A Low-Rank Coal-Derived Soil Amendment Promotes Plant Growth and Shapes Rhizosphere Microbial Communities of Lettuce (Lactuca sativa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lettuce Growth Experiments
2.3. Soil Sampling and Analysis
2.4. Rhizosphere Microbiome Analysis
2.4.1. Rhizosphere Soil Sampling and DNA Extraction
2.4.2. Sequencing Libraries Preparation, 16S rRNA, and ITS Sequencing
2.4.3. Sequencing Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Properties
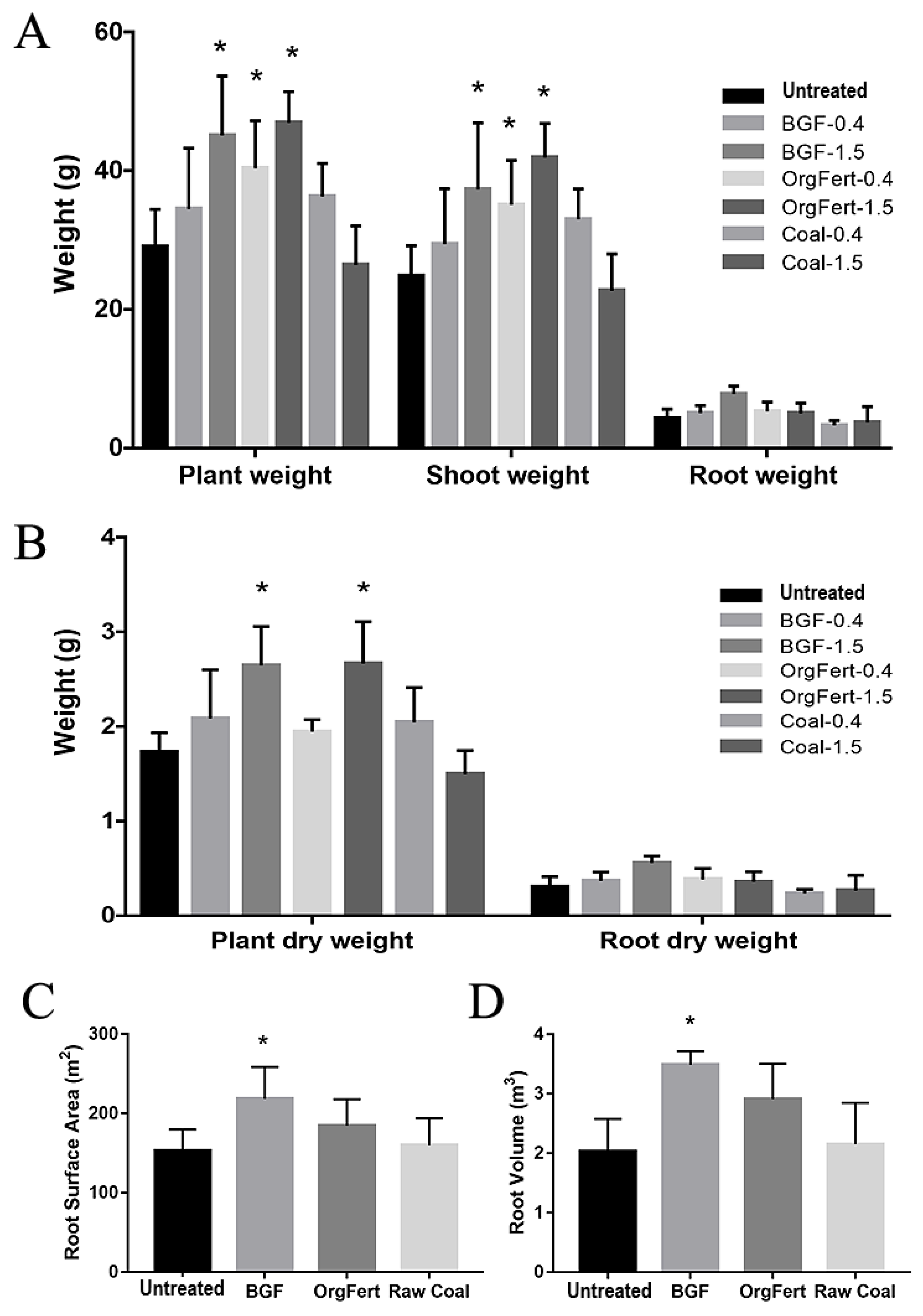
3.2. Effects on Lettuce Growth
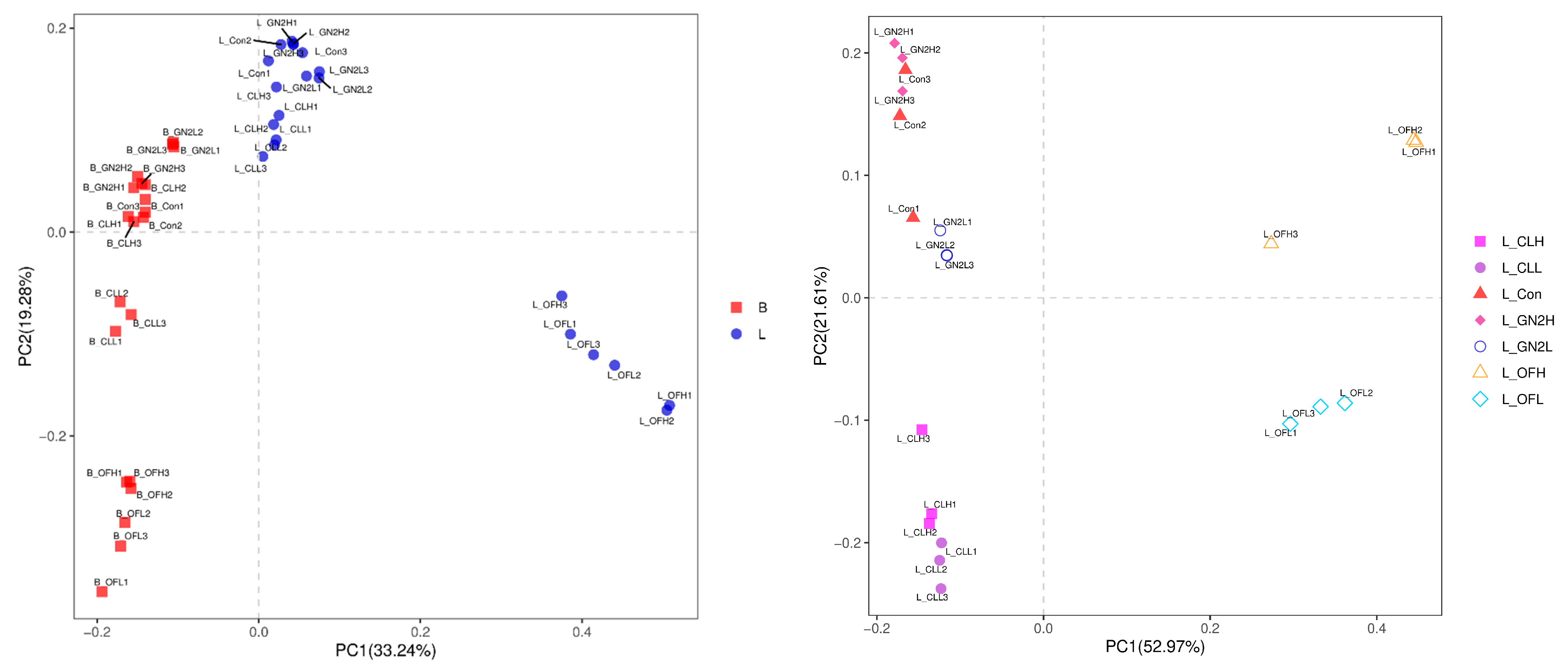
3.3. Rhizosphere Bacterial Community Analysis
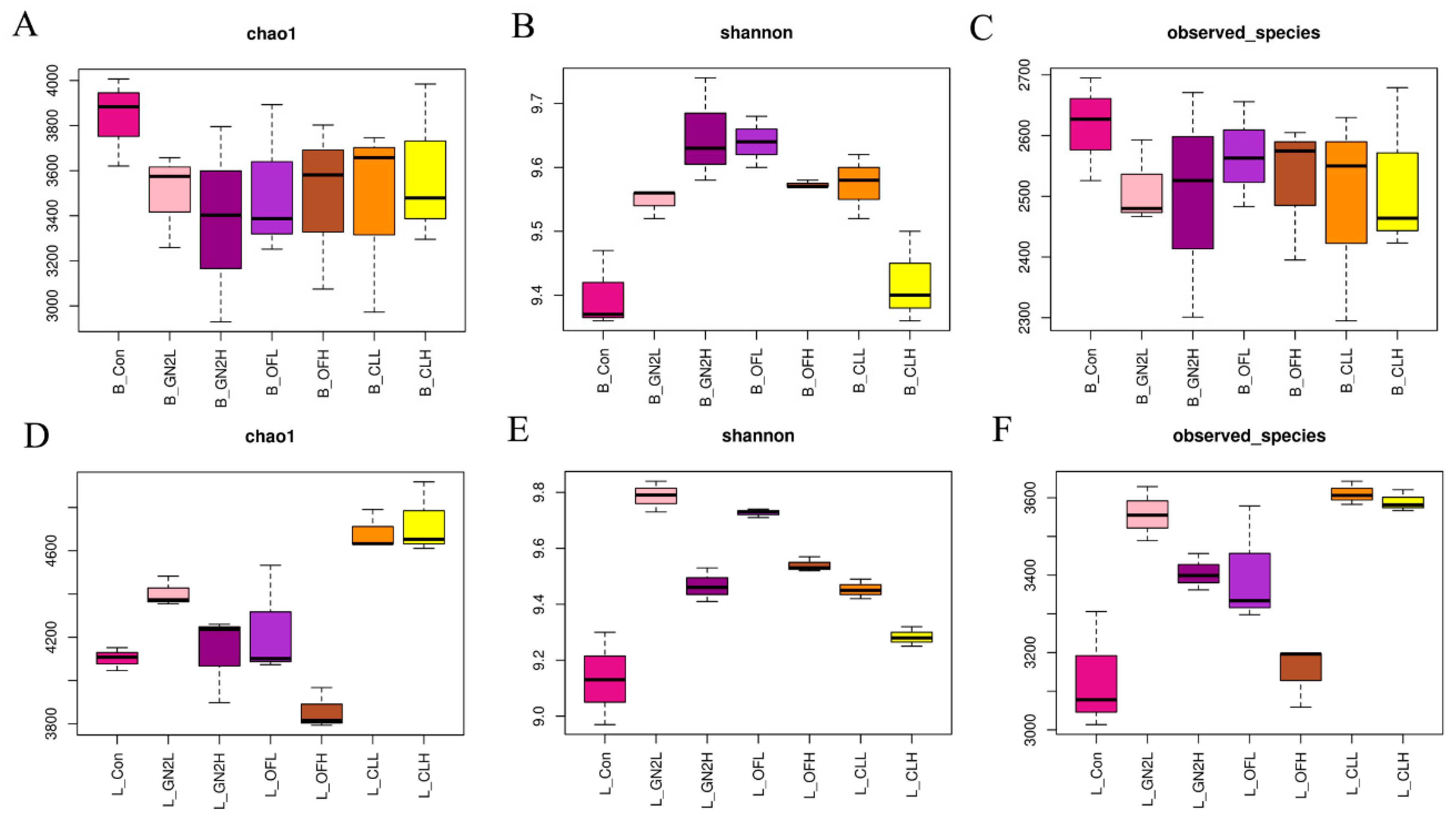
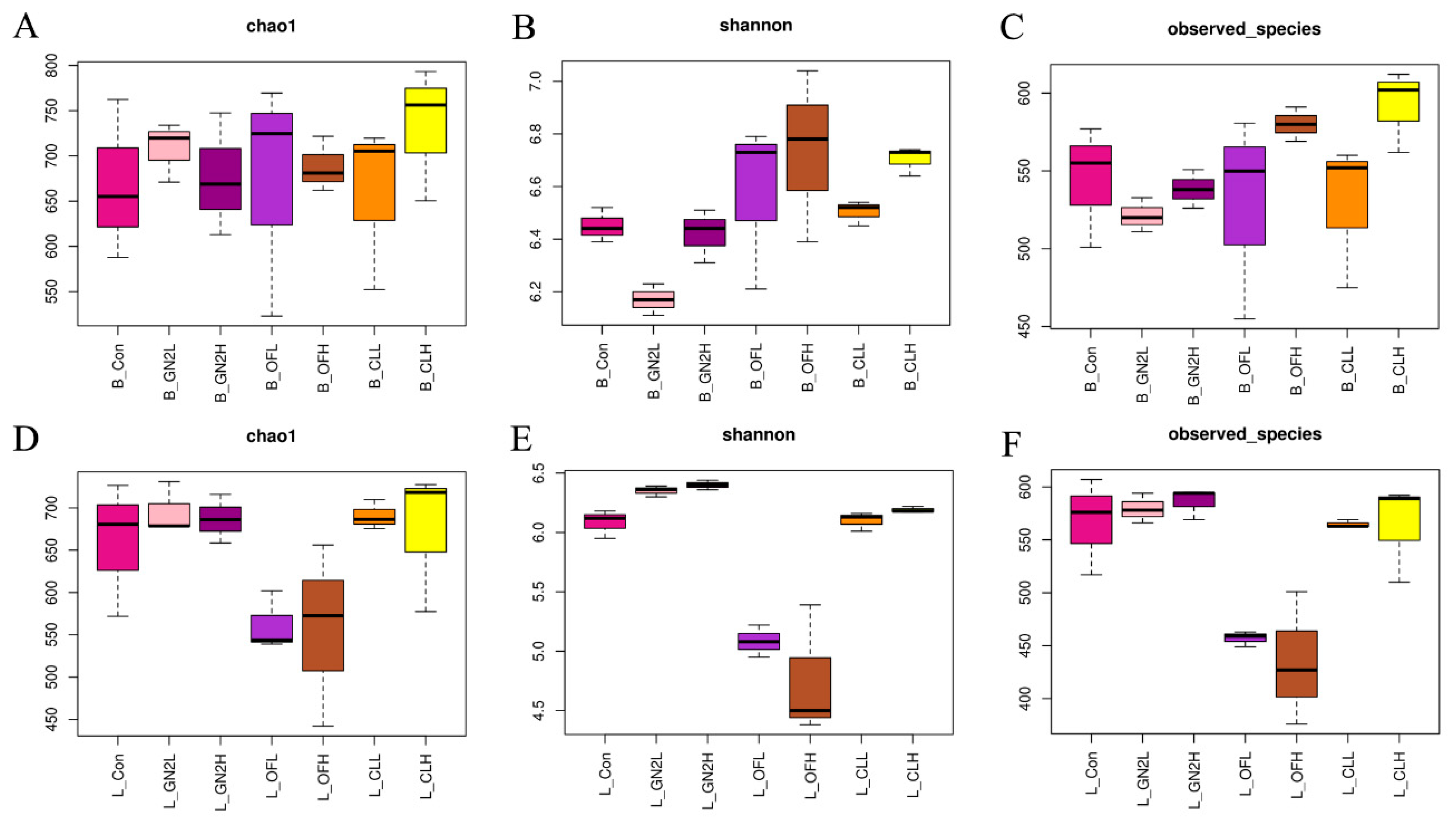
3.3.1. Abundance and Diversity of Rhizosphere Bacteria
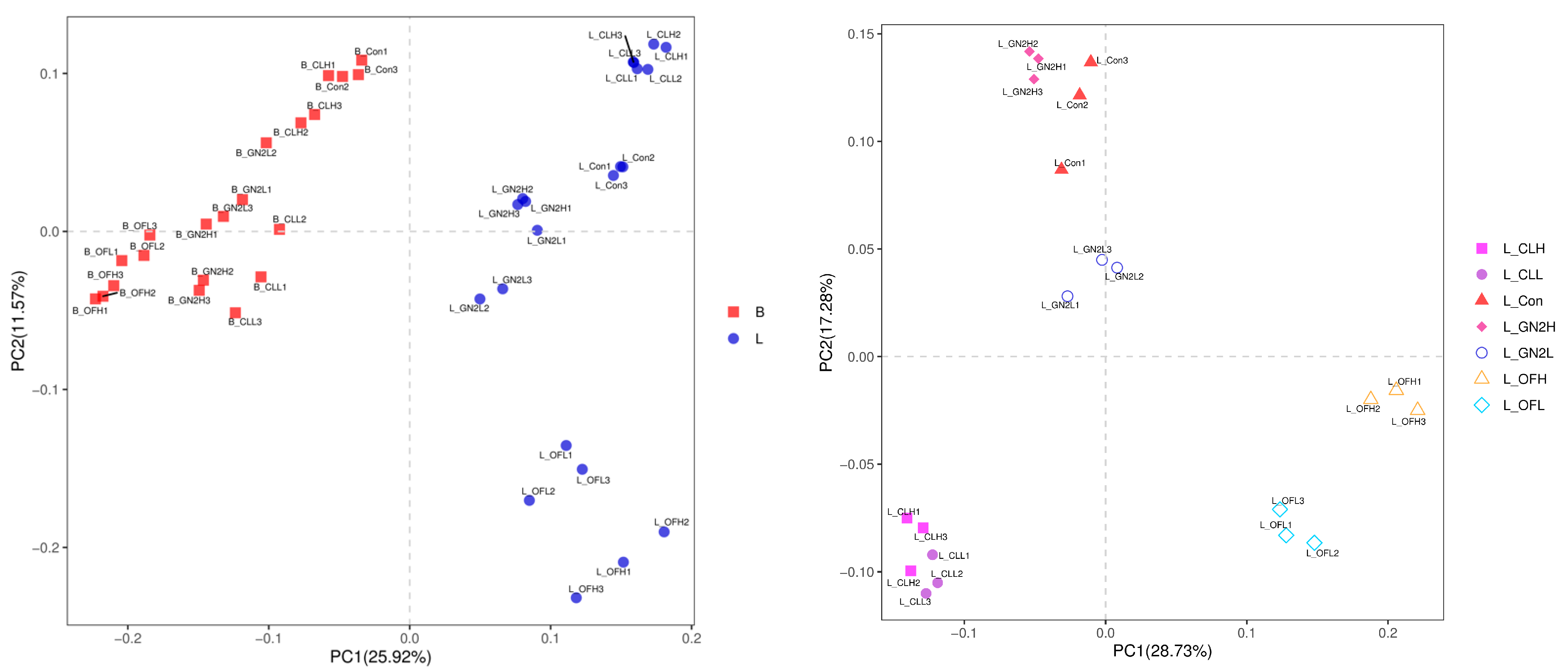
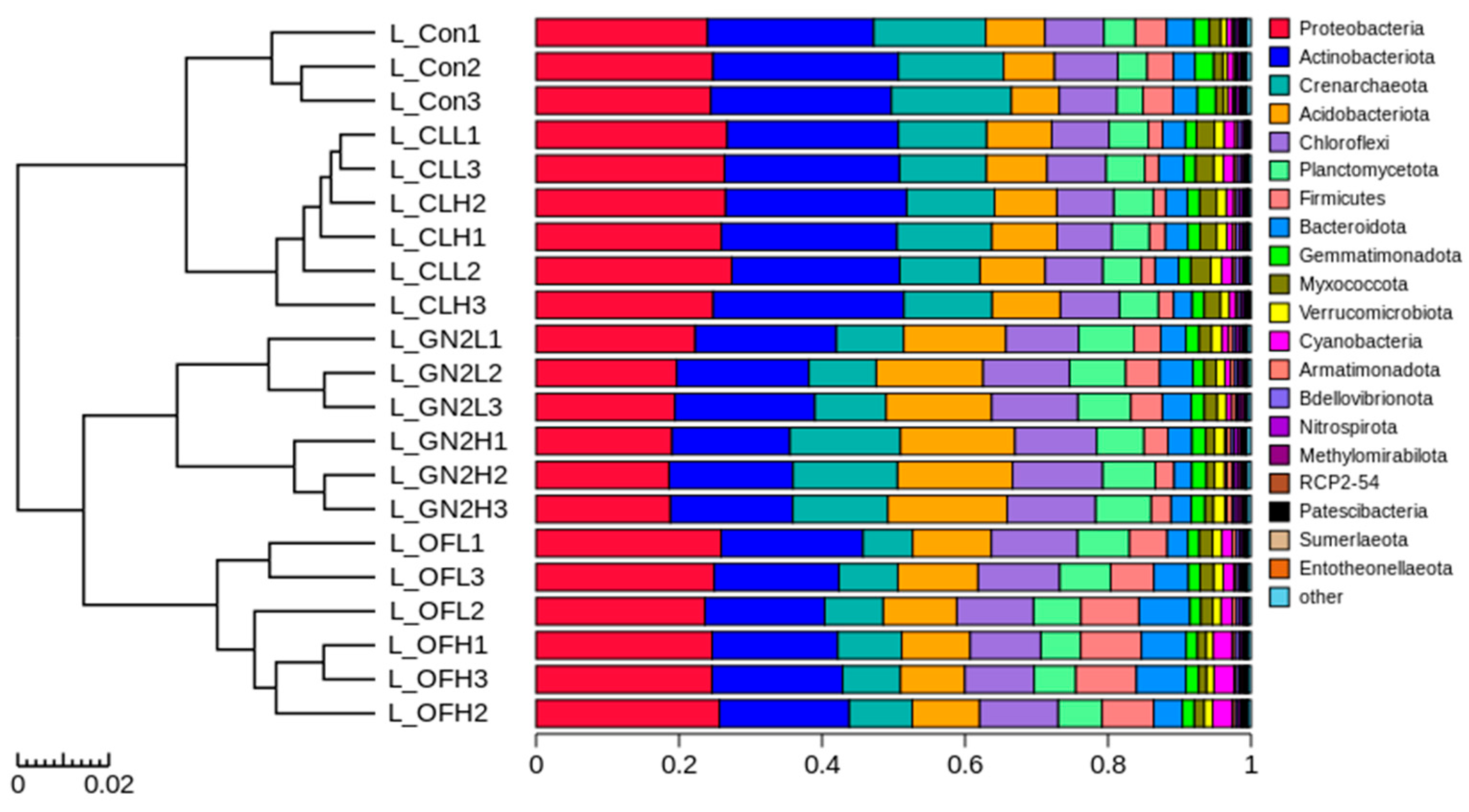
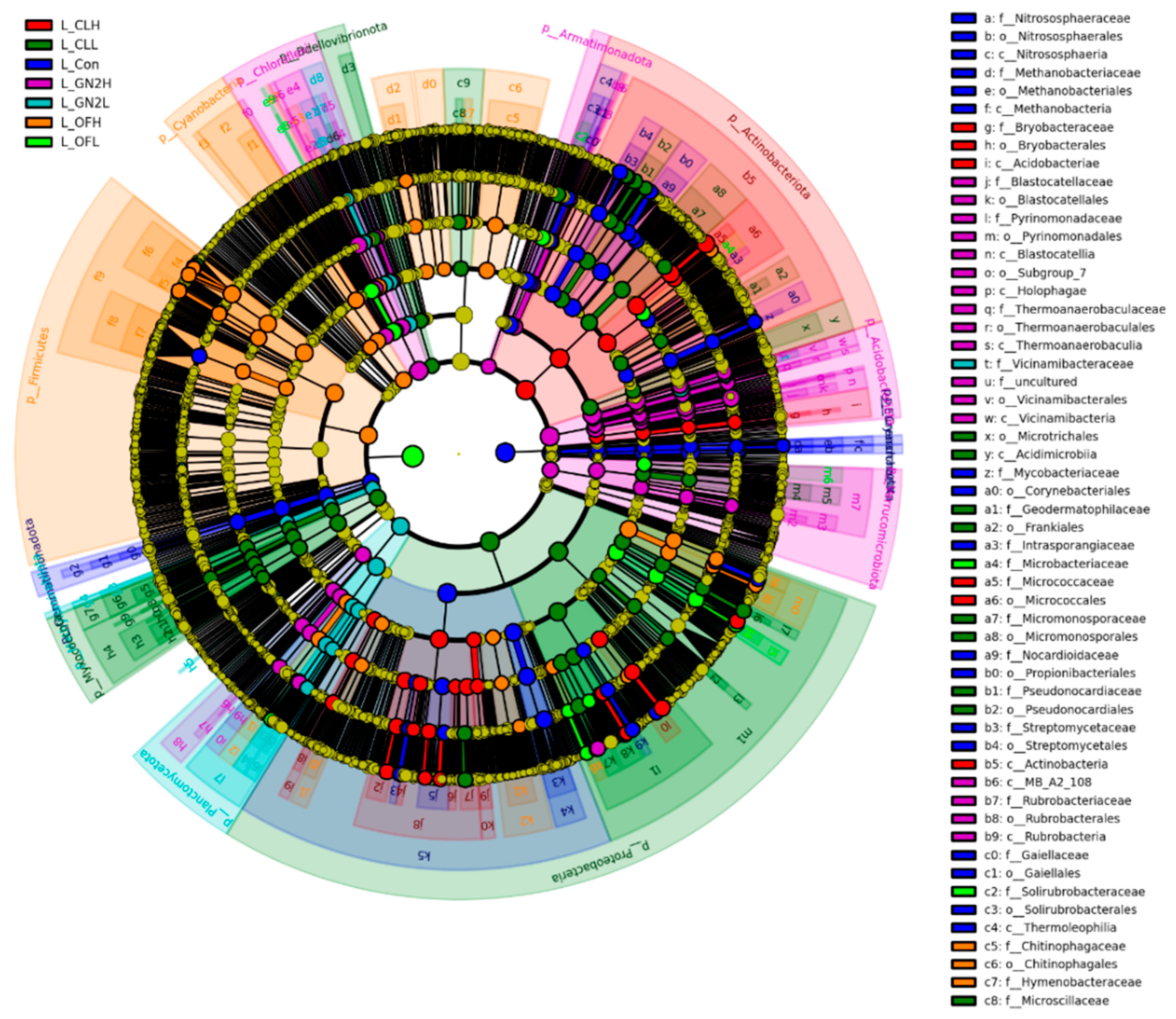
3.3.2. The Community Structure and Composition of the Rhizosphere Bacteria
3.4. Rhizosphere Fungal Community
3.4.1. Abundance and Diversity of Rhizosphere Fungi
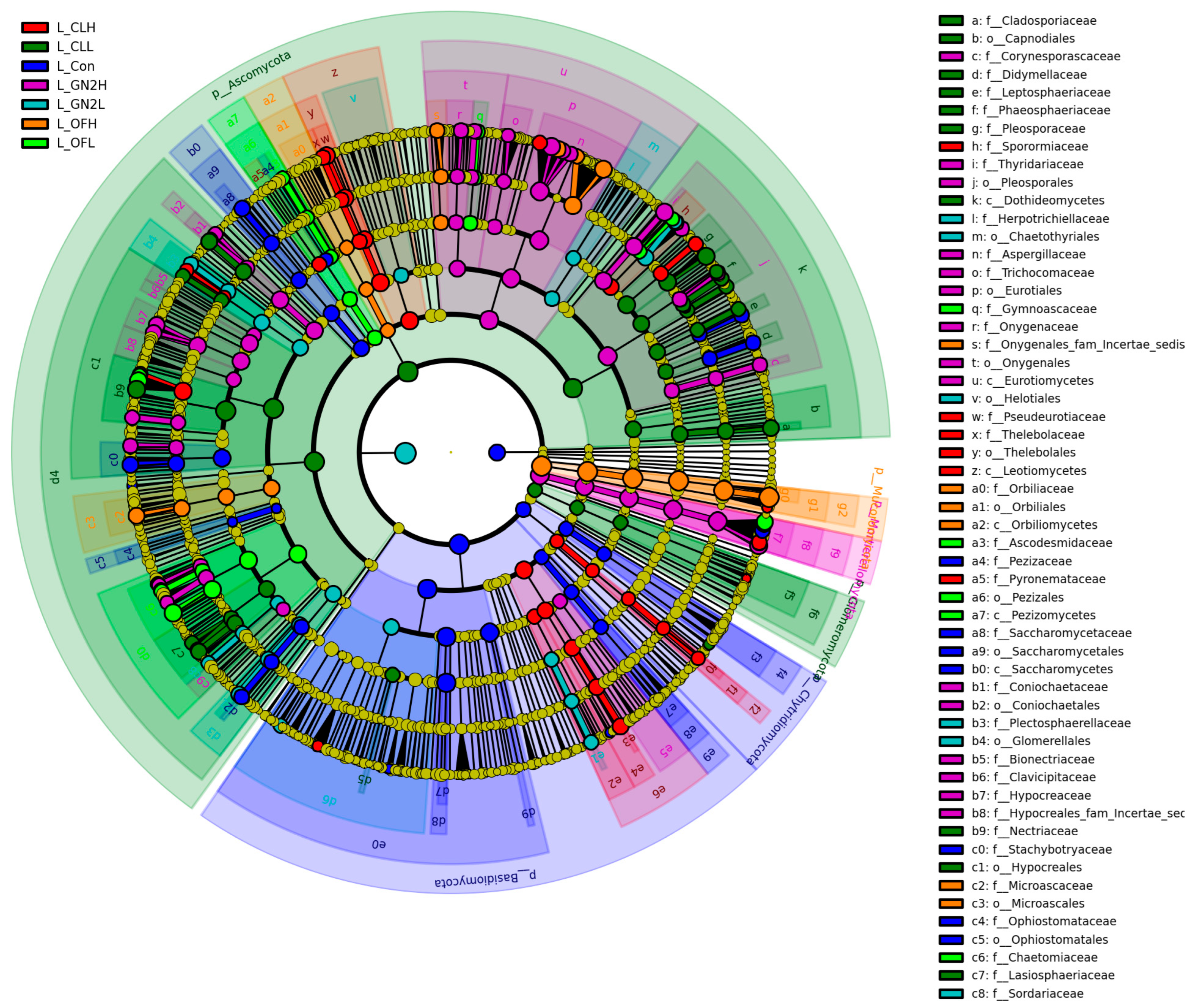
3.4.2. The Community Structure and Composition of the Rhizosphere Fungi
4. Discussion
4.1. The Effects of the Coal-Derived (BGF) Product on Plant Growth
4.2. The Effects of the Coal-Derived (BGF) Product on Soil Properties
4.3. The Effects of the Coal-Derived (BGF) Product on the Plant Rhizosphere Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerland, P.; Raftery, A.E.; Ševčíková, H.; Li, N.; Gu, D.; Spoorenberg, T.; Alkema, L.; Fosdick, B.K.; Chunn, J.; Lalic, N.; et al. World population stabilization unlikely this century. Science 2014, 346, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health; Springer: Cham, Switzerland, 2021; Volume 2. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Akimbekov, N.S.; Digel, I.; Tastambek, K.T.; Sherelkhan, D.K.; Jussupova, D.B.; Altynbay, N.P. Low-Rank Coal as a Source of Humic Substances for Soil Amendment and Fertility Management. Agriculture 2021, 11, 1261. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Rouhani, A.; Skousen, J.; Tack, F.M.G. An Overview of Soil Pollution and Remediation Strategies in Coal Mining Regions. Minerals 2023, 13, 1064. [Google Scholar] [CrossRef]
- Fallgren, P.H.; Chen, L.; Peng, M.; Urynowicz, M.A.; Jin, S. Facultative-anaerobic microbial digestion of coal preparation waste and use of effluent solids to enhance plant growth in a sandy soil. Int. J. Coal Sci. Technol. 2021, 8, 767–779. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Zhang, X.; Xiong, Y.; Huang, Q.; Jin, S.; Sun, S.; Chi, D.; Huang, G. Effects of lignite bioorganic product on sunflower growth, water and nitrogen productivity in saline-sodic farmlands at Northwest China. Agric. Water Manag. 2022, 271, 107806. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Fenton, O.; Szara, E.; Thornton, S.F.; Malina, G. Holistic Assessment of Biochar and Brown Coal Waste as Organic Amendments in Sustainable Environmental and Agricultural Applications. Water Air Soil Pollut. 2021, 232, 106. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Jeong, H.J.; Cha, J.-Y.; Choi, M.; Jang, K.-S.; Kim, W.-Y.; Kim, M.G.; Jeon, J.