Combined Biological and Chemical Control of Sclerotinia sclerotiorum on Oilseed Rape in the Era of Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Experiment
2.2. Field Experiment
2.3. Statistical Analysis
3. Results
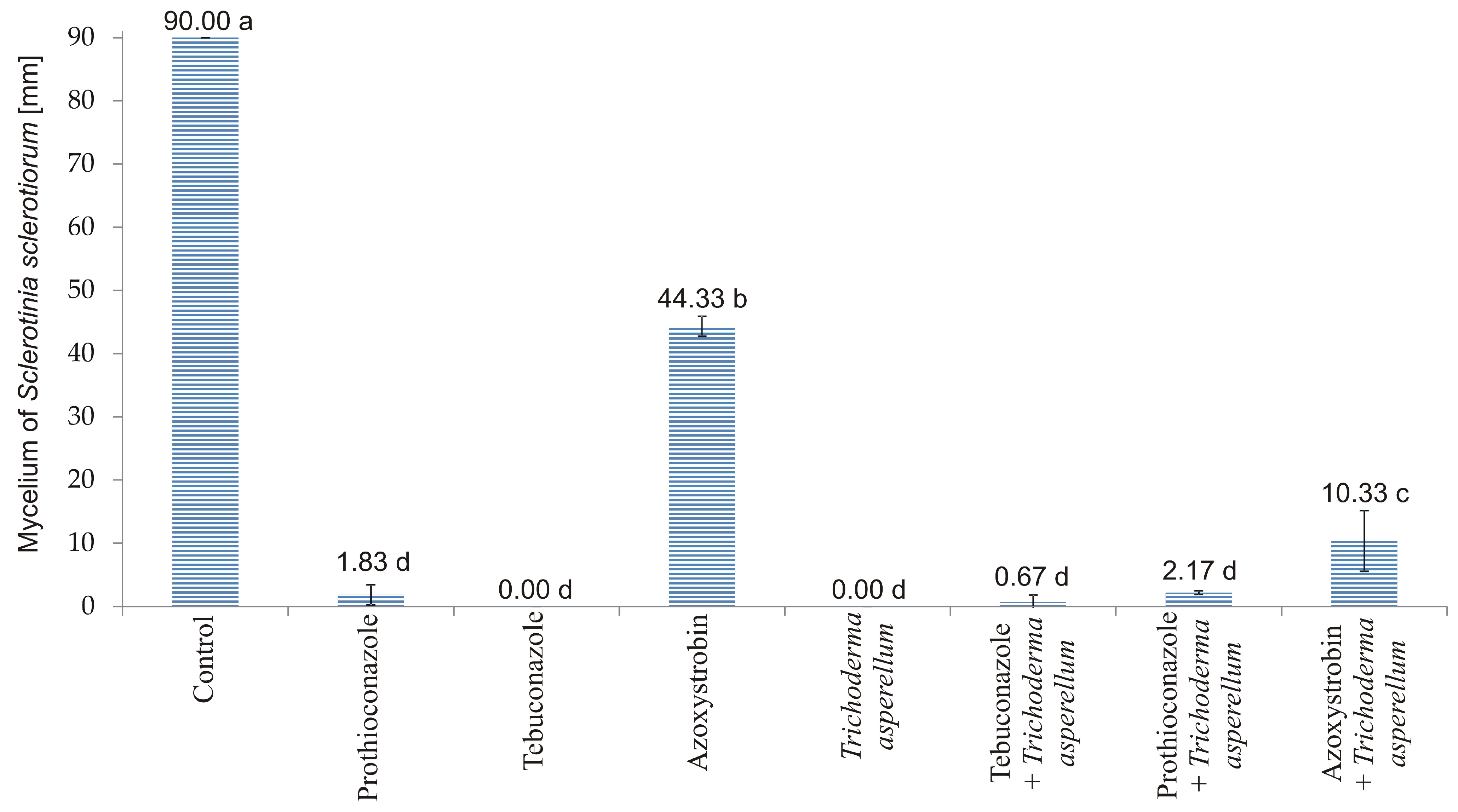
3.1. In Vitro Experiment
3.2. Field Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Month/Year | Weather Parameters | Decades | Average/Sum | ||
|---|---|---|---|---|---|
| I | II | III | |||
| 08-2021 | Average temperature [°C] | 17.94 | 19.31 | 15.63 | 17.62 |
| Average air humidity [%] | 75.91 | 70.79 | 85.26 | 77.32 | |
| Total rainfall [mm] | 23.10 | 6.30 | 1.00 | 30.40 | |
| 09-2021 | Average temperature [°C] | 16.52 | 15.88 | 14.11 | 15.50 |
| Average air humidity [%] | 70.60 | 81.67 | 80.60 | 77.62 | |
| Total rainfall [mm] | 0.10 | 3.00 | 6.30 | 9.40 | |
| 10-2021 | Average temperature [°C] | 12.44 | 9.27 | 9.63 | 10.45 |
| Average air humidity [%] | 77.02 | 86.65 | 75.11 | 79.59 | |
| Total rainfall [mm] | 4.90 | 12.40 | 7.10 | 24.40 | |
| 11-2021 | Average temperature [°C] | 7.98 | 5.97 | 3.10 | 5.68 |
| Average air humidity [%] | 88.31 | 94.47 | 93.24 | 92.01 | |
| Total rainfall [mm] | 8.70 | 0.80 | 8.70 | 18.20 | |
| 12-2021 | Average temperature [°C] | −0.15 | 2.08 | −2.20 | −0.09 |
| Average air humidity [%] | 89.33 | 95.80 | 93.33 | 92.82 | |
| Total rainfall [mm] | 4.80 | 1.90 | 12.00 | 18.70 | |
| 01-2022 | Average temperature [°C] | 3.06 | 0.33 | 1.70 | 1.70 |
| Average air humidity [%] | 92.05 | 91.68 | 90.54 | 91.42 | |
| Total rainfall [mm] | 25.70 | 3.20 | 10.70 | 39.60 | |
| 02-2022 | Average temperature [°C] | 4.29 | 4.69 | 3.98 | 4.32 |
| Average air humidity [%] | 90.30 | 78.27 | 78.58 | 82.38 | |
| Total rainfall [mm] | 19.40 | 13.10 | 6.50 | 39.00 | |
| 03-2022 | Average temperature [°C] | 0.82 | 4.71 | 7.07 | 4.20 |
| Average air humidity [%] | 75.86 | 54.68 | 58.86 | 63.13 | |
| Total rainfall [mm] | 0.00 | 0.00 | 0.00 | 0.00 | |
| 04-2022 | Average temperature [°C] | 5.49 | 7.95 | 10.37 | 7.94 |
| Average air humidity [%] | 67.39 | 69.20 | 69.92 | 68.84 | |
| Total rainfall [mm] | 9.80 | 10.80 | 2.50 | 23.10 | |
| 05-2022 | Average temperature [°C] | 14.02 | 16.46 | 14.04 | 14.84 |
| Average air humidity [%] | 62.10 | 54.98 | 74.61 | 63.90 | |
| Total rainfall [mm] | 3.50 | 18.80 | 28.90 | 51.20 | |
| 06-2022 | Average temperature [°C] | 17.93 | 19.09 | 22.40 | 19.81 |
| Average air humidity [%] | 72.98 | 68.01 | 63.43 | 68.14 | |
