The Effect of Long-Term Organic Amendments on Soil Organic Carbon Accumulation via Regulating Microbial Traits in a Paddy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
- (1)
- CF, chemical fertilizers;
- (2)
- CF + RS, chemical fertilizers and rice straw;
- (3)
- CF + GM, chemical fertilizers and green manure (milk vetch);
- (4)
- CF + PM, chemical fertilizers and pig manure.
2.2. Soil Sampling and Analysis
2.3. Soil Organic Carbon Fractions
2.4. Analysis of SC Chemical Composition
2.5. Phospholipid Fatty Acids (PLFAs) Analysis
2.6. Measurement of Microbial Carbon Use Efficiency
2.7. Soil Amino Sugars Analysis
2.8. Statistical Analysis
3. Results
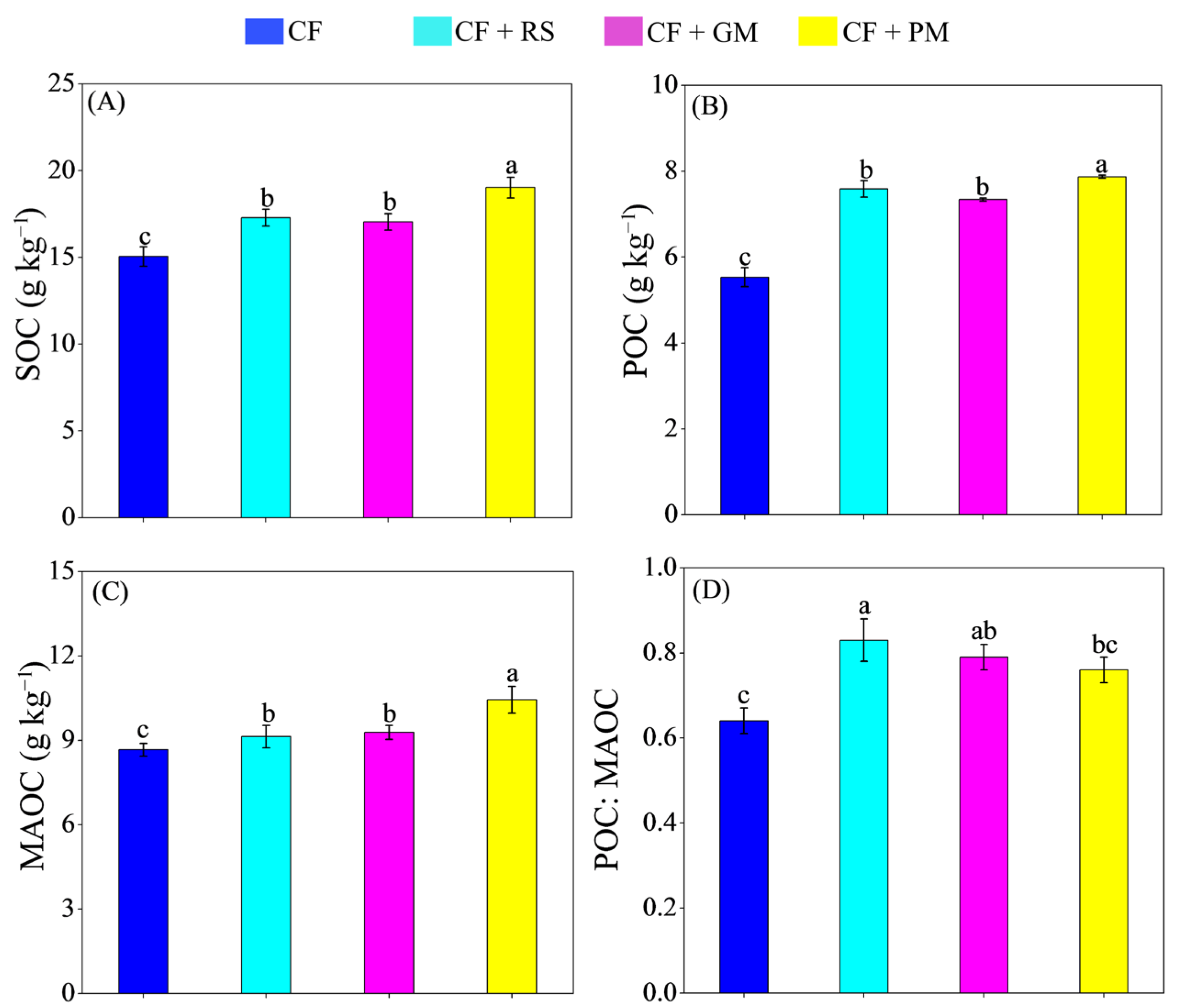
3.1. Soil Properties, Organic C Fractions, and Chemical Composition
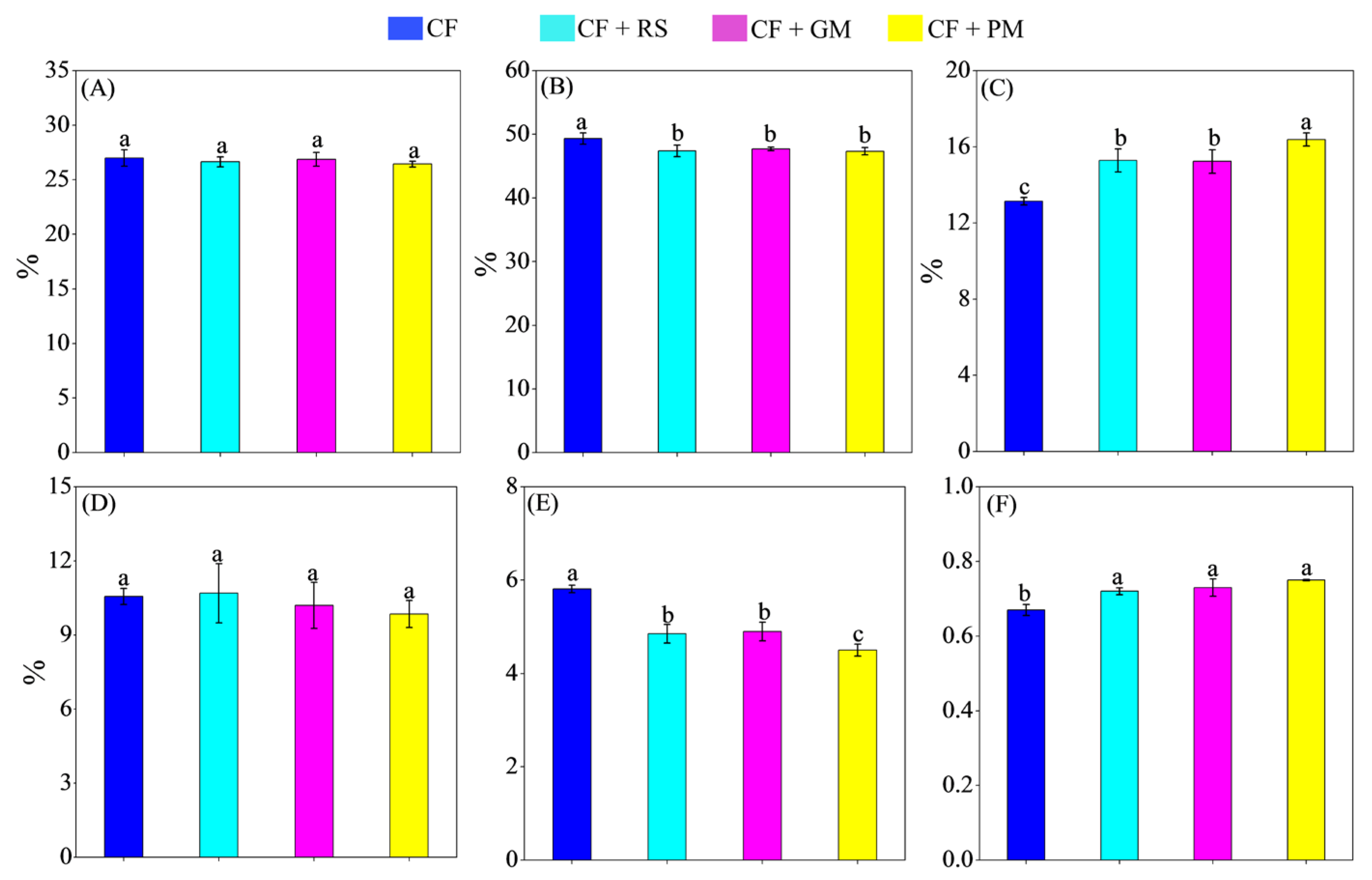
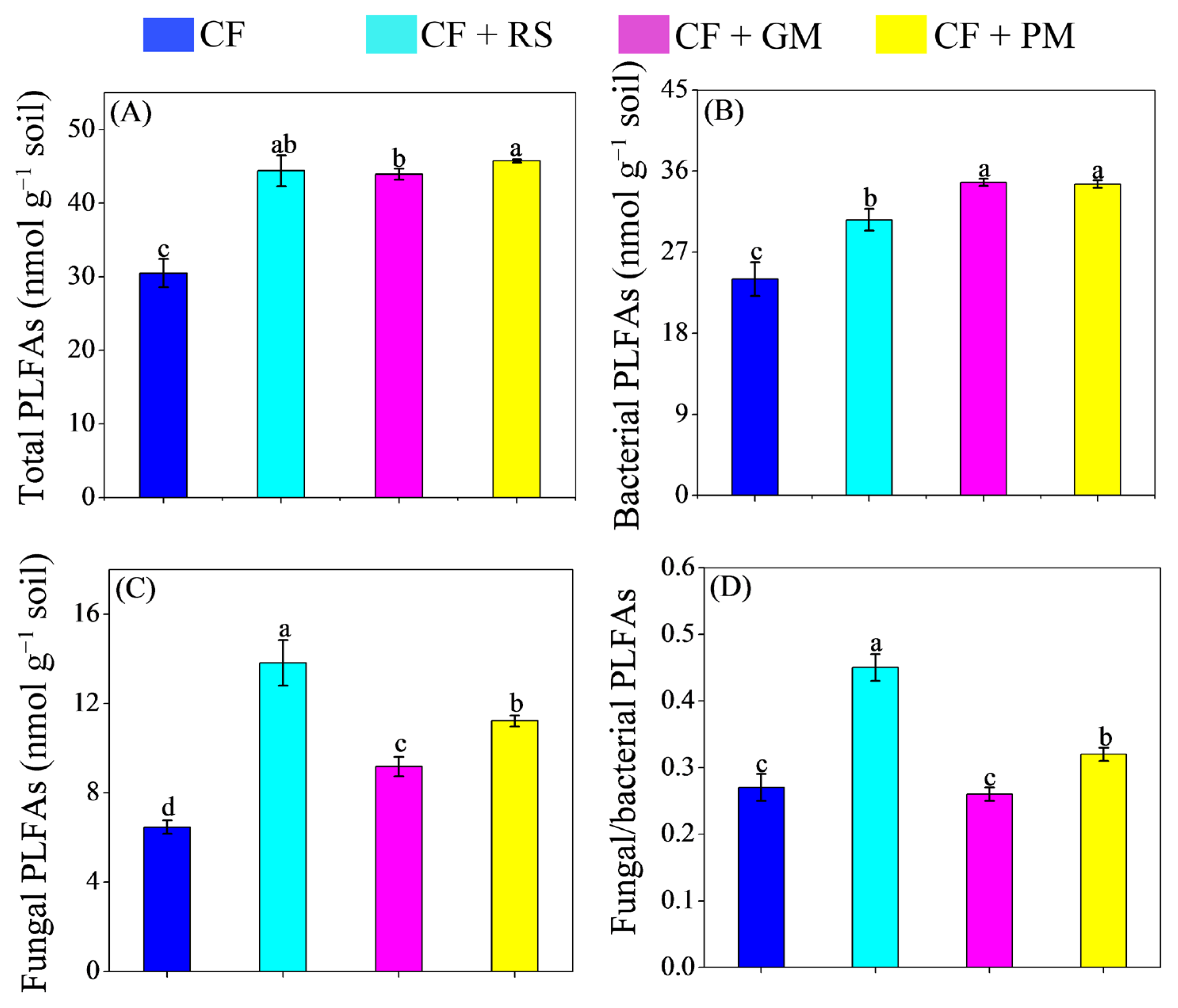
3.2. Microbial Community Composition
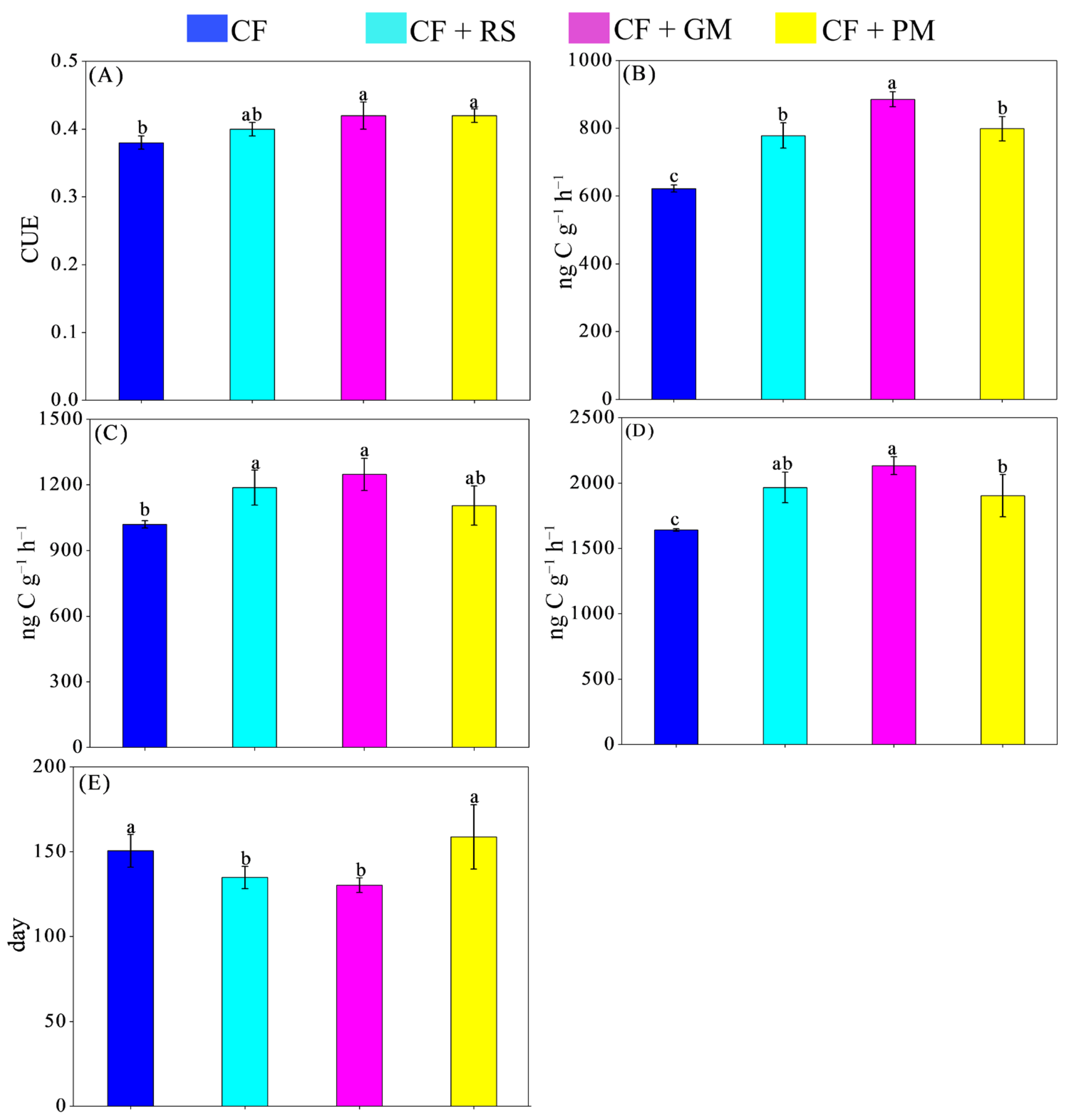
3.3. Microbial C Use Efficiency
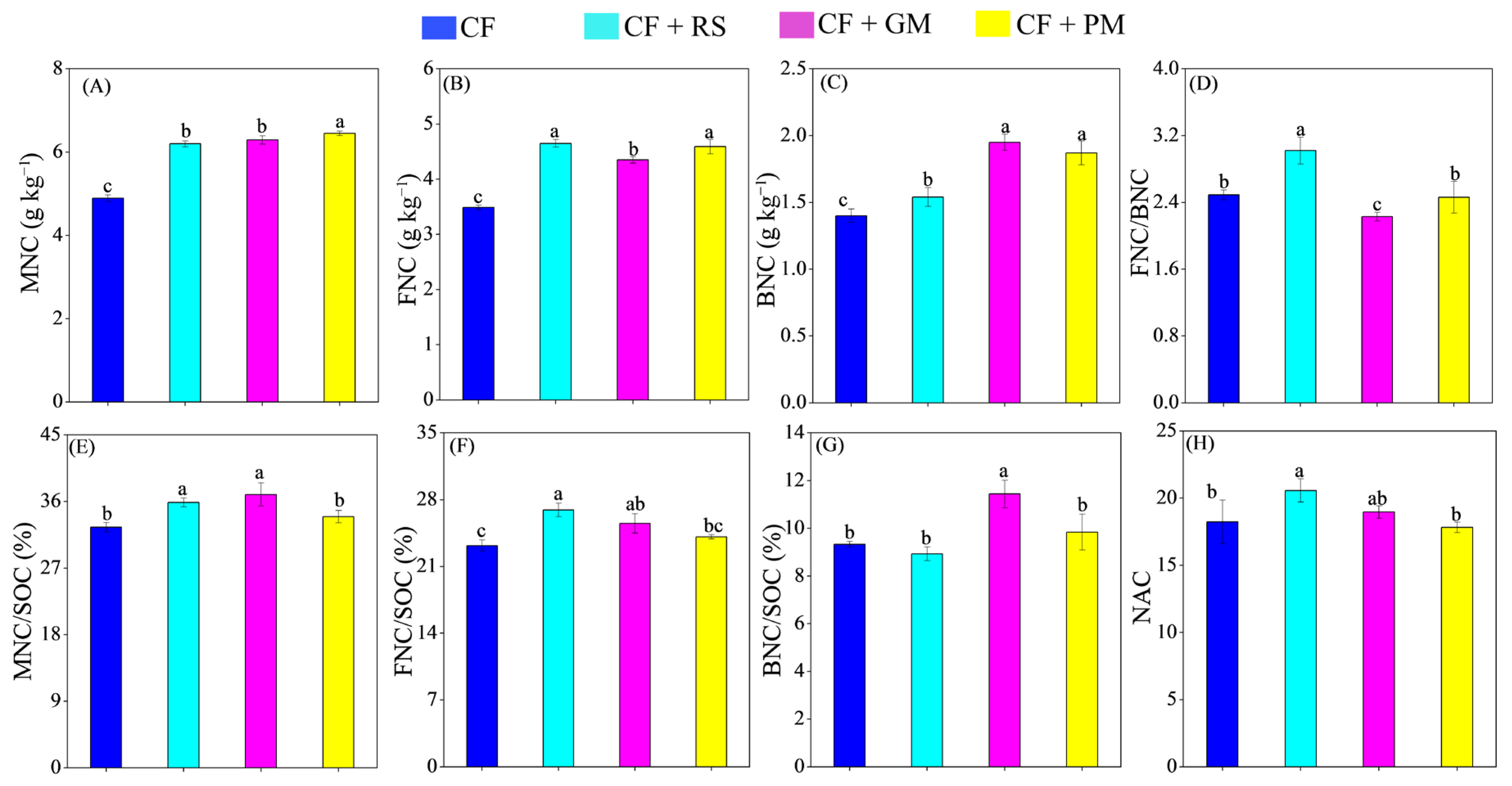
3.4. Microbial Necromass C
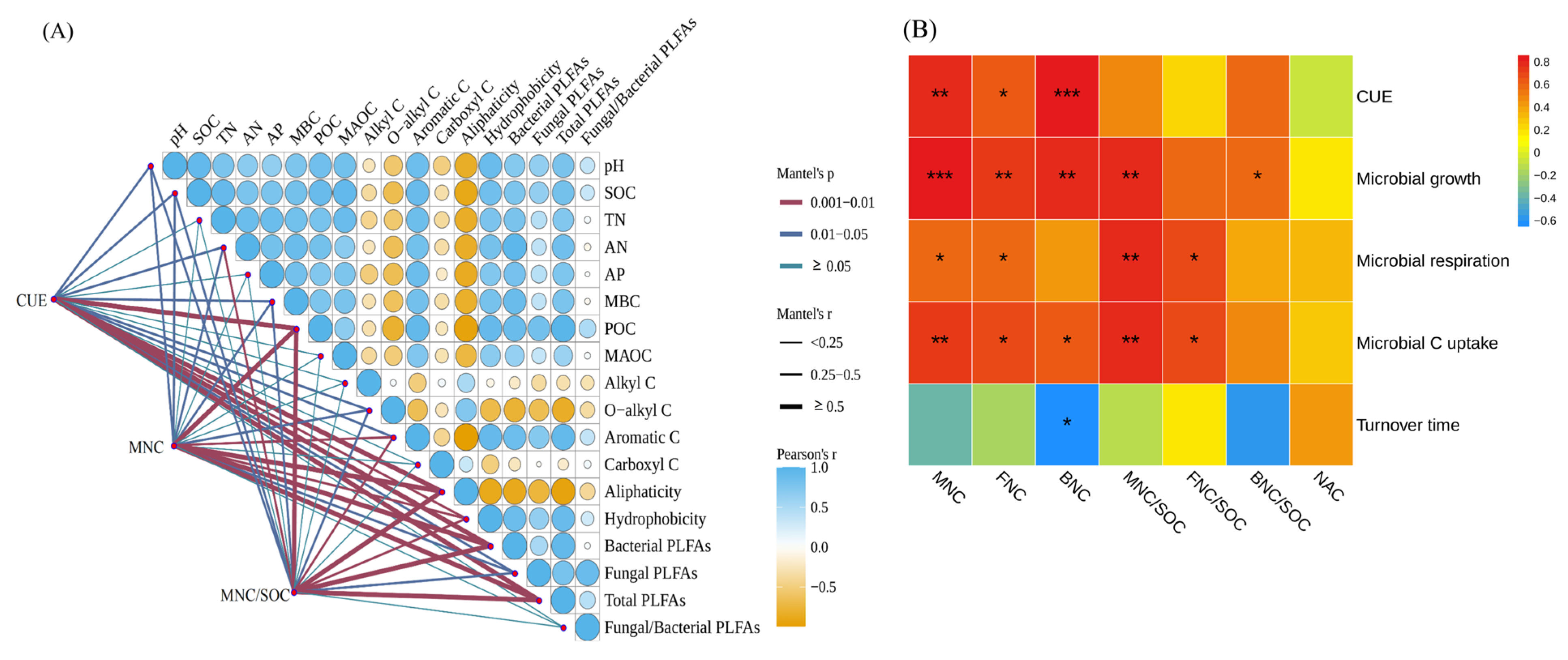
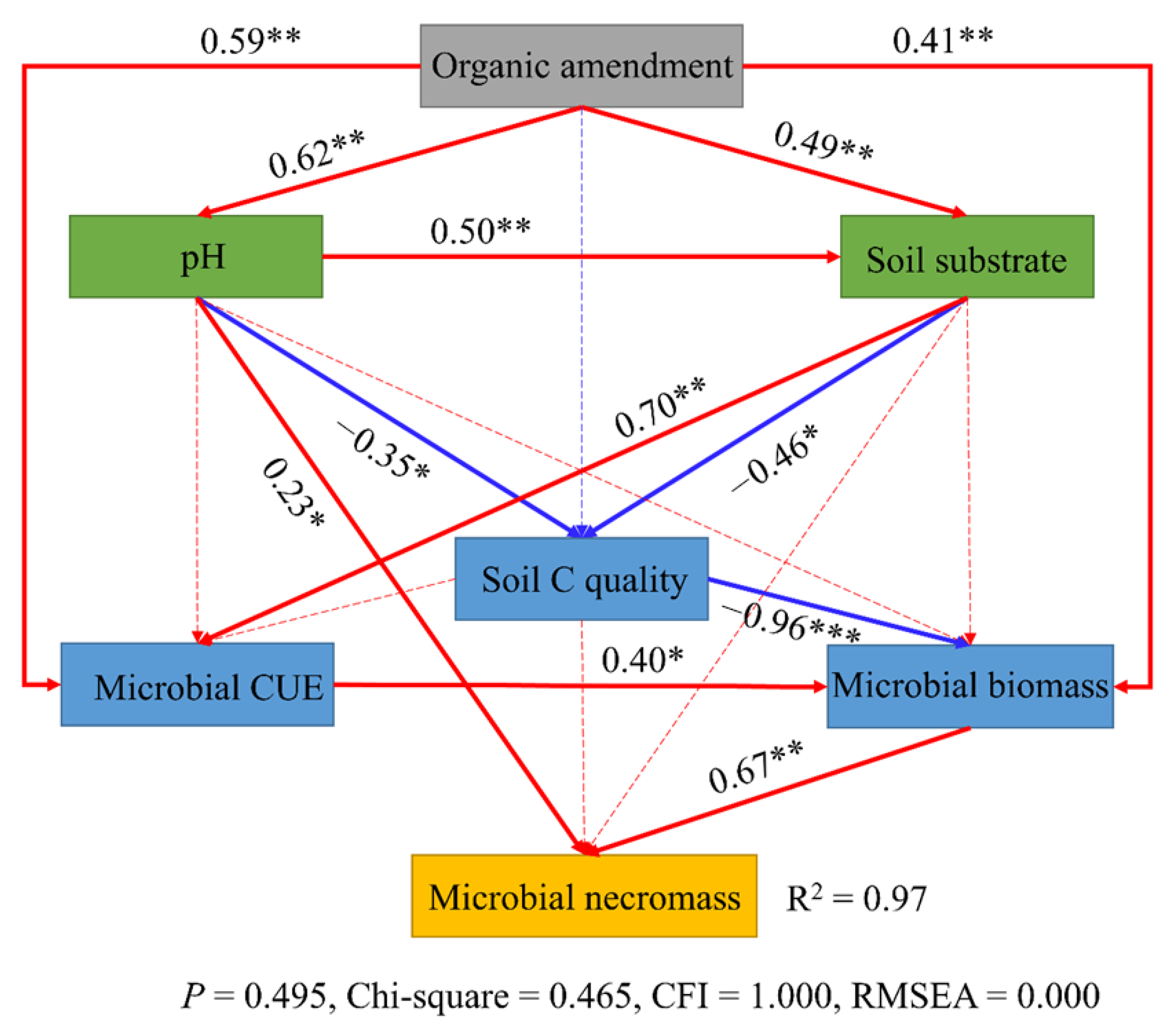
3.5. Correlations Among Soil Properties, Microbial Metabolic Traits, and Microbial Necromass
4. Discussion
4.1. Effects of Organic Amendments Soil Organic Carbon
4.2. Effects of Organic Amendments on Microbial C Use Efficiency
4.3. Effects of Organic Amendments on Microbial Necromass Carbon
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef]
- Lugato, E.; Lavallee, J.M.; Haddix, M.L.; Panagos, P.; Cotrufo, M.F. Different climate sensitivity of particulate and mineral-associated soil organic matter. Nat. Geosci. 2021, 14, 295–300. [Google Scholar] [CrossRef]
- Zhang, W.; Song, Y.; Ma, S.; Lu, J.; Zhu, J.; Wang, J.; Li, X. Rice-crayfish farming system promote subsoil microbial residual carbon accumulation and stabilization by mediating microbial metabolism process. Sci. Total Environ. 2024, 946, 174188. [Google Scholar] [CrossRef]
- Pan, G.; Li, L.; Wu, L.; Zhang, X. Storage and sequestration potential of topsoil organic carbon in China’s paddy soils. Glob. Chang. Biol. 2004, 10, 79–92. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, T.; van Groenigen, K.J.; Yang, Y.; Wang, P.; Cheng, K.; Zhu, Z.; Wang, J.; Li, Y.; Guggenberger, G.; et al. Rice paddy soils are a quantitatively important carbon store according to a global synthesis. Commun. Earth Environ. 2021, 2, 154. [Google Scholar] [CrossRef]
