Distinguishing True from False Estrus in Hanwoo Cows Using Neck-Mounted IMU Sensors: Quantifying Behavioral Differences to Reduce False Positives
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing Conditions, and Ethical Approval
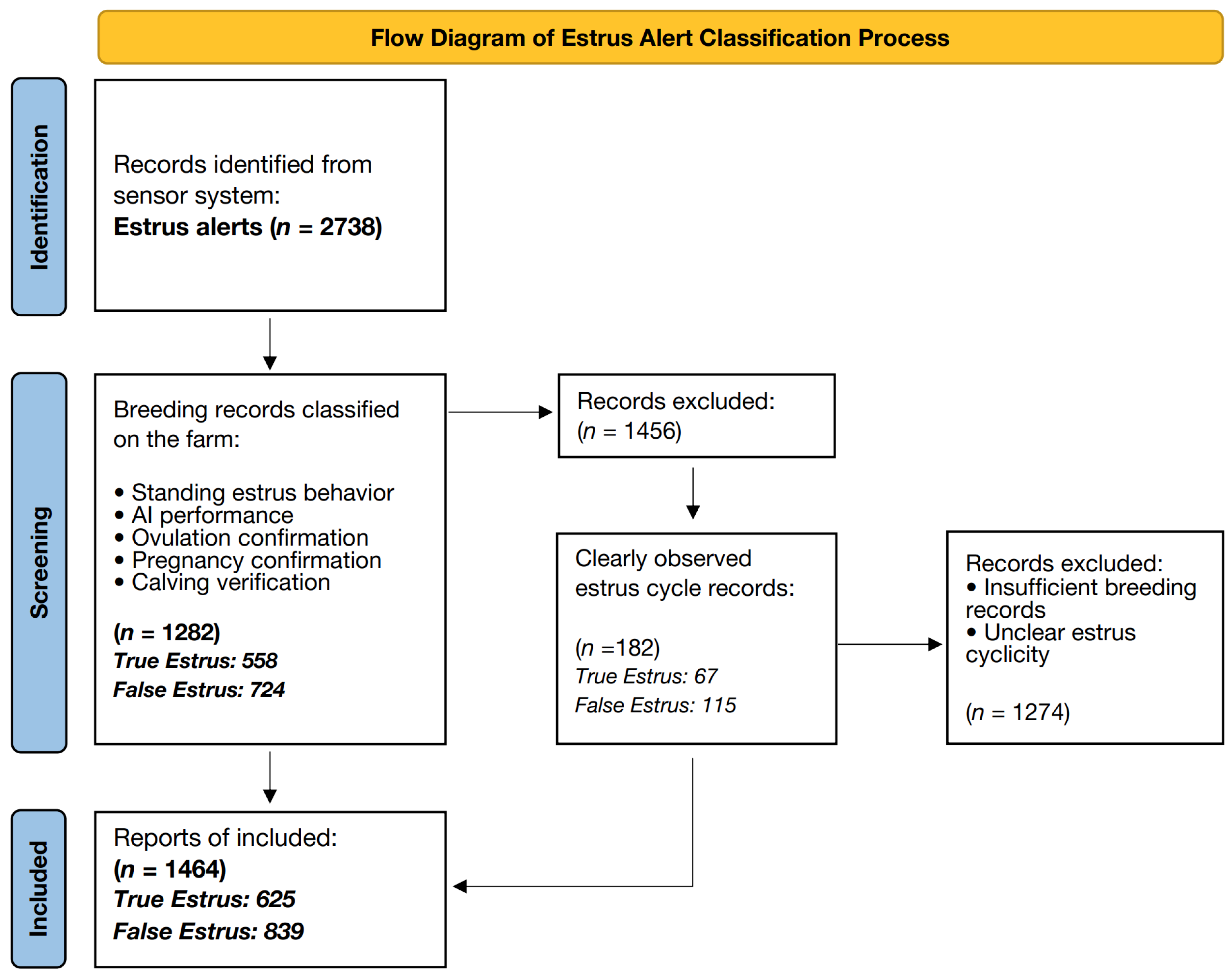
2.2. Estrus Detection System and Classification
2.3. Behavioral Data Collection and Processing
2.4. Statistical Analysis
3. Results
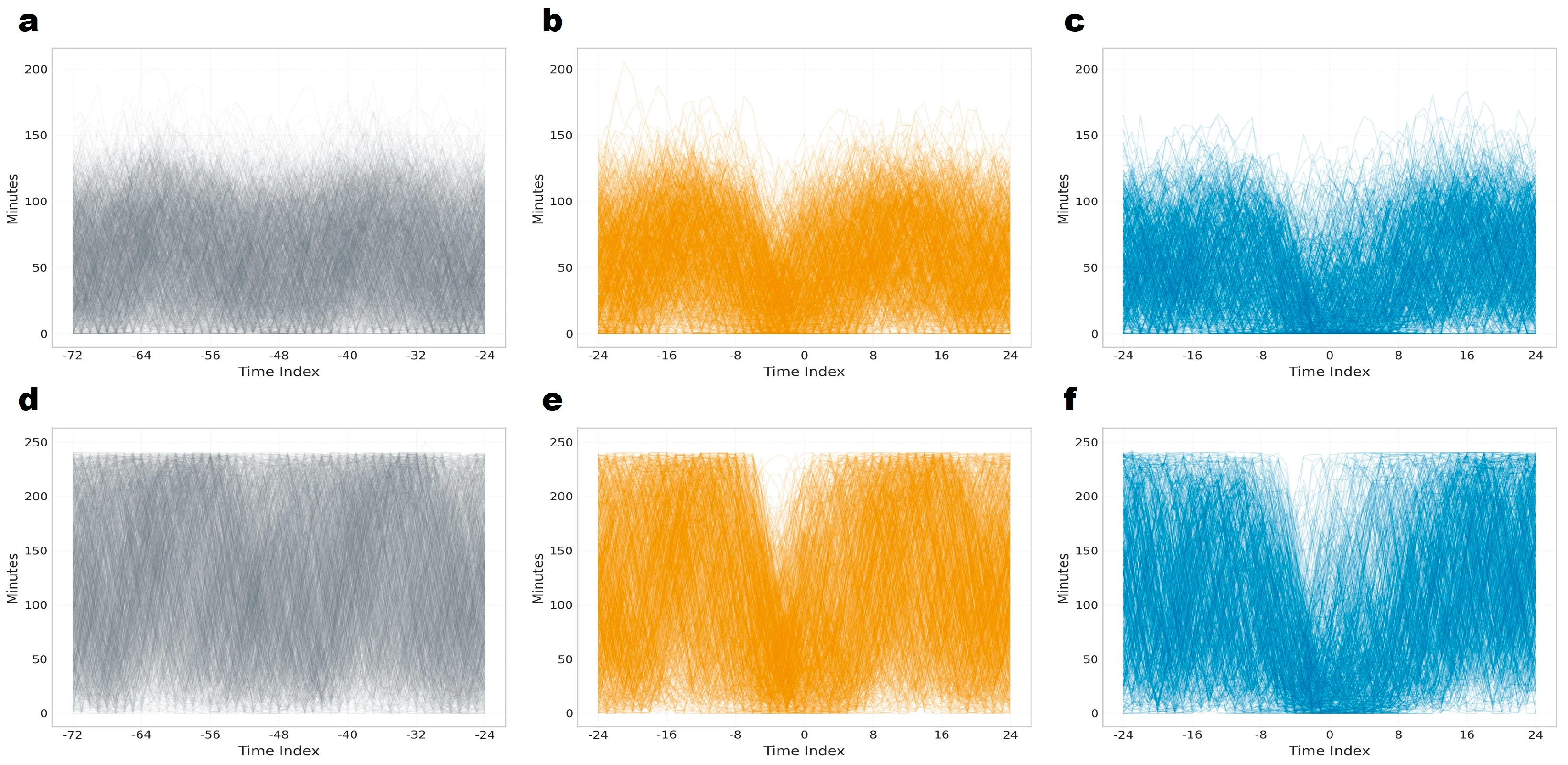
3.1. Circadian Distribution of Estrus Alert Generation
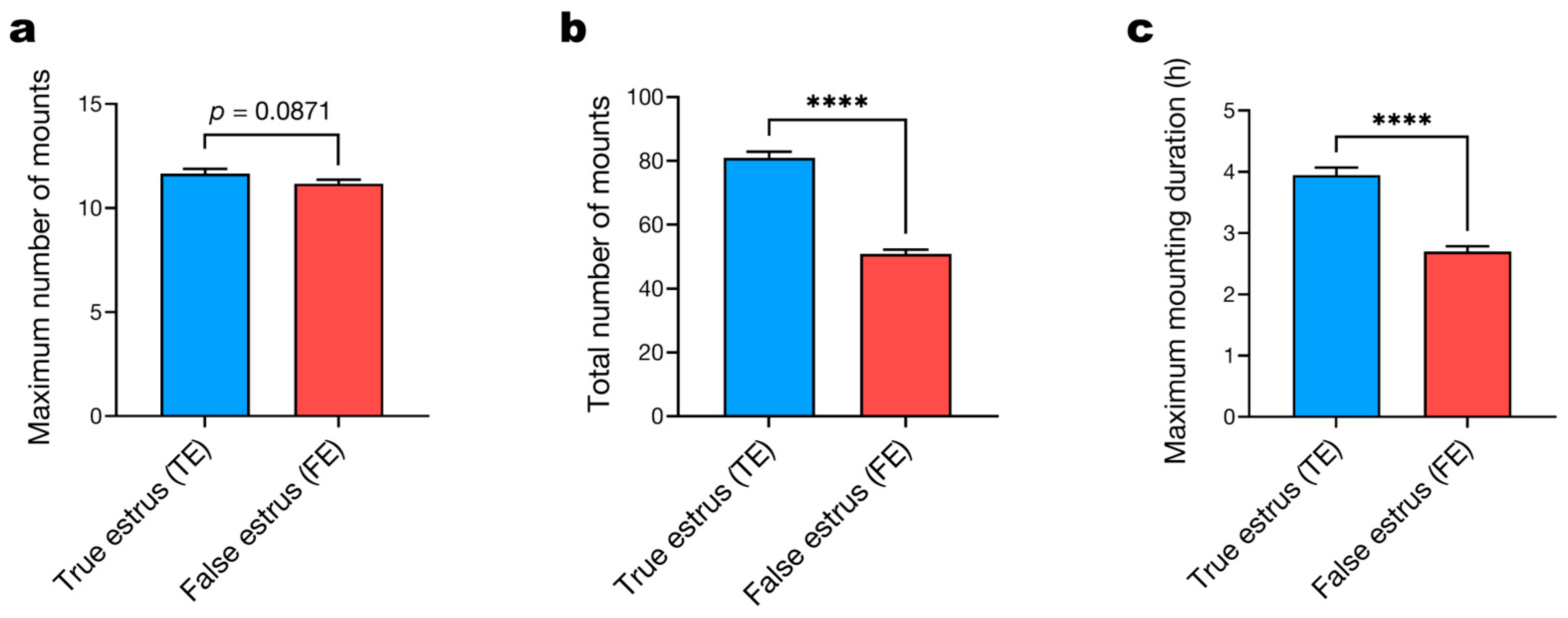
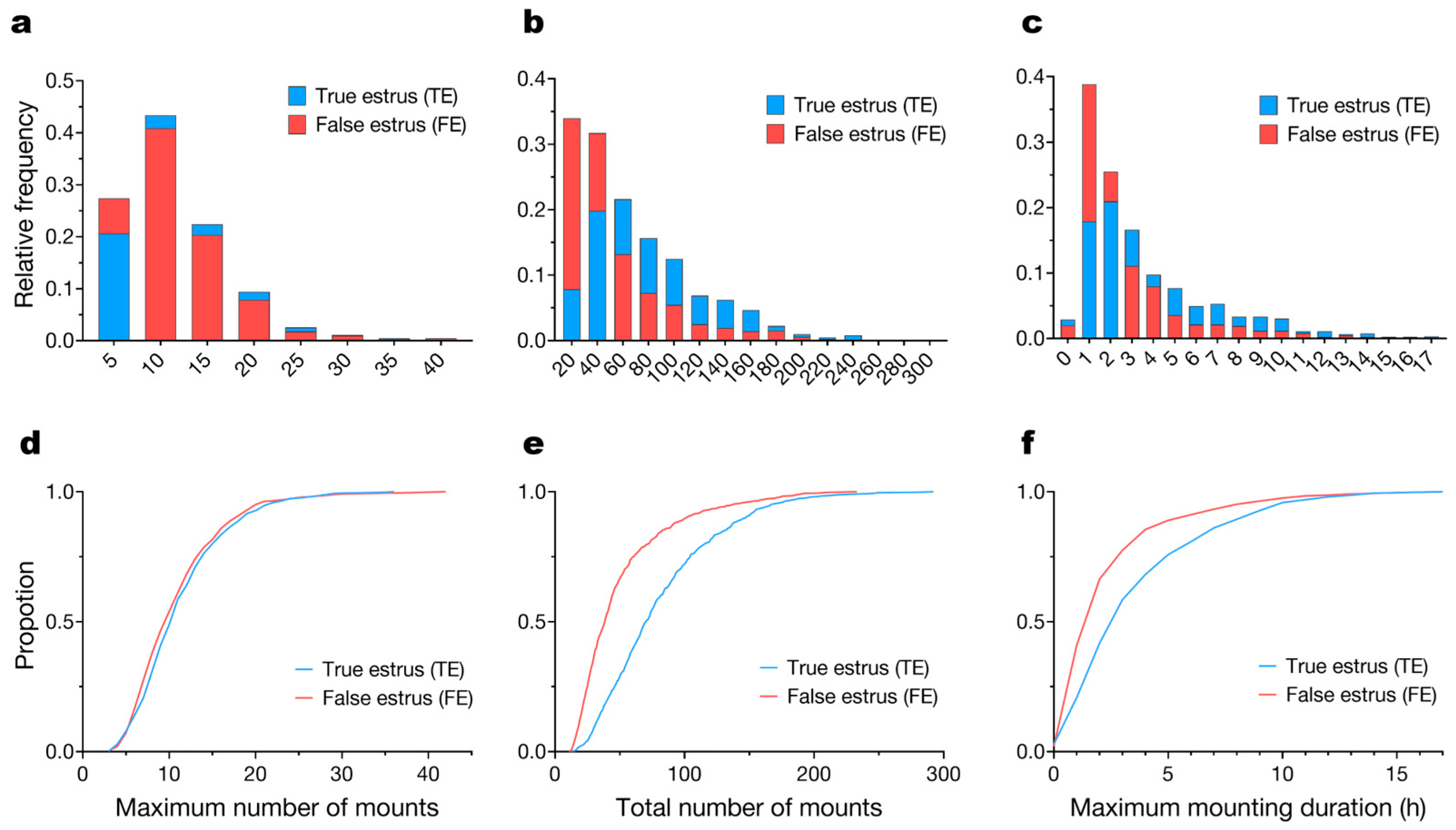
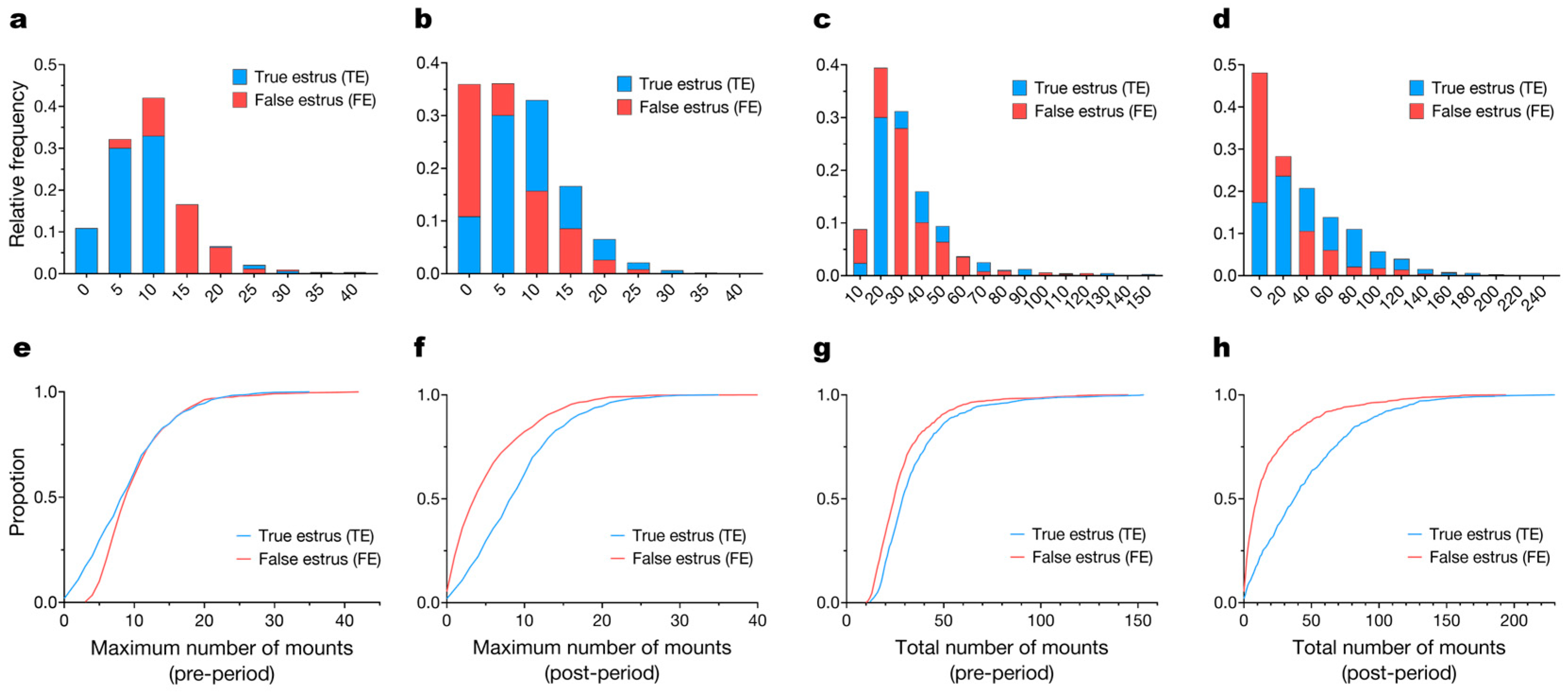
3.2. Mounting Behavior Parameters
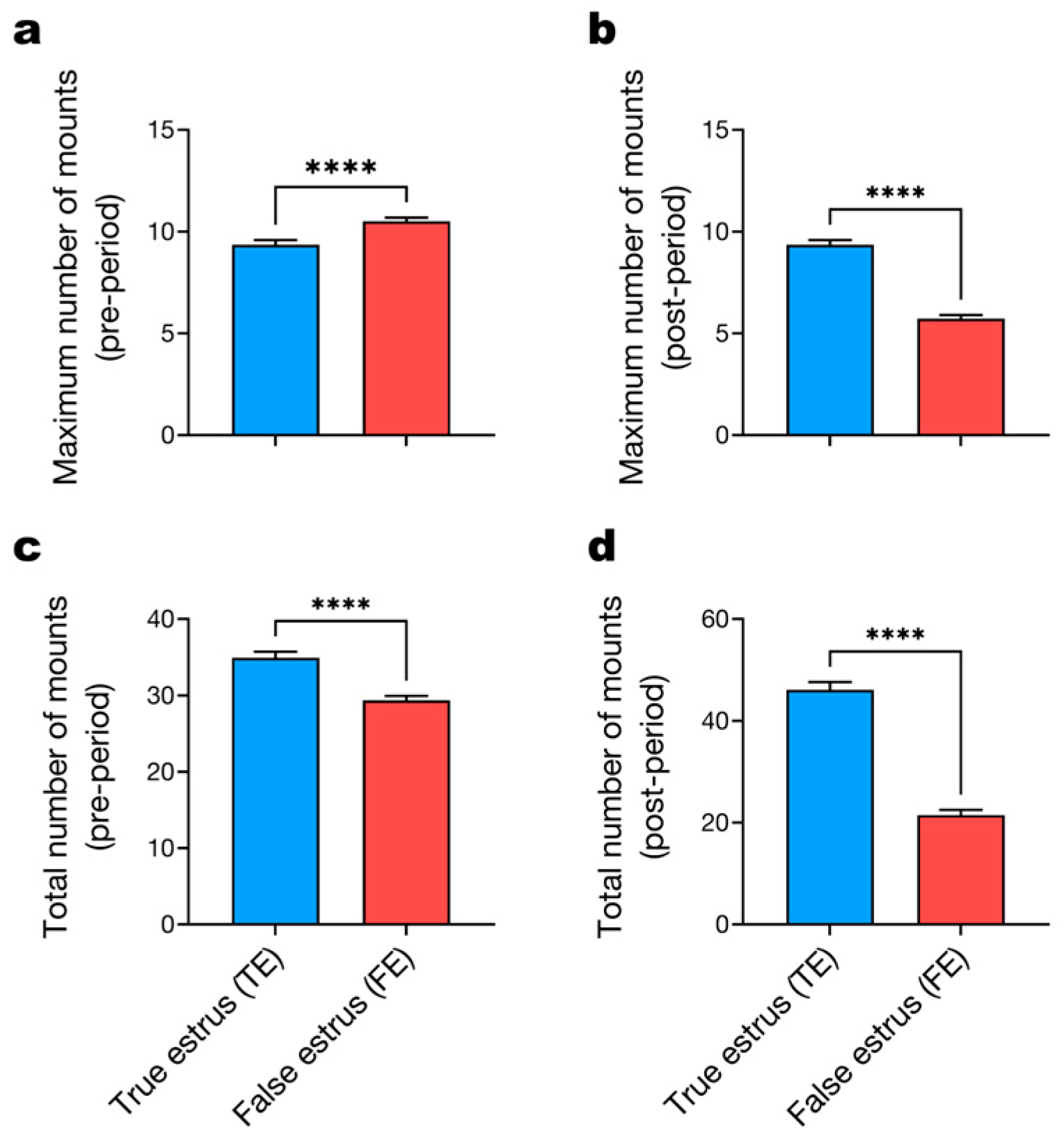
3.3. Temporal Patterns of Mounting Behavior
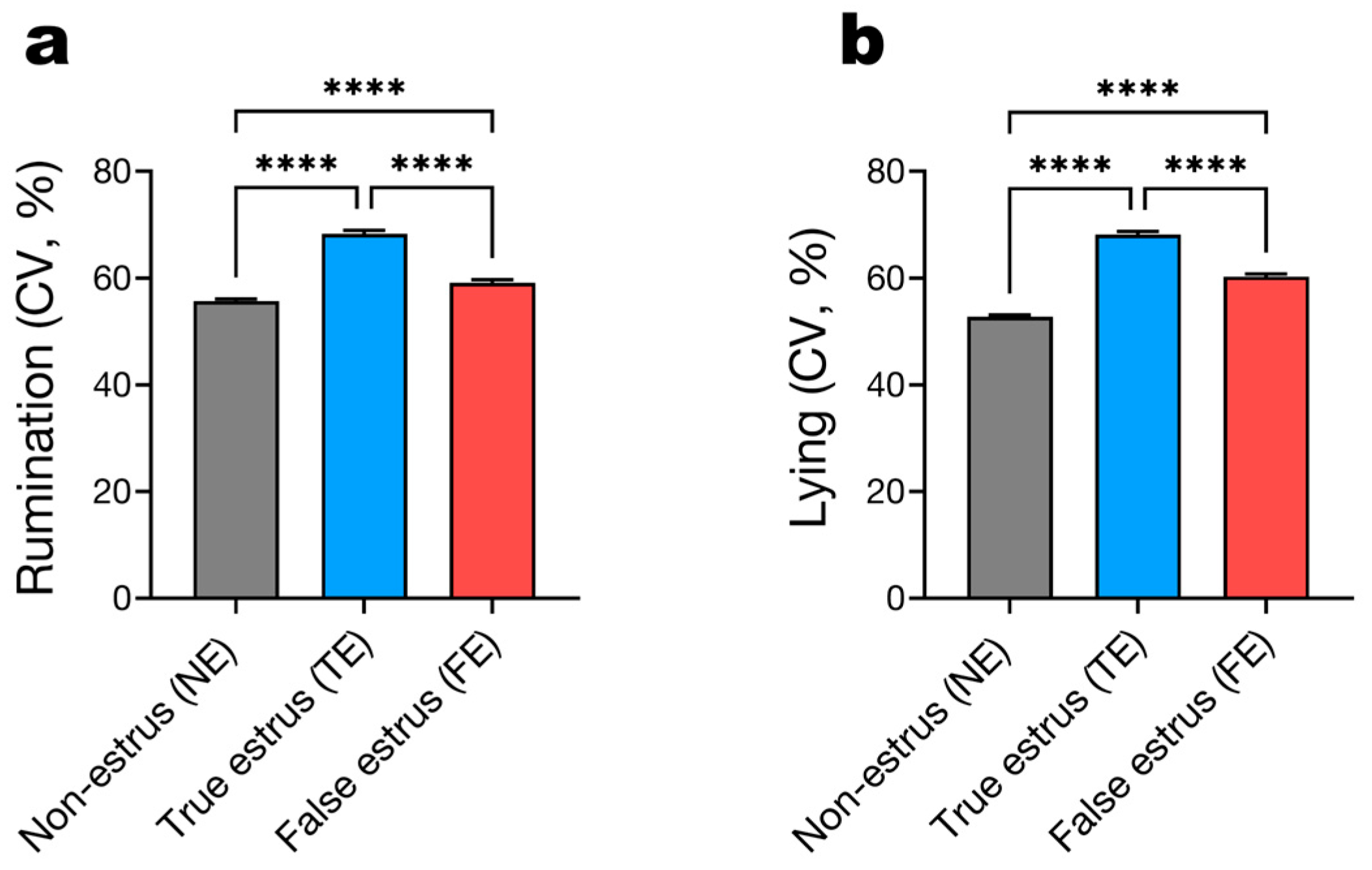
3.4. Behavioral Variability During Estrus Events
3.5. Temporal Dynamics of Rumination and Lying Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TE | True estrus |
| FE | False estrus |
| IMU | Inertial measurement unit |
| NE | Non-estrus |
| CV | Coefficient of variation |
| CDF | Cumulative distribution functions |
References
- Lucy, M.C. Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? J. Dairy Sci. 2001, 84, 1277–1293. [Google Scholar] [CrossRef]
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef]
- de Vries, A.; Conlin, B.J. Economic Value of Timely Determination of Unexpected Decreases in Detection of Estrus Using Control Charts. J. Dairy Sci. 2003, 86, 3516–3526. [Google Scholar] [CrossRef]
- Gerber, P.; Henderson, B.; Makkar, H. Mitigation of Greenhouse Gas Emissions in Livestock Production: A Review of Technical Options for Non-CO2 Emissions; FAO Animal Production and Health Paper (FAO): Rome, Italy, 2013. [Google Scholar]