| Total rainfall [mm] | 30.70 | 16.20 | 13.70 | 60.60 | |
| 07-2022 | Average temperature [°C] | 19.11 | 19.37 | 20.98 | 19.82 |
| Average air humidity [%] | 72.89 | 64.19 | 64.85 | 67.31 | |
| Total rainfall [mm] | 6.50 | 13.90 | 9.40 | 29.80 | |
| 08-2022 | Average temperature [°C] | 21.18 | 23.67 | 20.39 | 21.75 |
| Average air humidity [%] | 65.40 | 69.80 | 80.64 | 71.95 | |
| Total rainfall [mm] | 18.10 | 12.60 | 20.70 | 51.40 | |
| 09-2022 | Average temperature [°C] | 16.36 | 12.86 | 10.30 | 13.18 |
| Average air humidity [%] | 66.35 | 85.84 | 85.68 | 85.68 | |
| Total rainfall [mm] | 36.10 | 5.40 | 8.20 | 49.70 | |
| 10-2022 | Average temperature [°C] | 11.78 | 11.49 | 12.59 | 11.95 |
| Average air humidity [%] | 83.74 | 85.06 | 91.88 | 86.89 | |
| Total rainfall [mm] | 4.70 | 17.10 | 13.90 | 35.70 | |
| 11-2022 | Average temperature [°C] | 9.18 | 3.50 | 1.09 | 4.86 |
| Average air humidity [%] | 89.84 | 91.40 | 94.58 | 91.94 | |
| Total rainfall [mm] | 0.00 | 0.00 | 0.00 | 0.00 | |
| 12-2022 | Average temperature [°C] | 0.92 | −3.12 | 6.17 | 1.32 |
| Average air humidity [%] | 94.26 | 90.07 | 90.05 | 91.46 | |
| Total rainfall [mm] | 3.60 | 2.40 | 21.40 | 27.40 | |
| 01-2023 | Average temperature [°C] | 6.51 | 3.67 | 0.55 | 3.58 |
| Average air humidity [%] | 89.74 | 89.03 | 94.66 | 91.14 | |
| Total rainfall [mm] | 11.00 | 12.10 | 12.50 | 35.60 | |
| 02-2023 | Average temperature [°C] | −0.28 | 4.15 | 2.61 | 2.16 |
| Average air humidity [%] | 86.92 | 88.41 | 84.72 | 86.68 | |
| Total rainfall [mm] | 11.90 | 10.40 | 4.00 | 26.30 | |
| 03-2023 | Average temperature [°C] | 0.74 | 5.88 | 8.08 | 4.90 |
| Average air humidity [%] | 91.16 | 77.83 | 79.60 | 82.86 | |
| Total rainfall [mm] | 8.40 | 0.50 | 0.00 | 8.90 | |
| 04-2023 | Average temperature [°C] | 4.85 | 9.50 | 10.42 | 8.21 |
| Average air humidity [%] | 81.35 | 85.55 | 72.83 | 79.83 | |
| Total rainfall [mm] | 0.30 | 0.00 | 0.00 | 0.30 | |
| 05-2023 | Average temperature [°C] | 10.20 | 13.71 | 16.13 | 13.35 |
| Average air humidity [%] | 73.45 | 75.35 | 63.11 | 69.23 | |
| Total rainfall [mm] | 12.00 | 16.30 | 0.10 | 28.40 | |
| 06-2023 | Average temperature [°C] | 18.40 | 18.71 | 20.08 | 19.06 |
| Average air humidity [%] | 53.47 | 66.15 | 74.79 | 64.80 | |
| Total rainfall [mm] | 0.20 | 6.60 | 36.30 | 43.10 | |
| 07-2023 | Average temperature [°C] | 20.66 | 21.65 | 18.83 | 20.38 |
| Average air humidity [%] | 61.06 | 62.34 | 77.30 | 66.90 | |
| Total rainfall [mm] | 1.70 | 10.20 | 26.90 | 38.80 | |