- Chaudhary, S.; Dheri, G.S.; Brar, B.S. Long-term effects of NPK fertilizers and organic manures on carbon stabilization and management index under rice-wheat cropping system. Soil Tillage Res. 2017, 166, 59–66. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Shao, L.; Sun, H.; Niu, J.; Liu, X. Effects of straw and manure management on soil and crop performance in North China Plain. Catena 2020, 187, 104359. [Google Scholar] [CrossRef]
- Lessmann, M.; Ros, G.H.; Young, M.D.; de Vries, W. Global variation in soil carbon sequestration potential through improved cropland management. Glob. Chang. Biol. 2022, 28, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, S.; Zhou, G.; Cao, W. The potential of green manure to increase soil carbon sequestration and reduce the yield-scaled carbon footprint of rice production in southern China. J. Integr. Agric. 2022, 22, 2233–2247. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A.; Elser, J. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef]
- Adingo, S.; Yu, J.R.; Xuelu, L.; Li, X.; Jing, S.; Xiaong, Z. Variation of soil microbial carbon use efficiency (CUE) and its Influence mechanism in the context of global environmental change: A review. PeerJ 2021, 9, e12131. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Geyer, K.M.; Kyker-Snowman, E.; Grandy, A.S.; Frey, S.D. Microbial carbon use efficiency: Accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochemistry 2016, 127, 173–188. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, J.; Li, X.; Chen, Y.; Schimel, J.P.; Zhu, B. Effect of field warming on soil microbial carbon use efficiency—A meta-analysis. Soil Biol. Biochem. 2024, 197, 109531. [Google Scholar] [CrossRef]
- Geyer, K.M.; Dijkstra, P.; Sinsabaugh, R.; Frey, S.D. Clarifying the interpretation of carbon use efficiency in soil through methods comparison. Soil Biol. Biochem. 2019, 128, 79–88. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dungait, J.A.J.; Hou, R.; Deng, Y.; Hartley, I.P.; Yang, Y.; Kuzyakov, Y.; Zhang, F.; Cotrufo, M.F.; Zhou, J. Microbially mediated mechanisms underlie soil carbon accrual by conservation agriculture under decade-long warming. Nat. Commun. 2024, 15, 377. [Google Scholar] [CrossRef]
- Li, J.; Pei, J.; Dijkstra, F.A.; Nie, M.; Pendall, E. Microbial carbon use efficiency, biomass residence time and temperature sensitivity across ecosystems and soil depths. Soil Biol. Biochem. 2021, 154, 108117. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, J.; Shi, L.; Feng, W.; Dippold, M.A.; Zang, H.; Kurganova, I.; de Gerenyu, V.L.; Kalinina, O.; Giani, L.; et al. Microbial growth rates, carbon use efficiency and enzyme activities during post-agricultural soil restoration. Catena 2022, 214, 106226. [Google Scholar] [CrossRef]
