Evaluation of Pelargonic Acid as a Sustainable Defoliant in Cotton (Gossypium hirsutum L.) Production
Abstract
1. Introduction
- Analysis of physiological and yield-related parameters in response to the defoliation treatments.
2. Materials and Methods
2.1. Experimental Site and Weather Data
2.2. Experimental Design and Treatment
- PELACID: Pure pelargonic acid (Beloukha®, Belchim, Saronno, Italy);
- PLACEBO: A water-based placebo solution;
- PYRAFETH: Pyraflufen-ethyl (Revolution®, Sipcam, Milan, Italy), a commonly used commercial defoliant in conventional cotton farming.
2.3. Crop Management
2.4. Economic Parameters
2.5. Data Collection and Statistical Analysis
3. Results
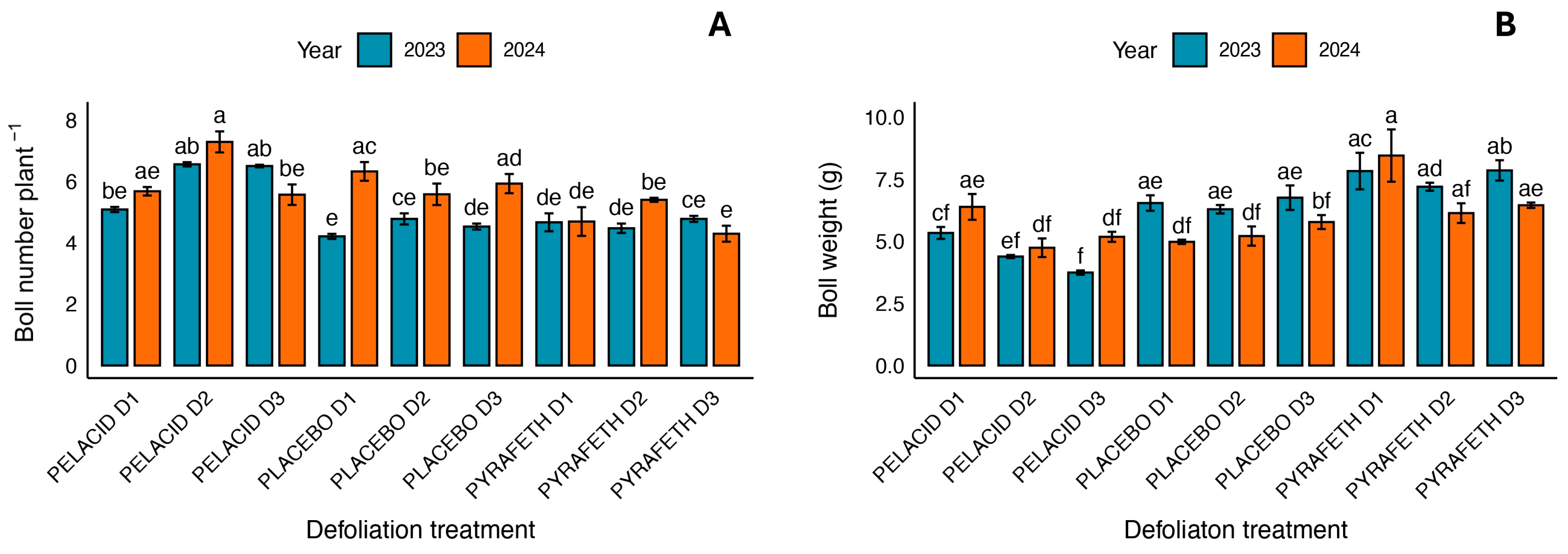
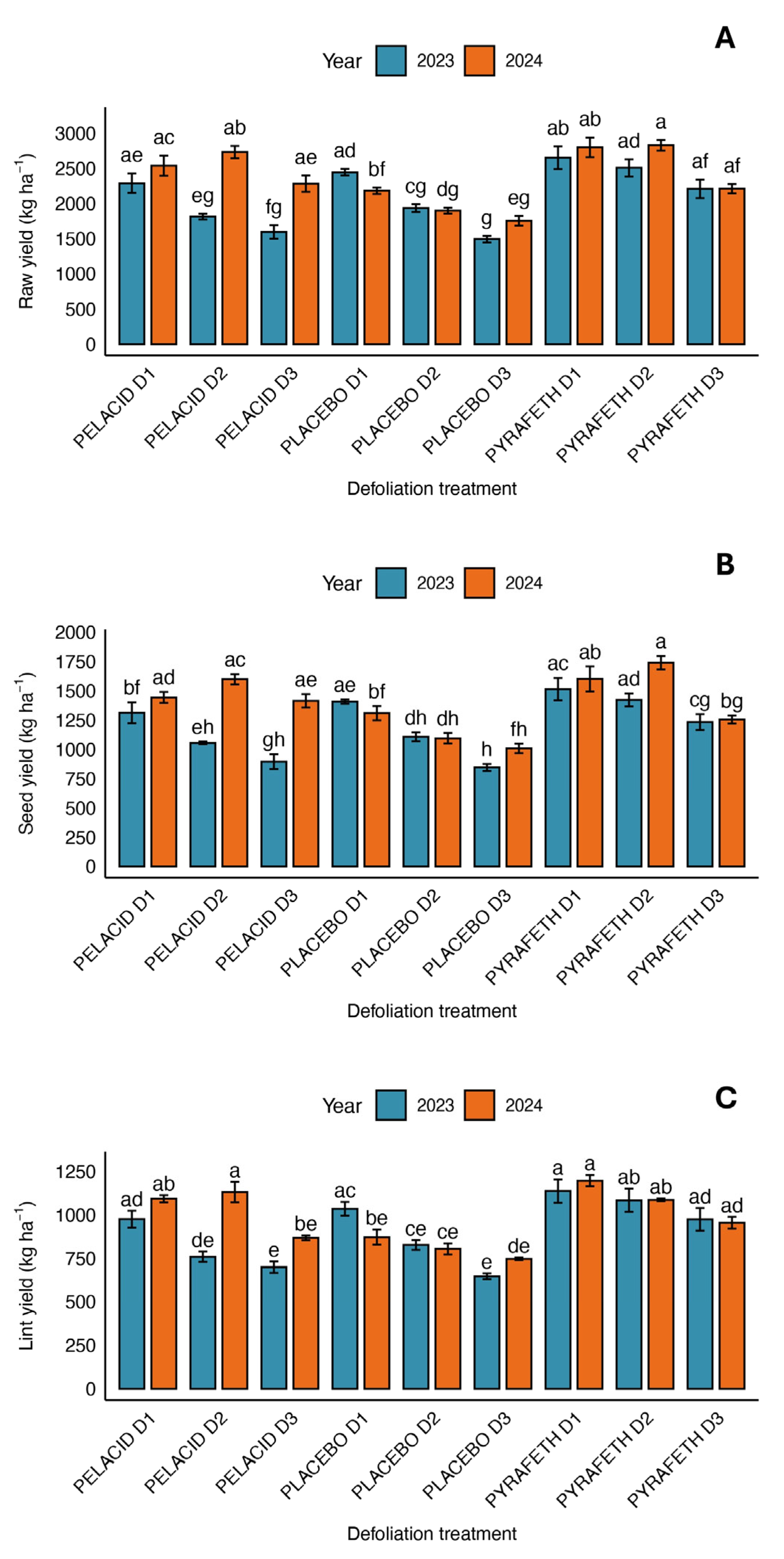
3.1. Morphological and Productive Traits
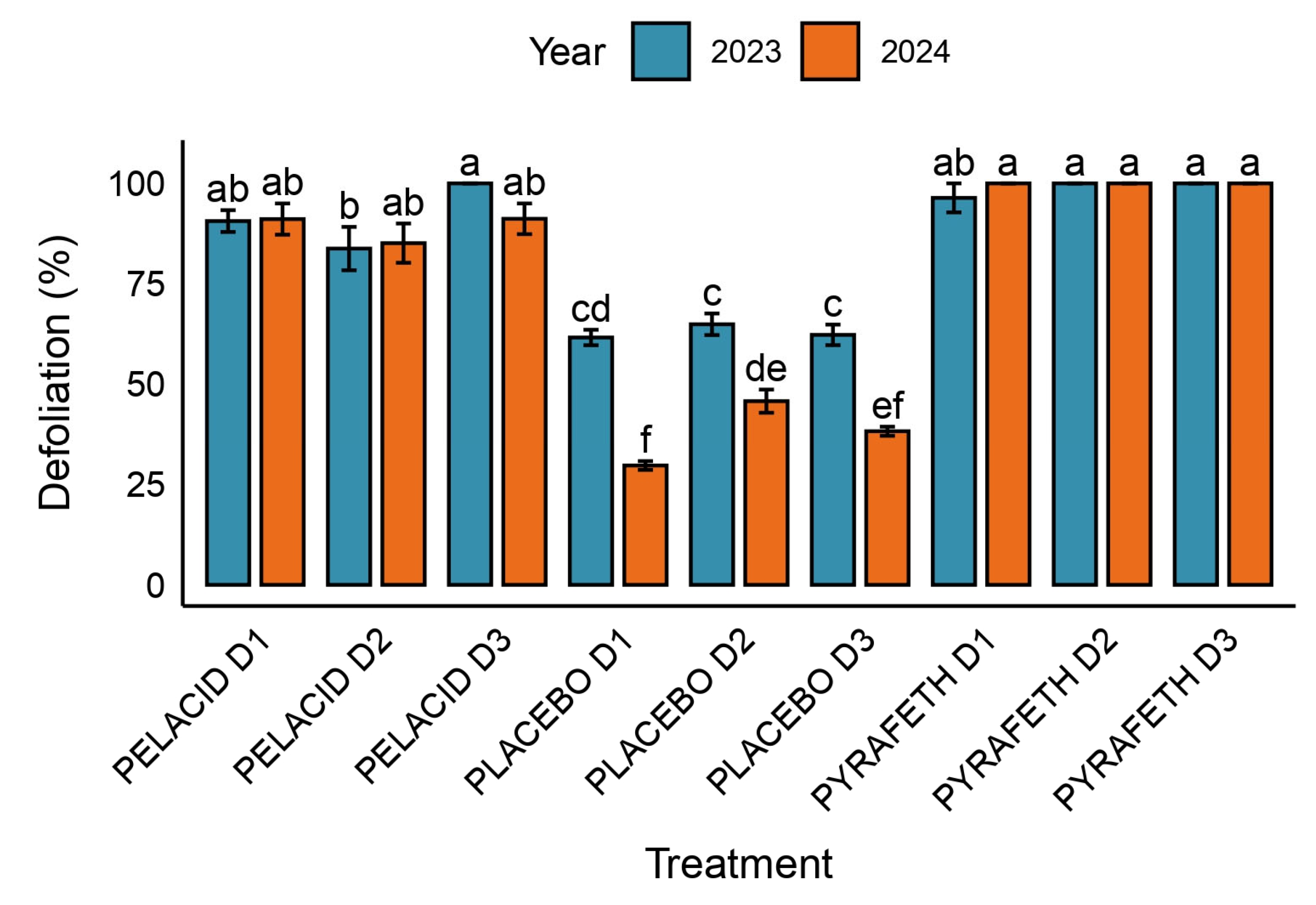
3.2. Defoliation Traits
4. Discussion
Limitations and Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CER | Cost-Effectiveness Ratio |
| ETc | reference crop evapotranspiration |
| FC | Field Capacity |
| ET0 | daily reference evapotranspiration |
| Kc | crop coefficients |
| PELACID | Pelargogic Acid |
| PLACEBO | Placebo solution (water) |
