Abstract
Background: Soybean (Glycine max (L.) Merr.), a nutrient-rich leguminous crop high in protein, lipids, and minerals, is extensively cultivated worldwide. The chemical composition of soybean seeds depends not only on the genetic characteristics of the cultivar but also on environmental conditions and agricultural practices. In recent years, biostimulants have gained increasing importance in crop production due to their ability to enhance physiological processes in plants and potentially influence nutrient accumulation. This study aimed to investigate how cultivar and biostimulant type influence the chemical composition of soybean seeds under varying weather conditions in Central Europe. Methods: A three-year field experiment (2017–2019) was conducted in eastern Poland (Central Europe) using a split-plot design. The experimental factors included three non-GMO soybean cultivars (Abelina, Merlin, and SG Anser) and two foliar biostimulants (Asahi SL and Improver). In addition to classical ANOVA, the multivariate analysis of the impact of the investigated factors included principal component analysis (PCA). Results: The applied factors significantly affected seed contents of fat, protein, dry matter, ash, fibre, and macronutrients (N, P, K). Cv. Merlin had the highest fat (22.65%) and fibre content (9.33%), while Abelina showed the highest protein (37.06%) and dry matter content (94.42%). Biostimulant application increased the accumulation of several seed components. Asahi SL significantly enhanced fat content (by 0.69%), protein content (by over 1.5%), and dry matter content (by nearly 0.2%) compared to the control. Improver was more effective in increasing nitrogen (by 0.24%), phosphorus (by 0.5%), and potassium (by 0.15%) contents. Weather conditions throughout the growing seasons significantly altered the impact of the biostimulants. The PCA analysis revealed distinct relationships among the chemical properties of seeds, meteorological factors, and the applied biostimulants.
1. Introduction
Changing climatic conditions and rising demand for nutrient-rich food have prompted the exploration of new strategies to enhance the yield and quality of crops, particularly those vital as food sources for humans and livestock [1,2,3,4]. Soybean seeds are a valuable source of protein, unsaturated fatty acids, and bioactive compounds [5,6,7]. They contain about 40% protein, 20% fat—half of which consists of unsaturated fatty acids—and only 5–8% fibre [8,9]. Soybean protein is known for its beneficial amino acid composition [10,11,12]. Chemical composition analysis also demonstrates that soybeans are rich in potassium, calcium, phosphorus, magnesium, and sodium [13,14]. Additionally, soybean seeds contain minerals and antioxidants [15]. The quality and chemical composition of soybean seeds depend not only on the growth conditions but also on the cultivar. Different cultivars of the same species may accumulate different nutrient levels, which are also influenced by environmental factors [16]. The accumulation of minerals in seeds and grains is controlled by several processes involving both physiological and molecular processes [15,16,17,18,19]. Soybean is a warmth-loving plant that requires an extended growing season and a high sum of effective temperatures (GDD—Growing Degree Days) for proper flowering and seed maturation [20,21,22]. The optimal temperature for soybean germination is approximately 25–30 °C, with a minimum temperature for growth of 10 °C [23]. Thermal conditions also influence protein and oil content in seeds [24]. Insufficient precipitation during critical developmental stages, particularly during flowering and pod formation, may lead to a significant reduction in yield [25]. Conversely, excessive rainfall during maturation may cause seed sprouting in pods, increasing the risk of reduced seed quality [26]. Consequently, climatic variability—including extreme weather events such as droughts or heavy rainfall—poses a major challenge in modern soybean agrotechnology [27]. In addition to climate, the soil type and its physicochemical properties are equally critical factors. Soybean prefers fertile, well-drained soils with a slightly acidic-to-neutral reaction (pH 6.0–7.0) and high humus content [28]. Optimal soil structure promotes root system development, which is crucial for nutrient uptake and effective symbiosis with nitrogen-fixing bacteria (Bradyrhizobium spp.), enabling atmospheric nitrogen fixation [29].
Deficiencies in micro- and macroelements in the soil, particularly phosphorus, potassium, molybdenum, and iron, can restrict the plant’s capacity for effective symbiosis and affect generative development. Furthermore, excessively compacted or waterlogged soil may restrict oxygen availability to roots, impairing plant development and increasing susceptibility to diseases [30].
Research indicates that plant tolerance to stresses (e.g., drought, high temperature, soil salinity) can be enhanced through the application of biostimulants [31,32,33,34,35]. These substances support plant growth, improve physiological functions, and can influence metabolism [36,37]. When applied, they increase yields and may also enhance the soybean seed protein, lipid, and mineral content, which is crucial for both the food and feed industries [38,39,40]. Biostimulant application is particularly justified for species highly sensitive to adverse climatic conditions, such as soybeans, which are sensitive to both low and high temperatures and excessive rainfall or drought [41]. Although soybeans are classified as thermophilic plants and require adequate warm days for maturation, climate change and advances in breeding have made it possible to successfully cultivate them in Central European countries, including Poland [42,43]. The increasing soybean cultivation area in Poland and the incorporation of biostimulants into modern production technologies prompted research into biostimulant application in soybean cultivation under stress-inducing climatic conditions. There is limited research on the effects of specific biostimulants, such as Asahi SL and Improver, on the quality of soybean seeds cultivated in Poland. Previous studies have primarily focused on their impact on yield, growth parameters, or plant stress resistance. The influence of these biostimulants on seed quality traits, such as protein content, macronutrient content, and overall nutritional value, is insufficiently documented. Furthermore, there is a paucity of research addressing the variability in weather conditions typical of Poland’s climate, such as spring droughts and erratic precipitation patterns. In the context of escalating climate change, a critical challenge in agriculture is not only to increase yields but also to enhance the quality of plant material and to improve plant resilience to abiotic stresses, such as drought, high temperatures, or fluctuations in soil moisture. Consequently, there is a pressing need to develop and implement agronomic methods to support crops that not only enhance yield but also maintain seed under stress conditions. Biostimulants, such as Asahi SL and Improver, offer a sustainable solution for sustainable agriculture under variable climatic conditions.
To date, research has predominantly focused on the effects of biostimulants on crop yield and growth parameters, whereas their impact on seed quality—particularly protein, lipid, and macronutrient content—under Poland’s variable weather conditions is poorly understood. The present study addresses this gap by determining the specific nutrient content in the seeds of three non-GMO soybean cultivars grown under the conditions of central-eastern Poland, based on biostimulant application.
In light of this, the following research hypothesis was formulated: ‘The application of the biostimulants Asahi SL and Improver significantly increases the protein and macronutrient mineral (P, K, Mg, Ca) content in the seeds of tested soybean cultivars compared to the control, under specific climatic stress conditions typical of Central Europe.’