References
- Clarkson, J.P.; Phelps, K.; Whipps, J.M.; Young, C.S.; Smith, J.A.; Watling, M. Forecasting sclerotinia disease on lettuce: A predictive model for carpogenic germination of Sclerotinia sclerotiorum sclerotia. Phytopathology 2007, 97, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, M.; Wójtowicz, A. Significance of Direct and Indirect Impacts of Temperature Increase Driven by Climate Change on Threat to Oilseed Rape Posed by Sclerotinia sclerotiorum. Pathogens 2023, 12, 1279. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.P.; Fawcett, L.; Anthony, S.G.; Young, C. A model for Sclerotinia sclerotiorum Infection and disease development in lettuce, based on the effects of temperature, relative humidity and ascospore density. PLoS ONE 2014, 9, e94049. [Google Scholar] [CrossRef] [PubMed]
- Peltier, A.J.; Bradley, C.A.; Chilvers, M.I.; Malvick, D.K.; Mueller, D.S.; Wise, K.A.; Esker, P.D. Biology, Yield Loss and Control of Sclerotinia Stem Rot of Soybean. J. Integr. Pest Manag. 2012, 3, B1–B7. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Brand, S. Overview of the joint research project “SkleroPro”-Evaluation of environmental factors affecting Sclerotinia sclerotiorum. In Proceedings of the Working Group Integrated Control in Oilseed Crops; Proceedings of the Online Meeting, Braunschweig, Germany, 17–18 May 2022; Volume 157, pp. 36–39. [Google Scholar]
- Koch, S.; Dunker, S.; Kleinhenz, B.; Rohring, M.; Tiedemann, A. A crop loss-related forecasting model for Sclerotinia stem rot in winter oilseed rape. Phytopathology 2007, 97, 1186–1194. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D. Two convenient methods to evaluate soybean for resistance to Sclerotinia sclerotiorum. Plant Dis. 2005, 89, 1268–1272. [Google Scholar] [CrossRef]
- Clarkson, J.P.; Phelps, K.; Whipps, J.M.; Young, C.S.; Smith, J.A.; Watling, M. Forecasting Sclerotinia Disease on Lettuce: Toward Developing a Prediction Model for Carpogenic Germination of Sclerotia. Phytopathology 2004, 94, 268–279. [Google Scholar] [CrossRef]
- Abdullah, M.T.; Ali, N.Y.; Suleman, P. Biological Control of Sclerotinia sclerotiorum (Lib.) de Bary with Trichoderma harzianum and Bacillus amyloliquefaciens. Crop Prot. 2008, 27, 1354–1359. [Google Scholar] [CrossRef]