| PYRAFETH | Pyraflufen-ethyl |
| PPO | protoporphyrinogen oxidase |
References
- Wakelyn, P.J.; Chaudhry, M.R. Organic Cotton: Production Practices and Post-Harvest Considerations. In Sustainable Textiles: Life Cycle and Environmental Impact; Woodhead Publishing: Cambridge, UK, 2009; pp. 231–301. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 29 November 2022).
- United States Department of Agriculture Cotton|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/production/commodity/2631000 (accessed on 28 September 2025).
- Vitale, G.S.; Scavo, A.; Zingale, S.; Tuttolomondo, T.; Santonoceto, C.; Pandino, G.; Lombardo, S.; Anastasi, U.; Guarnaccia, P. Agronomic Strategies for Sustainable Cotton Production: A Systematic Literature Review. Agriculture 2024, 14, 1597. [Google Scholar] [CrossRef]
- Vitale, G.S.; Iacuzzi, N.; Zingale, S.; Lombardo, S.; Tuttolomondo, T.; Guarnaccia, P. Environmental Sustainability of Cotton: A Systematic Literature Review of Life Cycle Assessments. J. Agric. Food Res. 2025, 22, 102069. [Google Scholar] [CrossRef]
- Xie, Z.; Xie, X.; Qin, Y.; Yang, D.; Zhou, Z.; Wang, Q.; Liu, A.; Tu, X. Advances in Cotton Harvesting Aids. Front. Plant Sci. 2025, 16, 1570251. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, S.; Somasundaram, S.; Shri Rangasami, S.; Anantharaju, P.; Vijayalakshmi, D.; Ragavan, T.; Dhamodharan, P. Managing Cotton Canopy Architecture for Machine Picking Cotton via High Plant Density and Plant Growth Retardants. J. Cotton Res. 2025, 8, 2. [Google Scholar] [CrossRef]
- Neupane, J.; Maja, J.M.; Miller, G.; Marshall, M.; Cutulle, M.; Luo, J. Effect of Controlled Defoliant Application on Cotton Fiber Quality. Appl. Sci. 2023, 13, 5694. [Google Scholar] [CrossRef]
- Yu, K.; Li, K.; Wang, J.; Gong, Z.; Liang, Y.; Yang, M.; Sun, H.; Zheng, J.; Li, X.; Wang, L.; et al. Optimizing the Proportion of Thidiazuron and Ethephon Compounds to Improve the Efficacy of Cotton Harvest Aids. Ind. Crops Prod. 2023, 191, 115949. [Google Scholar] [CrossRef]
- Liao, B.; Li, F.; Yi, F.; Du, M.; Tian, X.; Li, Z. Comparative Physiological and Transcriptomic Mechanisms of Defoliation in Cotton in Response to Thidiazuron versus Ethephon. Int. J. Mol. Sci. 2023, 24, 7590. [Google Scholar] [CrossRef]
- European Union Commission Directive-91/414-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/1991/414/oj/eng (accessed on 28 September 2025).