2. Materials and Methods
2.1. Description of the Experiment
A field experiment was conducted at Łączka, eastern Poland (52°15′ N, 21°95′ E) during the years 2017–2019. The experiment was a split-plot arrangement with three replicates. The experimental factors were as follows: factor A—soybean cultivars: A1—Abelina (developed by Saatzucht Donau Ges.m.b.H. & CoKG (Probstdorf, Austria), A2—SG Anser (developed by Saatzucht Donau Ges.m.b.H. & CoKG (Probstdorf, Austria), A3—Merlin (developed by Saatzucht Donau Ges.m.b.H. & CoKG (Probstdorf, Austria); factor B—types of applied biostimulants: B1—Control (no biostimulant), B2—Asahi SL (developed by UPL Polska sp.zoo, Poland Warsaw), B3—Improver (developed by Bio-Gen sp.zoo, Poland, Łódź). Asahi SL is a biostimulant based on synthetic phenolic compounds containing three active substances: sodium para-nitrophenolate (0.3%), sodium ortho-nitrophenolate (0.2%), and sodium 5-nitroguaiacolate (0.1%). The formulation primarily functions by regulating plant hormonal balance, thereby supporting processes such as photosynthesis, cell division, and energy metabolism. Improver is an anti-stress biostimulant containing a complex of potassium nitrophenolates: potassium 4-nitrophenolate (0.25–0.30%), potassium 2-nitrophenolate (0.14–0.20%), and potassium 5-nitroguaiacolate (0.07–0.10%). It enhances plant resistance mechanisms, increases tolerance to stress conditions, promotes enzymatic activity, and improves the absorption of mineral nutrients from the soil. The soybean cultivar Abelina is recommended for cultivation throughout Poland. It is characterised by rapid growth, uniform maturation, and a superior yield potential. The cultivars SG Anser and Merlin are recommended for cultivation in central and southern Poland. The experimental unit comprised individual plots, each measuring 9 m2 and representing a unique combination of cultivar and biostimulant treatment. Maize was grown as the preceding crop each year. Each treatment had three replicates arranged in a split-plot design. Main plots were assigned to cultivars and subplots to biostimulant treatments. Soybean seeds used for planting were purchased from Saatbau, a company that distributes soybean seeds in Poland. These seeds were certified to ensure freedom from GMO contamination.
The experiment was conducted on soil classified under the World Reference Base for Soil Resources (2014) [44] as belonging to the Haplic Luvisol group. Soil characteristics are presented in Table 1. Based on soil nutrient availability in each study year, mineral fertilisation rates were adjusted to supply 30 kg N, 30 kg P, and 90 kg K ha−1.

Table 1.
Selected soil properties in the 0–0.25 m layer prior to the experiment set-up (2017–2019).
The row spacing was set at 22 cm, and seeds were sown at a depth of approximately 4 cm, at a rate of 70 seeds per m2 on the following dates: 4 May 2017; 5 May 2018; and 1 May 2019. Within five days after sowing, the herbicide Stomp Aqua 455 CS was applied to the soil at a rate of 1.5 L per hectare. During the growing season, Focus Ultra 100 EC was used at a rate of 2 L per hectare. Biostimulants were applied foliarly at two growth stages: after the development of the third trifoliate leaf at the third node (BBCH 13–15) and at the beginning of flowering (BBCH 61). The rates were 0.6 dm3 ha−1 (diluted in 500 l water ha−1) for Asahi SL and 1.0 dm3 ha−1 (diluted in 700 l water ha−1) for Improver. In the control plots, spraying was conducted at the same times, using only pure water. Whole plants were harvested when fully mature (BBCH 99).
2.2. Weather Conditions
Agroclimatic parameters are crucial for interpreting plant growth conditions and plant responses to applied biostimulants. Over the study years, monthly precipitation sums, average relative air humidity, and cumulative effective temperature values (Growing Degree Days, GDD) were analysed for the soybean growing season (April–September) from 2017 to 2019 (Table 2).

Table 2.
Growing Degree Days (GDD, °C), average relative humidity (%) and atmospheric precipitation (mm) in 2017–2019.
Growing Degree Days (GDD) were calculated using the following formula:
where
GDD = ((T_max + T_min)/2) − T_base
T_max—maximum daily air temperature (°C);
T_min—minimum daily air temperature (°C);
T_base—base (threshold) temperature for the crop (°C), set at 10 °C for soybeans;
If GDD < 0, GDD is set to 0.
where
GDD_month = Σ(GDD_1 + GDD_2 + … + GDD_n)
GDD_1 … GDD_n—GDD values for consecutive days of the month;
n—number of days in the month.
This result represents the cumulative daily heat available for plant growth.
In 2018, the total GDD reached the highest value among the analysed years (1334.1 °C), indicating the warmest growing season, particularly in July and August, when GDD values were 314.3 °C and 312.1 °C, respectively. Conversely, the lowest cumulative GDD was recorded in 2017 (1026.1 °C), primarily due to a cooler April (GDD: 17.5 °C) and lower GDD values in the remaining months.
Precipitation distribution varied considerably across the years. The year 2017 was characterised by a more uniform distribution and the highest total precipitation (425 mm). In contrast, 2019 exhibited an uneven precipitation pattern, with a high sum in May (114 mm) and a low sum in July (40 mm), potentially affecting the flowering and pod-setting phases. The driest year was 2018 (320 mm), despite it being the warmest in terms of GDD. Over the three-year period (2017–2019), the average relative air humidity during the growing season (April–September) ranged from 66.7 to 72.3%. These values fall within or slightly exceed the optimal range for soybean cultivation (60–70%), suggesting generally favourable conditions for crop development.
2.3. Research Material and Statistical Analyses
The research material consisted of soybean seeds collected from plants at full maturity. Samples were collected from each plot representing each combination of factors and replicates. Chemical analyses were conducted at the Main Laboratory of Chemical Analyses of the Institute of Soil Science and Plant Cultivation—State Research Institute in Puławy. The laboratory is accredited (accreditation number: AB 339) by the Polish Centre for Accreditation, confirming that it meets the requirements of the PN-EN ISO/IEC 17025:2018-02 standard.