- Troian, R.F.; Steindorff, A.S.; Ramada, M.H.S.; Arruda, W.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: Evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotechnol. Lett. 2014, 36, 2095–2101. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-Plant-Pathogen Interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol Mechanisms of Trichoderma Strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [PubMed]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- El-Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of Novel Trichoderma asperellum Isolates to Select Effective Biocontrol Agents against Tomato Fusarium Wilt. Plant Pathol. J. 2015, 31, 50–60. [Google Scholar] [CrossRef]
- Hou, Y.P.; Mao, X.W.; Lin, S.P.; Song, X.S.; Duan, Y.B.; Wang, J.X.; Zhou, M.G. Activity of a novel succinate dehydrogenase inhibitor fungicide pyraziflumid against Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2018, 145, 22–28. [Google Scholar] [CrossRef]
- Braga, A.F.; Santos, L.d.C.; Mendes, S.P.d.S.C.; Pires, F.A.; Geraldine, A.M.; Ferreira, J.W.N. Interaction between Trichoderma aspeellum and Bacillus spp. in the biological control of disease in the soya bean. Rev. Ciênc. Agron. 2025, 56, e202294124. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef]
- Akrami, M.; Sabzi, M.; Baghbani Mehmandar, F.; Khodadadi, E. Effect of seed treatment with Trichoderma harzianum and Trichoderma asperellum species for controlling Fusarium rot of common bean. Ann. Biol. Res. 2012, 3, 2187–2189. [Google Scholar]
- Yoshioka, Y.; Ichikawa, H.; Naznin, H.A.; Kogure, A.; Hyakumachi, M. Systemic resistance induced in Arabidopsis thaliana by Trichoderma asperellum SKT-1, a microbial pesticide of seedborne diseases of rice. Pest. Manag. Sci. 2012, 68, 60–66. [Google Scholar] [CrossRef]
- Gupta, M.; Dohroo, N.P. Shelf-life study of formulations of fungal and bacterial antagonists as bioinoculants. Agric. Sci. Dig. 2014, 34, 81–284. [Google Scholar] [CrossRef]
- Melo, I.S.; Cassiolato, A.M.R.; Faull, J.L. Development of Mutants of Coniothyrium minitans with Improved Efficiency for Control of Sclerotinia sclerotiorum. JPPR 2011, 51, 180–183. [Google Scholar] [CrossRef]
- Jones, E.E.; Stewart, A. Coniothyrium minitans survival in soil and ability to infect sclerotia of Sclerotinia sclerotiorum. N. Z. Plant Prot. 2011, 64, 168–174. [Google Scholar] [CrossRef]
- PP1/181; Conduct and Reporting of Efficacy Evaluation Trials, Including Good Experimental Practice. European and Mediterranean Plant Protection Organization: Paris, France, 2021. Available online: https://pp1.eppo.int/standards/PP1-181-5 (accessed on 20 July 2025).