- European Union Commission Decision-2008/296-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dec/2008/296/oj/eng?utm_source=chatgpt.com (accessed on 28 September 2025).
- Snipes, C.E.; Cathey, G.W. Evaluation of Defoliant Mixtures in Cotton. Field Crops Res. 1992, 28, 327–334. [Google Scholar] [CrossRef]
- Song, X.; Zhang, L.; Zhao, W.; Xu, D.; Eneji, A.E.; Zhang, X.; Han, H.; Cao, L.; Zhang, W.; Lu, Z.; et al. The Relationship between Boll Retention and Defoliation of Cotton at the Fruiting Site Level. Crop Sci. 2022, 62, 1333–1347. [Google Scholar] [CrossRef]
- Trakulsrichai, S.; Chuayaupakarn, K.; Tansuwannarat, P.; Rittilert, P.; Tongpoo, A.; Sriapha, C.; Wananukul, W. Ethephon Poisoning: Clinical Characteristics and Outcomes. Toxics 2025, 13, 115. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, X.; Yang, X.; Yu, Q.; Deng, S.; Guan, Q.; Chen, D.; Zhang, M.; Gao, B.; Xu, S.; et al. Prenatal Exposure to Pesticides and Domain-Specific Neurodevelopment at Age 12 and 18 Months in Nanjing, China. Environ. Int. 2023, 173, 107814. [Google Scholar] [CrossRef]
- Reba, M.L.; Teague, T.G.; Vories, E.D. A Retrospective Review of Cotton Irrigation on a Production Farm in the Mid-South. J. Cotton Sci. 2014, 18, 137–144. [Google Scholar] [CrossRef]
- Karam, F.; Lahoud, R.; Masaad, R.; Daccache, A.; Mounzer, O.; Rouphael, Y. Water Use and Lint Yield Response of Drip Irrigated Cotton to the Length of Irrigation Season. Agric. Water Manag. 2006, 85, 287–295. [Google Scholar] [CrossRef]
- Buttar, G.S.; Aujla, M.S.; Thind, H.S.; Singh, C.J.; Saini, K.S. Effect of Timing of First and Last Irrigation on the Yield and Water Use Efficiency in Cotton. Agric. Water Manag. 2007, 89, 236–242. [Google Scholar] [CrossRef]
- Barth, M.J.; Mowrer, J. Evaluating Reduced Tillage, Cover Crops, and Living Mulches for Weed Management in Cotton; OAKTrust: Oldham, UK, 2022. [Google Scholar]
- Salehin, S.M.U.; Rajan, N.; Mowrer, J.E.; Bagavathiannan, M. Challenges and Opportunities of Southern Texas Organic Cotton in Southern Texas. Available online: https://soilcrop.tamu.edu/wp-content/uploads/sites/16/2025/02/04-2025-Organic-Cotton-in-Texas.pdf (accessed on 18 July 2025).
- Greer, L.; Dole, J.M. Defoliation of Woody Cut Stems with Preharvest, Less Toxic Chemical and Postharvest Environmental Methods. Horttechnology 2005, 15, 376–380. [Google Scholar] [CrossRef]
- Arboleya, J.E.; Masabni, J.G.; Particka, M.G.; Zandstra, B.H. Identification of Preharvesr Desiccants for Use in Onion Production. Horttechnology 2005, 15, 808–811. [Google Scholar] [CrossRef]
- European Food Safety Authority Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Fatty Acids C7 to C18 (Approved under Regulation (EC) No 1107/2009 as Fatty Acids C7 to C20). EFSA Journal 2013, 11, 3023. [CrossRef]
- Torres-Pagán, N.; Muñoz, M.; Barbero, S.; Mamone, R.; Peiró, R.; Carrubba, A.; Sánchez-Moreiras, A.M.; Gómez de Barreda, D.; Verdeguer, M. Herbicidal Potential of the Natural Compounds Carvacrol, Thymol, Eugenol, p-Cymene, Citral and Pelargonic Acid in Field Conditions: Indications for Better Performance. Agronomy 2024, 14, 537. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Herbicides Based on Pelargonic Acid: Herbicides of the Bioeconomy. Biofuels Bioprod. Biorefin. 2019, 13, 1476–1482. [Google Scholar] [CrossRef]
- Soltani, N.; Willemse, C.; Sikkema, P.H. White Bean and Weed Desiccation With Pelargonic Acid. J. Agric. Sci. 2024, 16, 1–17. [Google Scholar] [CrossRef]
- López-González, D.; Muñoz Usero, M.; Hermida-Ramón, J.M.; Álvarez-Rodríguez, S.; Araniti, F.; Teijeira, M.; Verdeguer, M.; Sánchez-Moreiras, A.M. Pelargonic Acid’s Interaction with the Auxin Transporter PIN1: A Potential Mechanism behind Its Phytotoxic Effects on Plant Metabolism. Plant Sci. 2024, 349, 112278. [Google Scholar] [CrossRef]
- Muñoz, M.; Torres-Pagán, N.; Jouini, A.; Araniti, F.; Sánchez-Moreiras, A.M.; Verdeguer, M. Control of Problematic Weeds in Mediterranean Vineyards with the Bioherbicide Pelargonic Acid. Agronomy 2022, 12, 2476. [Google Scholar] [CrossRef]
- Brancato, A.; Brocca, D.; Carrasco Cabrera, L.; De Lentdecker, C.; Erdos, Z.; Ferreira, L.; Greco, L.; Jarrah, S.; Kardassi, D.; Leuschner, R.; et al. Evaluation of Confirmatory Data Following the Article 12 MRL Review for Pyraflufen-Ethyl. EFSA J. 2018, 16, e05444. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 22nd Edition (2023)—AOAC INTERNATIONAL. Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 23 July 2025).
