Phosphorus Dynamics in High-Legacy Soils: Acid Phosphatase Activity, Extraction Techniques and Isotherm in Florida Potato Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. In Site Description and Experimental Design
2.2. Soil Sampling
2.3. Soil Extracellular Acid Phosphatase Activity
2.4. Phosphorus Sorption Parameters
- If PSR < 0.1 and SPSC > 0, the soil is a P sink.
- If PSR = 0.1 and SPSC = 0, the soil is neither a sink nor a source.
- If PSR > 0.1 and SPSC < 0, the soil is a P source.
2.5. Phosphorus Sorption Isotherms
2.6. Soil Organic Matter (SOM), pH, Mehlich-3 Nutrients, Total P, Morgan P, and Bray-1 P Tests
2.7. Statistical Analyses
3. Results
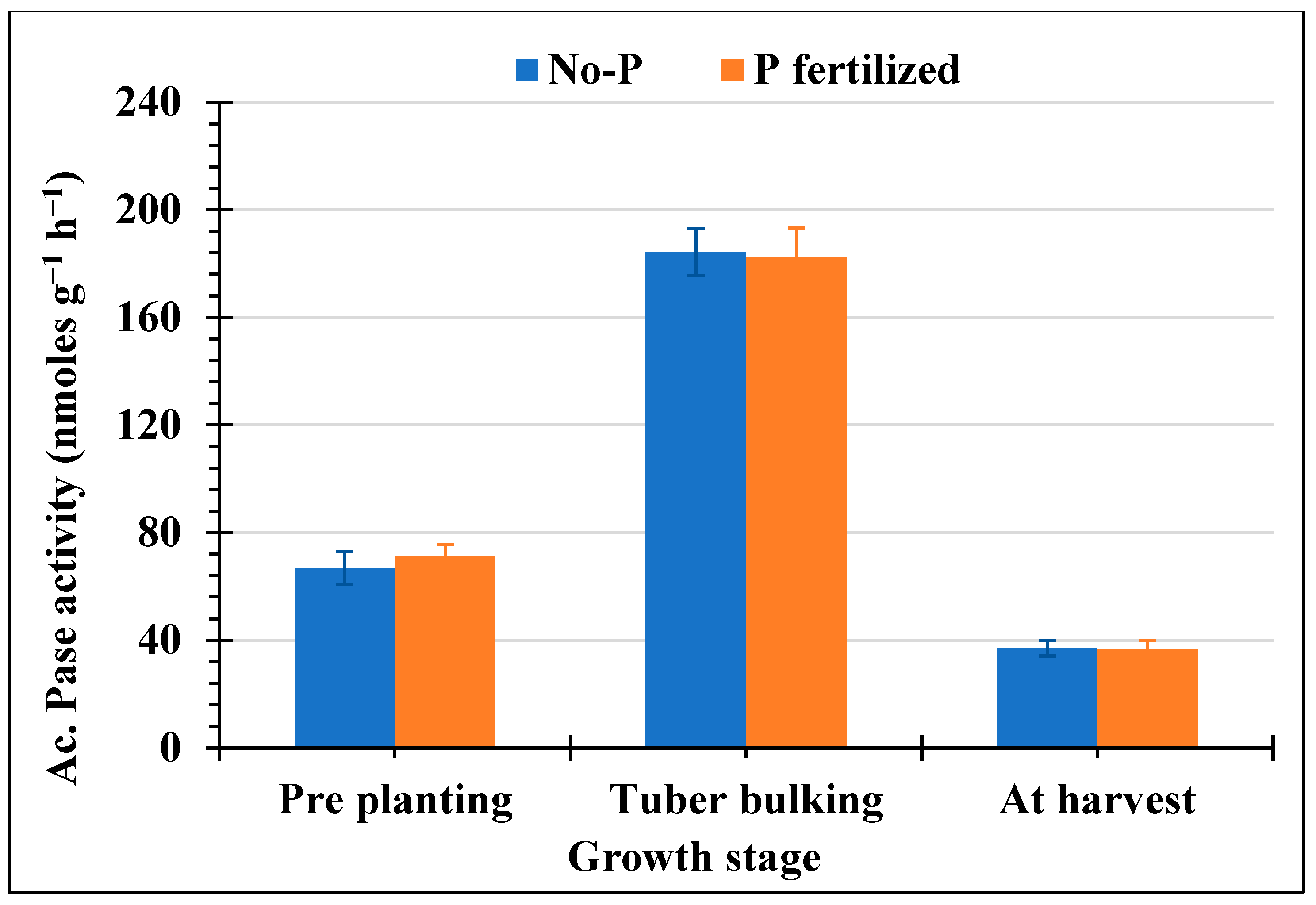
3.1. Acid Phosphatase Activity of Soil Microorganisms
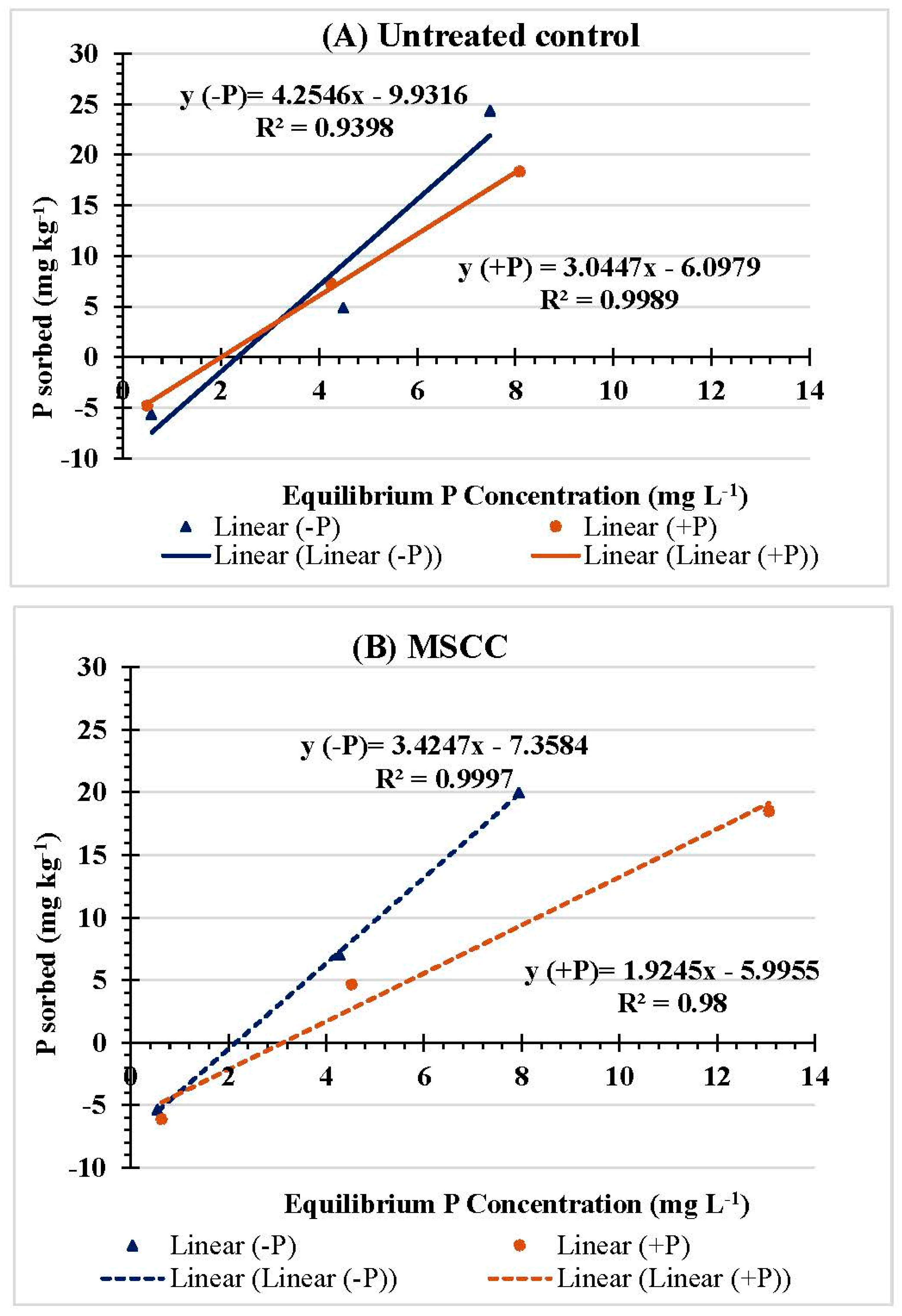
3.2. PSR, SPSC, and Multipoint Isotherm
3.3. Soil Organic Matter, Total P, Mehlich-3, Morgan, and Bray-1 P Tests
3.3.1. Comparison of Soil P Extraction Methods
3.3.2. Soil pH, OM, and Mehlich-3 Extractable Nutrients
4. Discussion
4.1. Phosphatase Activity of Soil Microorganisms and Its Relationship to Soil P Tests
4.2. PSR, SPSC, and Multipoint Isotherm
4.3. High AcPA Prompts Split P Applications for Environmental and Economic Sustainability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldham, L.; Jones, K. Inorganic Fertilizers for Crop Production; CALS Publications: New York, NY, USA, 2020; p. 23. Available online: https://scholarsjunction.msstate.edu/cals-publications/23/ (accessed on 20 June 2025).
- Ma, X.; Li, H.; Zhang, J.; Shen, J. Spatiotemporal pattern of acid phosphatase activity in soils cultivated with maize sensing to phosphorus-rich patches. Front. Plant Sci. 2021, 12, 650436. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Sun, Y.; Goll, D.S.; Ciais, P.; Peng, S.; Margalef, O.; Asensio, D.; Sardans, J.; Peñuelas, J. Spatial pattern and environmental drivers of acid phosphatase activity in Europe. Front. Big Data 2020, 2, 51. [Google Scholar] [CrossRef]
- Liu, G.D.; Simonne, E.H.; Morgan, K.T.; Hochmuth, G.; Agehara, S.; Mylavarapu, R.; Frey, C. Chapter 2. Fertilizer management for vegetable production in Florida. In 2025–2026 Vegetable Production Handbook of Florida; Dittmar, P., Agehara, S., Dufault, N.S., Eds.; UF/IFAS: Gainesville, FL, USA, 2023; Available online: https://edis.ifas.ufl.edu/publication/CV296 (accessed on 25 September 2025).