-R. Structural variation of humic-like substances and its impact on plant stimulation: Implication for structure-function relationship of soil organic matters. Sci. Total Environ. 2020, 725, 138409. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. Chapter Two—A Meta-Analysis and Review of Plant-Growth Response to Humic Substances: Practical Implications for Agriculture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 124, pp. 37–89. [Google Scholar]
- Guan, X.; Cheng, Z.; Li, Y.; Wang, J.; Zhao, R.; Guo, Z.; Zhao, T.; Huang, L.; Qiu, C.; Shi, W.; et al. Mixed organic and inorganic amendments enhance soil microbial interactions and environmental stress resistance of Tibetan barley on plateau farmland. J Environ. Manag. 2023, 330, 117137. [Google Scholar] [CrossRef]
- Miller, S.B.; Heuberger, A.L.; Broeckling, C.D.; Jahn, C.E. Non-Targeted Metabolomics Reveals Sorghum Rhizosphere-Associated Exudates are Influenced by the Belowground Interaction of Substrate and Sorghum Genotype. Int. J. Mol. Sci. 2019, 20, 431. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Little, K.R.; Rose, M.T.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Do lignite-derived organic amendments improve early-stage pasture growth and key soil biological and physicochemical properties? Crop Pasture Sci. 2014, 65, 899–910. [Google Scholar] [CrossRef]
- Della Lucia, M.C.; Bertoldo, G.; Broccanello, C.; Maretto, L.; Ravi, S.; Marinello, F.; Sartori, L.; Marsilio, G.; Baglieri, A.; Romano, A.; et al. Novel Effects of Leonardite-Based Applications on Sugar Beet. Front. Plant Sci. 2021, 12, 646025. [Google Scholar] [CrossRef]
