Abstract
This study examined the effect of long-term physical activity during the finishing period on meat and fat quality, and metabolic gene expression in obese Alentejano (AL) pigs. From 87.3 to 161.6 kg BW and for 130 days, eighteen pigs were assigned to either individual pens without an exercise area (NE, n = 9) or an outdoor park with an exercise area (WE, n = 9). Both groups received identical commercial diets at 85% ad libitum intake. Loin (Longissimus lumborum—LL), tenderloin (Psoas major—PM), and dorsal subcutaneous fat samples were obtained at slaughter, and analyzed for fatty acid composition and gene expression. Physical activity modulated the fatty acid profile and key metabolic genes in muscle and fat tissues. WE pigs showed higher palmitoleic (p = 0.031) and linolenic (p = 0.022) acids in LL, while Fatty acid synthase and Leptin in LL were downregulated (p = 0.071 and p = 0.018, respectively); Fatty acid binding protein 4 was downregulated (p = 0.003) and Stearoyl-CoA desaturase upregulated (p = 0.020) in the PM of WE pigs, indicating changes in lipid metabolism. Also, Myosin heavy chain 7 was upregulated (p = 0.016) in LL, suggesting oxidative muscle remodeling. These findings suggest that moderate, long-term physical activity during finishing induces modest but favorable metabolic adaptations in muscle and fat tissues without compromising meat quality in AL pigs, supporting its use in traditional rearing systems aimed at balancing animal welfare and product quality in local breeds.
1. Introduction
Animal development is influenced, besides other factors, by environmental conditions during rearing. Enriched or complex environments can mitigate stress responses, reduce pain perception and anxiety, and decrease aggressive behaviors, ultimately improving animal welfare [1,2]. The Alentejano (AL) pig, a traditional Portuguese breed with genetic and phenotypic similarities to the Iberian pig [3], is well-adapted to extensive and semi-extensive production systems. Characterized by slow growth and a high and precocious propensity for fat deposition, AL pigs are highly valued for their meat and meat products’ quality, and unique sensory attributes, which are strongly influenced by both genotype and rearing conditions [4,5]. Traditionally, AL pigs have been finished outdoors, where moderate to high physical activity [6], acorn-based diets, and late slaughter ages contribute to enhanced intramuscular fat (IMF) content and desirable meat quality traits [7]. However, growing demand for fresh pork and year-round production has led to increased use of confined, indoor systems, which restrict movement and may alter metabolic development [6]. This transition raises concerns regarding the impact of physical activity limitation on meat and fat quality in this obese breed.
Exercise is a key modulator of lipid metabolism, muscle plasticity, and carcass traits. Previous research has shown that the rearing system and physical activity can influence carcass composition and meat quality in pigs [8,9], likely due to differences in movement patterns and associated metabolic adaptations [6,10,11]. Despite these findings, in traditional breeds such as the obese AL pig, the molecular mechanisms linking physical activity to phenotypic outcomes remain poorly understood, particularly regarding gene expression related to lipid metabolism, muscle growth, and energy homeostasis.
Recent studies have highlighted the metabolic plasticity of the AL pig. For example, dietary interventions such as betaine supplementation have been shown to upregulate genes involved in lipogenesis, lipolysis, and cholesterol metabolism [12]. Likewise, crossbreeding studies revealed genotype-specific effects on meat and fat traits, further emphasizing the interplay between genetics and environment [5]. However, the specific contribution of long-term physical activity in regulating these pathways remains largely unexplored in AL pigs, despite its potential to synergize with genetic and nutritional factors to optimize product quality.
Given the increasing shift from traditional outdoor to indoor confined rearing systems for the AL pig, a breed valued for its distinctive fat deposition and meat quality, the impact of reduced physical activity on product traits and metabolic regulation remains not completely understood. Coupled with rising consumer demand for high-quality and ethically produced pork [13], this study aims to isolate the effects of long-term physical activity during the finishing phase on meat and fat quality, as well as on the expression of key metabolic genes in AL pigs. By integrating phenotypic analyses of the Longissimus lumborum, Psoas major, and dorsal subcutaneous fat with molecular data, this research seeks to elucidate tissue-specific adaptations to exercise in this breed. With these findings, we aim to contribute to developing sustainable production strategies that balance animal welfare, breed conservation, and evolving market expectations.
2. Materials and Methods
All experimental procedures were conducted in strict accordance with the ethical guidelines and regulations set by the Portuguese Animal Nutrition and Welfare Commission (DGAV—Directorate-General for Food and Veterinary, Lisbon, Portugal), and followed the 2010/63/EU Directive. The data used in this work were obtained by the MITTIC Project—Modernización e Innovación Tecnológica com base TIC em sectores estratégicos y tradicionales (QREN, Cooperación Transfronteriza Portugal-España and European Regional Development Fund—FEDER), which ended in 2016.
2.1. Chemicals and Solvents
High-purity chemicals and solvents were purchased from VWR (Radnor, PA, USA) and MilliporeSigma (St. Louis, MO, USA). Molecular biology reagents and kits were obtained from Thermo Fisher Scientific (Waltham, MA, USA).
2.2. Animal Husbandry and Experimental Design
This study was conducted in collaboration with the Alentejano Breeders Association (ANCPA) to closely mimic commercial rearing and feeding practices for AL pigs. The experimental design is detailed in a companion publication [6].
Briefly, eighteen purebred AL pigs (females and males surgically castrated within the first week of life) were used in a 130-day trial, between an initial average body weight (BW) of 87.3 kg and a final weight of 161.6 kg. The pigs were randomly assigned to two groups, similar in age, gender, and body weight distribution: nine pigs were allocated to 3 m2 open-air individual pens with feeding trough and drinking nipples, zinc sheds, and concrete floor (no-exercise group, NE), and nine to an outdoor park of 400 m2 (with-exercise group, WE). The individual pens confined the pigs’ physical activity mainly to standing up, remaining on their feet, and taking a few walking steps. The common area of the outdoor park had a battery of individual feeding stalls and was connected by a corridor to an area with an automatic waterer, forcing pigs to a minimum daily walking distance of 800 m between the feeding and drinking areas. The park also provided 50 m2 of shade space from a zinc shed, along with natural tree cover. Pigs were fed two commercial diets, one during growth (up to 100 kg BW), replaced by another during the finishing period (100–160 kg BW) (for composition, see Table S1). Diets were offered at 85% estimated ad libitum intake, and water was available ad libitum. Daily feed intake, refusals, and spillage were recorded. Access to pasture was not allowed (for further details, see [6]).
At 161.6 kg BW, pigs were slaughtered following CO2 stunning and exsanguination at a commercial slaughterhouse (Maporal, Reguengos de Monsaraz, Portugal). The left side of each carcass was processed into major commercial cuts [14], with individual cut weights recorded. Samples from loin (Longissimus lumborum, LL) between the last thoracic and the 5th lumbar vertebrae, the whole tenderloin (Psoas major, PM), as well as dorsal subcutaneous fat (DSF) obtained at the last rib level were collected, vacuum-sealed, and stored at −30 °C for further analysis.
2.3. Muscle and Dorsal Subcutaneous Fat Composition
Moisture (in LL, PM, and DSF), total protein and lipid content (muscles and DSF), pH, water loss, myoglobin content, total collagen (muscles), and surface color (muscles and DSF) were previously determined, as reported by Martins et al. (2021) [6].
The FA composition of muscle and fat tissues was determined from lipid extracts [15]. Methylated FA samples [16] were analyzed using a 6890 Hewlett Packard gas chromatograph equipped with a 75 m × 0.18 mm × 0.14 µm capillary column (SP-2560, Supelco, Bellefonte, PA, USA). A temperature program of 200 °C was used, with the split–splitless injector and the FID detector maintained at 250 and 270 °C, respectively. Hydrogen was the carrier gas, used at a flow rate of 1.2 mL/min. FA methyl esters (FAMEs) were identified based on retention times of reference standards (Supelco cat. n. 47801 [17] and 47885-U [18], Bellefonte, PA, USA), and the tissue FA composition was expressed as g/100 g of the total FAME identified (C12:0, C14:0, C16:0, C16:1 n-7, C17:0, C17:1 n-8, C18:0, C18:1 n-7, transC18:1 n-9, C18:1 n-9, C18:2 n-6, C18:3 n-3, C20:0, C20:1 n-9, C20:4 n-6, and C22:1 n-9). Based on the FA profile, saturation (SAT) [19], atherogenic (ATH), thrombogenic (THR) [20], and unsaturation indices (UNSAT) [21], the hypocholesterolemic/hypercholesterolemic fatty acid (FA) ratio (h/H FA ratio) [22], the ∆9-desaturase index activity for C16 and C18 [23], the total desaturation index (TDI) [24], the nutritive value index (NVI) [25], the health-promoting index (HPI) [26], the nutritional ratio [27], the iodine value [28], and the peroxidizability index (PI) [29] were calculated.
2.4. Total RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
Tissue samples from LL, PM, and DSF were snap-frozen in liquid nitrogen at the slaughterhouse and stored at −80 °C until analysis. Total RNA was extracted using the TRIzol® Plus RNA Purification Kit (Invitrogen, Waltham, MA, USA) from muscle and fat tissue (1 mL of TRIzol reagent per ~150 mg of tissue sample) following the manufacturer’s instructions, and stored at −80 °C. RNA integrity (NanoDrop® 2000 UV –Vis Spectrophotometer, Thermo Scientific, Waltham, MA, USA) was assessed by the OD260/OD280 nm absorption ratio calculation and was greater than 1.9, indicating highly pure RNA. For reverse transcription, 1 µg of total RNA was used in a 20 µL reaction following Maxima® First Strand cDNA Synthesis Kit for RT-qPCR (Thermo Scientific, Waltham, MA, USA) per the manufacturer’s instructions.