- Dang, C.; Morrissey, E.M. The size and diversity of microbes determine carbon use efficiency in soil. Environ. Microbiol. 2024, 26, e16633. [Google Scholar] [CrossRef]
- Feng, X.; Qin, S.; Zhang, D.; Chen, P.; Hu, J.; Wang, G.; Liu, Y.; Wei, B.; Li, Q.; Yang, Y.; et al. Nitrogen input enhances microbial carbon use efficiency by altering plant-microbe-mineral interactions. Glob. Chang. Biol. 2022, 28, 4845–4860. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xia, Y.; Rui, Y.; Ning, Z.; Hu, Y.; Tang, H.; He, H.; Li, H.; Kuzyakov, Y.; Ge, T.; et al. Microbial carbon use efficiency, biomass turnover, and necromass accumulation in paddy soil depending on fertilization. Agric. Ecosyst. Environ. 2020, 292, 106816. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, Y.; Wu, L.; Tian, Y.; Wang, Q.; Wang, B.; Xu, M.; Zhang, W. Long-term manuring increases microbial carbon use efficiency and mitigates priming effect via alleviated soil acidification and resource limitation. Biol. Fertil. Soils 2021, 57, 925–934. [Google Scholar] [CrossRef]
- Spohn, M.; Pötsch, E.M.; Eichorst, S.A.; Woebken, D.; Wanek, W.; Richter, A. Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol. Biochem. 2016, 97, 168–175. [Google Scholar] [CrossRef]
- Zhran, M.; Ge, T.; Tong, Y.; Deng, Y.; Wei, X.; Lynn, T.M.; Zhu, Z.; Wu, J.; Gunina, A. Assessment of depth-dependent microbial carbon-use efficiency in long-term fertilized paddy soil using an 18O–H2O approach. Land Degrad. Dev. 2020, 32, 199–207. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Zhang, Z.; Li, M.; Wang, D.; Zhang, P.; Li, N.; Yin, H. Microbial metabolic traits drive the differential contribution of microbial necromass to soil organic carbon between the rhizosphere of absorptive roots and transport roots. Soil Biol. Biochem. 2024, 197, 109529. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2967. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions-a review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef]
- Wu, G.; Su, Y.; Wang, J.; Lin, S.; Huang, Z.; Huang, G. Elevational patterns of microbial carbon use efficiency in a subtropical mountain forest. Biol. Fertil. Soils 2023, 60, 5–15. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Cha, X.; Zhou, T.; Pang, X.; Zhao, F.; Han, X.; Yang, G.; Wei, G.; Ren, C. The biogeography of soil microbiome potential growth rates. Nat. Commun. 2024, 15, 9472. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Liu, C.; He, C.; Chang, S.X.; Chen, X.; An, S.; Wang, D.; Yan, J.; Zhang, Y.; Li, P. Fertilization and tillage influence on soil organic carbon fractions: A global meta-analysis. Catena 2024, 246, 108404. [Google Scholar] [CrossRef]