- Kjeldahl, J. Neue Methode Zur Bestimmung Des Stickstoffs in Organischen Körpern. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Agricultural Chemicals; Contaminants; Drugs; Association of Official Analytical Chemists: Arington, VA, USA, 1990; Volume 1. [Google Scholar]
- Dane, J.H.; Topp, G.C.; Campbell, G.S.; Horton, R.; Jury, W.A.; Nielsen, D.R.; van Es, H.M.; Wierenga, P.J.; Al-Amoodi, L.; Dick, W.A. Methods of Soil Analysis, Part 4: Physical Methods; Soil Science Society of America, Inc.: Fitchburg, WI, USA, 2018; pp. 1–1692. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis, Part 3: Chemical Methods; Soil Science Society of America, Inc.: Fitchburg, WI, USA, 2018; pp. 1201–1229. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods; Soil Science Society of America, Inc.: Fitchburg, WI, USA, 2018; pp. 363–375. [Google Scholar] [CrossRef]
- Klute, A. Water Retention: Laboratory Methods. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods; Soil Science Society of America, Inc.: Fitchburg, WI, USA, 2018; pp. 635–662. [Google Scholar] [CrossRef]
- Flint, A.L.; Flint, L.E. 2.2 Particle Density. In Methods of Soil Analysis, Part 4: Physical Methods; Soil Science Society of America, Inc.: Fitchburg, WI, USA, 2018. [Google Scholar]
- Allen, R.G.; Pereira, L.S. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Filintas, A.; Nteskou, A.; Kourgialas, N.; Gougoulias, N.; Hatzichristou, E. A Comparison between Variable Deficit Irrigation and Farmers’ Irrigation Practices under Three Fertilization Levels in Cotton Yield (Gossypium hirsutum L.) Using Precision Agriculture, Remote Sensing, Soil Analyses, and Crop Growth Modeling. Water 2022, 14, 2654. [Google Scholar] [CrossRef]
- Moussouraki, M.A.; Tani, E.; Velliou, A.; Goufa, M.; Psychogiou, M.; Papadakis, I.E.; Abraham, E.M. Growth, Physiological and Biochemical Responses of Two Greek Cotton Cultivars to Salt Stress and Their Impact as Selection Indices for Salt Tolerance. Not. Bot. Horti Agrobot. 2019, 47, 706–715. [Google Scholar] [CrossRef]
- Vitale, G.S.; Iacuzzi, N.; Tortorici, N.; Indovino, G.; Franco, L.; Mosca, C.; Giovino, A.; Scavo, A.; Lombardo, S.; Tuttolomondo, T.; et al. Sustainable Cotton Production in Sicily: Yield Optimization Through Varietal Selection, Mycorrhizae, and Efficient Water Management. Agronomy 2025, 15, 1892. [Google Scholar] [CrossRef]
- Beloukha–Certis Belchim. Available online: https://certisbelchim.it/prodotti/beloukha/ (accessed on 21 September 2025).
- Loddo, D.; Jagarapu, K.K.; Strati, E.; Trespidi, G.; Nikolić, N.; Masin, R.; Berti, A.; Otto, S. Assessing Herbicide Efficacy of Pelargonic Acid on Several Weed Species. Agronomy 2023, 13, 1511. [Google Scholar] [CrossRef]
- PiraMax EC–Certis Belchim. Available online: https://certisbelchim.it/prodotti/piramax-ec/ (accessed on 21 September 2025).
- REVOLUTION|Erbicidi|SIPCAM. Available online: https://www.sipcam.com/it/it/prodotti/revolution (accessed on 21 September 2025).
- Cotton: World Markets and Trade|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/cotton-world-markets-and-trade-08122025 (accessed on 21 September 2025).