- Neumann, G.; Römheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 1999, 211, 121–130. [Google Scholar] [CrossRef]
- Wu, L.; Kobayashi, Y.; Wasaki, J.; Koyama, H. Organic acid excretion from roots: A plant mechanism for enhancing phosphorus acquisition, enhancing aluminum tolerance, and recruiting beneficial rhizobacteria. Soil Sci. Plant Nutr. 2018, 64, 697–704. [Google Scholar] [CrossRef]
- Yarzábal, L.A.; Chica, E.J. Role of rhizobacterial secondary metabolites in crop protection against agricultural pests and diseases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Kavka, M.; Korn, K.; Hazarika, M.; Bachmann-Pfabe, S.; Uptmoor, R. Potato root and leaf phosphatase activity in response to P deprivation. J. Plant Nutr. Soil Sci. 2021, 184, 668–677. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Teles, A.P.B.; Sartor, L.R.; Pavinato, P.S. Cover cropping may alter legacy phosphorus dynamics under long-term fertilizer addition. Front. Environ. Sci. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.P.; Tsai, C.F.; Rekha, P.D.; Ghate, S.D.; Huang, H.Y.; Hsu, Y.H.; Liaw, L.L.; Young, C.C. Agricultural management practices influence the soil enzyme activity and bacterial community structure in tea plantations. Bot. Stud. 2021, 62, 8. [Google Scholar] [CrossRef] [PubMed]
- Karandashov, V.; Bucher, M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 2005, 10, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bhomia, R.K.; Inglett, P.W.; Reddy, K.R. Soil and phosphorus accretion rates in sub-tropical wetlands: Everglades Stormwater Treatment Areas as a case example. Sci. Total Environ. 2015, 533, 297–306. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Mwamila, L.B.; Kergoat, K. The pH dependence of phosphate sorption and desorption in Swedish agricultural soils. Geoderma 2012, 189–190, 304–311. [Google Scholar] [CrossRef]
- Li, W.; Pierre-Louis, A.-M.; Kwon, K.D.; Kubicki, J.D.; Strongin, D.R.; Phillips, B.L. Molecular level investigations of phosphate sorption on corundum (α-Al2O3) by 31P solid state NMR, ATR-FTIR and quantum chemical calculation. Geochim. Cosmochim. Acta 2013, 107, 252–266. [Google Scholar] [CrossRef]