Expression of genes involved in FA transport and lipogenesis (FABP4, Fatty acid binding protein 4; ACLY, ATP-citrate lyase; ME1, Malic enzyme 1; ACACA, Acetyl-CoA carboxylase; FASN, Fatty acid synthase; SCD, Stearoyl-CoA desaturase; ELOV6, Elongation of long-chain fatty acids family member 6; LEP, Leptin), lipolysis/FA oxidation (LPL, Lipoprotein lipase; LIPE/HSL, Hormone-sensitive lipase; CPT1B, Muscle-type carnitine palmitoyltransferase 1B; ADIPOQ, Adiponectin; IL6, Interleukin-6), muscle growth and differentiation (MYF5, Myogenic factor 5; MYOD1, Myogenic differentiation 1; MYF6, Myogenic factor 6; MYOG, Myogenin; IGF1, Insulin-like growth factor 1; IGF2, Insulin-like growth factor 2; PAX7, Paired Box 7), muscle mass regulation (MSTN, Myostatin; MTOR, Mechanistic target of rapamycin kinase; MAP3K14, Mitogen-activated protein kinase kinase kinase 14; MAFBX/FBXO32, Atrogin-1; MURF-1/TRIM63, Muscle-specific RING finger-1), muscle contraction and structure (TNNT1, Troponin T1; MYH3, Myosin heavy chain 3; MYH7, Myosin heavy chain 7), as well as regulation of lipid and metabolism (PPARG, Peroxisome proliferator-activated receptor gamma; PPARA, Peroxisome proliferator-activated receptor alpha; EGF, Epidermal growth factor) and three housekeeping genes, heat shock protein 90 alpha family class B member 1 (HSP90AB1/HSPCB), hypoxanthine phosphoribosyltransferase 1 (HPRT1) and β-actin (ACTB) (Table S2), were investigated by real-time PCR. Amplification mixtures containing 12.5 µL of 2 × SYBR Green PCR Master Mix, 0.3 µM of each primer, and 12.5 ng of cDNA per sample were prepared in 96-well plates and run using a 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) for 40 amplification cycles comprising a 15 s denaturation at 95 °C, followed by a 50 s at 60 °C step. A no-template control was run with every assay, and samples were performed in triplicate as technical replicates. Cycle threshold (CT) values were determined using the fit-point method and the Applied Biosystems 7500 software with a fluorescence threshold and baseline automatically set. Melt curve analysis was conducted at the end of the PCR to validate the specificity of the primers.
PCR efficiency was estimated based on five-point standard curves of a two-fold dilution series (1:84-1:128) from pooled cDNA of each tissue. Overall, primer efficiency for each tissue fluctuated between acceptable values of 90 and 105%.
2.5. Calculations and Data Analyses
Results are presented as means ± standard error of the mean (SEM). Data normality was verified using the Shapiro–Wilk test. Statistical comparisons were performed using Student’s t-test (IBM SPSS Statistics for Windows, Version 24.0. IBM Corp.: Armonk, NY, USA). Target relative expression data were obtained using normalization factors based on the expression of endogenous control genes (HSP90AB1, HPRT1, and ACTB) with the geNorm application [30]. For multiple group comparisons, a multiple test correction (Benjamini and Hochberg False Discovery Rate) [31] was used. Differences were considered significant when p < 0.05 and trends considered at probability values between 0.05 and 0.10.
3. Results
All pigs remained in good health throughout the experimental period. In this period, average daily temperatures and relative humidity were recorded as follows: 21.1 °C (mean), 29.5 °C (max), 14.1 °C (min), and 51.4%, respectively.
3.1. Animal Performance, Carcass, and Cuts
Animal performance, carcass, and cut data were previously reported [6]. Briefly, at the end of the trial and with a similar daily feed intake, WE pigs presented a higher final weight (p = 0.027) and average daily gain (ADG) (p = 0.005), leading to a lower feed conversion ratio (p = 0.006) compared to NE ones (Figure 1). DSF thickness and its increase from 90 to 160 kg were also higher in WE pigs (p = 0.081 and p = 0.019, respectively). While LL thickness followed a similar trend, differences were not statistically significant. Additionally, hot carcass weight and carcass yield were higher in WE pigs (p = 0.029 and p = 0.104, respectively; see Figure 1), as was PM weight (+12.7%; p = 0.063).
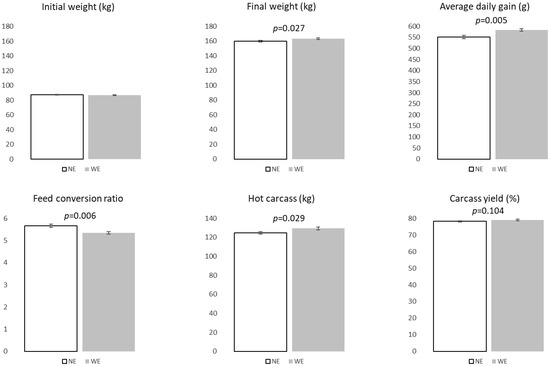
Figure 1.
Growth performance, carcass characteristics, and major cut weights of Alentejano pigs raised in individual pens without exercise (NE, n = 9) or outdoors with exercise (WE, n = 9) from 87.3 to 161.6 kg LW.
3.2. Tissue Composition
Tissue composition data are detailed elsewhere [6]. Briefly, chemical composition of both muscles was significantly different among treatments only in their total protein content, which was higher in LL (p = 0.044) and PM (p = 0.0002) for the WE pigs. Finally, the DSF physical–chemical composition was not affected by experimental treatments.
3.3. FA Profile in Muscles and Subcutaneous Fat Tissues
Oleic acid (C18:1 n-9) was the predominant FA in LL and PM from AL pigs. For LL, oleic acid proportions varied between 52.0 and 51.1 g/100 g (Table 1), and for PM, between 38.4 and 39.2 g/100 g (Table 3) (for NE and WE pigs, respectively), of the total FAMEs analyzed.

Table 1.
Major fatty acid profile (g/100 g) of total intramuscular lipids of Longissimus lumborum muscle in Alentejano pigs kept in individual pens without exercise (NE, n = 9) or outdoors with exercise (WE, n = 9) from 87.3 to 161.6 kg LW.
Composition of the major FAs from LL and PM was slightly affected by the experimental treatments. In LL, WE pigs exhibited ~9% higher palmitoleic (C16:1 n-7; p = 0.031) and ~18% higher polyunsaturated linolenic (C18:3 n-3; p = 0.022) acids than NE pigs (Table 1). On the other hand, palmitic (C16:0), stearic (C18:0), and oleic acid contents were not affected by the experimental treatments. Overall, LL proportions of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) were not significantly affected by experimental treatments, as well as the sum of n-6 and n-9 FAs, but the sum of n-3 FAs was 18% higher (p = 0.022). These FA profiles for NE and WE pigs led to identical LL lipid and lipid quality indices and ratios (Table 2). Only the h/H FA ratio, C16:1/C16, ∆9-desaturase index activity for C16, and nutritional ratio tended to be significantly different, respectively, at ~3% lower (p = 0.089), and ~5 (p = 0.053), 7 (p = 0.053), and 5% higher (p = 0.091) in WE pigs when compared to NE pigs. In PM, WE pigs showed a ~9% decrease in the stearic acid (p = 0.085) and a ~24% increase in the linolenic acid (p = 0.058) proportion, with the latter contributing to the increase in the sum of n-3 FAs (Table 3). Nevertheless, these changes were not sufficient to significantly affect the sum of SFAs, MUFAs, and PUFAs, as well as the indices and ratios between both experimental groups (Table 4). Finally, and as observed in LL, PM palmitic, stearic, and oleic acid contents were not affected by the experimental treatments (see Table 3).

Table 2.
Lipid and lipid quality indices and ratios of Longissimus lumborum muscle in Alentejano pigs kept in individual pens without exercise (NE, n = 9) or outdoors with exercise (WE, n = 9) from 87.3 to 161.6 kg LW.

Table 3.
Major fatty acid profile (g/100 g) of total intramuscular lipids of Psoas major muscle in Alentejano pigs kept in individual pens without exercise (NE, n = 9) or outdoors with exercise (WE, n = 9) from 87.3 to 161.6 kg LW.

Table 4.
Lipid and lipid quality indices and ratios of Psoas major muscle in Alentejano pigs kept in individual pens without exercise (NE, n = 9) or outdoors with exercise (WE, n = 9) from 87.3 to 161.6 kg LW.
The most abundant FA present on the total lipids of DSF from AL pigs was also oleic acid (Table 5). This FA presented a ~3% decrease in WE when compared to NE pigs, although not attaining statistical significance (p = 0.111). This trend contributed to the decrease in n-9 proportions (p = 0.095), which was ~3% lower in the DSF of WE pigs. Conversely, linoleic acid (C18:2 n-6) increased ~4% in WE pigs (p = 0.072). Finally, as observed in both muscles, DSF palmitic acid, stearic, SFA, MUFA, and PUFA contents were not affected by the experimental treatments (Table 5), leading to identical lipid and lipid quality indices and ratios (Table 6). Only the ATH index, nutritive value index, and nutritional ratio tended to be significantly different, respectively, being ~4% lower (p = 0.088 and p = 0.098), and ~4% higher (p = 0.095) in WE pigs when compared to NE pigs.