- Olego, M.Á.; Cuesta Lasso, M.; Quiroga, M.J.; Visconti, F.; López, R.; Garzón-Jimeno, E. Effects of Leonardite Amendments on Vineyard Calcareous Soil Fertility, Vine Nutrition and Grape Quality. Plants 2022, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Pietramellara, G.; Mbagwu, J.S.C. Effects of coal derived humic substances on water retention and structural stability of Mediterranean soils. Soil Use Manag. 1996, 12, 209–213. [Google Scholar] [CrossRef]
- Ece, A.; Saltali, K.; Eryigit, N.; Uysal, F. The Effects of Leonardite Applications on Climbing Bean (Phaseolus vulgaris L.) Yield and the Some Soil Properties. J. Agron. 2007, 6, 480–483. [Google Scholar] [CrossRef]
- da Silva, M.S.R.d.A.; de Carvalho, L.A.L.; Braos, L.B.; de Sousa Antunes, L.F.; da Silva, C.S.R.d.A.; da Silva, C.G.N.; Pinheiro, D.G.; Correia, M.E.F.; Araújo, E.d.S.; Colnago, L.A.; et al. Effect of the application of vermicompost and millicompost humic acids about the soybean microbiome under water restriction conditions. Front. Microbiol. 2022, 13, 1000222. [Google Scholar] [CrossRef]
- Qin, K.; Leskovar, D.I. Lignite-derived humic substances modulate pepper and soil-biota growth under water deficit stress. J. Plant Nutr. Soil Sci. 2018, 181, 655–663. [Google Scholar] [CrossRef]
- Akimbekov, N.; Qiao, X.; Digel, I.; Abdieva, G.; Ualieva, P.; Zhubanova, A. The Effect of Leonardite-Derived Amendments on Soil Microbiome Structure and Potato Yield. Agriculture 2020, 10, 147. [Google Scholar] [CrossRef]
- Arjumend, T.; Abbasi, M.K.; Rafique, E. Effects of lignite-derived humic acid on some selected soil properties, growth and nutrient uptake of wheat (Triticum aestivum L.) grown under greenhouse conditions. Pak. J. Bot 2015, 47, 2231–2238. [Google Scholar]