- Peng, H.; Jiang, A.; Wang, H. Adsorption and desorption characteristics of phosphorus on sediments in Panzhihua Section of Jinsha River, China. In Proceedings of the IOP Conference Series: Earth and Environmental Scienc, Shenyang, China, 14–15 November 2020; Volume 651, p. 042059. Available online: https://iopscience.iop.org/issue/1755-1315/651/4 (accessed on 2 July 2025).
- Christensen, M.L.; Cvitanich, C.; Quist-Jensen, C.A.; Thau, M.; Malmgren-Hansen, B. Precipitation and recovery of phosphorus from the wastewater hydrolysis tank. Sci. Total Environ. 2022, 813, 151875. Available online: https://www.sciencedirect.com/science/article/pii/S0048969721069515 (accessed on 25 September 2025). [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Liao, X.; Liu, G.; Hogue, B.; Li, Y. Phosphorus availability and environmental risks in potato fields in North Florida. Soil Use Manag. 2015, 31, 308–312. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y. Phosphorus-sorption characteristics of calcareous soils and limestone from the southern Everglades and adjacent farmlands. Soil Sci. Soc. Am. J. 2001, 65, 1404–1412. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Sobucki, L.; Ramos, R.F.; Meireles, L.A.; Antoniolli, Z.I.; Jacques, R.J.S. Contribution of enzymes to soil quality and the evolution of research in Brazil. Rev. Bras. Cienc. Solo 2021, 45, e0210109. [Google Scholar] [CrossRef]
- Wang, L.; Kaur, M.; Zhang, P.; Li, J.; Xu, M. Effect of different agricultural farming practices on microbial biomass and enzyme activities of celery growing field soil. Int. J. Environ. Res. Public Health 2021, 18, 12826. [Google Scholar] [CrossRef]
- Das, S.K.; Varma, A. Role of enzymes in maintaining soil health. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Janes-Bassett, V.; Blackwell, M.S.A.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A meta-analysis of phosphatase activity in agricultural settings in response to phosphorus deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2016; ISBN 9780133254488. [Google Scholar]