Table 5.
Major fatty acid profile (g/100 g) of total lipids of dorsal subcutaneous fat in Alentejano pigs kept in individual pens without exercise (NE, n = 9) or outdoors with exercise (WE, n = 9) from 87.3 to 161.6 kg LW.

Table 6.
Lipid and lipid quality indices of dorsal subcutaneous fat in Alentejano pigs kept in individual pens without exercise (NE, n = 9) or outdoors with exercise (WE, n = 9) from 87.3 to 161.6 kg LW.
3.4. Gene Expression
FA synthesis and transport-related genes in both muscles showed few significant differences between groups (Figure 2). However, FASN and LEP expression decreased in the LL of WE pigs (~47 and 53%; p = 0.071 and p = 0.018, respectively) when compared to NE pigs. In PM, FABP4 was ~33% lower (p = 0.003), while SCD increased by ~147% (p = 0.020) in WE pigs when compared to NE pigs. Expression of lipolysis/FA oxidation-related genes showed a similar pattern (Figure 3). In LL, only one of the target genes showed statistical differences, ADIPOQ (p = 0.041), which was associated with a 48% decrease in WE pigs when compared to NE pigs. Furthermore, CPT1B tended to decrease (~21%, p = 0.051) in LL, and the same happened in PM, with WE pigs showing a ~33% lower (p = 0.012) relative expression compared to NE pigs.
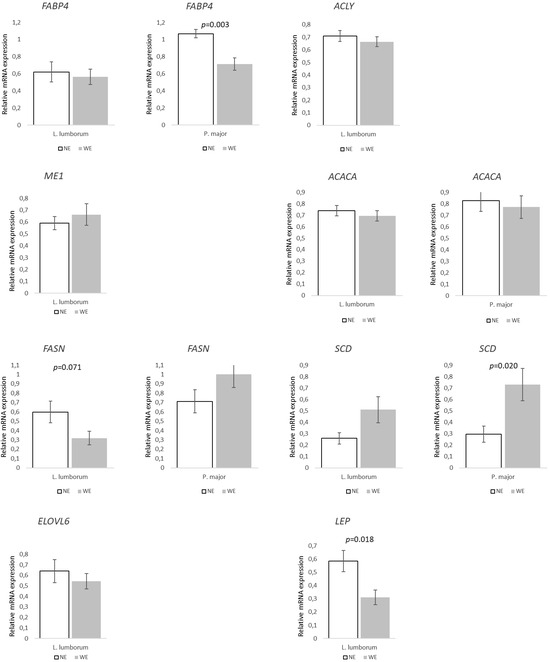
Figure 2.
Relative expression of genes involved in fatty acid transport and lipogenesis in Longissimus lumborum and Psoas major muscles of Alentejano pigs raised in individual pens without exercise (NE, n = 6) or outdoors with exercise (WE, n = 6) from 87.3 to 161.6 kg LW. FABP4 (Fatty acid binding protein 4), ACLY (ATP-citrate lyase), ME1 (Malic enzyme 1), ACACA (Acetyl-CoA carboxylase), FASN (Fatty acid synthase), SCD (Stearoyl-CoA desaturase), ELOVL6 (Elongation of long-chain fatty acids family member 6), and LEP (Leptin). Values are means with their standard errors represented by vertical bars.
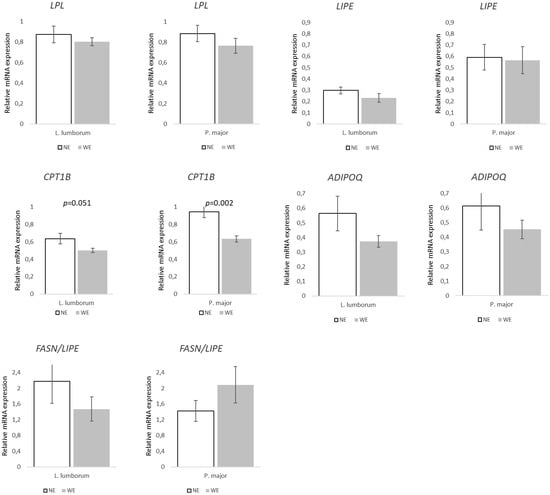
Figure 3.
Relative expression of genes related to lipolysis/fatty acid oxidation and FASN/LIPE ratio in Longissimus lumborum and Psoas major muscles of Alentejano pigs raised in individual pens without exercise (NE, n = 6) or outdoors with exercise (WE, n = 6) from 87.3 to 161.6 kg LW. LPL (Lipoprotein lipase), LIPE (Hormone-sensitive lipase), CPT1B (Muscle-type carnitine palmitoyltransferase 1), ADIPOQ (Adiponectin), and FASN (Fatty acid synthase)/LIPE. Values are means with their standard errors represented by vertical bars.
Regarding the genes associated with muscle growth and differentiation (Figure 4), they were not differently expressed between groups regardless of the muscle. Similarly, the expression of genes associated with muscle mass regulation (Figure 5) did not statistically differ between LL muscles from WE and NE pigs. On the other hand, within the genes associated to muscle contraction and structure (Figure 6), both TNNT1 (p = 0.058) and MYH7 (p = 0.016) revealed increases of ~50 and 69% in the LL of WE pigs when compared to NE pigs.
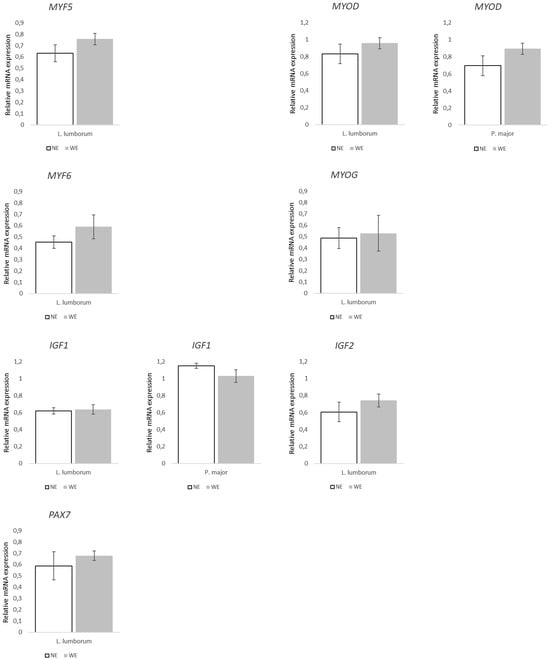
Figure 4.
Relative expression of genes associated with muscle growth and differentiation markers in Longissimus lumborum and Psoas major muscles of Alentejano pigs raised in individual pens without exercise (NE, n = 6) or outdoors with exercise (WE, n = 6) from 87.3 to 161.6 kg LW. MYF5 (Myogenic factor 5), MYOD (Myogenic differentiation 1), MYF6 (Myogenic factor 6), MYOG (Myogenin), IGF1 (Insulin-like growth factor 1), IGF2 (Insulin-like growth factor 2), and PAX7 (Paired Box 7). Values are means with their standard errors represented by vertical bars.
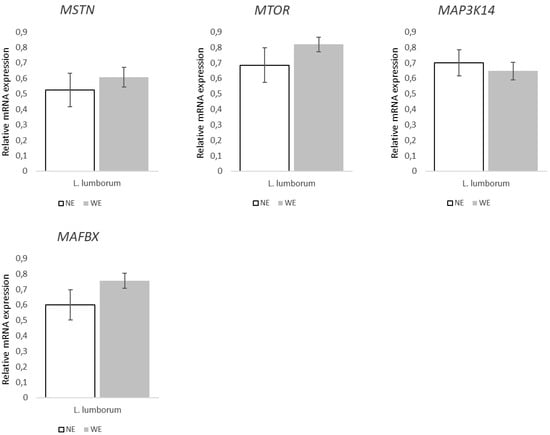
Figure 5.
Relative expression of muscle mass regulation factors in Longissimus lumborum muscle of Alentejano pigs raised in individual pens without exercise (NE, n = 6) or outdoors with exercise (WE, n = 6) from 87.3 to 161.6 kg LW. MSTN (Myostatin), MTOR (Mammalian target of rapamycin), MAP3K14 (Mitogen-activated protein kinase kinase kinase 14), MAFBX (Atrogin-1/FBXO32), and MURF-1 (Muscle-specific RING finger-1). Values are means with their standard errors represented by vertical bars.
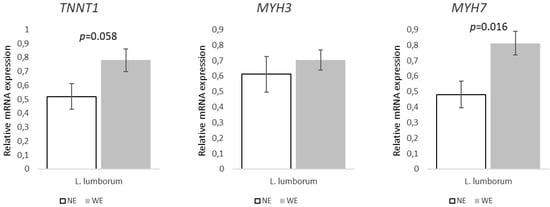
Figure 6.