- Sugier, D.; Kołodziej, B.; Bielińska, E. The effect of leonardite application on Arnica montana L. yielding and chosen chemical properties and enzymatic activity of the soil. J. Geochem. Explor. 2013, 129, 76–81. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Sołek-Podwika, K.; Filipek-Mazur, B.; Tabak, M. Comparative effects of lignite-derived humic acids and FYM on soil properties and vegetable yield. Geoderma 2017, 303, 85–92. [Google Scholar] [CrossRef]
- Cubillos Hinojosa, J.G.; Valero Valero, N.O.; Peralta, A.d.J. Effect of a low rank coal inoculated with coal solubilizing bacteria for the rehabilitation of a saline-sodic soil in field conditions. Rev. Fac. Nac. Agron. Medellín 2017, 70, 8271–8283. [Google Scholar] [CrossRef]
- Bekele, A.; Roy, J.L.; Young, M.A. Use of biochar and oxidized lignite for reconstructing functioning agronomic topsoil: Effects on soil properties in a greenhouse study. Can. J. Soil Sci. 2015, 95, 269–285. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Cheng, D. Activated-Lignite-Based Super Large Granular Slow-Release Fertilizers Improve Apple Tree Growth: Synthesis, Characterizations, and Laboratory and Field Evaluations. J. Agric. Food Chem. 2017, 65, 5879–5889. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Rose, M.T.; Wong, V.; Cavagnaro, T.R.; Patti, A.F. Hybrid brown coal-urea fertiliser reduces nitrogen loss compared to urea alone. Sci. Total Environ. 2017, 601–602, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Rose, M.T.; Wong, V.N.L.; Cavagnaro, T.R.; Patti, A.F. Nitrogen Dynamics in Soil Fertilized with Slow Release Brown Coal-Urea Fertilizers. Sci. Rep. 2018, 8, 14577. [Google Scholar] [CrossRef]