The fat content, dry matter content, ash content, and crude fibre content were determined using gravimetric methods in accordance with reference documents (respectively: PB 54.1, Issue II, 1 August 2013; PB 035, Issue II, 6 February 2017; PB 038, Issue VII, 16 February 2021; and PB 51.1, Issue II, 1 August 2013). Nitrogen and phosphorus content was measured using Continuous Flow Analysis (CFA) with spectrophotometric detection (PB 033, Issue IV, 24 February 2020), potassium content was measured by Flame Atomic Absorption Spectrometry (FAAS) (PB 032, Issue IV, 24 February 2020), and total protein content (calculated) was assessed using CFA with spectrophotometric detection (PB 033, Issue IV, 24 February 2020). Detailed information on the chemical analysis methods is provided on the Institute’s website [45].
The analysis results—including fat content, dry matter, ash, fibre, protein, and macronutrients (nitrogen, phosphorus, and potassium)—were statistically processed using two-factor analysis of variance based on the split-plot model:
where
yijl = m + ai + gj + eij1 + bl + abil + eijl2
yijl—value of the examined trait;
m—population mean;
ai—effect of the i-th level of factor A (cultivar);
gj—effect of the j-th replicate;
eij1—error 1 (resulting from factor A × replicate interaction);
bl—effect of the l-th level of factor B (biostimulant);
eijl2—random error.
Tukey’s test was used to compare means at a significance level of α = 0.05. All calculations were performed using Statistica (v.13.3) software with the ‘Zestaw Przyrodnika’ (Naturalist’s Toolkit) add-on [46]. To assess comprehensively the influence of genotypic, environmental, and agronomic factors on soybean seed quality, a principal component analysis (PCA) was conducted. Only components with eigenvalues greater than 1 were retained for further analysis. The results are presented in the form of a biplot. The application of PCA also enabled the evaluation of interrelationships among key nutritional components of the seeds.
3. Results
Fat content was significantly influenced by the growing season conditions, biostimulant used, and soybean cultivar. The conditions most conducive to fat accumulation in soybean seeds occurred in 2018, when the average fat content in seeds was the highest (23.18%). Cv. Merlin had a significantly higher average fat content (22.65%) compared with Abelina (21.82%) but did not differ significantly from SG Anser (Table 3). Differences between cultivars were caused by environmental conditions, as indicated by the significant cultivar × study year interaction. In the first, wetter study year (2017), cv. Merlin produced the best results for fat content (22.15%). In the following years, seeds of the tested cultivars accumulated similar amounts of fat. The effect of the biostimulants was influenced by growing seasons. Significant differences between biostimulants were observed in 2017, when the fat content in soybean seeds treated with Improver was significantly higher (22.16%) compared with the control (20.48%) and Asahi SL (21.12%). However, in 2018 and 2019, Asahi SL contributed to a higher fat accumulation than the other treatments. Analysis of three-year data confirmed a significant cultivar × biostimulant interaction, indicating varied cultivar responses to biostimulants. Cv. Abelina treated with Asahi SL accumulated significantly more fat than the control and SG Anser. In the latter cultivar, the highest fat content (22.92%) was observed in plants treated with Improver compared with Asahi SL (22.06%) and the control (21.56%). In contrast, the fat content in cv. Merlin was not significantly affected by biostimulants (Table 3).

Table 3.
Basic chemical composition of soybean seeds as influenced by cultivar, biostimulant treatment, and study year.
The dry matter content in soybean seeds was significantly affected by study years, cultivars, and application of biostimulants (Table 3). Significant interactions were confirmed between years and biostimulants, as well as between cultivars and biostimulants. Soybean seeds harvested in 2018 had the highest dry matter content (95%), which was significantly higher than in 2017 (93.94%) and 2019 (93.91%). Regardless of other experimental factors, seeds of cv. Abelina (94.42%) and Merlin (94.33%) showed significantly higher dry matter content compared with Anser (94.11%). The application of Asahi SL and Improver significantly increased the dry matter content of soybean seeds compared with the untreated control (94.21% on average). However, the effect of biostimulants was influenced by environmental conditions, as indicated by the significant year × biostimulant interaction. A significant increase in dry matter content following biostimulant application was observed in 2017, whereas in 2018 and 2019, dry matter content in soybean seeds treated with biostimulants did not differ significantly from that in the untreated control.
Different responses were observed in soybean cultivars to treatment with biostimulants in terms of the dry matter content, as evidenced by the significant cultivar × biostimulant interaction. Application of Asahi SL and Improver significantly increased the dry matter content only in cv. SG Anser seeds compared with the untreated control, cultivars, and biostimulant treatments (Table 3). The highest ash content was determined in seeds harvested in the warm and dry year of 2018 (5.32%), followed by 2019 (5.19%), with the lowest content recorded in 2017 (5.05%). Cv. Merlin had the highest average ash content (5.17%), while Abelina had the lowest (5.09%). The ash content in the seeds of cv. SG Anser did not differ significantly from that of the other cultivars. The impact of environmental conditions on cultivar response was confirmed by a significant cultivar × study year interaction. In 2017, cv. Merlin produced the lowest ash content (4.88%), though it was the lowest (5.13%) in cv. Abelina in 2018. No significant differences among cultivars were found in 2019. The highest ash content was associated with an application of Asahi SL (5.29%), it being significantly higher compared with Improver (5.20%), and the lowest was found in the untreated control (5.07%). A significant cultivar × biostimulant interaction was also observed. Asahi SL contributed to an increased ash content in the seeds of cv. Abelina and SG Anser compared with both the control and Improver treatments. In cv. Merlin, both Asahi SL and Improver significantly increased ash content compared with the control, the increase being 0.13 and 0.15%, respectively.
The ash content in soybean seeds was significantly affected by seasonal conditions, cultivars, and biostimulant treatments. The highest ash content was determined in seeds harvested in the warm and dry year of 2018 (5.32%), followed by 2019 (5.19%), with the lowest content recorded in 2017 (5.05%). Cv. Merlin had the highest average ash content (5.17%) while Abelina had the lowest (5.09%). The ash content in the seeds of cv. SG Anser did not differ significantly from that of the other cultivars. The impact of environmental conditions on cultivar response was confirmed by a significant cultivar × study year interaction. In 2017, cv. Merlin produced the lowest ash content (4.88%), it being the lowest (5.13%) in cv. Abelina in 2018. The highest ash content was associated with an application of Asahi SL (5.29%), it being significantly higher compared with Improver (5.20%), and the lowest was found in the untreated control (5.07%). A significant cultivar × biostimulant interaction was also observed. Asahi SL contributed to an increased ash content in the seeds of cv. Abelina and SG Anser compared with both the control and Improver treatments. In cv. Merlin, both Asahi SL and Improver significantly increased ash content compared with the control, the increase being 0.13 and 0.15%, respectively (Table 3).