- Archer, S.C.; Green, M.J.; Huxley, J.N. Association between milk yield and serial locomotion score assessments in UK dairy cows. J. Dairy Sci. 2010, 93, 4045–4053. [Google Scholar] [CrossRef]
- Firk, R.; Stamer, E.; Junge, W.; Krieter, J. Automation of oestrus detection in dairy cows: A review. Livest. Prod. Sci. 2002, 75, 219–232. [Google Scholar] [CrossRef]
- Roelofs, J.; López-Gatius, F.; Hunter, R.H.F.; van Eerdenburg, F.J.C.M.; Hanzen, C. When is a cow in estrus? Clinical and practical aspects. Theriogenology 2010, 74, 327–344. [Google Scholar] [CrossRef]
- López-Gatius, F. Revisiting the Timing of Insemination at Spontaneous Estrus in Dairy Cattle. Animals 2022, 12, 3565. [Google Scholar] [CrossRef]
- Roelofs, J.B.; van Eerdenburg, F.J.C.M.; Soede, N.M.; Kemp, B. Various behavioral signs of estrous and their relationship with time of ovulation in dairy cattle. Theriogenology 2005, 63, 1366–1377. [Google Scholar] [CrossRef]
- Santos, C.A.d.; Landim, N.M.D.; Araújo, H.X.d.; Paim, T.d.P. Automated Systems for Estrous and Calving Detection in Dairy Cattle. AgriEngineering 2022, 4, 475–482. [Google Scholar] [CrossRef]
- Orihuela, A.n. Some factors affecting the behavioural manifestation of oestrus in cattle: A review. Appl. Anim. Behav. Sci. 2000, 70, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gwazdauskas, F.C.; Lineweaver, J.A.; McGilliard, M.L. Environmental and Management Factors Affecting Estrous Activity in Dairy Cattle. J. Dairy Sci. 1983, 66, 1510–1514. [Google Scholar] [CrossRef]