Relative expression of genes associated with muscle contraction and structure in Longissimus lumborum muscle of Alentejano pigs raised in individual pens without exercise (NE, n = 6) or outdoors with exercise (WE, n = 6) from 87.3 to 161.6 kg LW. TNNT1 (Troponin T1), MYH3 (Myosin heavy chain 3), and MYH7 (Myosin heavy chain 7). Values are means with their standard errors represented by vertical bars.
Expression of genes associated with the regulation of lipid and muscle metabolism (Figure 7) revealed differences only in the LL muscle. PPARG decreased ~33% (p = 0.032) and EGF increased 50% (p = 0.061) in WE pigs when compared to NE pigs.
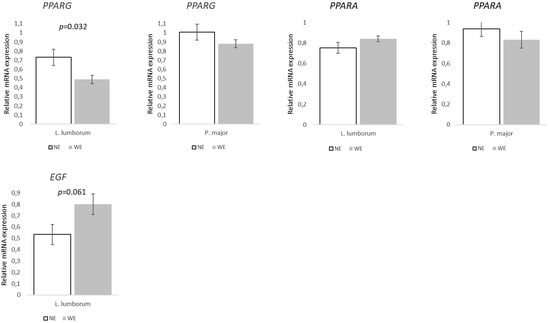
Figure 7.
Relative expression of genes involved in lipid and muscle metabolism in Longissimus lumborum and Psoas major muscles of Alentejano pigs raised in individual pens without exercise (NE, n = 6) or outdoors with exercise (WE, n = 6) from 87.3 to 161.6 kg LW. PPARG (Peroxisome proliferator-activated receptor gamma), PPARA (Peroxisome proliferator-activated receptor alpha), and EGF (Epidermal growth factor). Values are means with their standard errors represented by vertical bars.
Finally, in DSF, the relative expression in the selected set of genes related to FA transport and lipogenesis (Figure 8) showed a pattern towards WE pigs, with increases of ~37% on ACACA (p = 0.078), 27% on FASN (p = 0.082), and 47% on SCD (p = 0.073). Regarding the genes associated with lipolysis/FA oxidation (Figure 9), only LIPE presented significant differences between groups, with a ~20% decrease (p = 0.010) in WE pigs. Additionally, the FASN/LIPE ratio was ~60% higher (p = 0.0007) in WE pigs compared to NE pigs.
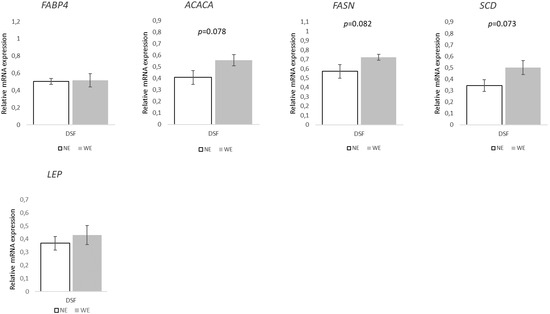
Figure 8.
Relative expression of key lipogenic genes and fatty acid transporters in dorsal subcutaneous fat tissue of Alentejano pigs raised in individual pens without exercise (NE, n = 6) or outdoors with exercise (WE, n = 6) from 87.3 to 161.6 kg LW. FABP4 (Fatty acid binding protein 4), ACACA (Acetyl-CoA carboxylase), FASN (Fatty acid synthase), SCD (Stearoyl-CoA desaturase), and LEP (Leptin). Values are means with their standard errors represented by vertical bars.
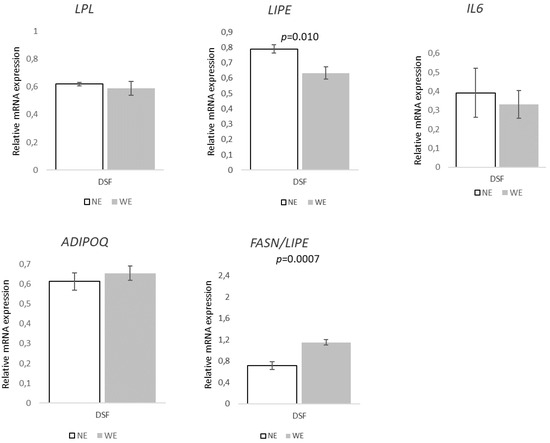
Figure 9.
Relative expression of genes associated with lipolysis/fatty acid oxidation, inflammatory markers, and FASN/LIPE ratio in dorsal subcutaneous fat tissues of Alentejano pigs raised in individual pens without exercise (NE, n = 6) or outdoors with exercise (WE, n = 6) from 87.3 to 161.6 kg LW. LPL (Lipoprotein lipase), LIPE (Hormone-sensitive lipase), IL6 (Interleukin-6), ADIPOQ (Adiponectin), and FASN (Fatty acid synthase)/LIPE. Values are means with their standard errors represented by vertical bars.
4. Discussion
Extensive pig production systems that promote movement, exploratory behavior, and foraging can influence growth and meat quality [32], especially in local breeds like the AL pig [6]. Although AL and Iberian pigs naturally walk long distances for food [7], modern systems increasingly restrict outdoor access to reduce production time and costs. Besides Martins et al. (2021) [6], no studies have investigated long-term physical activity effects on meat traits in AL pigs. This exploratory study addresses this gap by evaluating how sustained exercise affects meat and fat quality, as well as the expression of key metabolic genes in muscle (LL and PM) and subcutaneous fat (DSF) in AL pigs slaughtered at ~160 kg BW.
As noted by Bee et al. [33], comparisons between outdoor and indoor systems often involve confounding factors (physical activity and environmental stimuli) which jointly affect animal performance and product quality. To minimize such effects, our pigs were reared in adjacent pens or an outdoor park, fed identical diets at 85% ad libitum, had free access to water, and no access to pasture [6].
Animals remained healthy throughout the trial. Although some previous studies reported limited effects of exercise on growth and carcass traits, especially with low exercise intensity or duration [34,35], our study found that long-term physical activity significantly improves growth performance in AL pigs [6]. This was shown by higher final body weight, increased ADG, and better feed conversion ratio in WE pigs compared to NE pigs. These improvements align with previous reports in outdoor-reared pigs, where welfare and physical activity positively influenced performance [10,32,36]. Higher carcass yield and DSF thickness observed in WE pigs [6] suggest enhanced lipid deposition, which is consistent with increased backfat and carcass fat in pigs reared under enriched or outdoor conditions [8,9,37]. Such fat distribution may be beneficial in traditional pork systems valuing external fat cover and muscle quality. Finally, increased muscle protein in WE pigs [6] likely reflects enhanced anabolism associated with sustained activity [38,39]. Lower plasma cortisol levels in WE pigs when compared to NE pigs [6] may have also contributed, as cortisol is known to inhibit protein synthesis [40].
The effects of physical activity on muscle FA composition were subtle but biologically relevant. In the LL muscle of WE pigs, palmitoleic and linolenic acid proportions increased, while linolenic acid showed a near-significant rise in the PM muscle (p = 0.058). The higher palmitoleic acid could reflect exercise-induced upregulated SCD activity, which catalyzes the conversion of palmitic (C16:0) to palmitoleic acid, consistent with traditional breed adaptations in more active rearing systems [41,42]. Increased linolenic acid aligns with previous findings that physical activity and outdoor rearing enhance PUFA content in pork, particularly n-3 FAs [9,33], improving membrane fluidity and insulin sensitivity [43]. This rise in n-3 linolenic acid observed in WE pigs is also beneficial for meat nutritional quality and consumer health, as higher n-3 PUFA intake is associated with reduced cardiovascular risk [44,45].
Notably, the WE pigs’ FA profiles partly matched the reference values established for outdoor-reared Iberian pigs [46]. Specifically, muscle and subcutaneous fat (see below) samples from WE pigs showed favorable trends, including modest shifts in the FA composition and an increase in n-3 PUFA levels, without exceeding limits that would compromise quality classification. Although these pigs were not pasture-fed or supplemented with acorns, the physiological adaptations resulting from sustained physical activity appear to reproduce some of the beneficial FA profile traits typical of extensive, high-quality systems such as “cebo de campo”. These findings are in line with the hypothesis that physical activity, independent of dietary inputs, can positively influence fat quality in local pig breeds like AL pigs.
Desaturation indices (C16:1/C16 ratio and ∆9-desaturase activity for C16) and lipid quality indices (h/H FA and nutritional ratios) tended to increase in the LL of WE pigs (p = 0.053, 0.053, 0.089, and 0.091, respectively). These trends suggest the enhanced synthesis of unsaturated FAs (UFAs), as previously reported in Iberian pigs [41], and is further supported by SCD upregulation in both LL and PM muscles (p = 0.120 and 0.020, respectively).
The significant upregulation of SCD in PM, but not in LL, may reflect their distinct metabolic profiles. Studies in rodents have shown that SCD1 expression and activity are markedly increased in oxidative muscles, but less so in glycolytic muscles or the liver [47]. As a highly oxidative muscle with dense mitochondrial content, PM is specialized for sustained activity through FA oxidation, demanding more MUFAs, like oleic acid, for mitochondrial β-oxidation [42,48]. SCD converts SFAs, like stearic acid, into MUFAs, such as oleic acid [49], and may be upregulated to meet this demand [50]. Oleic acid is among the principal FAs oxidized by skeletal muscle during aerobic exercise [51,52], and the steady oleic acid proportion, despite the increased SCD expression in the PM of WE pigs, suggests continuous utilization. Additionally, a trend toward higher linolenic acid and a near-significant reduction in stearic acid (p = 0.085) supports increased desaturation activity and oxidative demand in this tissue.