- Vrba, J.; Kopáček, J.; Bittl, T.; Nedoma, J.; Štrojsová, A.; Nedbalová, L.; Kohout, L.; Fott, J. A key role of aluminium in phosphorus availability, food web structure, and plankton dynamics in strongly acidified lakes. Biologia 2006, 61, S441–S451. [Google Scholar] [CrossRef]
- Nair, V.D.; Graetz, D.A. Phosphorus saturation in Spodosols impacted by manure. J. Environ. Qual. 2002, 31, 1279–1285. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Guillard, K.; Dest, W.M. Extractable soil phosphorus concentrations and creeping bentgrass response on sand greens. Crop. Sci. 2003, 43, 272–281. [Google Scholar] [CrossRef]
- Reddy, K.R.; DeLaune, R.D.; Inglett, P.W. Biogeochemistry of Wetlands: Science and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–712. [Google Scholar]
- McIntosh, J.L. Bray and Morgan soil extractants modified for testing acid soils from different parent materials. Agron. J. 1969, 61, 259–265. [Google Scholar] [CrossRef]
- Richardson, C.J.; Vaithiyanathan, P. Phosphorus sorption characteristics of Everglades soils along a eutrophication gradient. Soil Sci. Soc. Am. J. 1995, 59, 1782–1788. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Cui, S.; Qin, Y.; Yu, J.; Shi, X.; Jia, L.; Fan, M. Improving tuber yield and phosphorus use efficiency using split phosphorus application to potatoes in Inner Mongolia. Am. J. Potato Res. 2020, 97, 318–324. [Google Scholar] [CrossRef]
- Ekelöf, J.; Lundell, J.; Asp, H.; Jensen, E. Recovery of phosphorus fertilizer in potato as affected by application strategy and soil type. J. Plant Nutr. Soil Sci. 2014, 177, 369–377. [Google Scholar] [CrossRef]
- Mihovilovich, E.; Carli, C.; De Mendiburu, F.; Hualla, V.; Bonierbale, M. Tuber Bulking Maturity Assessment of Elite and Advanced Potato Clones; International Potato Center: Lima, Peru, 2014; ISBN 978-92-9060-441-9. [Google Scholar] [CrossRef]
- Mulder, D. Minor Elements in Fruit Growing. In Convegno Nazionale di Frutticoltura; Tip. Marguerrettaz-Diemoz: Saint-Vincent, Aosta Valley, Italy, 1953; pp. 118–198. (In French) [Google Scholar]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Qiu, Y.; Fall, T.; Su, Z.; Bortolozo, F.; Mussoline, W.; England, G.; Dinkins, D.; Morgan, K.; Clark, M.; Liu, G. Effect of phosphorus fertilization on yield of chipping potato grown on high legacy phosphorus soil. Agronomy 2022, 12, 812. [Google Scholar] [CrossRef]