The fibre content in soybean seeds was significantly affected by cultivars, biostimulant treatment, and study years (Table 3). The highest average fibre content was observed in 2018 (9.19%), it was significantly lower in 2017 (9.08%), and the lowest in 2019 (8.79%). Regardless of all the remaining factors, cv. Merlin seeds had the highest average fibre content (9.33%) compared with other cultivars. However, this trait was influenced by environmental conditions. In the dry year of 2018, cv. Merlin again accumulated the highest amount of fibre (9.41%), and it was the lowest in Abelina (8.99%). Cv. SG Anser did not differ significantly from the others (9.18%). In 2019, cv. SG Anser produced seeds with the lowest fibre content, whereas in 2017, no significant differences between cultivars were found for this trait. An application of Asahi SL significantly reduced fibre content in soybean seeds compared with the control, regardless of cultivar or study year. On the other hand, Improver had no significant effect on fibre concentration. A significant cultivar × biostimulant interaction indicated different cultivar responses to the tested biostimulants. In cv. Abelina, fibre content increased after Improver had been applied (9.3%). For SG Anser, both Asahi SL and Improver contributed to lower fibre content compared with the control. In contrast, cv. Merlin showed no significant response to either biostimulant treatment.
Application of biostimulants increased seed protein content compared with the untreated control, regardless of other experimental factors. Asahi SL was more effective than Improver in enhancing protein content. However, the effect of biostimulants was dependent on environmental conditions, as indicated by the significant biostimulant × study year interaction. In 2017, the highest protein concentration was determined in seeds from plants in plots treated with Improver (36.38%), whereas in 2018 and 2019, seeds from units treated with Asahi SL contained significantly more protein (37.59 and 36.89%, respectively). Of the cultivars, Abelina had the highest average protein content (37.06%) compared with the other genotypes. However, this trait was further modified by the biostimulant treatment. For cv. Abelina and SG Anser, Asahi SL application resulted in the highest protein levels (37.97 and 36.24%, respectively). In contrast, for cv. SG Anser, Improver was a better choice, suggesting a cultivar-specific response to the applied biostimulants (Table 4).

Table 4.
Macronutrient content in soybean seeds as influenced by cultivar, biostimulant, and study year.
Nitrogen content in the soybean seeds was significantly affected by the growing season and the application of biostimulants. The highest average nitrogen concentration was found in 2018 (5.75%), regardless of the other experimental factors, indicating that the warm and dry conditions of that year were most conducive to nitrogen accumulation. Conversely, 2017 was the least favourable year in this regard. An application of biostimulants increased nitrogen content compared with the untreated control, regardless of the other factors. However, the effect of biostimulants varied with environmental conditions, as demonstrated by a significant year × biostimulant interaction. In both the cool year of 2017 and the dry year of 2018, Asahi SL contributed to a significant increase in nitrogen content compared with both the control and Improver. In 2019, Improver was more effective in increasing nitrogen levels. The effect of cultivars on nitrogen content emerged through its interaction with biostimulants. An application of Asahi SL significantly increased nitrogen concentration in the seeds of cv. Abelina (5.46%) and SG Anser (5.33%), while for Merlin, the most notable increase was observed following Improver treatment (5.56%, Table 4).
Phosphorus content in soybean seeds was significantly affected by growing season conditions, cultivars, and biostimulants (Table 4). The effect of biostimulants on phosphorus accumulation varied depending on environmental conditions, indicating a significant year × biostimulant interaction. On average, regardless of the experimental treatments, the lowest phosphorus content was determined in 2018 (0.62%), whereas the highest level was observed in 2017 (0.67%), suggesting that the cool 2017 season contributed to phosphorus accumulation. Of the cultivars, Merlin accumulated the least phosphorus (0.63%) in its seeds. In 2018 and 2019, the application of Asahi SL resulted in significantly higher phosphorus content compared with the control and Improver-treated plots. In contrast, under the cool conditions of 2017, phosphorus accumulation associated with the application of Improver (0.67%) was similar to the control (0.65%). Cultivars displayed a different response to biostimulant treatments, as shown by a significant cultivar × biostimulant interaction. For cv. Abelina, both biostimulants significantly enhanced phosphorus content compared with the control, with Improver contributing to the highest phosphorus concentration (0.71%).
Potassium content in soybean seeds was significantly influenced by growing seasons, cultivars, and biostimulant application (Table 4). The highest potassium concentration was recorded in 2017 (1.70%), followed by 2018 (1.54%). Of the test cultivars, SG Anser accumulated the lowest amount of potassium (1.56%). No significant differences in potassium content were found between cv. Abelina and Merlin. The application of biostimulants, regardless of the other experimental factors, resulted in a higher potassium content in soybean seeds compared with the control. However, the effectiveness of biostimulants was dependent on the growing season. In both 2017 and 2018, Improver application led to the highest potassium concentrations (1.79 and 1.62%, respectively), followed by Asahi SL (1.69 and 1.56%) and the control (1.62 and 1.42%, respectively). In 2019, the use of biostimulants also resulted in higher potassium content compared with the control, although no significant differences were found between Asahi SL and Improver. The accumulation of potassium was further affected by cultivar response to biostimulant application. While all the cultivars showed increased potassium content in response to biostimulants compared with the control, significant differences between Asahi SL and Improver were observed only for cv. Abelina (Table 4).
To better understand the relationships among the applied biostimulants, soybean cultivars, growing seasons, and the chemical composition of soybean seeds, a principal component analysis (PCA) was performed (Figure 1). The first two principal components (PC1 and PC2) together accounted for 73.41% of the total variance, with PC1 explaining 52.60% and PC2 explaining 20.81%. Chemical constituents such as total protein, nitrogen, fat, dry matter, and ash were strongly correlated with PC1, indicating an association with the environmental conditions of the 2018 season and the application of the biostimulant Asahi SL.
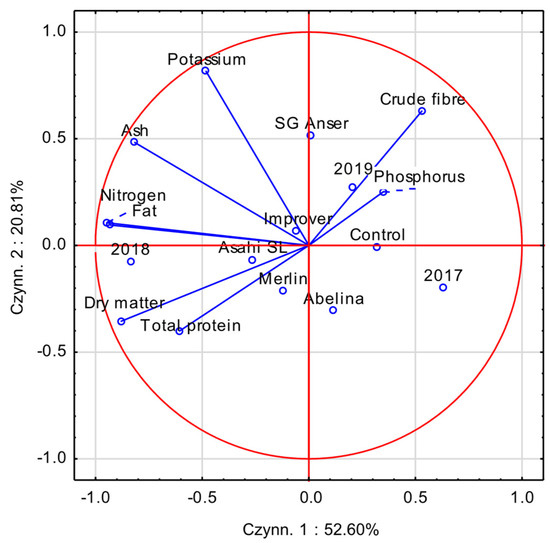
Figure 1.