Conversely, the lower proportion of gadoleic acid (C20:1 n-9) in the LL muscle of WE pigs, an FA synthesized via the elongation of oleic acid mainly through ELOVL5 and ELOVL6 [53], likely reflects a shift in metabolic priorities induced by exercise. This shift may include a reduced lipid storage capacity and the downregulation of key lipogenic genes in this predominantly glycogenic muscle [54,55] (see below). These muscle-specific responses highlight the importance of fiber-type composition in modulating lipid metabolism and gene expression in response to physical activity.
In the glycolytic LL muscle, the downregulation of lipogenic and adipogenic genes, such as FASN, LEP, and PPARG, in WE pigs suggests reduced de novo FA synthesis. Although direct evidence in pigs is lacking, exercise has been shown to decrease lipogenic enzyme activity in other mammals, like the rat liver [56]. This downregulation may reflect a metabolic shift toward enhanced FA catabolism rather than (MUFA) elongation and storage [33,57], as well as increased dependence on circulating FAs as energy substrates during exercise [44,57]. Supporting this, plasma triacylglycerol levels were consistently lower in WE pigs than in NE ones (−17, −7, and −28% at weeks 11 and 18, and slaughter, respectively), suggesting increased uptake and oxidation of blood-borne FAs by muscle tissue [6]. These findings align with reports that glycolytic muscles are less responsive to exercise-induced lipid gene modulation and rely more on glycogenolysis for energy [54].
These transcriptional changes, though modest, suggest a shift in muscle function and fiber-type composition, with the upregulation of the slow-twitch fiber markers MYH7 and TNNT1 (p = 0.016 and 0.058, respectively) in LL indicating partial oxidative remodeling [58], but not enough to induce SCD upregulation, as seen in the PM. EGF upregulation may contribute to this transition by promoting satellite cell-mediated repair and fiber differentiation, as shown in exercised muscle models [59]. The reported increased muscle protein content may also reflect a fiber-type shift, as exercise induces a transition toward more oxidative, slow-twitch fibers, associated with higher protein density and endurance [48,60,61]. Although histochemical analysis was not performed in this trial, MYH7 upregulation in WE pigs supports this molecular shift. Such metabolic and structural adaptations at the fiber level, particularly toward oxidative fibers, may affect pork sensory traits like tenderness, the water-holding capacity, and juiciness [61,62].
Our findings align with recent studies [63] showing distinct gene expression patterns between oxidative and glycolytic muscles in pigs. These differences reflect the enrichment of lipid metabolism and mitochondrial function pathways in oxidative muscles, supporting their role in sustained FA oxidation and metabolic efficiency. Such differentiation impacts metabolic adaptability and meat quality traits, especially intramuscular fat content and composition. While diet predominantly shapes tissue FA profiles [64], physical activity also modulates the balance between SFAs and UFAs, influencing the nutritional quality and healthfulness of pork [33]. Moreover, recent systems biology and transcriptomic analyses highlight mitochondrial function’s role in feed efficiency and skeletal muscle metabolism in pigs. Genes linked to mitochondrial translation elongation (not tested), electron transport, and FA β-oxidation are upregulated with exercise, paralleling human muscle adaptations [55,64]. In this context, our results of improved feed conversion and increased muscle protein in WE pigs [6] suggest that enhanced mitochondrial activity may contribute to greater metabolic efficiency and adaptive muscle remodeling toward a more oxidative profile during long-term physical activity.
The marked downregulation of FABP4 in the PM muscle of WE pigs likely reflects a beneficial metabolic adaptation to long-term physical activity. High FABP4 expression is linked to obesity-related metabolic stress, inflammation, and impaired insulin sensitivity, while its reduction (whether through exercise or other interventions) is associated with improved metabolic health and enhanced lipid oxidative capacity [65,66]. The oxidative, metabolically flexible PM muscle’s responsiveness to environmental changes [33] likely explains the observed FABP4 downregulation in WE pigs [65,66]. Conversely, the glycolytic LL muscle, which is less dependent on lipid oxidation, showed no significant FABP4 change, highlighting muscle-type specificity in metabolic plasticity. These findings suggest that physical activity promotes a healthier metabolic profile in oxidative muscle by reducing FABP4 expression, facilitating FA oxidation and overall muscle function in this fatty pig breed. Additionally, pen-reared pigs, such as those in the NE group, with higher plasma cortisol levels [6], may upregulate FABP4 due to chronic stress, possibly supporting lipid storage in sedentary conditions.
CPT1B facilitates FA transport into mitochondria for β-oxidation [67]. The upregulation of this gene in both muscles of NE pigs (p = 0.051 and 0.002 in LL and PM, respectively) suggests a compensatory response to reduced physical activity and intracellular FA accumulation [33,39]. In oxidative muscles like the PM, decreased physical activity and mitochondrial respiration demand may cause FA accumulation [33,61], triggering the upregulation of CPT1B to enhance beta-oxidation despite limited capacity [39]. Similarly, the upregulation of PPARG in NE pigs may reflect insulin resistance and lipid-induced stress, as PPARG regulates adipocyte differentiation and lipid storage [68,69]. The upregulation of these genes in NE pigs might also be a response to low-grade inflammation from inactivity or FA accumulation. Conversely, active pigs show lower PPARG and CPT1B expression, suggesting improved mitochondrial efficiency and reduced lipid overload, diminishing the need for compensatory mechanisms [70].
In DSF, the expression of ACACA, FASN, and SCD showed consistent but non-significant increases in WE pigs (p = 0.078, 0.082, and 0.073, respectively), reflecting its role as a lipid reservoir and the increased DSF thickness observed in this group [6]. These findings align with previous reports that subcutaneous and IM fat depots are regulated by distinct molecular mechanisms and respond differentially to exercise in a tissue-specific manner [71]. Oxidative muscles like the PM prioritize FA oxidation, DSF acts as a dynamic lipid reservoir influenced by hormonal and metabolic changes, while glycolytic muscles such as LL rely mainly on glycogenolysis, with limited lipid storage or oxidative capacity [71,72,73]. The fatty AL pig’s genotype favors lipid storage via esterification and de novo synthesis, evidenced by the upregulation of genes like FASN, ACLY, and ME1 [74,75], a tendency reinforced rather than counteracted by exercise [76]. Supporting this, FASN expression in DSF rose by ~27% (p = 0.082), while HSL, a key enzyme in lipolysis [77], decreased by 20% (p = 0.010) in WE pigs versus NE pigs. The resulting higher FASN to LIPE ratio in the WE pigs suggests net lipid accumulation, consistent with the increase in DSF thickness [6]. Lower plasma cortisol in the WE pigs likely also suppressed lipolysis, as glucocorticoids stimulate LIPE expression and fat mobilization [40]. Although sensory quality was not directly assessed, the literature indicates that the rearing system and physical activity can affect sensory traits. Free-range or outdoor rearing has been linked to improved tenderness, juiciness, and overall consumer acceptance [10,62]. However, results across studies are mixed, varying by genotype, sex, and muscle type [10,34]. In this study, the higher muscle protein content and better FA profile in the WE pigs may enhance the sensory and nutritional quality, aligning with consumer preferences for traditional or free-range pork [9,11]. Further research with direct sensory and structural evaluations is needed to confirm the potential benefits.
5. Conclusions
This study demonstrates that sustained physical activity during the finishing phase promotes not only significant improvements in growth performance, carcass yield, and muscle protein content [6], but also modest yet favorable metabolic adaptations in the muscles and adipose tissues of AL pigs. These changes include the upregulation of certain oxidative muscle markers and gene expression patterns, suggesting a more favorable lipid metabolism. Although the effects on the FA composition were subtle, increases in linolenic and palmitoleic acids, together with trends toward improved lipid quality without compromising meat quality, suggest potential nutritional benefits relevant to both producers and consumers. While only a limited number of statistically significant differences were observed, this work provides the first molecular and phenotypic evidence of potential metabolic adaptations to long-term physical activity in AL pigs. Collectively, these findings support the role of physical activity as a practical, non-dietary intervention to improve performance, meat quality, and animal welfare in traditional rearing systems.
Further research integrating direct animal welfare and activity indices, alongside genetic, nutritional, and environmental variables, is needed to validate and refine these strategies, ensuring both breed sustainability and alignment with the evolving consumer demands for high-quality, ethically produced pork.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15192047/s1, Table S1: Components and chemical composition of the experimental diets; Table S2: Porcine-specific primers used for real-time PCR [58,67,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98].
Author Contributions
Conceptualization, J.M.M. and A.F.; methodology, J.M.M., A.A., D.S., and J.A.N.; animal management, D.S., R.C., A.F., J.M.M., and J.A.N.; data analysis, J.M.M. and A.A.; writing—original draft preparation, J.M.M.; writing—review, J.M.M., A.A., D.S., J.A.N., R.C., and A.F.; writing—editing, J.M.M.; project administration and funding acquisition, A.F. and J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the MITTIC Project—Modernización e Innovación Tecnológica com base TIC em sectores estratégicos y tradicionales (QREN, Cooperación Transfronteriza Portugal-España and European Regional Development Fund—FEDER, 0606_MITTIC_4_E) and by National Funds through FCT—Fundação para a Ciência e a Tecnologia under the Project UIDB/05183.
