to normal amounts [>4%] (
Figure 4A) and resulted in a significant increase in height compared to untreated controls (
Figure 4B). In addition, we observed that nitrogen rescue produced a visible difference in color that corresponds with an increase in measured NDVI (
Figure 4C). Together, these responses suggest that nitrogen deficiency was a limiting factor in the growth of plants in the chlorotic patches.
At harvest, we observed a significant increase in yield for all nitrogen treatments (
Figure 4D). This suggests that the burst of growth observed for nitrogen-treated plants resulted in an increased ability to focus energy on seed production. Even the lowest level of nitrogen rescue produced an average of 10 bushels more soybeans per acre. This significant increase in yield will have a major impact for growers, as it indicates that nitrogen-deficient soybeans can be rescued if action is taken by the time the plants flower.
These results suggest that side dressing nitrogen-deficient soybeans with moderate levels of nitrogen at or before the R1 stage could be economically feasible depending on leaf nitrogen levels, nitrogen costs, and the price of soybeans. Because of the danger of harming plants with high levels of nitrogen application, our experiments suggest that the application of relatively low levels of rescue nitrogen (40–80 pounds per acre) is the safest option. Thus, our general recommendation to soybean growers with chlorotic patches with leaf nitrogen levels below 3% is to apply 40–80 pounds per acre of nitrogen to the affected area.
As it is only advisable to apply rescue applications to areas that show clear signs of nitrogen deficiency, we are continuing to pursue methods for growers to accurately prescribe variable rate applications. In conjunction with our 2021 test plots at the Tampa Creek location, we were able to perform a simplified variable rate nitrogen application to the remainder of the field as needed (100 pounds per acre in regions predicted to have low nitrogen levels). This basic test application produced promising results with the patchy canopy color becoming more uniform and greener. However, additional experiments are needed to facilitate the development of mechanisms to confidently identify low-nitrogen areas and prescribe rescue applications.
4. Discussion
This study shows that nod- soybeans can be used to generate a range of leaf nitrogen levels (
Figure 1). This type of ground truthing is necessary to calibrate effective models for identifying nitrogen deficiency in soybean and other crops. Our proof-of-concept experiments showed a clear correlation between the amount of nitrogen applied and the amount of nitrogen present in the leaf (
Figure 1). Although the Williams 82 variety used in parts of this study is not an elite cultivar, the visible leaf chlorosis produced should be comparable to other cultivars. However, we recommend that additional studies be performed across a wider range of soil types and climates to refine the leaf nitrogen prediction model.
Our results suggest that a model for the prediction of leaf nitrogen levels could be used to develop a tool that growers can use for the identification of nitrogen-deficient plants. However, there are other deficiencies that produce chlorosis in soybean leaves. The most common source of leaf chlorosis for soybean is iron deficiency in high-pH soils (especially calcareous soils with high moisture and nitrate contents [
22]). Several tools for rating iron deficiency chlorosis in soybeans have been developed using both ground- and aerial-based images [
23,
24,
25,
26]. Thus, there needs to be coordination between iron-deficiency and nitrogen-deficiency research to produce a more comprehensive prediction tool.
These results provide insight into how to use digital image processing to inform producers’ decisions. Our experiments clearly demonstrated that nitrogen-deficient soybean patches can be rescued with the application of nitrogen at the R1 stage. The robust recovery of these plants confirms that nitrogen deficiency was responsible for the chlorosis and stunting of nod- mutants and patches in grower’s fields. In both cases, providing this critical nutrient allowed plants to grow enough to significantly increase the yield (
Figure 3D and
Figure 4D). We observed that a relatively low amount of nitrogen (40–80 pounds per acre) was sufficient to see significant growth and greening (
Figure 4). We hypothesize that this boost in nitrogen availability allowed plants to expand their root networks to obtain resources and expand their canopies and the associated photosynthetic capacity.
Based on these results, we recommend the development of mechanisms to allow growers to perform prescribed nitrogen applications to areas that show clear signs of nitrogen deficiency. Our general recommendation for chlorotic patches with leaf nitrogen levels below 3% is to apply 40–50 pounds of nitrogen to the affected area (equivalent to 45–56 kg per hectare). We recommend against the broad application of high levels of nitrogen to fields, as this has negative economic and environmental consequences. Although we side-dressed the nitrogen about 6–8 inches away from the nitrogen-deficient plants in these experiments, it should also be relatively safe to broadcast moderate amounts of nitrogen. Thus, this study provides proof of concept for the identification of nitrogen-deficient soybeans using aerial images and prescribed rescue application of nitrogen. To this end, we were able to perform a simplified treatment plan of 100 lbs/ac nitrogen application to parts of a field predicted to have low nitrogen levels based on aerial image analysis. This trial produced visual improvement in canopy color, but additional experiments are needed to validate the economic return on a large scale.