The PCA biplot illustrating multivariate relationships between the chemical composition of soybean seeds and the experimental factors under investigation.
The interrelationships among the traits showed that seeds with higher fat content also contained higher levels of dry matter, ash, nitrogen, and protein, while simultaneously having lower amounts of phosphorus and crude fibre. Phosphorus and crude fibre vectors were associated with the control treatment and the 2019 season, suggesting that the environmental conditions in 2019, combined with the absence of biostimulant application, favoured greater accumulation of these components in the seeds.
Cultivars also differed in terms of their chemical composition. Cv. Abelina is positioned close to the variables associated with high protein and dry matter content, indicating its high biological quality. The placement of cv. Merlin near the Asahi SL vector may suggest a favourable response of this genotype to the biostimulant. In contrast, SG Anser is more strongly correlated with potassium content compared with the other cultivars, which may indicate its greater capacity for mineral accumulation.
The year 2018, characterised by warmer and drier conditions, was strongly associated with higher nitrogen, fat, and protein levels, suggesting that such conditions promoted the synthesis of these compounds. On the other hand, the year 2017, which was cooler and wetter, is positioned in the opposite direction to the fat and nitrogen vectors and was associated with lower levels of these components but higher levels of phosphorus and crude fibre. The vector for phosphorus and crude fibre content is also correlated with the control treatment and the 2019 season, suggesting that the environmental conditions of 2019, combined with the absence of biostimulant application, may have promoted greater natural accumulation of these components in the seeds.
4. Discussion
Soybean cultivation in Central Europe, including Poland, has become increasingly feasible due to advances in plant breeding and climate change, including rising temperatures and shifting precipitation patterns [47,48]. This crop, owing to its high nutritional value derived from considerable amounts of protein, fat, carbohydrates, and minerals [49,50], offers a viable alternative to native high-protein crops.
The results of this study confirm the significant influence of weather conditions and biostimulant application on soybean seed quality, consistent with findings reported in the literature [51,52,53,54,55,56]. In our research, the highest fat content was recorded in 2018, a season characterised by the highest Growing Degree Days (GDD), indicating a positive effect of higher temperatures on lipid accumulation. Similar findings were reported by Bellaloui et al. [57,58] and Mourtzinis et al. [59], who emphasised the critical role of temperature during the seed-filling stage.
Bellaloui et al. [60] also demonstrated that higher temperatures during seed filling result in increased fat accumulation and altered fatty acid profiles, with a corresponding reduction in protein content. Moreover, under heat and water stress, these authors observed strong activation of lipid transport and biosynthesis processes, particularly in stress-tolerant cultivars [58].
Alsajri et al. [61] established functional relationships between temperature and seed quality in a controlled experiment, examining the effects of different day/night temperatures of 21/13, 25/17, 29/21, 33/25, and 37/29 °C. Their findings indicate that the optimal temperature for seed quality was 25 °C during the day and 17 °C at night. Further increases to 33/25 and 37/29 °C led to a significant decline in oil content and a concurrent rise in protein levels.
According to Piper and Boote [62], oil content increases by approximately 0.4–0.5% for each 1 °C rise in mean temperature during the seed-filling period, although temperatures exceeding ~28 °C significantly reduce oil content while increasing protein concentration. However, the underlying mechanisms are complex and depend on water stress and photoperiod.
Our study revealed genotypic differences in the capacity for fat accumulation. Cv. Merlin exhibited the highest average oil content, underscoring the importance of genetic factors in determining this trait. The findings of Carrera et al. [63,64] confirm the significant influence of genotype on soybean seed oil content. Their analysis of 20 cultivars revealed statistically significant differences (p < 0.001) in oil concentration, regardless of cultivation location, ranging from 18.2 to 21.2%. These results highlight genetic variability as a key factor influencing seed lipid composition, with practical implications for breeding programmes and cultivar selection strategies.
The application of Asahi SL significantly increased oil content in soybean seeds across all genotypes, aligning with the findings of Kocira et al. [32], who demonstrated that biostimulants containing seaweed extracts and amino acids enhance lipid biosynthesis through improved photosynthesis, assimilate transport, and enzymatic activity. Although Improver did not have a statistically significant effect on oil content, a slightly increasing trend was observed, suggesting its potential role in mitigating environmental stress (e.g., drought) by supporting seed metabolism.
The application of Asahi SL consistently led to increased oil content regardless of cultivar, consistent with previous research on biostimulants like Fylloton (a seaweed and amino acid-based product), which reported oil increases of 8–23% depending on genotype and rate [65]. This mechanism may involve the stimulation of photosynthesis, transport assimilation, and lipid enzyme activation. By contrast, Improver did not show a statistically significant effect on oil content, although a positive trend was noted. Compared with findings by Gawęda et al. [65], where seaweed-based (Kelpak SL) and amino acid-based (Terra Sorb Complex) biostimulants increased oil content by ~1 percentage point, Improver appears to offer more supportive than stimulatory effects. Its lack of hormone-like plant extracts and simpler formulation may account for the weaker lipid accumulation effect. Nevertheless, its observed influence suggests a potential role in stabilising plant performance under suboptimal environmental conditions.
Dry matter content in seeds was highest in 2018, likely due to conditions promoting faster seed maturation and reduced water content. Vollmann et al. [66] indicated that warm, dry conditions lead to a reduction in seed moisture and increased levels of solid constituents. Biostimulants also influenced ash content; Asahi SL significantly enhanced mineral accumulation, possibly by improving nutrient uptake and ion transport [67]. Improver had a beneficial effect primarily on potassium content, especially during dry seasons, reinforcing its anti-stress properties. Potassium plays a crucial role in plant water regulation and tolerance to abiotic stress [19].
The highest levels of protein and nitrogen in seeds were recorded in 2018, suggesting that elevated temperatures combined with moderate water deficits during early growth stages favour protein biosynthesis [68]. The highest protein content was found in cv. Abelina, particularly following Asahi SL application, possibly due to more efficient nitrogen fixation and assimilation. Genotypic variability in response to biostimulants confirms the need for customised agronomic strategies. For cv. Abelina, Asahi SL was more effective (higher protein, nitrogen, and phosphorus content), whereas Merlin responded better to Improver (higher nitrogen and potassium content), indicating specific genotype × biochemistry interactions.