- Sveberg, G.; Refsdal, A.O.; Erhard, H.W.; Kommisrud, E.; Aldrin, M.; Tvete, I.F.; Buckley, F.; Waldmann, A.; Ropstad, E. Behavior of lactating Holstein-Friesian cows during spontaneous cycles of estrus. J. Dairy Sci. 2011, 94, 1289–1301. [Google Scholar] [CrossRef]
- Reith, S.; Hoy, S. Review: Behavioral signs of estrus and the potential of fully automated systems for detection of estrus in dairy cattle. Animal 2018, 12, 398–407. [Google Scholar] [CrossRef]
- Perez Marquez, H.J.; Ambrose, D.J.; Bench, C.J. Behavioral changes to detect estrus using ear-sensor accelerometer compared to in-line milk progesterone in a commercial dairy herd. Front. Anim. Sci. 2023, 4, 1149085. [Google Scholar] [CrossRef]
- Aungier, S.P.M.; Roche, J.F.; Sheehy, M.; Crowe, M.A. Effects of management and health on the use of activity monitoring for estrus detection in dairy cows. J. Dairy Sci. 2012, 95, 2452–2466. [Google Scholar] [CrossRef] [PubMed]
- NIAS. Korean Feeding Standard for Hanwoo, 4th ed.; National Institute of Animal Science: Wanju, Republic of Korea, 2022. [Google Scholar]
- Kim, S.-J.; Jin, X.-C.; Bharanidharan, R.; Kim, N.-Y. Monitoring Multiple Behaviors in Beef Calves Raised in Cow–Calf Contact Systems Using a Machine Learning Approach. Animals 2024, 14, 3278. [Google Scholar] [CrossRef] [PubMed]
- Stangaferro, M.L.; Wijma, R.; Caixeta, L.S.; Al-Abri, M.A.; Giordano, J.O. Use of rumination and activity monitoring for the identification of dairy cows with health disorders: Part I. Metabolic and digestive disorders. J. Dairy Sci. 2016, 99, 7395–7410. [Google Scholar] [CrossRef]