Institutional Review Board Statement
The study was conducted according to the regulations and ethical guidelines set by the Portuguese Animal Nutrition and Welfare Commission (DGAV—Directorate-General for Food and Veterinary, Lisbon, Portugal) following the 2010/63/EU Directive.
Data Availability Statement
Data will not be shared due to privacy issues.
Acknowledgments
The authors would like to thank Alentejano pig Breeders National Association—ANCPA, Portugal, for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACACA | Acetyl-CoA carboxylase |
| ACLY | ATP-citrate lyase |
| ACTB | β-actin |
| ADG | Average daily gain |
| ADIPOQ | Adiponectin |
| AL | Alentejano |
| ATH index | Atherogenic index |
| BW | Body weight |
| CPT1B | Muscle-type carnitine palmitoyltransferase 1 |
| DSF | Dorsal subcutaneous fat |
| EGF | Epidermal growth factor |
| ELOVL6 | Elongation of long-chain fatty acids family member 6 |
| FA | Fatty acid |
| FABP4 | Fatty acid binding protein 4 |
| FASN | Fatty acid synthase |
| h/H FA ratio | Hypocholesterolemic/hypercholesterolemic fatty acid ratio |
| HPI | Health-promoting index |
| HPRT1 | Hypoxanthine phosphoribosyltransferase 1 |
| HSP90AB1/HSPCB | Heat shock protein 90 alpha family class B member 1 |
| IGF1 | Insulin-like growth factor 1 |
| IGF2 | Insulin-like growth factor 2 |
| IL6 | Interleukin-6 |
| IMF | Intramuscular fat |
| LEP | Leptin |
| LIPE | Hormone-sensitive lipase |
| LL | Longissimus lumborum |
| LPL | Lipoprotein lipase |
| MAFBX | Atrogin-1/FBXO32 |
| MAP3K14 | Mitogen-activated protein kinase kinase kinase 14 |
| ME1 | Malic enzyme 1 |
| MSTN | Myostatin |
| MTOR | Mammalian target of rapamycin |
| MUFA | Monounsaturated fatty acid |
| MURF1 | Muscle-specific RING finger-1 |
| MYF5 | Myogenic factor 5 |
| MYF6 | Myogenic factor 6 |
| MYH3 | Myosin heavy chain 3 |
| MYH7 | Myosin heavy chain 7 |
| MYOD | Myogenic differentiation 1 |
| MYOG | Myogenin |
| NE | No-exercise group |
| NVI | Nutritive value index |
| PAX7 | Paired Box 7 |
| PI | Peroxidizability index |
| PM | Psoas major |
| PPARA | Peroxisome proliferator-activated receptor alpha |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| PUFA | Polyunsaturated fatty acid |
| SAT index | Saturation index |
| SCD | Stearoyl-CoA desaturase |
| SFA | Saturated fatty acid |
| TDI | Total desaturation index |
| THR index | Thrombogenic index |
| TNNT1 | Troponin T1 |
| UFA | Unsaturated fatty acid |
| UNSAT index | Unsaturation index |
| WE | With-exercise group |
References
- Olsson, I.A.S.; Jonge, F.H.d.; Schuurman, T.; Helmond, F.A. Poor rearing conditions and social stress in pigs: Repeated social challenge and the effect of behavioral and physiological responses to stressors. Behav. Process. 1999, 46, 201–215. [Google Scholar] [CrossRef]
- Beattie, V.E.; O’Connell, N.E.; Moss, B.W. Influence of environmental enrichment on the behavior, performance, and meat quality of domestic pigs. Livest. Prod. Sci. 2000, 65, 71–79. [Google Scholar] [CrossRef]
- Muñoz, M.; Bozzi, R.; García, F.; Núñez, Y.; Geraci, C.; Crovetti, A.; García-Casco, J.; Alves, E.; Škrlep, M.; Charneca, R.; et al. Diversity across major and candidate genes in European local pig breeds. PLoS ONE 2018, 13, e0207475. [Google Scholar] [CrossRef]
- Pugliese, C. Quality of meat and cured products of Mediterranean autochthonous pigs. In Options Méditerranéennes: Série A, Séminaires Méditerranéens, Proceedings of the 7th International Symposium on the Mediterranean Pig, Cordoba, Spain, 14–16 October 2010; De Pedro, E.J., Cabezas, A.B., Eds.; CIHEAM: Zaragoza, Spain, 2012; Volume 101, pp. 267–273. [Google Scholar]
- Martins, J.M.; Fialho, R.; Albuquerque, A.; Neves, J.; Freitas, A.; Nunes, J.T.; Charneca, R. Portuguese Local Pig Breeds: Genotype Effects on Meat and Fat Quality Traits. Animals 2020, 10, 905. [Google Scholar] [CrossRef]
- Martins, J.M.; Silva, D.; Albuquerque, A.; Neves, J.; Charneca, R.; Freitas, A. Physical Activity Effects on Blood Parameters, Growth, Carcass, and Meat and Fat Composition of Portuguese Alentejano Pigs. Animals 2021, 11, 156. [Google Scholar] [CrossRef]
- López-Bote, C.J. Sustained utilization of the Iberian pig breed. Meat Sci. 1998, 49, S17–S27. [Google Scholar] [CrossRef]
- Enfält, A.-C.; Lundström, K.; Hansson, I.; Lundeheim, N.; Nyström, P.-E. Effects of outdoor rearing and sire breed (Duroc or Yorkshire) on carcass composition and sensory and technological meat quality. Meat Sci. 1997, 45, 1–15. [Google Scholar] [CrossRef]
- Lebret, B.; Guillard, A.-S. Outdoor rearing of cull sows: Effects on carcass, tissue composition and meat quality. Meat Sci. 2005, 70, 247–257. [Google Scholar] [CrossRef]
- Edwards, S.A. Product quality attributes associated with outdoor pig production. Livest. Prod. Sci. 2005, 94, 5–14. [Google Scholar] [CrossRef]
- Nilzén, V.; Babol, J.; Dutta, P.C.; Lundeheim, N.; Enfält, A.C.; Lundström, K. Free range rearing of pigs with access to pasture grazing—Effect on fatty acid composition and lipid oxidation products. Meat Sci. 2001, 58, 267–275. [Google Scholar] [CrossRef]
- Albuquerque, A.; Neves, J.A.; Redondeiro, M.; Laranjo, M.; Félix, M.R.; Freitas, A.; Tirapicos, J.L.; Martins, J.M. Long term betaine supplementation regulates genes involved in lipid and cholesterol metabolism of two muscles from an obese pig breed. Meat Sci. 2017, 124, 25–33. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- NP-2931; Swine Slaughtered for Direct Consumption—Cutting Half Carcass. Instituto Português da Qualidade: Lisboa, Portugal, 2006.