Biostimulant application also affected phosphorus and potassium content. Asahi SL was more effective for phosphorus, particularly during warmer seasons, whereas Improver more effectively increased potassium content under stress conditions. These findings align with those of Du Jardin [36] and Szparaga et al. [33], who highlighted the role of biostimulants in enhancing the uptake of mineral nutrients, particularly under limited water and nutrient availability.
Principal component analysis (PCA) revealed strong multivariate relationships among seed chemical composition, meteorological conditions, cultivars, and applied biostimulants. The analysis identified a distinct cluster of variables associated with the 2018 season and Asahi SL application, including high levels of oil, protein, dry matter, nitrogen, and ash. This supports the hypothesis that the warm and dry conditions of 2018 enhanced the effectiveness of Asahi SL, leading to greater nutrient accumulation in the seeds. This PCA structure is consistent with findings by Mourtzinis et al. [69], who demonstrated that the R5–R8 growth stages (from seed filling to full maturity) are critical for dry matter and oil accumulation. Their study showed that higher average temperatures during this period correlate with increased oil content and yield, directly supporting our observations from 2018.
In contrast, the cluster represented by PC2, encompassing the 2019 season, the control treatment, and fibre and potassium content, suggests that the absence of biostimulation under moderate conditions (balanced temperature and rainfall) permits the natural accumulation of these components. Pipolo et al. [70] observed that lower temperatures slow biomass accumulation, potentially favouring a proportional increase in fibre at the expense of oil. Furthermore, the PCA results revealed significant interactions among season, genotype, and biostimulant. Our findings indicate that cv. Abelina, SG Anser, and Merlin exhibited distinct responses to Asahi SL and Improver across seasons, emphasising the need for genotype-specific agronomic strategies. These findings are consistent with those of Whaley et al. [71], who confirmed differential genotype responses to environmental conditions. In line with the approach of Ding et al. [72], who utilised PCA to identify traits determining seed vigour, our analysis supports PCA as an effective tool for identifying key factors influencing the chemical composition of soybean seeds.
5. Conclusions
The results of the three-year field experiment indicate that the chemical composition of soybean seeds is significantly influenced by cultivar genotype, biostimulant application, and weather conditions, particularly temperature, measured as Growing Degree Days (GDD). Among the tested cultivars, Merlin exhibited the greatest stability in terms of fat and fibre accumulation, while Abelina was characterised by the highest levels of protein and dry matter, notable after Asahi SL application. The effectiveness of biostimulants depended on environmental conditions and genotype. Asahi SL enhanced the accumulation of protein, nitrogen, and phosphorus, particularly in warmer and drier growing seasons, whereas Improver had a more pronounced effect on potassium content under environmental stress conditions.
Principal component analysis (PCA) confirmed strong associations among fat, protein, nitrogen, and the warmer conditions of the 2018 season (with the highest GDD), underscoring the role of temperature in nutrient synthesis. In contrast, phosphorus and fibre correlated with cooler, wetter conditions and non-application of biostimulants.
The observed interactions suggest that biostimulant effectiveness is shaped by the combination of genotype × weather conditions × biostimulant, indicating that agronomic strategies should be tailored accordingly. The selection of a biostimulant should be matched to the specific cultivar and anticipated weather conditions.
Application of biostimulants, particularly Asahi SL, can significantly enhance the nutritional value of soybean seeds, with direct implications for the food, feed, and oil industries. However, the choice of product should consider the cultivar’s characteristics and expected climatic conditions. Asahi SL may thus be recommended for warm and dry seasons, as well as for cultivars with high protein potential, while Improver may be more suitable for cultivation under stress-prone conditions (e.g., limited water or potassium availability).
This study advances understanding of the interactions among biostimulants, climatic factors, and genotypic variation: an area of increasing importance in the context of climate change. These data may provide a foundation for developing predictive models of biostimulant effectiveness based on weather indicators (GDD and precipitation) and cultivar traits.
Author Contributions
Conceptualisation, K.R., E.R. and J.C.; Methodology, K.R. and J.C.; Software, E.R. and K.R.; Writing—original draft preparation, E.R. and K.R.; Writing—review and editing, E.R. and K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kozak, M.; Malarz, W.; Kotecki, A.; Černý, I.; Serafin-Andrzejewska, M. The effect of different sowing rate and Asahi SL biostimulator on chemical composition of soybean seeds and postharvest residues. Oilseed Crops 2008, 29, 217–230. [Google Scholar]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Chapter 2—Soy protein: Impacts, production, and applications. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–45. [Google Scholar] [CrossRef]
- Księżak, J.; Bojarszczuk, J. The evaluation of productivity effects of soybean cultivation [Glycine max (L.) Merr.] depending on soil tillage method. Agron. Sci. 2023, 78, 99–112. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, G. Biofortification of pulses and legumes to enhance nutrition. Heliyon 2020, 6, e03682. [Google Scholar] [CrossRef]
- Karolkowski, A.; Guichard, E.; Briand, L.; Salles, C. Volatile compounds in pulses: A review. Foods 2021, 10, 3140. [Google Scholar] [CrossRef]
- Pedrosa, M.M.; Guillamón, E.; Arribas, C. Autoclaved and extruded legumes as a source of bioactive phytochemicals: A review. Foods 2021, 10, 379. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Patil, G.; Mian, R.; Vuong, T.; Pantalone, V.; Song, Q.; Chen, P.; Shannon, J.; Carter, T.C.; Nguyen, H.T. Molecular mapping and genomics of soybean seed protein: A review and perspective for the future. Theor. Appl. Genet. 2017, 130, 1975–1991. [Google Scholar] [CrossRef] [PubMed]