- Wang, L.; Deng, Y.; Kong, F.; Duan, B.; Saeed, M.; Xin, M.; Wang, X.; Gao, L.; Shen, G.; Wang, J.; et al. Evaluating the Effects of Defoliant Spraying Time on Fibre Yield and Quality of Different Cotton Cultivars. J. Agric. Sci. 2023, 161, 205–216. [Google Scholar] [CrossRef]
- Barker, A.L.; Pawlak, J.; Duke, S.O.; Beffa, R.; Tranel, P.J.; Wuerffel, J.; Young, B.; Porri, A.; Liebl, R.; Aponte, R.; et al. Discovery, Mode of Action, Resistance Mechanisms, and Plan of Action for Sustainable Use of Group 14 Herbicides. Weed Sci. 2023, 71, 173–188. [Google Scholar] [CrossRef]
- Jin, D.; Wang, X.; Xu, Y.; Gui, H.; Zhang, H.; Dong, Q.; Sikder, R.K.; Yang, G.; Song, M. Chemical Defoliant Promotes Leaf Abscission by Altering ROS Metabolism and Photosynthetic Efficiency in Gossypium Hirsutum. Int. J. Mol. Sci. 2020, 21, 2738. [Google Scholar] [CrossRef]
- Sha, S.; Cheng, M.; Hu, K.; Zhang, W.; Yang, Y.; Xu, Q. Toxic Effects of Pb on Spirodela polyrhiza (L.): Subcellular Distribution, Chemical Forms, Morphological and Physiological Disorders. Ecotoxicol. Environ. Saf. 2019, 181, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, B.; Hu, B.; Wan, Y.; Wang, J.; Jia, M. Effects of Defoliation at Different Fertility Stages on Material Accumulation, Physiological Indices and Yield of Cotton. Agriculture 2024, 14, 258. [Google Scholar] [CrossRef]
- Grichar, W.J.; Dotray, P.A.; Baughman, T.A. Peanut Variety Response to Postemergence Applications of Carfentrazone-Ethyl and Pyraflufen-Ethyl. Crop Prot. 2010, 29, 1034–1038. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, Y.; Liu, Z.; Sun, S.; Wu, S.; Du, J.; Chen, Y.; Zhang, X.; Chen, D. Boll/Leaf Ratio Improves the Source–Sink Relationship and Lint Yield during the Boll Setting Stage of Cotton. Field Crops Res. 2024, 310, 109342. [Google Scholar] [CrossRef]
- Guo, S.; Liu, T.; Han, Y.; Wang, G.; Du, W.; Wu, F.; Li, Y.; Feng, L. Changes in Within-Boll Yield Components Explain Cotton Yield and Quality Variation across Planting Dates under a Double Cropping System of Cotton-Wheat. Field Crops Res. 2023, 293, 108853. [Google Scholar] [CrossRef]
- Constable, G.A.; Bange, M.P. The Yield Potential of Cotton (Gossypium hirsutum L.). Field Crops Res. 2015, 182, 98–106. [Google Scholar] [CrossRef]
- Huot, C.; Philp, J.N.M.; Zhou, Y.; Denton, M.D. Root Penetration Is Associated with Root Diameter and Root Growth Rate in Tropical Forage Grasses. Grasses 2025, 4, 4. [Google Scholar] [CrossRef]
- Guo, C.; Bao, X.; Sun, H.; Zhu, L.; Zhang, Y.; Zhang, K.; Bai, Z.; Zhu, J.; Liu, X.; Li, A.; et al. Optimizing Root System Architecture to Improve Cotton Drought Tolerance and Minimize Yield Loss during Mild Drought Stress. Field Crops Res. 2024, 308, 109305. [Google Scholar] [CrossRef]
- Guo, C.; Sun, H.; Bao, X.; Zhu, L.; Zhang, Y.; Zhang, K.; Li, A.; Bai, Z.; Liu, L.; Li, C. Increasing Root-Lower Characteristics Improves Drought Tolerance in Cotton Cultivars at the Seedling Stage. J. Integr. Agric. 2024, 23, 2242–2254. [Google Scholar] [CrossRef]
- Ghimire, O.P.; Kuraparthy, V.; Jones, M.; Campbell, B.T.; Bridges, W.C.; Alege, F.P.; Delhom, C.; Narayanan, S. Better Root Length Distribution in the Deep Soil Profile Enhances Upland Cotton Performance. Field Crops Res. 2025, 325, 109805. [Google Scholar] [CrossRef]
- Poiger, T.; Müller, J.; Kasteel, R.; Buerge, I.J. Degradation and Sorption of the Herbicide Pelargonic Acid in Subsoils below Railway Tracks Compared to a Range of Topsoils. Environ. Sci. Eur. 2024, 36, 4. [Google Scholar] [CrossRef]