- Riaboff, L.; Couvreur, S.; Madouasse, A.; Roig-Pons, M.; Aubin, S.; Massabie, P.; Chauvin, A.; Bédère, N.; Plantier, G. Use of Predicted Behavior from Accelerometer Data Combined with GPS Data to Explore the Relationship between Dairy Cow Behavior and Pasture Characteristics. Sensors 2020, 20, 4741. [Google Scholar] [CrossRef]
- Wang, J.; Bell, M.; Liu, X.; Liu, G. Machine-Learning Techniques Can Enhance Dairy Cow Estrus Detection Using Location and Acceleration Data. Animals 2020, 10, 1160. [Google Scholar] [CrossRef]
- Carslake, C.; Occhiuto, F.; Vázquez-Diosdado, J.A.; Kaler, J. Repeatability and Predictability of Calf Feeding Behaviors—Quantifying Between- and Within-Individual Variation for Precision Livestock Farming. Front. Vet. Sci. 2022, 9, 827124. [Google Scholar] [CrossRef]
- Piñeiro, J.M.; Menichetti, B.T.; Barragan, A.A.; Relling, A.E.; Weiss, W.P.; Bas, S.; Schuenemann, G.M. Associations of postpartum lying time with culling, milk yield, cyclicity, and reproductive performance of lactating dairy cows. J. Dairy Sci. 2019, 102, 3362–3375. [Google Scholar] [CrossRef]
- Braun, U.; Zürcher, S.; Hässig, M. Eating and rumination activity in 10 cows over 10 days. Res. Vet. Sci. 2015, 101, 196–198. [Google Scholar] [CrossRef]
- Vanhoudt, A.; van Winden, S.; Fishwick, J.C.; Bell, N.J. Monitoring cow comfort and rumen health indices in a cubicle-housed herd with an automatic milking system: A repeated measures approach. Ir. Vet. J. 2015, 68, 12. [Google Scholar] [CrossRef] [PubMed]
- Reith, S.; Brandt, H.; Hoy, S. Simultaneous analysis of activity and rumination time, based on collar-mounted sensor technology, of dairy cows over the peri-estrus period. Livest. Sci. 2014, 170, 219–227. [Google Scholar] [CrossRef]