- Bian, Q.; Zhao, L.; Cheng, K.; Jiang, Y.; Li, D.; Xie, Z.; Sun, B.; Wang, X. Divergent accumulation of microbe- and plant-derived carbon in different soil organic matter fractions in paddy soils under long-term organic amendments. Agric. Ecosyst. Environ. 2024, 366, 108934. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Zhu, X.; Jackson, R.D.; DeLucia, E.H.; Tiedje, J.M.; Liang, C. The soil microbial carbon pump: From conceptual insights to empirical assessments. Glob. Chang. Biol. 2020, 26, 6032–6039. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kastner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Whalen, E.D.; Grandy, A.S.; Sokol, N.W.; Keiluweit, M.; Ernakovich, J.; Smith, R.G.; Frey, S.D. Clarifying the evidence for microbial- and plant-derived soil organic matter, and the path toward a more quantitative understanding. Glob. Chang. Biol. 2022, 28, 7167–7185. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Kuzyakov, Y.; Liu, D.; Luo, J.; Lindsey, S.; Wang, W.; Fan, J.; Ding, W. Manure over crop residues increases soil organic matter but decreases microbial necromass relative contribution in upland Ultisols: Results of a 27-year field experiment. Soil Biol. Biochem. 2019, 134, 15–24. [Google Scholar] [CrossRef]
- Zhou, R.R.; Liu, Y.; Dungait, J.A.J.; Kumar, A.; Wang, J.S.; Tiemann, L.K.; Zhang, F.S.; Kuzyakov, Y.; Tian, J. Microbial necromass in cropland soils: A global meta-analysis of management effects. Glob. Chang. Biol. 2023, 29, 1998–2014. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, 2nd ed.; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Yu, Q.; Hu, X.; Ma, J.; Ye, J.; Sun, W.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy fields. Soil Tillage Res. 2020, 196, 104483. [Google Scholar] [CrossRef]
- Lu, R. Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass by fumigation extraction C-an antomated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Helfrich, M.; Ludwig, B.; Buurman, P.; Flessa, H. Effect of land use on the composition of soil organic matter in density and aggregate fractions as revealed by solid-state 13C NMR spectroscopy. Geoderma 2006, 136, 331–341. [Google Scholar] [CrossRef]
- Simpson, M.J.; Simpson, A.J. The Chemical Ecology of Soil Organic Matter Molecular Constituents. J. Chem. Ecol. 2012, 38, 768–784. [Google Scholar] [CrossRef]