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bannon, C.; Craske, J.; Hilliker, A. Analysis of fatty acid methyl esters with high accuracy and reliability. IV. Fats with fatty acids containing four or more carbon atoms. J. Am. Oil Chem. Soc. 1985, 62, 1501–1507. [Google Scholar] [CrossRef]
- Supelco. Fatty Acid/FAME Application Guide. Analysis of Foods for Nutritional Needs; Sigma-Aldrich: Bellefonte, PA, USA, 2008; pp. 1–24. [Google Scholar]
- Supelco. Comparison of 37 Component FAME Standard on Four Capillary GC Columns; Sigma-Aldrich: Bellefonte, PA, USA, 1996; pp. 1–4. [Google Scholar]
- Goluch, Z.; Wereńska, M.; Wołoszyn, J.; Rybarczyk, A.; Okruszek, A.; Teleszko, M.; Haraf, G. Effect of BioPlus YC probiotic on the fatty acid profile and lipid indices in pork. J. Elem. 2020, 25, 973–991. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Cava, R.; Estévez, M.; Morcuende, D.; Antequera, T. Evolution of fatty acids from intramuscular lipid fractions during ripening of Iberian hams as affected by α-tocopheryl acetate supplementation in diet. Food Chem. 2003, 81, 199–207. [Google Scholar] [CrossRef]
- Fernández, M.; Ordóñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; Hoz, L.d.l. Fatty acid compositions of selected varieties of Spanish dry ham related to their nutritional implications. Food Chem. 2007, 101, 107–112. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Siebert, B.D.; Bottema, C.D.K.; Pitchford, W.S. Breed comparison of the fatty acid composition of muscle phospholipids in Jersey and Limousin cattle. J. Anim. Sci. 1998, 76, 766–773. [Google Scholar] [CrossRef][Green Version]
- Green, C.D.; Ozguden-Akkoc, C.G.; Wang, Y.; Jump, D.B.; Olson, L.K. Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species. J. Lipid Res. 2010, 51, 1871–1877. [Google Scholar] [CrossRef]
- Sari, M.; Onk, K.; Sisman, T.; Tilki, M.; Yakan, A. Effects of different fattening systems on technological properties and fatty acid composition of goose meat. Eur. Poult. Sci. 2015, 79, 1–12. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and Sensory Properties of Dairy Products from Cows with Various Milk Fatty Acid Compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; Cava, R. Extensively reared Iberian pigs versus intensively reared white pigs for the manufacture of frankfurters. Meat Sci. 2006, 72, 356–364. [Google Scholar] [CrossRef]
- Lo Fiego, D.P.; Minelli, G.; Volpelli, L.A.; Ulrici, A.; Macchioni, P. Calculating the iodine value for Italian heavy pig subcutaneous adipose tissue from fatty acid methyl ester profiles. Meat Sci. 2016, 122, 132–138. [Google Scholar] [CrossRef]
- Witting, L.A. Lipid peroxidation in vivo. J. Am. Oil Chem. Soc. 1965, 42, 908–913. [Google Scholar] [CrossRef]
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lebret, B. Effects of feeding and rearing systems on growth, carcass composition and meat quality in pigs. Animal 2008, 2, 1548–1558. [Google Scholar] [CrossRef]
- Bee, G.; Guex, G.; Herzog, W. Free-range rearing of pigs during the winter: Adaptations in muscle fiber characteristics and effects on adipose tissue composition and meat quality traits. J. Anim. Sci. 2004, 82, 1206–1218. [Google Scholar] [CrossRef]
- Enfält, A.-C.; Lundström, K.; Hansson, I.; Karlsson, A.; Essén-Gustavsson, B.; Håkansson, J. Moderate indoor exercise: Effect on production and carcass traits, muscle enzyme activities and meat quality in pigs. Anim. Sci. 1993, 57, 127–135. [Google Scholar] [CrossRef]
- Dostálová, A.; Svitáková, A.; Bureš, D.; Vališ, L.; Volek, Z. Effect of an Outdoor Access System on the Growth Performance, Carcass Characteristics, and Longissimus lumborum Muscle Meat Quality of the Prestice Black-Pied Pig Breed. Animals 2020, 10, 1244. [Google Scholar] [CrossRef]
- Dworschák, E.; Barna, É.; Gergely, A.; Czuczy, P.; Hóvári, J.; Kontraszti, M.; Gaál, Ö.; Radnóti, L.; Bíró, G.; Kaltenecker, J. Comparison of some components of pigs kept in natural (free-range) and large-scale conditions. Meat Sci. 1995, 39, 79–86. [Google Scholar] [CrossRef]
- Gentry, J.G.; McGlone, J.J.; Miller, M.F.; Blanton, J.R. Environmental effects on pig performance, meat quality, and muscle characteristics. J. Anim. Sci. 2004, 82, 209–217. [Google Scholar] [CrossRef]
- Goldspink, D.F. Exercise-related changes in protein turnover in mammalian striated muscle. J. Exp. Biol. 1991, 160, 127–148. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise Metabolism and the Molecular Regulation of Skeletal Muscle Adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Gregory, N.G.; Grandin, T. Animal Welfare and Meat Science; Grandin, T., Ed.; CABI Publishing: New York, NY, USA, 1998; p. 298. [Google Scholar]
- Daza, A.; Rey, A.I.; Olivares, A.; Cordero, G.; Toldrá, F.; López-Bote, C.J. Physical activity-induced alterations on tissue lipid composition and lipid metabolism in fattening pigs. Meat Sci. 2009, 81, 641–646. [Google Scholar] [CrossRef]
- Estany, J.; Ros-Freixedes, R.; Tor, M.; Pena, R.N. A Functional Variant in the Stearoyl-CoA Desaturase Gene Promoter Enhances Fatty Acid Desaturation in Pork. PLoS ONE 2014, 9, e86177. [Google Scholar] [CrossRef]
- Anderson, B.M.; Ma, D.W.L. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef]
- Coelho, D.F.; Pereira-Lancha, L.O.; Chaves, D.S.; Diwan, D.; Ferraz, R.; Campos-Ferraz, P.L.; Poortmans, J.R.; Junior, A.H.L. Effect of high-fat diets on body composition, lipid metabolism and insulin sensitivity, and the role of exercise on these parameters. Braz. J. Med. Biol. Res. 2011, 44, 966–972. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación. ORDEN APA/3653/2007, de 13 de diciembre, por la que se publican los valores de ácidos grasos aplicables a las designaciones de alimentación «Bellota» y «Recebo», para la campaña 2007–2008. In Boletín Oficial del Estado (BOE); Boletín Oficial del Estado: Madrid, Spain, 2007; Volume 300, p. 51655. [Google Scholar]
- Dobrzyn, P.; Pyrkowska, A.; Jazurek, M.; Szymanski, K.; Langfort, J.; Dobrzyn, A. Endurance training-induced accumulation of muscle triglycerides is coupled to upregulation of stearoyl-CoA desaturase. J. Appl. Physiol. 2010, 109, 1653–1661. [Google Scholar] [CrossRef]
- McAllister, R.M.; Reiter, B.L.; Amann, J.F.; Laughlin, M.H. Skeletal muscle biochemical adaptations to exercise training in miniature swine. J. Appl. Physiol. 1997, 82, 1862–1868. [Google Scholar] [CrossRef][Green Version]
- Doran, O.; Moule, S.K.; Teye, G.A.; Whittington, F.M.; Hallett, K.G.; Wood, J.D. A reduced protein diet induces stearoyl-CoA desaturase protein expression in pig muscle but not in subcutaneous adipose tissue: Relationship with intramuscular lipid formation. Br. J. Nutr. 2006, 95, 609–617. [Google Scholar] [CrossRef]
- Cheng, H.; Song, S.; Kim, G.-D. Frozen/thawed meat quality associated with muscle fiber characteristics of porcine longissimus thoracis et lumborum, psoas major, semimembranosus, and semitendinosus muscles. Sci. Rep. 2021, 11, 13354. [Google Scholar] [CrossRef]
- Bruininx, E.; Borne, J.v.d.; Heugten, E.v.; Milgen, J.v.; Verstegen, M.; Gerrits, W. Oxidation of Dietary Stearic, Oleic, and Linoleic Acids in Growing Pigs Follows a Biphasic Pattern. J. Nutr. 2011, 141, 1657–1663. [Google Scholar] [CrossRef]
- Miller, G.M.; Conrad, J.H.; Keenan, T.W.; Featherston, W.R. Fatty Acid Oxidation in Young Pigs. J. Nutr. 1971, 101, 1343–1349. [Google Scholar] [CrossRef]
- Liu, J.; Sebastià, C.; Jové-Juncà, T.; Quintanilla, R.; González-Rodríguez, O.; Passols, M.; Castelló, A.; Sánchez, A.; Ballester, M.; Folch, J.M. Identification of genomic regions associated with fatty acid metabolism across blood, liver, backfat and muscle in pigs. Genet. Sel. Evol. 2024, 56, 66. [Google Scholar] [CrossRef]
- Damon, M.; Wyszynska-Koko, J.; Vincent, A.; Hérault, F.; Lebret, B. Comparison of Muscle Transcriptome between Pigs with Divergent Meat Quality Phenotypes Identifies Genes Related to Muscle Metabolism and Structure. PLoS ONE 2012, 7, e33763. [Google Scholar] [CrossRef]
- Carmelo, V.A.O.; Kadarmideen, H.N. Genome Regulation and Gene Interaction Networks Inferred From Muscle Transcriptome Underlying Feed Efficiency in Pigs. Front. Genet. 2020, 11, 650. [Google Scholar] [CrossRef]
- Griffiths, M.A.; Fiebig, R.; Gore, M.T.; Baker, D.H.; Esser, K.; Oscai, L.; Ji, L.L. Exercise Down-Regulates Hepatic Lipogenic Enzymes in Food-Deprived and Refed Rats. J. Nutr. 1996, 126, 1959–1971. [Google Scholar] [CrossRef]
- Frayn, K.N.; Arner, P.; Yki-Jarvinen, H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006, 42, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, A.; Óvilo, C.; Núñez, Y.; Benítez, R.; López-Garcia, A.; García, F.; Félix, M.R.; Laranjo, M.; Charneca, R.; Martins, J.M. Transcriptomic Profiling of Skeletal Muscle Reveals Candidate Genes Influencing Muscle Growth and Associated Lipid Composition in Portuguese Local Pig Breeds. Animals 2021, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Liu, Y.; Hao, R.; Zhang, L.; Zhang, X.; Li, S.; Li, S.; Tong, H. The mechanism of EGF in promoting skeletal muscle post-injury regeneration. Differentiation 2025, 143, 100862. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.S.; Henckel, P.; Oksbjerg, N.; Sørensen, M.T. Adaptations in muscle fibre characteristics induced by physical activity in pigs. Anim. Sci. 1998, 66, 733–740. [Google Scholar] [CrossRef]