- Cheon Si, N.; Yoo Geum, Z.; Kim Chan, H.; Jung Ji, Y.; Kim Dong, H.; Jeon Jung, H. Study on behavioral change of estrus in Hanwoo (Korean native cattle). J. Korea Acad. Ind. Coop. Soc. 2020, 21, 825–832. [Google Scholar] [CrossRef]
- Mayo, L.M.; Silvia, W.J.; Ray, D.L.; Jones, B.W.; Stone, A.E.; Tsai, I.C.; Clark, J.D.; Bewley, J.M.; Heersche, G., Jr. Automated estrous detection using multiple commercial precision dairy monitoring technologies in synchronized dairy cows. J. Dairy Sci. 2019, 102, 2645–2656. [Google Scholar] [CrossRef]
- Cheon, S.N.; Park, G.-W.; Park, K.-H.; Jeon, J.H. Peri-estrus activity and mounting behavior and its application to estrus detection in Hanwoo (Korea Native Cattle). J. Anim. Sci. Technol. 2023, 65, 748–758. [Google Scholar] [CrossRef]
- Crowe, M.A.; Mullen, M. Regulation and Function of Gonadotropins Throughout the Bovine Oestrous Cycle. In Gonadotropin; Vizcarra, J.A., Ed.; IntechOpen: London, UK, 2013. [Google Scholar][Green Version]
- Blackshaw, J.K.; Blackshaw, A.W.; McGlone, J.J. Buller steer syndrome review. Appl. Anim. Behav. Sci. 1997, 54, 97–108. [Google Scholar] [CrossRef]
- Williams, G.L.; Cardoso, R.C. Neuroendocrine Control of Estrus and Ovulation. In Bovine Reproduction; Wiley: Hoboken, NJ, USA, 2021; pp. 269–291. [Google Scholar]
- Weber, W.E.; Looney, C.R.; Rook, W.L. 136 Application of Ear-attached Sensor Technology (CowManager) to Detect Estrus in a Commercial Beef Cow AI Program. J. Anim. Sci. 2022, 100, 6. [Google Scholar] [CrossRef]
- Fauvel, K.; Masson, V.; Fromont, É.; Faverdin, P.; Termier, A. Towards Sustainable Dairy Management—A Machine Learning Enhanced Method for Estrus Detection. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Anchorage, AK, USA, 4–8 August 2019; pp. 3051–3059. [Google Scholar]