- EPPO Standards. PP 1/78 Root, Stem, Foliar and Pod Diseases of Oilseed Rape. EPPO Bull. 2021, 52, 30–38. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Zamani-Noor, N. Baseline Sensitivity and Control Efficacy of Various Group of Fungicides against Sclerotinia sclerotiorum in Oilseed Rape Cultivation. Agronomy 2021, 11, 1758. [Google Scholar] [CrossRef]
- Sumida, C.H.; Daniel, J.F.S.; Araujod, A.P.C.S.; Peitl, D.C.; Abreu, L.M.; Dekker, R.F.H.; Canteri, M.G. Trichoderma asperelloides antagonism to nine Sclerotinia sclerotiorum strains and biological control of white mold disease in soybean plants. Biocontrol Sci. Technol. 2018, 28, 142–156. [Google Scholar] [CrossRef]
- Elias, L.M.; Domingues, M.V.P.F.; Moura, K.E.; Harakava, R.; Patrício, F.R.A. Selection of Trichoderma isolates for biological control of Sclerotinia minor and S. sclerotiorum in lettuce. Summa phytopathol. 2016, 42, 216–221. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Abdel-Hafez, S.I.; Abdel-Rahim, I.R. Isolation of Trichoderma and evaluation of their antagonistic potential against Alternaria porri. J. Phytopathol. 2014, 162, 567–574. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.; Sharma, S. Breeding for resistance to Sclerotinia sclerotiorum in oilseed rape: Challenges and opportunities. Field Crops Res. 2017, 211, 1–12. [Google Scholar]
- Jajor, E.; Korbas, M.; Horoszkiewicz-Janka, J.; Wójtowicz, M. Wpływ ochrony fungicydowej i warunków meteorologicznych na porażenie odmian rzepaku przez Sclerotinia sclerotiorum. Prog. Plant Prot. 2010, 50, 1334–1339. [Google Scholar]
- Wu, B.M.; Subbarao, K.V. Effects of soil temperature, moisture, and burial depths on carpogenic germination of Sclerotinia sclerotiorum and S. minor. Phytopathology 2008, 98, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Dillard, H.R.; Ludwig, J.W.; Hunter, J.E. Conditioning sclerotia of Sclerotinia sclerotiorum for carpogenic germination. Plant Dis. 1995, 79, 411–415. [Google Scholar] [CrossRef]
- Huang, H.; Kozub, G. Temperature requirements for carpogenic germination of sclerotia of Sclerotinia sclerotiorum isolates of different geographic origin. Bot. Bull. Acad. Sin. 1991, 32, 279–286. [Google Scholar]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Hu, X.; Roberts, D.P.; Xie, L.; Yu, C.; Li, Y.; Qin, L.; Hu, L.; Zhang, Y.; Liao, X. Use of formulated Trichoderma sp. Tri-1 in combination with reduced rates of chemical pesticide for control of Sclerotinia sclerotiorum on oilseed rape. Crop Prot. 2016, 79, 124–127. [Google Scholar] [CrossRef]
- Ma, H.X.; Chen, Y.; Wang, J.X.; Yu, W.Y.; Tang, Z.H.; Chen, C.; Zhou, M.G. Activity of carbendazim, dimethachlon, iprodione, procymidone and boscalid against Sclerotinia stem rot in Jiangsu Province of China. Phytoparasitica 2009, 37, 421–429. [Google Scholar] [CrossRef]