- Yeasmin, S.; Singh, B.; Smernik, R.J.; Johnston, C.T. Effect of land use on organic matter composition in density fractions of contrasting soils: A comparative study using 13C NMR and DRIFT spectroscopy. Sci. Total Environ. 2020, 726, 138395. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, N.; Wang, G.; Xu, J.; Wu, J.; Brookes, P.C. Effects of different soil weights, storage times and extraction methods on soil phospholipid fatty acid analyses. Geoderma 2009, 150, 171–178. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Joergensen, R.G. Phospholipid fatty acids in soil—Drawbacks and future prospects. Biol. Fertil. Soils 2021, 58, 1–6. [Google Scholar] [CrossRef]
- Spohn, M.; Klaus, K.; Wanek, W.; Richter, A. Microbial carbon use efficiency and biomass turnover times depending on soil depth-Implications for carbon cycling. Soil Biol. Biochem. 2016, 96, 74–81. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Sietiö, O.-M.; Meyer, N.; Poeplau, C.; Adamczyk, S.; Biasi, C.; Kalu, S.; Räsänen, M.; Ambus, P.; Fritze, H.; et al. Microbial carbon use efficiency along an altitudinal gradient. Soil Biol. Biochem. 2022, 173, 108799. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Han, B.; Yao, Y.; Wang, Y.; Su, X.; Ma, L.; Chen, X.; Li, Z. Microbial traits dictate soil necromass accumulation coefficient: A global synthesis. Glob. Ecol. Biogeogr. 2023, 33, 151–161. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, J.; Chen, L.; Chu, H.; He, J.-S.; Zhang, Y.; Feng, X. Aridity and NPP constrain contribution of microbial necromass to soil organic carbon in the Qinghai-Tibet alpine grasslands. Soil Biol. Biochem. 2021, 156, 108213. [Google Scholar] [CrossRef]
- Schroeder, J.; Peplau, T.; Pennekamp, F.; Gregorich, E.; Tebbe, C.C.; Poeplau, C. Deforestation for agriculture increases microbial carbon use efficiency in subarctic soils. Biol. Fertil. Soils 2022, 60, 17–34. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Tang, L.; Ren, F.; Wang, X.; Zhu, P.; Sun, N.; Xu, M. Manure application decreases soil organic carbon priming by increasing mineral protection and nitrogen availability. Geoderma 2023, 439, 116676. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Mayes, M.A.; Allison, S.D.; Frey, S.D.; Shi, Z.; Hu, X.M.; Luo, Y.; Melillo, J.M. Reduced carbon use efficiency and increased microbial turnover with soil warming. Glob. Chang. Biol. 2018, 25, 900–910. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Mao, X.; Sun, T.; Zhu, L.; Wanek, W.; Cheng, Q.; Wang, X.; Zhou, J.; Liu, X.; Ma, Q.; Wu, L.; et al. Microbial adaption to stoichiometric imbalances regulated the size of soil mineral-associated organic carbon pool under continuous organic amendments. Geoderma 2024, 445, 116883. [Google Scholar] [CrossRef]