- Walker, S.; Smith, R.; Routly, J.; Jones, D.; Morris, M.; Dobson, H. Lameness, activity time-budgets, and estrus expression in dairy cattle. J. Dairy Sci. 2008, 91, 4552–4559. [Google Scholar] [CrossRef] [PubMed]
- Perez Marquez, H.J.; Ambrose, D.J.; Schaefer, A.L.; Cook, N.J.; Bench, C.J. Evaluation of infrared thermography combined with behavioral biometrics for estrus detection in naturally cycling dairy cows. Animal 2021, 15, 100205. [Google Scholar] [CrossRef]
- Sheldon, I.; Dobson, H. Reproductivechallengesfacing the cattle industry at the beginningof the 21st century. Reproduction 2003, 61, 1–13. [Google Scholar]
- Abeni, F.; Galli, A. Monitoring cow activity and rumination time for an early detection of heat stress in dairy cow. Int. J. Biometeorol. 2017, 61, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Mičiaková, M.; Strapák, P.; Strapáková, E.; Szencziová, I. Evaluating Rumination Time Changes During Estrus in Dairy Cows. Dairy 2025, 6, 5. [Google Scholar] [CrossRef]
- Codl, R.; Ducháček, J.; Vacek, M.; Pytlík, J.; Stádník, L.; Vrhel, M. Relationship between daily activities duration and oestrus in dairy cows over the year. Acta Vet. Brno 2022, 91, 11–16. [Google Scholar] [CrossRef]
- Toušová, R.; Ducháček, J.; Codl, R.; Pytlík, J.; Ptáček, M.; Gašparík, M.; Stádník, L. Rumination Time Monitoring as a Possible Tool to Improve Diagnostics of Heat and Parturition. Acta Univ. Agric. Silvic. Mendel. Brun. 2020, 68, 109–117. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, C.; Gomes Lopes, M.; Loor, J.J.; Shao, Y.; Jia, B.; Shao, G.; Huang, B. Characterization of the physical and ruminal activities related to estrus in dairy cows raised on Chinese commercial farms. Vet. Arh. 2023, 93, 143–158. [Google Scholar] [CrossRef]