- Gossen, B.D.; Rimmer, S.R.; Holley, J.D. First report of resistance to benomyl fungicide in Sclerotinia sclerotiorum. Plant Dis. 2001, 85, 1206. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Denton-Giles, M. The control of sclerotinia stem rot on oilseed rape (Brassica napus): Current practices and future opportunities. Plant Pathol. 2016, 65, 859–877. [Google Scholar] [CrossRef]
- Zheng, X.; Koopmann, B.; Ulber, B.; Von Tiedemann, A. A global survey on diseases and pests in oilseed rape—Current challenges and innovative strategies of control. Front. Agron. 2020, 2, 590908. [Google Scholar] [CrossRef]
- Kowalska, J.; Remlein-Starosta, D. Efficacy of microbiological treatments and trap crop against pests of winter oilseed rape. In Proceedings of the 4th ISOFAR Scientific Conference: ‘Building Organic Bridges’, at the Organic World Congress 2014, Istanbul, Turkey, 13–15 October 2014; Rahmann, G., Aksoy, U., Eds.; 2014; pp. 913–916. Available online: https://orgprints.org/id/eprint/23269/1/23269_MM.pdf (accessed on 12 September 2025).
- McQuilken, M.P.; Mitchell, S.J.; Budge, S.P.; Whipps, J.M.; Fenlon, J.S.; Archer, S.A. Effect of Coniothyrium minitans on sclerotial survival and apothecial production of Sclerotinia sclerotiorum in field-grown oilseed rape. Plant Pathol. 1995, 44, 883–896. [Google Scholar] [CrossRef]
- Tiedemann, A.V.; Ulber, B. Climate change and the risk of Sclerotinia diseases in oilseed rape. J. Plant Pathol. 2008, 90, 319–326. [Google Scholar]
| No. | Combination | Dose per 200 L of Water |
|---|---|---|
| 1 | Untreated | - |
| 2 | Prothioconazole (300 g/L) | 0.5 L |
| 3 | Tebuconazole (250 g/L) | 1.0 L |
| 4 | Azoxystrobin (250 g/L) | 0.8 L |
| 5 | T. asperellum T34 strain | 10 kg |
| 6 | Tebuconazole (250 g/L) + T. asperellum T34 strain | 1.0 L + 10 kg |
| 7 | Prothioconazole (300 g/L) + T. asperellum T34 strain | 0.5 L + 10 kg |
| 8 | Azoxystrobin (250 g/L) + T. asperellum T34 strain | 0.8 L + 10 kg |
| No. | Combination | Content of Active Ingredient per l/kg of Product | Application Timing | Dose per Hectare (L/kg) |
|---|---|---|---|---|
| 1 | Untreated | - | - | - |
| 2 | Metconazole | 60 g·L−1 | BBCH 16 | 0.7 |
| Difenoconazole + tebuconazole | 100 g·L−1 + 250 g·L−1 | BBCH 39 | 0.8 | |
| Fluopyram + prothioconazole | 125 g·L−1 + 125 g·L−1 | BBCH 65 | 1.0 | |
| 3 | Trichoderma asperellum | 10 g·kg−1 | BBCH 00 (during sowing) | 10.0 |
| Metconazole | 60 g·L−1 | BBCH 16 | 0.7 | |
| Difenoconazole + tebuconazole | 100 g·L−1 + 250 g·L−1 | BBCH 39 | 0.8 | |
| Fluopyram + prothioconazole | 125 g·L−1 + 125 g·L−1 | BBCH 65 | 1.0 | |
| 4 | Trichoderma asperellum | 1 × 107 CFU/kg | BBCH 00 (during sowing) | 10.0 |
| Metconazole | 60 g·L−1 | BBCH 16 | 0.7 | |
| Difenoconazole + tebuconazole | 100 g·L−1 + 250 g·L−1 | BBCH 39 | 0.8 | |