- Rocci, K.S.; Lavallee, J.M.; Stewart, C.E.; Cotrufo, M.F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 793, 148569. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar] [CrossRef]
- Luo, R.; Kuzyakov, Y.; Zhu, B.; Qiang, W.; Zhang, Y.; Pang, X. Phosphorus addition decreases plant lignin but increases microbial necromass contribution to soil organic carbon in a subalpine forest. Glob. Chang. Biol. 2022, 28, 4194–4210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Mou, R.; Wang, L.; Liu, J.; Tang, Y.; Chen, J.; Heděnec, P.; Xu, Z.; Tan, B.; Cui, X.; et al. Fertilization effects on soil organic matter chemistry. Soil Tillage Res. 2025, 246, 106364. [Google Scholar] [CrossRef]
- Liu, M.; Lin, H.; Li, J. Are there links between nutrient inputs and the response of microbial carbon use efficiency or soil organic carbon? A meta-analysis. Soil Biol. Biochem. 2025, 201, 109656. [Google Scholar] [CrossRef]
- Wang, C.; Qu, L.; Yang, L.; Liu, D.; Morrissey, E.; Miao, R.; Liu, Z.; Wang, Q.; Fang, Y.; Bai, E. Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon. Glob. Chang. Biol. 2021, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, C.; Zhou, S.; Kuzyakov, Y. Nitrogen addition to soil affects microbial carbon use efficiency: Meta-analysis of similarities and differences in 13C and 18O approaches. Glob. Chang. Biol. 2022, 28, 4977–4988. [Google Scholar] [CrossRef]
- Duan, X.; Rui, Y.; Xia, Y.; Hu, Y.; Ma, C.; Qiao, H.; Zeng, G.; Su, Y.; Wu, J.; Chen, X. Higher microbial C use efficiency in paddy than in adjacent upland soils: Evidence from continental scale. Soil Tillage Res. 2024, 235, 105891. [Google Scholar] [CrossRef]
- Cui, Y.; Hu, J.; Peng, S.; Delgado-Baquerizo, M.; Moorhead, D.L.; Sinsabaugh, R.L.; Xu, X.; Geyer, K.M.; Fang, L.; Smith, P.; et al. Limiting Resources Define the Global Pattern of Soil Microbial Carbon Use Efficiency. Adv. Sci. 2024, 11, 2308176. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, E.; Jia, J.; Wang, Y.; Kang, E.; Yi, W.; Jiang, Z.; Dai, G.; Feng, X. Does microbial carbon use efficiency differ between particulate and mineral-associated organic matter? Funct. Ecol. 2024, 38, 1510–1522. [Google Scholar] [CrossRef]
- Wang, M.; Dungait, J.A.J.; Wei, X.; Ge, T.; Hou, R.; Ouyang, Z.; Zhang, F.; Tian, J. Long-term warming increased microbial carbon use efficiency and turnover rate under conservation tillage system. Soil Biol. Biochem. 2022, 172, 108770. [Google Scholar] [CrossRef]