- de Aquino, R.F.B.A.; Cavalcante, A.G.; Clemente, J.M.; Macedo, W.R.; Novais, R.F.; de Aquino, L.A. Split fertilization of phosphate in onion as strategy to improve the phosphorus use efficiency. Sci. Hortic. 2021, 290, 110494. [Google Scholar] [CrossRef]
- Johnston, A.M.; Bruulsema, T.W. 4R Nutrient stewardship for improved nutrient use efficiency. Procedia Eng. 2014, 83, 365–370. [Google Scholar] [CrossRef]

| P Rate (kg ha−1) | Treatments | Soil pH | SOM (%) | P | Ca | Al | Fe | K | Mg | Mn |
|---|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | ||||||||||
| 0 | Untreated control | 5.98 ± 0.2 | 0.47 ± 0.05 | 403.79 ± 69.5 | 789.92 ± 109.4 | 867.54 ± 231.7 | 361.47 ± 52.3 | 49.88 ± 16.8 | 110.68 ± 23.4 | 29.14 ± 5.9 |
| MSCC | 5.8 ± 0.1 | 0.52 ± 0.09 | 423.7 ± 68.9 | 763.3 ± 71.5 | 850.4 ± 162.5 | 379.4 ± 25.3 | 60 ± 15.6 | 101.2 ± 9.9 | 30 ± 4.7 | |
| MSCC + Telone-C35 | 5.9 ± 0.1 | 0.6 ± 0.12 | 410 ± 16.4 | 904.8 ± 244.3 | 881 ± 94 | 371.3 ± 17.3 | 58 ± 16.9 | 114 ± 14.6 | 31.9 ± 4.1 | |
| 49 | Untreated control | 6 ± 0.2 | 0.51 ± 0.01 | 403.2 ± 34.9 | 798.6 ± 68 | 784.3 ± 57.2 | 350.5 ± 29.6 | 51.8 ± 15.3 | 115.4 ± 6.3 | 30.5 ± 1.7 |
| MSCC | 5.8 ± 0.1 | 0.56 ± 0.06 | 457.6 ± 50.7 | 876.5 ± 82.8 | 946.3 ± 91.7 | 410.5 ± 15.1 | 62.8 ± 6.6 | 120.2 ± 16.2 | 33.9 ± 6.4 | |
| MSCC + Telone-C35 | 6 ± 0.1 | 0.52 ± 0.09 | 426.8 ± 44.4 | 832.5 ± 44.8 | 859.7 ± 44.7 | 364.6 ± 28 | 58.6 ± 8.9 | 116.8 ± 13 | 33.3 ± 2.9 | |
| Activities | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|
| Summer | Fall | Spring | Summer | Fall | Spring | |
| MSCC Season | ||||||
| First soil sampling (pre-planting) | 19-Jun | 15-May | ||||
| Planting MSCC | 22-Jun | 23-Jun | ||||
| Shoot/leave sampling | 13-Aug | 27-Sep | ||||
| Root sampling | 13-Aug | N/A | ||||
| Second soil sampling (harvest) | 13-Aug | 14-Oct | ||||
| Termination MSCC | 22-Sep | 15-Oct | ||||
| Potato Season | ||||||
| Application of Telone-C35 | 16-Dec | 15-Dec | ||||
| First soil sampling (pre-planting) | 13-Jan | 11-Jan | ||||
| Pre-planting fertilization | 17-Jan | 19-Jan | ||||
| Planting potato | 22-Jan | 21-Jan | ||||
| Emergence fertilization | 20-Feb | 15-Feb | ||||