- Kotecki, A.; Lewandowska, S. Studia nad Uprawą soi Zwyczajnej (Glycine max (L.) Merrill) w Południowo-Zachodniej Polsce; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2020. [Google Scholar]
- Fallen, B.D.; Hatcher, C.N.; Allen, F.L.; Kopsell, D.A.; Saxton, A.M.; Chen, P.; Kantartzi, S.K.; Cregan, P.B.; Hyten, D.L.; Pantalone, V.R. Amino acid content in soybean seeds QTL detected using the universal soybean linkage panel 1.0 with 1536 SNPs. Plant Genet. Genom. Biotechnol. 2017, 1, 68–79. [Google Scholar] [CrossRef]
- Niwińska, B.; Witaszek, K.; Niedbała, G.; Pilarski, K. Seeds of n-GM soybean varieties cultivated in Poland and their processing products as high-protein feeds in cattle nutrition. Agriculture 2020, 10, 174. [Google Scholar] [CrossRef]
- Olszewski, J.; Dzienis, G.; Okorski, A.; Goś, W.; Pszczółkowska, A. Fungal colonization of the anatomical parts of soybean seeds supplied with different nitrogen rates and inoculated with Bradyrhizobium japonicum. Agriculture 2025, 15, 857. [Google Scholar] [CrossRef]
- Van Eys, J.; Offner, A.; Bach, A. Manual of quality analyses for soybean products in the feed industry. Am. Soybean Assoc. 2005, 18, 47. [Google Scholar]
- Galben, R.D.; Urdă, C.; Rezi, R.; Gheorghieş, V.; Negrea, A.; Russu, F.; Balaş, S.; Varga, A.I.; Duda, M.M. Seed composition of soybean and its significance for human health. Hop. Med. Plants 2021, 29, 157–163. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.; Bellaloui, N. Soybean seed physiology, quality, and chemical composition under soil moisture stress. Food Chem. 2019, 278, 92–100. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Baud, S.; Dubreucq, B.; Miquel, M.; Rochat, C.; Lepiniec, L. Storage reserve accumulation in Arabidopsis: Metabolic and developmental control of seed filling. Arab. Book 2008, 6, e0113. [Google Scholar] [CrossRef]
- Capelin, M.A.; Madella, L.A.; Panho, M.C.; Meira, D.; Barrionuevo, F.; Rodrigues, A.P.D.C.; Benin, G. Physiological quality and seed chemical composition of soybean seeds under different altitudes. Bragantia 2022, 81, e1022. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C. Seed Biology Updates; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Westgate, M.E. Meta-analysis of environmental effects on soybean seed composition. Field Crops Res. 2009, 110, 147–156. [Google Scholar] [CrossRef]
- Phengsouvana, V.; Attanandana, T.; Yost, R.S. Lime application to two acidic upland soils for soybean production in Champasak Province, Lao PDR. Agric. Nat. Resour. 2009, 43, 19–27. [Google Scholar]
- Timilsina, A.P.; Baigorria, G.A.; Wilhite, D.; Shulski, M.; Heeren, D.; Romero, C.; Fensterseifer, C.A. Soybean response under climatic scenarios with changed mean and variability under rainfed and irrigated conditions in major soybean-growing states of the USA. J. Agric. Sci. 2023, 161, 157–174. [Google Scholar] [CrossRef]
- Staniak, M.; Szpunar-Krok, E.; Kocira, A. Responses of soybean to selected abiotic stresses—Photoperiod, temperature and water. Agriculture 2023, 13, 146. [Google Scholar] [CrossRef]
- Puthur, J.T.; Dhankher, O.P. Bioenergy Crops, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Chlapecka, J. Soybean Irrigation Initiation Timing Using Evapotranspiration and Soil Moisture Sensor Cues. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 2015. Available online: https://arch.astate.edu/all-etd/693 (accessed on 10 May 2021).
- Singh, R.J.; Nelson, R.L.; Chung, G. Soybean (Glycine max (L.) Merr.). In Genetic Resources, Chromosome Engineering, and Crop Improvement; Singh, R.J., Ed.; Oilseed Crops; CRC Press: Boca Raton, FL, USA, 2006; Volume 4, pp. 13–50. [Google Scholar]
- Księżak, J.; Bojarszczuk, J. The productivity of selected soybean cultivars grown using various cultivation methods. J. Water Land. Dev. 2024, 62, 88–96. [Google Scholar] [CrossRef]
- Hungria, M.; Mendes, I.C. Nitrogen fixation with soybean: The perfect symbiosis? In Biological Nitrogen Fixation; de Bruijin, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1009–1024. [Google Scholar]
- Bender, R.R.; Haegele, J.W.; Below, F.E. Nutrient uptake, partitioning, and remobilization in modern soybean varieties. Agron. J. 2015, 107, 563–573. [Google Scholar] [CrossRef]
- Mangena, P. Water stress: Morphological and anatomical changes in soybean (Glycine max L.) plants. In Plant, Abiotic Stress and Responses to Climate Change; IntechOpen: London, UK, 2018; pp. 9–31. [Google Scholar]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of growth, yield, and the nutraceutical and antioxidative potential of soybean through the use of synthetic biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Rymuza, K.; Radzka, E.; Cała, J. The effect of applied biostimulants on the yielding of three non-genetically modified soybean cultivars. Agriculture 2023, 13, 900. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Łangowski, Ł.; Goñi, O.; Marques, F.S.; Hamawaki, O.T.; da Silva, C.O.; Nogueira, A.P.O.; Teixeira, M.A.J.; Glasenapp, J.S.; Pereira, M.; O’Connell, S. Ascophyllum nodosum extract (Sealicit™) boosts soybean yield through reduction of pod shattering-related seed loss and enhanced seed production. Front. Plant Sci. 2021, 12, 631768. [Google Scholar] [CrossRef]
- Franzoni, G.; Bulgari, R.; Florio, F.E.; Gozio, E.; Villa, D.; Cocetta, G.; Ferrante, A. Effect of biostimulant raw materials on soybean (Glycine max) crop, when applied alone or in combination with herbicides. Front. Agron. 2023, 5, 1238273. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Zhou, M. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Karges, K.; Bellingrath-Kimura, S.D.; Watson, C.A.; Stoddard, F.L.; Halwani, M.; Reckling, M. Agro-economic prospects for expanding soybean production beyond its current northerly limit in Europe. Eur. J. Agron. 2022, 133, 126415. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Bobrecka-Jamro, D.; Pikuła, W.; Jańczak-Pieniążek, M. Effect of nitrogen fertilization and inoculation with Bradyrhizobium japonicum on nodulation and yielding of soybean. Agronomy 2023, 13, 1341. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources. International soil classification system for naming soils and creating legends for soil. In World Soil Resources Report; FAO: Rome, Italy, 2014; Volume 106. [Google Scholar]
- Instytut Uprawy Nawożenia i Gleboznawstwa—Państwowy Instytut Badawczy. Karta Glebowa. Available online: https://www.iung.pl/o-instytucie/struktura/dzialy-wspomagania/karta-glach/ (accessed on 14 April 2024).
- TIBCO Software Inc. Statistica (Data Analysis Software System), version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. Available online: https://www.statsoft.pl/ (accessed on 12 January 2025).