- Nagasawa, K.; Wang, B.; Nishiya, K.; Ushijima, K.; Zhu, Q.; Fukushima, M.; Ichijo, T. Effects of humic acids derived from lignite and cattle manure on antioxidant enzymatic activities of barley root. J. Environ. Sci. Health Part B 2016, 51, 81–89. [Google Scholar] [CrossRef]
- David, J.; Šmejkalová, D.; Hudecová, Š.; Zmeškal, O.; von Wandruszka, R.; Gregor, T.; Kučerík, J. The physico-chemical properties and biostimulative activities of humic substances regenerated from lignite. SpringerPlus 2014, 3, 156. [Google Scholar] [CrossRef]
- Jomhataikool, B.; Faungnawakij, K.; Kuboon, S.; Kraithong, W.; Chutipaichit, S.; Fuji, M.; Eiad-Ua, A. Effect of humic acid extracted from Thailand’s leonardite on rice growth. J. Met. Mater. Miner. 2019, 29, 1–7. [Google Scholar]
- Qian, S.; Ding, W.; Li, Y.; Liu, G.; Sun, J.; Ding, Q. Characterization of humic acids derived from Leonardite using a solid-state NMR spectroscopy and effects of humic acids on growth and nutrient uptake of snap bean. Chem. Speciat. Bioavailab. 2015, 27, 156–161. [Google Scholar] [CrossRef]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Kazmi, M.H. Lignite-Derived Humic Acid Effect on Growth of Wheat Plants in Different Soils. Pedosphere 2011, 21, 124–131. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Sharif, M.; Khattak, R.A.; Sarir, M.S. Effect of different levels of lignitic coal derived humic acid on growth of maize plants. Commun. Soil Sci. Plant Anal. 2002, 33, 3567–3580. [Google Scholar] [CrossRef]