- Zhan, D.; Yang, Y.; Hu, Y.; Zhang, Y.; Luo, H.; Zhang, W. Contributions of Nonleaf Organs to the Yield of Cotton Grown with Different Water Supply. Sci. World J. 2014, 2014, 602747. [Google Scholar] [CrossRef] [PubMed]
- Faircloth, J.C.; Edmisten, K.L.; Wells, R.; Stewart, A.M. The Influence of Defoliation Timing on Yields and Quality of Two Cotton Cultivars. Crop Sci. 2004, 44, 165–172. [Google Scholar] [CrossRef]
- Faircloth, J.C.; Edmisten, K.L.; Wells, R.; Stewart, A.M. Timing Defoliation Applications for Maximum Yields and Optimum Quality in Cotton Containing a Fruiting Gap. Crop Sci. 2004, 44, 158–164. [Google Scholar] [CrossRef]
- Manuchehri, M.R.; Dotray, P.A.; Grichar, W.J.; Baughman, T.A. Peanut Response to Pyraflufen-Ethyl Applied Postemergence. Weed Technol. 2017, 31, 464–469. [Google Scholar] [CrossRef]
- Lederer, B.; Fujimori, T.; Tsujino, Y.; Wakabayashi, K.; Böger, P. Phytotoxic Activity of Middle-Chain Fatty Acids II: Peroxidation and Membrane Effects. Pestic. Biochem. Physiol. 2004, 80, 151–156. [Google Scholar] [CrossRef]
- Koudahe, K.; Sheshukov, A.Y.; Aguilar, J.; Djaman, K. Irrigation-Water Management and Productivity of Cotton: A Review. Sustainability 2021, 13, 10070. [Google Scholar] [CrossRef]
- Mi, Y.; Wang, Y.; Wu, F.; Han, Y.; Yang, B.; Lei, Y.; Xiong, S.; Zhi, X.; Li, Y. Yield Variation in Early-Maturing Cotton in Response to Sowing Dates and Growing Seasons Is Associated with Differential Resource Utilization. Eur. J. Agron. 2025, 168, 127637. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, C.; Li, K.; Meng, L.; Song, X.; Qi, H.; Wang, Y.; Zhang, Z.; Yu, X.; Li, F.; et al. Quantifying the Influencing Factors and Predictive Analysis of Cotton Defoliation and Maturation Based on Machine Learning. Comput. Electron. Agric. 2025, 237, 110555. [Google Scholar] [CrossRef]
- Álvarez, F.; Arena, M.; Auteri, D.; Leite, S.B.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; Crivellente, F.; et al. Updated Peer Review of the Pesticide Risk Assessment of the Active Substance Pelargonic Acid (Nonanoic Acid). EFSA J. 2025, 23, e9408. [Google Scholar] [CrossRef]
- Alvarez, F.; Arena, M.; Auteri, D.; Borroto, J.; Brancato, A.; Carrasco Cabrera, L.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Pelargonic Acid (Nonanoic Acid). EFSA J. 2021, 19, e06813. [Google Scholar] [CrossRef]
- Snipes, C.E.; Baskin, C.C. Influence of Early Defoliation on Cotton Yield, Seed Quality, and Fiber Properties. Field Crops Res. 1994, 37, 137–143. [Google Scholar] [CrossRef]


| Soil Characteristics | Unit | Value | Method |
|---|---|---|---|
| Sand | % | 16.6 | [31] |
| Loam | % | 27.8 | [31] |
| Clay | % | 55.6 | [31] |
| N total | g kg−1 | 1 | Kjeldahl [32] |
| P | mg kg−1 | 2.18 | Ferrari [33] |
| K | mg kg−1 | 203.3 | Dirks and Scheffer [33] |
| Organic matter | % | 1.1 | Walkley and Black [33] |
| Electrical Conductivity | mS/m | 15 | [34] |
| Cation Exchange Capacity (CEC) | cmolc kg−1 | 14.8 | [35] |
| pH | 7.6 | In water solution | |
| Bulk density | kg m3 | 1200 | [36] |
| Field capacity at −0.03 MPa | % | 27 | [37] |
| Wilting point at −1.5 MPa | % | 11 | [38] |
| Defoliation Treatment | Active Ingredient | Dose (L ha−1) |
|---|---|---|
| PELACID D1 | Pure pelargonic acid 680 g L−1 | 12 |
| PELACID D2 | Pure pelargonic acid 680 g L−1 | 16 * |
| PELACID D3 | Pure pelargonic acid 680 g L−1 | 18 |
| PLACEBO D1 | Water | 30 ** |
| PLACEBO D2 | Water | 50 |
| PLACEBO D3 | Water | 60 |
| PYRAFETH D1 | Pyraflufen-ethyl 10.6 g L−1 | 1.5 |