| Layby fertilization | 3-Mar | 1-Mar | ||||
| Shoot/leave sampling | N/A | 17-Mar | ||||
| Second soil sampling (flowering) | 23-Mar | 22-Mar | ||||
| Harvest potato | 20-May | 30-Apr | ||||
| Third soil sampling (harvest) | 15-May | 10-May | ||||
| Year | 2019 | 2020 | 2021 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crop | MSCC | Potato | MSCC | Potato | ||||||
| Growth Stage | Pre-Plant | Harvest | Pre-Plant | Tuber Bulking | Harvest | Harvest | Pre-Plant | Tuber Bulking | Harvest | |
| P rate (kg ha−1) | Treatment | Mehlich3 P in kg ha−1 | ||||||||
| 0 | Untreated control | 403.79 ± 69.5 | 448.06 ± 45 | 388.66 ± 36.1 | 423.96 ± 89 | 432.93 ± 65.1 | 385.01 ± 50.7 | 397.9 ± 58.3 | 409.67 ± 34.8 | 400.42 ± 54.6 |
| MSCC | 423.68 ± 68.9 | 433.77 ± 35.8 | 394.26 ± 48.4 | 427.88 ± 47.4 | 439.93 ± 34.8 | 385.01 ± 26 | 379.97 ± 45.6 | 407.43 ± 41.5 | 418.36 ± 54.3 | |
| MSCC + Telone-C35 | 409.95 ± 16.4 | 446.38 ± 20.2 | 371.28 ± 4 | 423.4 ± 22.6 | 405.19 ± 22 | 366.8 ± 32.3 | 387.53 ± 16.5 | 377.45 ± 34 | 389.22 ± 12.1 | |
| 49 | Untreated control | 403.23 ± 34.9 | 437.97 ± 41 | 417.8 ± 78 | 474.12 ± 99.4 | 421.16 ± 22.4 | 410.23 ± 53.8 | 409.95 ± 77.5 | 505.78 ± 58.8 | 493.45 ± 59.4 |
| MSCC | 457.59 ± 50.7 | 430.97 ± 30.8 | 418.92 ± 62.7 | 487.29 ± 52.4 | 490.65 ± 31.8 | 442.46 ± 25.7 | 417.8 ± 67 | 496.82 ± 31.4 | 506.06 ± 40.8 | |
| MSCC + Telone-C35 | 426.76 ± 44.4 | 421.72 ± 42.5 | 414.71 ± 47.2 | 514.47 ± 45.9 | 437.41 ± 45.1 | 397.06 ± 43.7 | 414.71 ± 26.7 | 480.85 ± 35.3 | 497.38 ± 40.6 | |
| Year | Growth Stage | P Rate (kg ha−1) | PSR | SPSC |
|---|---|---|---|---|
| 2020 | Pre-planting | 0 | 0.325 | −118.550 |
| 49 | 0.328 | −129.392 | ||
| Tuber bulking | 0 | 0.358 | −136.575 | |
| 49 | 0.399 | −163.892 | ||
| Harvest | 0 | 0.383 | −140.167 | |
| 49 | 0.409 | −151.683 | ||
| 2021 | Pre-planting | 0 | 0.325 | −120.083 |
| 49 | 0.350 | −131.842 | ||
| Tuber bulking | 0 | 0.346 | −126.000 | |
| 49 | 0.417 | −167.300 | ||
| Harvest | 0 | 0.339 | −126.367 | |
| 49 | 0.411 | −168.242 |
| Treatment | P Rate (mg kg−1) | Linear Adsorption | Linearized Langmuir | |||||
|---|---|---|---|---|---|---|---|---|
| S0 (mg kg−1) | EPC0 (mg L−1) | Kd (L kg−1) | R2 | b (L mg−1) | Smax (mg kg−1) | R2 | ||