- Kim Thi Tran, C.; Rose, M.T.; Cavagnaro, T.R.; Patti, A.F. Lignite amendment has limited impacts on soil microbial communities and mineral nitrogen availability. Appl. Soil Ecol. 2015, 95, 140–150. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Trojan, D.; Roux, S.; Herbold, C.; Rattei, T.; Woebken, D. Genomic insights into the Acidobacteria reveal strategies for their success in terrestrial environments. Env. Microbiol 2018, 20, 1041–1063. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
| Treatment | pH | Electrical Conductivity (mmhos/cm) | Organic Matter (%) | NO3-N (ppm) | P(ppm) | K (ppm) |
|---|---|---|---|---|---|---|
| Soil before planting | ||||||
| Untreated | 7.1 ± 0.1 | 1.1 ± 0.0 | 1.6 ± 0.2 | 102.4 ± 1.5 | 9.6 ± 0.5 | 287.0 ± 10.5 |
| BGF-0.4 | 7.0 ± 0.0 | 1.3 ± 0.1 | 1.8 ± 0.1 | 118.1 ± 2.7 | 9.9 ± 0.3 | 295.4 ± 5.6 |
| BGF-1.5 | 7.0 ± 0.0 | 1.3 ± 0.1 | 2.4 ± 0.1 * | 115.8 ± 2.3 | 14.5 ± 0.7 * | 310.9 ± 8.3 |
| OrgFert-0.4 | 6.0 ± 0.1 * | 2.8 ± 0.2 * | 2.1 ± 0.0 * | 251.4 ± 8.3 * | 19.1 ± 1.3 * | 661.2 ± 6.7 * |
| OrgFert-1.5 | 5.7 ± 0.0 * | 4.6 ± 0.4 * | 2.7 ± 0.3 * | 370.9 ± 14.8 * | 26.1 ± 1.5 * | 1320.7 ± 24.4 * |
| Coal-0.4 | 6.9 ± 0.0 | 1.0 ± 0.1 | 1.9 ± 0.3 | 115.5 ± 9.6 | 8.0 ± 0.1 | 259.4 ± 4.8 |
| Coal-1.5 | 6.4 ± 0.0 * | 1.1 ± 0.0 | 2.8 ± 0.1 * | 115.2 ± 2.3 | 6.9 ± 0.3 | 269.3 ± 14.4 |
| Soil after harvest | ||||||
| Untreated | 7.3 ± 0.2 | 1.0 ± 0.5 | 1.8 ± 0.5 | 68.8 + 8.2 | 16.5 + 3.9 | 261.1 + 25.6 |
| BGF-0.4 | 6.9 ± 0.1 | 0.9 ± 0.5 | 1.8 ± 0.6 | 63.3 + 5.7 | 18.5 + 2.9 | 261.2 + 30.3 |