- Martin Vivanco, A.; Sietiö, O.-M.; Meyer, N.; Mganga, K.Z.; Kalu, S.; Adamczyk, S.; Celis Huayllasco, S.; Alegre Orihuela, J.; Karhu, K. Microbial carbon use efficiency and soil organic carbon stocks across an elevational gradient in the Peruvian Andes. Appl. Soil Ecol. 2024, 195, 105228. [Google Scholar] [CrossRef]
- Manzoni, S.; Čapek, P.; Porada, P.; Thurner, M.; Winterdahl, M.; Beer, C.; Brüchert, V.; Frouz, J.; Herrmann, A.M.; Lindahl, B.D.; et al. Reviews and syntheses: Carbon use efficiency from organisms to ecosystems—Definitions, theories, and empirical evidence. Biogeosciences 2018, 15, 5929–5949. [Google Scholar] [CrossRef]
- Soares, M.; Rousk, J. Microbial growth and carbon use efficiency in soil: Links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol. Biochem. 2019, 131, 195–205. [Google Scholar] [CrossRef]
- Pei, J.; Li, J.; Mia, S.; Singh, B.; Wu, J.; Dijkstra, F.A. Biochar aging increased microbial carbon use efficiency but decreased biomass turnover time. Geoderma 2021, 382, 114710. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 2020, 11, 3684. [Google Scholar] [CrossRef]
- Duan, P.; Fu, R.; Nottingham, A.T.; Domeignoz-Horta, L.A.; Yang, X.; Du, H.; Wang, K.; Li, D. Tree species diversity increases soil microbial carbon use efficiency in a subtropical forest. Glob. Chang. Biol. 2023, 29, 7131–7144. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Shen, Q.; Ling, N.; Nan, Z. Microbial carbon use efficiency in different ecosystems: A meta-analysis based on a biogeochemical equilibrium model. Glob. Chang. Biol. 2023, 29, 4758–4774. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Bai, E.; Wasner, D.; Hagedorn, F. Global prediction of soil microbial growth rates and carbon use efficiency based on the metabolic theory of ecology. Soil Biol. Biochem. 2024, 190, 109315. [Google Scholar] [CrossRef]
- Schnecker, J.; Baldaszti, L.; Gündler, P.; Pleitner, M.; Sandén, T.; Simon, E.; Spiegel, F.; Spiegel, H.; Urbina Malo, C.; Zechmeister-Boltenstern, S.; et al. Seasonal dynamics of soil microbial growth, respiration, biomass, and carbon use efficiency in temperate soils. Geoderma 2023, 440, 116693. [Google Scholar] [CrossRef]
- Schneider, T.; Keiblinger, K.M.; Schmid, E.; Sterflinger-Gleixner, K.; Ellersdorfer, G.; Roschitzki, B.; Richter, A.; Eberl, L.; Zechmeister-Boltenstern, S.; Riedel, K. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J. 2012, 6, 1749–1762. [Google Scholar] [CrossRef]