| Untreated control | 0 | 7.47 | 13.51 | 0.72 | 0.88 | 0.08 | 171.38 | 0.74 |
| 49 | 4.00 | 3.53 | 1.18 | 0.92 | 0.34 | 776.88 | 0.74 | |
| MSCC | 0 | 4.98 | 9.65 | 0.72 | 0.92 | 0.09 | 399.97 | 0.71 |
| 49 | 5.71 | 11.25 | 0.91 | 0.86 | 0.06 | 129.17 | 0.63 | |
| MSCC + Telone-C35 | 0 | 5.11 | 6.48 | 0.83 | 0.90 | 0.07 | 127.74 | 0.75 |
| 49 | 6.12 | 6.59 | 0.98 | 0.90 | 0.10 | 236.39 | 0.67 | |
| Main Effects | Morgan P | Bray-1 P | Mehlich-3 P | Total P | |
|---|---|---|---|---|---|
| kg ha−1 | |||||
| P Application (PR) | |||||
| 0 | 69.22 b z | 288.52 b | 396.44 b | 457.19 b | |
| 49 | 99.55 a | 347.58 a | 469.20 a | 497.90 a | |
| p-value | 0.0011 | 0.0027 | 0.0077 | 0.0187 | |
| SE | ±1.70 | ±14.04 | ±18.70 | ±12.93 | |
| LSD | 7.72 | 20.51 | 36.15 | 27.84 | |
| Growth Stage (GS) | |||||
| Pre-planting | 75.60 b | 304.76 b | 401.31 b | 460.40 a | |
| Tuber bulking | 87.72 a | 326.45 a | 446.33 a | 496.73 a | |
| At harvest | 89.82 a | 322.94 a | 450.82 a | 475.50 a | |
| p-value | 0.0120 | 0.0191 | 0.0047 | 0.2432 | |
| SE | ±2.30 | ±14.06 | ±18.74 | ±16.47 | |
| LSD | 8.35 | 14.04 | 24.55 | 46.99 | |
| Two-Way Interaction | Morgan P | Bray-1 P | Mehlich-3 P | Total P | |
| kg ha−1 | |||||
| GS | PR | ||||
| Pre-planting | 0 | 71.03 b | 290.72 b | 388.47 a | 457.02 a |
| 49 | 80.17 a | 318.81 a | 414.15 a | 463.78 a | |
| Tuber bulking | 0 | 66.75 b | 289.65 b | 398.18 b | 472.57 b |
| 49 | 108.69 a | 363.25 a | 494.48 a | 520.90 a | |
| At harvest | 0 | 69.86 b | 285.19 b | 402.67 b | 441.97 b |
| 49 | 109.78 a | 360.69 a | 498.97 a | 509.03 a | |
| p-value | <0.0001 | <0.0001 | <0.0001 | 0.1830 | |
| SE | ±2.87 | ±14.58 | ±19.80 | ±19.46 | |
| LSD | 7.56 | 18.85 | 33.31 | 42.32 | |
| Three-Way Interaction | Soil pH | M-3 P | |||
|---|---|---|---|---|---|
| Year | Growth Stage | P rate (kg ha−1) | (kg ha−1) | (mg kg−1) | |
| 2020 | Pre-planting | 0 | 6.2 a z | 385 a | 171.62 a |
| 49 | 6.1 a | 417 a | 186.08 a | ||
| Tuber bulking | 0 | 5.3 a | 425 b | 189.62 b | |
| 49 | 5.3 a | 492 a | 219.46 a | ||
| At harvest | 0 | 4.9 b | 426 a | 190.04 a | |
| 49 | 5.1 a | 450 a | 200.63 a | ||
| 2021 | Pre-planting | 0 | 5.9 A | 388 A | 173.29 A |
| 49 | 5.9 A | 414 A | 184.75 A | ||