| PYRAFETH D2 | Pyraflufen-ethyl 10.6 g L−1 | 2 |
| PYRAFETH D3 | Pyraflufen-ethyl 10.6 g L−1 | 2.5 *** |
| Phase | Description | Kc | Depth of Soil Explored by Roots (cm) |
|---|---|---|---|
| Initial | Germination: from dry seed (00) to emergence of hypocotyl with cotyledons (09) | 0.4–0.5 | 30 |
| Development | Leaf development: from cotyledons completely unfolded (10) to canopy closure (39) | 0.7–0.8 | 50 |
| Mid-season | Inflorescence emergence: from first detectable bud (51) to about 90% of capsules having reached their final size (79) | 1.05–1.25 | 50 |
| End-season | Senescence: from about 10% of discolored or abscessed leaves (91) to above-ground parts of dead plants | 0.65–0.70 | 50 |
| Source of Variation | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| df | Boll Number Plant−1 | Average Boll Weight (g) | Root Diameter (mm) | Raw Yield (kg ha−1) | Lint Yield (kg ha−1) | Seed Yield (kg ha−1) | Defoliation | |
| Y | 1 | 3.26 × 10−4 *** | 0.19 ns | 6.98 × 10−8 *** | 5.14 × 10−5 *** | 3.22 × 10−3 ** | 1.79 × 10−6 *** | 4.33 × 10−7 *** |
| T | 8 | 7.58 × 10−8 *** | 2.95 × 10−8 *** | 1.60 × 10−7 *** | 1.09 × 10−10 *** | 1.74 × 10−11 *** | 1.02 × 10−10 *** | 2 × 10−16 *** |
| Y × T | 36 | 6.17 × 10−4 *** | 1.19 × 10−2 * | 1.33 × 10−3 ** | 4.51 × 10−4 *** | 2.01 × 10−4 *** | 7.85 × 10−4 *** | 6.09 × 10−7 *** |
| Year | Treatment * | Dose (L ha−1) | Defoliant Cost (€ ha−1) | Lint Yield (kg ha−1) | Lint Yield Price (€ kg−1) | Lint Gross Revenue (€ ha−1) | Net Lint Revenue (€ ha−1) | Cost-Effectiveness Ratio |
|---|---|---|---|---|---|---|---|---|
| 2023 | PELACID D1 | 12 | 240 | 975 | 1.315 | 1282.13 | 1042.13 | 4.34 |
| 2023 | PELACID D2 | 16 | 320 | 759 | 1.315 | 998.09 | 678.09 | 2.12 |
| 2023 | PELACID D3 | 18 | 360 | 699 | 1.315 | 919.19 | 559.19 | 1.55 |
| 2023 | PYRAFETH D1 | 1.5 | 56.25 | 1136 | 1.315 | 1493.84 | 1437.59 | 25.56 |
| 2023 | PYRAFETH D2 | 2 | 75 | 1083 | 1.315 | 1424.15 | 1349.15 | 17.99 |
| 2023 | PYRAFETH D3 | 2.5 | 93.75 | 974 | 1.315 | 1280.81 | 1187.06 | 12.66 |
| 2024 | PELACID D1 | 12 | 240 | 1093 | 1.315 | 1437.30 | 1197.30 | 4.99 |
| 2024 | PELACID D2 | 16 | 320 | 1131 | 1.315 | 1487.27 | 1167.27 | 3.65 |
| 2024 | PELACID D3 | 18 | 360 | 868 | 1.315 | 1141.42 | 781.42 | 2.17 |
| 2024 | PYRAFETH D1 | 1.5 | 56.25 | 1196 | 1.315 | 1572.74 | 1516.49 | 26.96 |
| 2024 | PYRAFETH D2 | 2 | 75 | 1087 | 1.315 | 1429.41 | 1354.41 | 18.06 |
| 2024 | PYRAFETH D3 | 2.5 | 93.75 | 954 | 1.315 | 1254.51 | 1160.76 | 12.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, G.S.; Lombardo, S.; Pandino, G.; Guarnaccia, P. Evaluation of Pelargonic Acid as a Sustainable Defoliant in Cotton (Gossypium hirsutum L.) Production. Agriculture 2025, 15, 2134. https://doi.org/10.3390/agriculture15202134
Vitale GS, Lombardo S, Pandino G, Guarnaccia P. Evaluation of Pelargonic Acid as a Sustainable Defoliant in Cotton (Gossypium hirsutum L.) Production. Agriculture. 2025; 15(20):2134. https://doi.org/10.3390/agriculture15202134
Chicago/Turabian StyleVitale, Giuseppe Salvatore, Sara Lombardo, Gaetano Pandino, and Paolo Guarnaccia. 2025. "Evaluation of Pelargonic Acid as a Sustainable Defoliant in Cotton (Gossypium hirsutum L.) Production" Agriculture 15, no. 20: 2134. https://doi.org/10.3390/agriculture15202134
APA StyleVitale, G. S., Lombardo, S., Pandino, G., & Guarnaccia, P. (2025). Evaluation of Pelargonic Acid as a Sustainable Defoliant in Cotton (Gossypium hirsutum L.) Production. Agriculture, 15(20), 2134. https://doi.org/10.3390/agriculture15202134