- Bhople, P.; Keiblinger, K.; Djukic, I.; Liu, D.; Zehetner, F.; Zechmeister-Boltenstern, S.; Georg Joergensen, R.; Murugan, R. Microbial necromass formation, enzyme activities and community structure in two alpine elevation gradients with different bedrock types. Geoderma 2021, 386, 114922. [Google Scholar] [CrossRef]
- Khan, K.S.; Mack, R.; Castillo, X.; Kaiser, M.; Joergensen, R.G. Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 2016, 271, 115–123. [Google Scholar] [CrossRef]
- Zheng, T.; Miltner, A.; Liang, C.; Nowak, K.M.; Kästner, M. Turnover of bacterial biomass to soil organic matter via fungal biomass and its metabolic implications. Soil Biol. Biochem. 2023, 180, 108995. [Google Scholar] [CrossRef]
- Vidal, A.; Klöffel, T.; Guigue, J.; Angst, G.; Steffens, M.; Hoeschen, C.; Mueller, C.W. Visualizing the transfer of organic matter from decaying plant residues to soil mineral surfaces controlled by microorganisms. Soil Biol. Biochem. 2021, 160, 108347. [Google Scholar] [CrossRef]
| Organic Materials | Application Rate (kg ha–1) | OC Content (g kg–1) | N Content (g kg–1) | C/N | C Input (kg ha–1) | N Input (kg ha–1) |
|---|---|---|---|---|---|---|
| Rice straw | 6300 | 488 | 7.8 | 62.6 | 3074 | 49.1 |
| Green manure | 7900 | 392 | 28.2 | 13.9 | 3097 | 222.8 |
| Pig manure | 7000 | 439 | 17.5 | 25.1 | 3073 | 122.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Chen, Z.; Ma, J.; Ma, J.; Zou, P.; Sun, W.; Wang, F.; Yu, Q.; Wang, Q. The Effect of Long-Term Organic Amendments on Soil Organic Carbon Accumulation via Regulating Microbial Traits in a Paddy Soil. Agriculture 2025, 15, 2308. https://doi.org/10.3390/agriculture15212308
Ye J, Chen Z, Ma J, Ma J, Zou P, Sun W, Wang F, Yu Q, Wang Q. The Effect of Long-Term Organic Amendments on Soil Organic Carbon Accumulation via Regulating Microbial Traits in a Paddy Soil. Agriculture. 2025; 15(21):2308. https://doi.org/10.3390/agriculture15212308
Chicago/Turabian StyleYe, Jing, Zhaoming Chen, Jinchuan Ma, Junwei Ma, Ping Zou, Wanchun Sun, Feng Wang, Qiaogang Yu, and Qiang Wang. 2025. "The Effect of Long-Term Organic Amendments on Soil Organic Carbon Accumulation via Regulating Microbial Traits in a Paddy Soil" Agriculture 15, no. 21: 2308. https://doi.org/10.3390/agriculture15212308
APA StyleYe, J., Chen, Z., Ma, J., Ma, J., Zou, P., Sun, W., Wang, F., Yu, Q., & Wang, Q. (2025). The Effect of Long-Term Organic Amendments on Soil Organic Carbon Accumulation via Regulating Microbial Traits in a Paddy Soil. Agriculture, 15(21), 2308. https://doi.org/10.3390/agriculture15212308