| 5 | Coniothyrium minitans | 1 × 107 CFU/kg | before sowing | 2.0 |
| Metconazole | 60 g·L−1 | BBCH 16 | 0.7 | |
| Difenoconazole + tebuconazole | 100 g·L−1 + 250 g·L−1 | BBCH 39 | 0.8 | |
| Fluopyram + prothioconazole | 125 g·L−1 + 125 g·L−1 | BBCH 65 | 1.0 | |
| 6 | Coniothyrium minitans | 1 × 107 CFU/kg | before sowing | 2.0 |
| Metconazole | 60 g·L−1 | BBCH 16 | 0.7 | |
| Difenoconazole + tebuconazole | 100 g·L−1 + 250 g·L−1 | BBCH 39 | 0.8 |
| No. | Treatment * | Infestation DI [%] | Efficacy [%] | Infestation DI [%] | Efficacy [%] | |
|---|---|---|---|---|---|---|
| 2022 | 2023 | |||||
| 1 | Untreated | 34.2 a | - | 36.9 a | ||
| 2 | Metconazole | 16.0 b | 53 | 14.7 b | 60 | |
| Difenoconazole + tebuconazole | ||||||
| Fluopyram + prothioconazole | ||||||
| 3 | Trichoderma asperellum | 5.3 b | 85 | 7.1 b | 81 | |
| Metconazole | ||||||
| Difenoconazole + tebuconazole | ||||||
| Fluopyram + prothioconazole | ||||||
| 4 | Trichoderma asperellum | 18.7 b | 46 | 22.2 c | 40 | |
| Metconazole | ||||||
| Difenoconazole + tebuconazole | ||||||
| 5 | Coniothyrium minitans | 11.1 b | 68 | 11.6 b | 69 | |
| Metconazole | ||||||
| Difenoconazole + tebuconazole | ||||||
| Fluopyram + prothioconazole | ||||||
| 6 | Coniothyrium minitans | 20.9 b | 39 | 20.1 c | 43 | |
| Metconazole | ||||||
| Difenoconazole + tebuconazole | ||||||
| No. | Treatment * | Infestation DI [%] | Efficacy [%] | Infestation DI [%] | Efficacy [%] | |
|---|---|---|---|---|---|---|
| 2022 | 2023 | |||||
| 1 | Untreated | 24.0 a | - | 33.8 a | ||
| 2 | Metconazole | 5.8 b | 76 | 4.4 b | 87 | |
| Difenoconazole + tebuconazole | ||||||
| Fluopyram + prothioconazole | ||||||
| 3 | Trichoderma asperellum | 0.0 b | 100 | 0.4 b | 99 | |
| Metconazole | ||||||
| Difenoconazole + tebuconazole | ||||||
| Fluopyram + prothioconazole | ||||||
| 4 | Trichoderma asperellum | 4.9 b | 82 | 4.0 b | 88 | |
| Metconazole | ||||||
| Difenoconazole + tebuconazole | ||||||
| 5 | Coniothyrium minitans | 3.6 b | 85 | 4.4 b | 87 | |
| Metconazole | ||||||
| Difenoconazole + tebuconazole | ||||||
| Fluopyram + prothioconazole | ||||||
| 6 | Coniothyrium minitans | 4.9 b | 82 | 8.9 b | 74 | |
| Metconazole | ||||||
| Difenoconazole + tebuconazole | ||||||
| No. | Treatment * | Yield (t/ha) | TGW (g) | Oil Content (%) | |||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 1 | Untreated | 4.9 b | 5.7 a | 4.33 a | 4.75 a | 47.8 b | 48.5 a |
| 2 | Metconazole | 5.0 a | 6.1 a | 4.32 a | 5.12 a | 48.5 ab | 48.7 a |
| Difenoconazole + tebuconazole | |||||||
| Fluopyram + prothioconazole | |||||||
| 3 | Trichoderma asperellum | 5.2 a | 6.1 a | 4.40 a | 5.16 a | 48.9 a | 48.9 a |
| Metconazole | |||||||
| Difenoconazole + tebuconazole | |||||||
| Fluopyram + prothioconazole | |||||||
| 4 | Trichoderma asperellum | 5.1 a | 6.0 a | 4.40 a | 4.81 a | 48.6 ab | 48.5 a |