5. Conclusions
Nitrogen plays a critical role in plant growth and development, and its availability can impact crop yield. Despite the ability to fix atmospheric nitrogen in nodules, soybean plants can experience nitrogen deficiency in some environments, resulting in stunting, chlorosis, and decreased seed production. Nitrogen-deficient soybean patches can be identified using both ground-based and aerial measurements, allowing growers to apply nitrogen fertilizer. These results show that the timely application of nitrogen fertilizer allows nitrogen-deficient plants to grow taller, green up, and produce more seeds. Thus, the development of grower-friendly protocols for the identification and rescue of nitrogen-deficient soybean plants is justified.
Author Contributions
Conceptualization, C.N.H. and K.K.; formal analysis, K.K.; funding acquisition, C.N.H. and K.K.; investigation, C.N.H., B.F. and K.K.; methodology, C.N.H. and K.K.; project administration, C.N.H.; writing—original draft, L.R.H.; writing—review and editing, C.N.H., B.F. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by South Carolina Soybean Board grants to C.N.H. and K.K. from 2018–2022.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank students in the Hancock laboratory for assisting with data collection. We are grateful to the South Carolina growers that provided us access to their fields to perform these experiments. We thank the Stacey lab at the University of Missouri for providing the nod-soybean strain.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish these results.
Abbreviations
The following abbreviations are used in this manuscript:
| nod- | Nodulation deficient |
| Wms 82 | Williams 82 |
| N | Nitrogen |
| R1 | Reproductive stage 1 |
| lbs | Pounds |
| NDVI | Normalized Difference Vegetation Index |
| bu | Bushels |
| ac | Acre |
References
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen Uptake, Fixation and Response to Fertilizer N in Soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Tamagno, S.; Balboa, G.R.; Assefa, Y.; Kovács, P.; Casteel, S.; Salvagiotti, F.; García, F.O.; Stewart, W.; Ciampitti, I.A. Nutrient Partitioning and Stoichiometry in Soybean: A synthesis-analysis. Field Crops Res. 2017, 200, 18–27. [Google Scholar] [CrossRef]
- La Menza, N.C.; Monzon, J.P.; Specht, J.E.; Grassini, P. Is Soybean Yield Limited by Nitrogen Supply? Field Crops Res. 2017, 213, 204–212. [Google Scholar] [CrossRef]
- Leggett, M.; Diaz-Zorita, M.; Koivunen, M.; Bowman, R.; Pesek, R.; Stevenson, C.; Leister, T. Soybean Response to Inoculation with Bradyrhizobium Japonicum in the United States and Argentina. Agron. J. 2017, 109, 1031–1038. [Google Scholar] [CrossRef]
- Sallam, A.; Scott, H. Effects of Prolonged Flooding on Soybeans during Early Vegetative Growth1. Soil Sci. 1987, 144, 61–66. [Google Scholar] [CrossRef]
- Barker, K.; Huisingh, D.; Johnston, S. Antagonistic Interaction between Heterodera Glycines and Rhizobium Japonicum on Soybean. Phytopathology 1972, 62, 1201–1205. [Google Scholar] [CrossRef]
- Huang, J.-s.; Barker, K.; Van Dyke, C. Suppression of Binding between Rhizobia and Soybean Roots by Heterodera Glycines. Phytopathology 1984, 74, 1381–1384. [Google Scholar] [CrossRef]
- Ko, M.; Barker, K.; Huang, J. Nodulation of Soybeans as Affected by Half-Root Infection with Heterodera Glycines. J. Nematol. 1984, 16, 97–105. [Google Scholar]
- Hussey, R.; Barker, K. Influence of Nematodes and Light Sources on Growth and Nodulation of Soybean. J. Nematol. 1976, 8, 48. [Google Scholar]