- Morton, J.M.; Wynn, P.C. Assessing ovulation detection performance in commercial dairy herds using progesterone concentrations from limited numbers of strategically collected milk samples. J. Dairy Sci. 2010, 93, 3019–3030. [Google Scholar] [CrossRef]
- Schilkowsky, E.M.; Granados, G.E.; Sitko, E.M.; Masello, M.; Perez, M.M.; Giordano, J.O. Evaluation and characterization of estrus alerts and behavioral parameters generated by an ear-attached accelerometer-based system for automated detection of estrus. J. Dairy Sci. 2021, 104, 6222–6237. [Google Scholar] [CrossRef]
- Daoerji, F.; Huijuan, W. Research of Estrus Detection Models in Dairy Cows by Activity. Sci. Discov. 2018, 6, 102–109. [Google Scholar] [CrossRef]


| Type | Specification |
|---|---|
| Model | FHL2 |
| Sensor | six-axis IMU (accelerometer + gyroscope) |
| Sampling Rate | 25 Hz |
| Data transmission | Bluetooth 5.0 |
| Power Supply | Li-Ion 18,650 battery/3.6 V 7000 mAh (3500 mAh × 2EA) |
| Data Storage | 128 Mb flash memory |
| Dimensions | 127 mm × 67 mm × 33 mm (L × W × H) |
| Weight | 216 g |
| Criterion | Description |
|---|---|
| Standing Estrus Behavior | The cow stood immobile when mounted by another cow for at least 2 s, observed a minimum of five times within a 24 h period |
| Artificial insemination Performance | Record of artificial insemination performed by an artificial insemination technician following observed estrus signs |
| Ovulation Confirmation | Detection of ovulation by rectal palpation or transrectal ultrasonography, with follicles palpated before ovulation and corpus luteum presence confirmed afterward |