| Metconazole | |||||||
| Difenoconazole + tebuconazole | |||||||
| 5 | Coniothyrium minitans | 5.1 a | 6.0 a | 4.56 a | 4.76 a | 48.8 a | 49.0 a |
| Metconazole | |||||||
| Difenoconazole + tebuconazole | |||||||
| Fluopyram + prothioconazole | |||||||
| 6 | Coniothyrium minitans | 5.0 a | 6.0 a | 4.35 a | 4.92 a | 48.5 ab | 48.7 a |
| Metconazole | |||||||
| Difenoconazole + tebuconazole | |||||||
| No. | Treatment * | Yield (t/ha) | TGW (g) | Oil content (%) | |||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 1 | Untreated | 4.4 b | 5.0 a | 4.95 a | 4.71 b | 47.5 b | 51.4 a |
| 2 | Metconazole | 4.8 a | 5.4 a | 5.09 a | 5.90 a | 48.2 ab | 51.7 a |
| Difenoconazole + tebuconazole | |||||||
| Fluopyram + prothioconazole | |||||||
| 3 | Trichoderma asperellum | 4.8 a | 5.4 a | 5.25 a | 5.02 a | 48.5 a | 51.6 a |
| Metconazole | |||||||
| Difenoconazole + tebuconazole | |||||||
| Fluopyram + prothioconazole | |||||||
| 4 | Trichoderma asperellum | 4.7 a | 5.3 a | 5.08 a | 5.01 a | 47.9 ab | 51.6 a |
| Metconazole | |||||||
| Difenoconazole + tebuconazole | |||||||
| 5 | Coniothyrium minitans | 4.8 a | 5.3 a | 4.95 a | 4.97 a | 48.1 ab | 51.5 a |
| Metconazole | |||||||
| Difenoconazole + tebuconazole | |||||||
| Fluopyram + prothioconazole | |||||||
| 6 | Coniothyrium minitans | 4.7 a | 5.2 a | 5.04 a | 4.72 a | 48.0 ab | 51.5 a |
| Metconazole | |||||||
| Difenoconazole + tebuconazole | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danielewicz, J.; Jajor, E.; Horoszkiewicz, J.; Korbas, M.; Sobiech, Ł.; Grzanka, M.; Sawinska, Z.; Bocianowski, J.; Cholewa, J. Combined Biological and Chemical Control of Sclerotinia sclerotiorum on Oilseed Rape in the Era of Climate Change. Agriculture 2025, 15, 2147. https://doi.org/10.3390/agriculture15202147
Danielewicz J, Jajor E, Horoszkiewicz J, Korbas M, Sobiech Ł, Grzanka M, Sawinska Z, Bocianowski J, Cholewa J. Combined Biological and Chemical Control of Sclerotinia sclerotiorum on Oilseed Rape in the Era of Climate Change. Agriculture. 2025; 15(20):2147. https://doi.org/10.3390/agriculture15202147
Chicago/Turabian StyleDanielewicz, Jakub, Ewa Jajor, Joanna Horoszkiewicz, Marek Korbas, Łukasz Sobiech, Monika Grzanka, Zuzanna Sawinska, Jan Bocianowski, and Jakub Cholewa. 2025. "Combined Biological and Chemical Control of Sclerotinia sclerotiorum on Oilseed Rape in the Era of Climate Change" Agriculture 15, no. 20: 2147. https://doi.org/10.3390/agriculture15202147
APA StyleDanielewicz, J., Jajor, E., Horoszkiewicz, J., Korbas, M., Sobiech, Ł., Grzanka, M., Sawinska, Z., Bocianowski, J., & Cholewa, J. (2025). Combined Biological and Chemical Control of Sclerotinia sclerotiorum on Oilseed Rape in the Era of Climate Change. Agriculture, 15(20), 2147. https://doi.org/10.3390/agriculture15202147