| Tuber bulking | 0 | 5.1 A | 398 B | 177.63 B | |
| 49 | 5.1 A | 494 A | 220.58 A | ||
| At harvest | 0 | 5.9 A | 403 B | 179.63 B | |
| 49 | 5.9 A | 499 A | 222.58 A | ||
| SE | ±0.04 | ±20.10 | ±8.97 | ||
| LSD | 0.09 | 35 | 15.43 | ||
| Two-Way Interaction | Soil OM | Ca | Al | Fe | K | Mg | Mn | |
|---|---|---|---|---|---|---|---|---|
| Year | Growth Stage | % | kg ha−1 | |||||
| 2020 | Pre-planting | 0.51 a z | 727 c | 892 a | 373 b | 44 c | 74 c | 24 c |
| Tuber bulking | 0.50 a | 908 b | 862 a | 411 a | 459 a | 101 b | 37 a | |
| At harvest | 0.45 b | 1021 a | 779 b | 384 b | 196 b | 162 a | 35 b | |
| 2021 | Pre-planting | 1.02 B | 739 B | 850 A | 386 B | 74 B | 76 A | 27 C |
| Tuber bulking | 1.07 A | 808 A | 824 A | 415 A | 129 A | 71 AB | 32 A | |
| At harvest | 1.02 B | 765 AB | 848 A | 419 A | 103AB | 65 B | 30 B | |
| SE | ±0.03 | ±29 | ±43 | ±17 | ±12 | ±4 | ±0.83 | |
| LSD | 0.04 | 22 | 40 | 18 | 35 | 10 | 1.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fall, T.; Inglett, K.; Ogram, A.V.; Inglett, P.; Schaffer, B.; Li, Y.; Morgan, K.; Liu, G. Phosphorus Dynamics in High-Legacy Soils: Acid Phosphatase Activity, Extraction Techniques and Isotherm in Florida Potato Fields. Agriculture 2025, 15, 2048. https://doi.org/10.3390/agriculture15192048
Fall T, Inglett K, Ogram AV, Inglett P, Schaffer B, Li Y, Morgan K, Liu G. Phosphorus Dynamics in High-Legacy Soils: Acid Phosphatase Activity, Extraction Techniques and Isotherm in Florida Potato Fields. Agriculture. 2025; 15(19):2048. https://doi.org/10.3390/agriculture15192048
Chicago/Turabian StyleFall, Thioro, Kanika Inglett, Andrew V. Ogram, Patrick Inglett, Bruce Schaffer, Yuncong Li, Kelly Morgan, and Guodong Liu. 2025. "Phosphorus Dynamics in High-Legacy Soils: Acid Phosphatase Activity, Extraction Techniques and Isotherm in Florida Potato Fields" Agriculture 15, no. 19: 2048. https://doi.org/10.3390/agriculture15192048
APA StyleFall, T., Inglett, K., Ogram, A. V., Inglett, P., Schaffer, B., Li, Y., Morgan, K., & Liu, G. (2025). Phosphorus Dynamics in High-Legacy Soils: Acid Phosphatase Activity, Extraction Techniques and Isotherm in Florida Potato Fields. Agriculture, 15(19), 2048. https://doi.org/10.3390/agriculture15192048