- Herridge, D.; Roughley, R.; Brockwell, J. Effect of Rhizobia and Soil Nitrate on the Establishment and Functioning of the Soybean Symbiosis in the Field. Aust. J. Agric. Res. 1984, 35, 149–161. [Google Scholar] [CrossRef]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of Nitrate on Nodule and Root Growth of Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef]
- Beard, B.H.; Hoover, R.M. Effect of Nitrogen on Nodulation and Yield of Irrigated Soybeans 1. Agron. J. 1971, 63, 815–816. [Google Scholar] [CrossRef]
- Nguyen, C.X.; Dohnalkova, A.; Hancock, C.N.; Kirk, K.R.; Stacey, G.; Stacey, M.G. Critical Role for Uricase and Xanthine Dehydrogenase in Soybean Nitrogen Fixation and Nodule Development. Plant Genome 2023, 16, e20171. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Kaur, G.; Orlowski, J.M.; Shapiro, C.A.; Lee, C.D.; Wortmann, C.; Holshouser, D.; Nafziger, E.D.; Kandel, H.; Niekamp, J. Soybean Response to Nitrogen Application across the United States: A Synthesis-Analysis. Field Crops Res. 2018, 215, 74–82. [Google Scholar] [CrossRef]
- Wood, C.; Torbert, H.; Weaver, D. Nitrogen Fertilizer Effects on Soybean Growth, Yield, and Seed Composition. J. Prod. Agric. 1993, 6, 354–360. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Schmitt, M.A.; Randall, G.W.; Lamb, J.A.; Orf, J.H.; Gollany, H.T. Swine Manure Application to Nodulating and Nonnodulating Soybean. Agron. J. 2000, 92, 987–992. [Google Scholar] [CrossRef]
- Gill, T.; Gill, S.K.; Saini, D.K.; Chopra, Y.; de Koff, J.P.; Sandhu, K.S. A Comprehensive review of High Throughput Phenotyping and Machine Learning for Plant Stress Phenotyping. Phenomics 2022, 2, 156–183. [Google Scholar] [CrossRef]
- Liang, W.-z.; Kirk, K.R.; Greene, J.K. Estimation of Soybean Leaf Area, Edge, and Defoliation Using Color Image Analysis. Comput. Electron. Agric. 2018, 150, 41–51. [Google Scholar] [CrossRef]
- Bernard, R.; Cremeens, C. Registration of ‘Williams 82′ soybean. Regist. Crop Cultiv. 1988, 28, 1027–1028. [Google Scholar] [CrossRef]
- Hwang, W.J.; Kim, M.Y.; Kang, Y.J.; Shim, S.; Stacey, M.G.; Stacey, G.; Lee, S.-H. Genome-Wide Analysis of Mutations in a Dwarf Soybean Mutant Induced by Fast Neutron Bombardment. Euphytica 2015, 203, 399–408. [Google Scholar] [CrossRef]
- Kirk, K. Batch Load Image Processor, version 1.1; Clemson University: Clemson, SC, USA, 2022.
- Merry, R.; Dobbels, A.A.; Sadok, W.; Naeve, S.; Stupar, R.M.; Lorenz, A.J. Iron Deficiency in Soybean. Crop Sci. 2022, 62, 36–52. [Google Scholar] [CrossRef]
- Naik, H.S.; Zhang, J.; Lofquist, A.; Assefa, T.; Sarkar, S.; Ackerman, D.; Singh, A.; Singh, A.K.; Ganapathysubramanian, B. A Real-Time Phenotyping Framework Using Machine Learning for Plant Stress Severity Rating in Soybean. Plant Methods 2017, 13, 23. [Google Scholar] [CrossRef]
- Zhang, J.; Naik, H.S.; Assefa, T.; Sarkar, S.; Reddy, R.C.; Singh, A.; Ganapathysubramanian, B.; Singh, A.K. Computer Vision and Machine Learning for Robust Phenotyping in Genome-Wide Studies. Sci. Rep. 2017, 7, 44048. [Google Scholar] [CrossRef]
- Bai, G.; Jenkins, S.; Yuan, W.; Graef, G.L.; Ge, Y. Field-Based Scoring of Soybean Iron Deficiency Chlorosis Using RGB Imaging and Statistical Learning. Front. Plant Sci. 2018, 9, 1002. [Google Scholar] [CrossRef]
- Dobbels, A.A.; Lorenz, A.J. Soybean Iron Deficiency Chlorosis High-Throughput Phenotyping Using an Unmanned Aircraft System. Plant Methods 2019, 15, 97. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).