| BGF-1.5 | 7.4 ± 0.4 | 2.0 ± 0.2 * | 2.4 ± 0.4 | 71.5 + 5.0 | 16.8 + 2.0 | 303.2 + 12.9 |
| OrgFert-0.4 | 7.4 ± 0.6 | 0.8 ± 0.4 | 2.5 ± 0.3 | 63.7 + 5.6 | 12.8 + 3.3 | 254.9 + 26.6 |
| OrgFert-1.5 | 6.8 ± 0.4 | 2.3 ± 0.4 * | 2.9 ± 0.2 * | 55.7 + 11.6 | 19.2 + 4.2 | 275.9 + 15.7 |
| Coal-0.4 | 6.8 ± 0.2 | 1.4 ± 0.4 | 2.1 ± 0.5 | 49.4 + 8.2 | 15.4 + 7.2 | 286.5 + 14.4 |
| Coal-1.5 | 7.0 ± 0.4 | 1.7 ± 0.9 | 1.7 ± 0.4 | 56.5 + 10.1 | 20.8 + 3.8 | 295.9 + 15.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.-F.; Fallgren, P.H.; Jin, S.; Reardon, K.F. A Low-Rank Coal-Derived Soil Amendment Promotes Plant Growth and Shapes Rhizosphere Microbial Communities of Lettuce (Lactuca sativa). Agriculture 2025, 15, 2310. https://doi.org/10.3390/agriculture15212310
Huang X-F, Fallgren PH, Jin S, Reardon KF. A Low-Rank Coal-Derived Soil Amendment Promotes Plant Growth and Shapes Rhizosphere Microbial Communities of Lettuce (Lactuca sativa). Agriculture. 2025; 15(21):2310. https://doi.org/10.3390/agriculture15212310
Chicago/Turabian StyleHuang, Xing-Feng, Paul H. Fallgren, Song Jin, and Kenneth F. Reardon. 2025. "A Low-Rank Coal-Derived Soil Amendment Promotes Plant Growth and Shapes Rhizosphere Microbial Communities of Lettuce (Lactuca sativa)" Agriculture 15, no. 21: 2310. https://doi.org/10.3390/agriculture15212310
APA StyleHuang, X.-F., Fallgren, P. H., Jin, S., & Reardon, K. F. (2025). A Low-Rank Coal-Derived Soil Amendment Promotes Plant Growth and Shapes Rhizosphere Microbial Communities of Lettuce (Lactuca sativa). Agriculture, 15(21), 2310. https://doi.org/10.3390/agriculture15212310