- Gondret, F.; Combes, S.; Lefaucheur, L.; Lebret, B. Effects of exercise during growth and alternative rearing systems on muscle fibers and collagen properties. Reprod. Nutr. Dev. 2005, 45, 69–86. [Google Scholar] [CrossRef]
- Jonsäll, A.; Johansson, L.; Lundström, K. Sensory quality and cooking loss of ham muscle (M. biceps femoris) from pigs reared indoors and outdoors. Meat Sci. 2001, 57, 245–250. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Y.; Wang, X.; Chen, Q.; Lu, S. Identification of potential tissue-specific biomarkers involved in pig fat deposition through integrated bioinformatics analysis and machine learning. Heliyon 2024, 10, e31311. [Google Scholar] [CrossRef]
- Ciconello, F.N.; Tuggle, C.K.; Gomes, J.D.; Silva, B.P.M.d.; Durval, M.d.C.; Oliveira, C.S.d.; Nascimento, L.E.; Freitas, L.S.; Koltes, J.E.; Cesar, A.S.M. Pig models reveal the interplay between fatty acids and cytokines in skeletal muscle. Sci. Rep. 2025, 15, 19528. [Google Scholar] [CrossRef]
- Makowski, L.; Hotamisligil, G.S. Fatty acid binding proteins—The evolutionary crossroads of inflammatory and metabolic responses. J. Nutr. 2004, 134, 2464s–2468s. [Google Scholar] [CrossRef]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef]
- Peffer, P.L.; Lin, X.; Odle, J. Hepatic beta-oxidation and carnitine palmitoyltransferase I in neonatal pigs after dietary treatments of clofibric acid, isoproterenol, and medium-chain triglycerides. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2005, 288, R1518–R1524. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kaufman, R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016, 57, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARγ and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; Bosscher, K.D. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q.; Wu, Y.; Zhang, Y.; Zhang, B.; Zhang, H. Identification of candidate genes that specifically regulate subcutaneous and intramuscular fat deposition using transcriptomic and proteomic profiles in Dingyuan pigs. Sci. Rep. 2022, 12, 2844. [Google Scholar] [CrossRef]
- Herpin, P.; Vincent, A.; Fillaut, M.; Bonito, B.P.; Hocquette, J.F. Mitochondrial and peroxisomal fatty acid oxidation capacities increase in the skeletal muscles of young pigs during early postnatal development but are not affected by cold stress. Reprod. Nutr. Dev. 2003, 43, 155–166. [Google Scholar] [CrossRef]
- Lebret, B.; Serviento, A.M.; Renaudeau, D. Pork quality traits and associated muscle metabolic changes in pigs under chronic prenatal and postnatal heat stress. J. Anim. Sci. 2023, 101, skad305. [Google Scholar] [CrossRef]
- Garrido, N.; Albuquerque, A.; Charneca, R.; Costa, F.; Marmelo, C.; Ramos, A.; Martin, L.; Martins, J.M. Transcriptomic Profiling of Subcutaneous Backfat in Castrated and Intact Alentejano Pigs Finished Outdoors with Commercial and Fiber-Rich Diets. Genes 2023, 14, 1722. [Google Scholar] [CrossRef]
- Albuquerque, A.; Óvilo, C.; Núñez, Y.; Benítez, R.; López-Garcia, A.; García, F.; Félix, M.R.; Laranjo, M.; Charneca, R.; Martins, J.M. Comparative Transcriptomic Analysis of Subcutaneous Adipose Tissue from Local Pig Breeds. Genes 2020, 11, 422. [Google Scholar] [CrossRef]
- Poklukar, K.; Čandek-Potokar, M.; Lukač, N.B.; Tomažin, U.; Škrlep, M. Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review. Animals 2020, 10, 424. [Google Scholar] [CrossRef]
- Mersmann, H.J. Lipoprotein and hormone-sensitive lipases in porcine adipose tissue. J. Anim. Sci. 1998, 76, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Duran-Montgé, P.; Theil, P.K.; Lauridsen, C.; Esteve-Garcia, E. Dietary fat source affects metabolism of fatty acids in pigs as evaluated by altered expression of lipogenic genes in liver and adipose tissues. Animal 2009, 3, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.R.; Li, M.Z.; Zhang, K.; Chen, L.; Jiang, A.A.; Wang, J.Y.; Li, X.W. Evaluation of endogenous control genes for gene expression studies across multiple tissues and in the specific sets of fat- and muscle-type samples of the pig. J. Anim. Breed. Genet. 2011, 128, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tang, R.; Wang, W.; Liu, D.; Wang, K. Effects of dietary protein/carbohydrate ratio on fat deposition and gene expression of peroxisome proliferator activated receptor γ and heart fatty acid-binding protein of finishing pigs. Livest. Sci. 2011, 140, 111–116. [Google Scholar] [CrossRef]
- Tan, B.; Yin, Y.; Liu, Z.; Tang, W.; Xu, H.; Kong, X.; Li, X.; Yao, K.; Gu, W.; Smith, S.B.; et al. Dietary l-arginine supplementation differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. J. Nutr. Biochem. 2011, 22, 441–445. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.H.; Jiang, H.; Xiao, S.Q.; Wang, S.; Ma, Q.; Sun, G.J.; Li, F.J.; Deng, Q.; Dai, L.S.; et al. Detection of differentially expressed genes in the longissimus dorsi of Northeastern Indigenous and Large White pigs. Genet. Mol. Res. GMR 2011, 10, 779–791. [Google Scholar] [CrossRef]
- Weber, T.E.; Kerr, B.J.; Spurlock, M.E. Regulation of hepatic peroxisome proliferator-activated receptor α expression but not adiponectin by dietary protein in finishing pigs. J. Anim. Physiol. Anim. Nutr. 2008, 92, 569–577. [Google Scholar] [CrossRef]
- Kennedy, T.G.; Brown, K.D.; Vaughan, T.J. Expression of the Genes for the Epidermal Growth Factor Receptor and its Ligands in Porcine Oviduct and Endometrium. Biol. Reprod. 1994, 50, 751–756. [Google Scholar] [CrossRef]
- Benítez, R.; Fernández, A.; Isabel, B.; Núñez, Y.; Mercado, E.D.; Gómez-Izquierdo, E.; García-Casco, J.; López-Bote, C.; Óvilo, C. Modulatory Effects of Breed, Feeding Status, and Diet on Adipogenic, Lipogenic, and Lipolytic Gene Expression in Growing Iberian and Duroc Pigs. Int. J. Mol. Sci. 2018, 19, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Leng, W.; Pi, D.; Tu, Z.; Zhu, H.; Shi, H.; Li, S.; Hou, Y.; Hu, C.-A.A. Aspartate inhibits LPS-induced MAFbx and MuRF1 expression in skeletal muscle in weaned pigs by regulating Akt, AMPKα and FOXO1. Innate Immun. 2017, 23, 34–43. [Google Scholar] [CrossRef]
- Shan, T.; Ren, Y.; Liu, Y.; Zhu, L.; Wang, Y. Breed difference and regulation of the porcine Sirtuin 1 by insulin. J. Anim. Sci. 2010, 88, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, M.; Cao, X.; Du, X.; Meng, F.; Bu, G.; Kong, F.; Huang, A.; Zeng, X. Mechanistic insight into the role of immunocastration on eliminating skatole in boars. Theriogenology 2019, 131, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kalbe, C.; Mau, M.; Rehfeldt, C. Developmental changes and the impact of isoflavones on mRNA expression of IGF-I receptor, EGF receptor and related growth factors in porcine skeletal muscle cell cultures. Growth Horm. IGF Res. 2008, 18, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Gondret, F.; Guével, B.; Com, E.; Vincent, A.; Lebret, B. A comparison of subcutaneous adipose tissue proteomes in juvenile piglets with a contrasted adiposity underscored similarities with human obesity. J. Proteom. 2012, 75, 949–961. [Google Scholar] [CrossRef]
- Wang, X.-q.; Yang, W.-J.; Yang, Z.; Shu, G.; Wang, S.-b.; Jiang, Q.-y.; Yuan, L.; Wu, T.-s. The Differential Proliferative Ability of Satellite Cells in Lantang and Landrace Pigs. PLoS ONE 2012, 7, e32537. [Google Scholar] [CrossRef]
- Fan, H.; Cinar, M.U.; Phatsara, C.; Tesfaye, D.; Tholen, E.; Looft, C.; Schellander, K. Molecular mechanism underlying the differential MYF6 expression in postnatal skeletal muscle of Duroc and Pietrain breeds. Gene 2011, 486, 8–14. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Li, N.; Plastow, G.; Liu, Z.L.; Hu, X.X.; Wu, C.X. Identification of three SNPs in the porcine myostatin gene (MSTN). Anim. Biotechnol. 2002, 13, 173–178. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Wu, L.; Wei, H.; Liu, Y.; Li, T.; Tan, B.; Kong, X.; Yao, K.; Chen, S.; et al. Effects of dietary protein restriction on muscle fiber characteristics and mTORC1 pathway in the skeletal muscle of growing-finishing pigs. J. Anim. Sci. Biotechnol. 2016, 7, 47. [Google Scholar] [CrossRef]
- Hou, G.Y.; Zhou, H.L.; Cao, T.; Xun, W.J.; Wang, D.J.; Shi, L.G.; Guan, S.; Wang, D.F.; Li, M. Expression and variation of Myf5 and MyoD1 genes in different tissues of Wuzhishan pigs. Genet. Mol. Res. 2015, 14, 3729–3735. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. Arginine Promotes Slow Myosin Heavy Chain Expression via Akirin2 and the AMP-Activated Protein Kinase Signaling Pathway in Porcine Skeletal Muscle Satellite Cells. J. Agric. Food Chem. 2018, 66, 4734–4740. [Google Scholar] [CrossRef]
- Xue, H.-l.; Zhou, Z.-x.; Bo, W. PCR-SSCP genetic polymorphism and its genetic effect of 3′ side of pig. Chin. J. Ecol. 2007, 26, 505–508. [Google Scholar]
- Ronis, M.J.J.; Chen, Y.; Shankar, K.; Gomez-Acevedo, H.; Cleves, M.A.; Badeaux, J.; Blackburn, M.L.; Badger, T.M. Formula feeding alters hepatic gene expression signature, iron and cholesterol homeostasis in the neonatal pig. Physiol. Genom. 2011, 43, 1281–1293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).