| Return to Estrus | Observation of estrus signs 18–24 days post-insemination, indicating a true estrous cycle despite conception failure |
| Pregnancy Confirmation | Confirmation of pregnancy 30–45 days post-insemination using rectal palpation, ultrasound, or blood test. |
| Calving Verification | Verification of successful calving at term, providing ultimate confirmation of the preceding estrus event |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Jin, X.-C.; Bharanidharan, R.; Kim, N.-Y. Distinguishing True from False Estrus in Hanwoo Cows Using Neck-Mounted IMU Sensors: Quantifying Behavioral Differences to Reduce False Positives. Agriculture 2025, 15, 2307. https://doi.org/10.3390/agriculture15212307
Kim S-J, Jin X-C, Bharanidharan R, Kim N-Y. Distinguishing True from False Estrus in Hanwoo Cows Using Neck-Mounted IMU Sensors: Quantifying Behavioral Differences to Reduce False Positives. Agriculture. 2025; 15(21):2307. https://doi.org/10.3390/agriculture15212307
Chicago/Turabian StyleKim, Seong-Jin, Xue-Cheng Jin, Rajaraman Bharanidharan, and Na-Yeon Kim. 2025. "Distinguishing True from False Estrus in Hanwoo Cows Using Neck-Mounted IMU Sensors: Quantifying Behavioral Differences to Reduce False Positives" Agriculture 15, no. 21: 2307. https://doi.org/10.3390/agriculture15212307
APA StyleKim, S.-J., Jin, X.-C., Bharanidharan, R., & Kim, N.-Y. (2025). Distinguishing True from False Estrus in Hanwoo Cows Using Neck-Mounted IMU Sensors: Quantifying Behavioral Differences to Reduce False Positives. Agriculture, 15(21), 2307. https://doi.org/10.3390/agriculture15212307