- Pörtner, H.-O.; Roberts, D.C.; Poloczanska, E.S.; Mintenbeck, K.; Tignor, M.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. Intergovernmental Panel on Climate Change (IPCC). In Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK, 2023; pp. 8–21. [Google Scholar] [CrossRef]
- Nendel, C.; Reckling, M.; Debaeke, P.; Schulz, S.; Berg-Mohnicke, M.; Constantin, J.; Fronzek, S.; Hoffmann, M.; Jakšić, S.; Kersebaum, K.; et al. Future area expansion outweighs increasing drought risk for soybean in Europe. Glob. Change Biol. 2023, 29, 1340–1358. [Google Scholar] [CrossRef]
- Ouédraogo, E.R.; Konaté, K.; Sanou, A.; Sama, H.; Compaoré, E.W.R.; Sytar, O.; Hilou, A.; Brestic, M.; Dicko, M.H. Assessing the quality of Burkina Faso soybeans based on fatty acid composition and pesticide residue contamination. Molecules 2022, 27, 6260. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Wondołowska-Grabowska, A. Quality evaluation indices for soybean oil in relation to cultivar, application of N fertiliser and seed inoculation with Bradyrhizobium japonicum. Foods 2022, 11, 762. [Google Scholar] [CrossRef]
- Yaklich, R.W.; Vinyard, B.T. A method to estimate soybean seed protein and oil concentration before harvest. J. Am. Oil Chem. Soc. 2004, 81, 1021–1027. [Google Scholar] [CrossRef]
- Staniak, M.; Czopek, K.; Stępień-Warda, A.; Kocira, A.; Przybyś, M. Cold stress during flowering alters plant structure, yield and seed quality of different soybean genotypes. Agronomy 2021, 11, 2059. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Boote, K.J.; Allen, L.H.; Gallo-Meagher, M.; Davis, J.M. Elevated temperature and carbon dioxide effects on soybean seed composition and transcript abundance. Crop Sci. 2003, 43, 1548–1557. [Google Scholar] [CrossRef]
- Ody, L.P.; Baisch, J.S.; Ugalde, G.; Grohs, M.; Dorneles, A.B.; Neu, G.R.F.; Santos, M.S.N.; Ferreira, P.A.A.; Tres, M.V.; Zabot, G.L. Early sowing and soil scarification improve protein and oil contents in soybean grains cultivated in lowlands. J. Soil. Sci. Plant Nutr. 2024, 24, 1015–1029. [Google Scholar] [CrossRef]
- Wilson, R.F. Seed composition. In Soybeans: Improvement, Production, and Uses; Boerma, H.R., Specht, J.E., Eds.; The American Society of Agronomy, Inc.: Madison, WI, USA, 2004; pp. 621–677. [Google Scholar]
- Lanna, A.; José, I.C.; Oliveira, M.G.; Barros, E.; Alves, M. Effect of temperature on polyunsaturated fatty acid accumulation in soybean seeds. Braz. J. Plant Physiol. 2005, 17, 213–222. [Google Scholar] [CrossRef]
- Bellaloui, N.; Mengistu, A.; Walker, E.R.; Young, L.D. Soybean seed composition as affected by seeding rates and row spacing. Crop Sci. 2014, 54, 1782–1795. [Google Scholar] [CrossRef]
- Bellaloui, N.; McClure, A.M.; Mengistu, A.; Abbas, H.K. The influence of agricultural practices, the environment, and cultivar differences on soybean seed protein, oil, sugars, and amino acids. Plants 2020, 9, 378. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Gaspar, A.; Naeve, S.; Conley, S. Planting date, maturity, and temperature effects on soybean seed yield and composition. Agron. J. 2017, 109, 2040–2049. [Google Scholar] [CrossRef]
- Bellaloui, N.; Smith, J.R.; Ray, J.D.; Gillen, A.M. Effect of maturity on seed composition in the early soybean production system as measured on near-isogenic soybean lines. Crop Sci. 2009, 49, 608–620. [Google Scholar] [CrossRef]
- Alsajri, F.A.; Wijewardana, C.; Irby, J.T.; Bellaloui, N.; Krutz, L.J.; Golden, B.; Gao, W.; Reddy, K.R. Developing functional relationships between temperature and soybean yield and seed quality. Agron. J. 2020, 112, 194–204. [Google Scholar] [CrossRef]
- Piper, E.; Boote, K. Temperature and cultivar effects on soybean seed oil and protein concentrations. J. Am. Oil Chem. Soc. 1999, 76, 1233–1241. [Google Scholar] [CrossRef]
- Carrera, C.; Martínez, M.J.; Dardanelli, J.; Balzarini, M. Environmental variation and correlation of seed components in nontransgenic soybeans: Protein, oil, unsaturated fatty acids, tocopherols, and isoflavones. Crop Sci. 2011, 51, 800–809. [Google Scholar] [CrossRef]
- Carrera, C.; Martínez, M.J.; Dardanelli, J.; Balzarini, M. Water deficit effect on the relationship between temperature during the seed fill period and soybean seed oil and protein concentrations. Crop Sci. 2009, 49, 990–998. [Google Scholar] [CrossRef]
- Gawęda, D.; Haliniarz, M.; Andruszczak, S.; Wacławowicz, R. The effect of herbicides and biostimulant application on the seed yield and seed quality of soybean (Glycine max (L.) Merr.). Agronomy 2024, 14, 2174. [Google Scholar] [CrossRef]
- Vollmann, J.; Fritz, C.N.; Wagentristl, H.; Ruckenbauer, P. Environmental and genetic variation of soybean seed protein content under Central European growing conditions. J. Sci. Food Agric. 2000, 80, 1300–1306. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and economical efect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef]
- Dornbos, D.L.; Mullen, R.E. Soybean seed protein and oil contents and fatty acid composition adjustments by drought and temperature. J. Am. Oil Chem. Soc. 1992, 69, 228–231. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Conley, S.P. Delineating soybean maturity groups across the United States. Agron. J. 2017, 109, 1397–1403. [Google Scholar] [CrossRef]
- Pipolo, A.; Sinclair, T.; Camara, G. Protein and oil concentration of soybean seed cultured in vitro using nutrient solutions of differing glutamine concentration. Ann. Appl. Biol. 2004, 144, 223–227. [Google Scholar] [CrossRef]
- Whaley, R.; Eskandari, M. Genotypic main effect and genotype-by-environment interaction effect on seed protein concentration and yield in food-grade soybeans (Glycine max (L.) Merrill). Euphytica 2019, 215, 33. [Google Scholar] [CrossRef]
- Ding, W.; Lin, J.; Li, C.; Zhu, Z.; Wu, C.; Cao, J.; Liu, D.; Zhang, Y.; Yang, Q.; Xing, A.; et al. Development of a comprehensive evaluation system and models to determine soybean seed vigor. Ind. Crops Prod. 2025, 224, 120329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).