Comparison of the Effects of Prohexadione Calcium and Uniconazole on Sweet Potato Storage and Texture Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Flour Sample Preparation
2.3. Determination of Soluble Sugar, Starch, and Apparent Amylose Content in Storage Roots
2.4. Dry Matter Weight, Percentage of Rotting, and Weight Loss
2.5. Texture Properties
2.6. Statistical Analysis
3. Results
3.1. Soluble Sugar, Starch, and Apparent Amylose Content
3.2. Dry Matter Weight of Storage Roots
3.3. Percentage of Rotting
3.4. Percentage Weight Loss
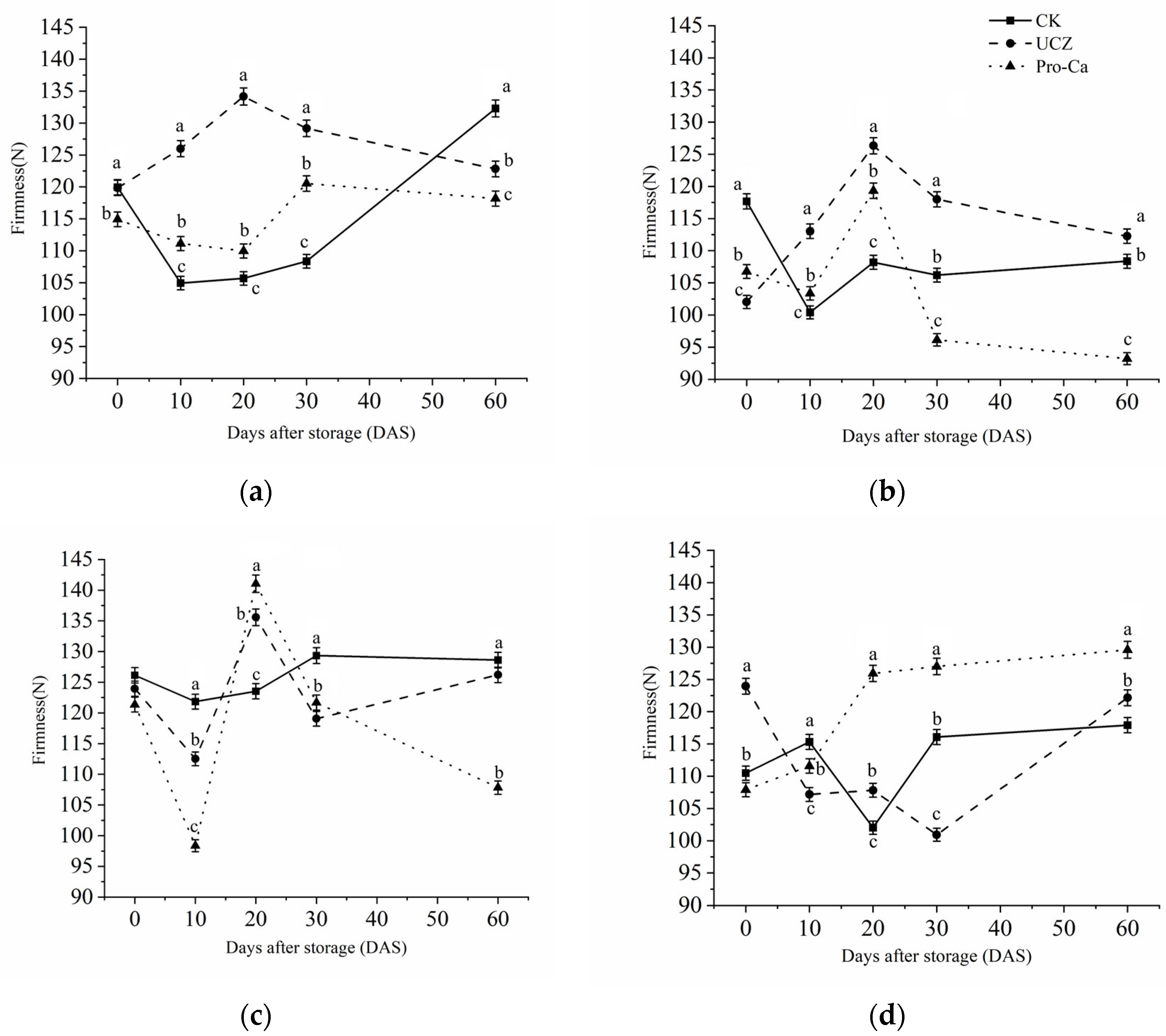
3.5. Firmness
3.6. Maximum Adhesion Force
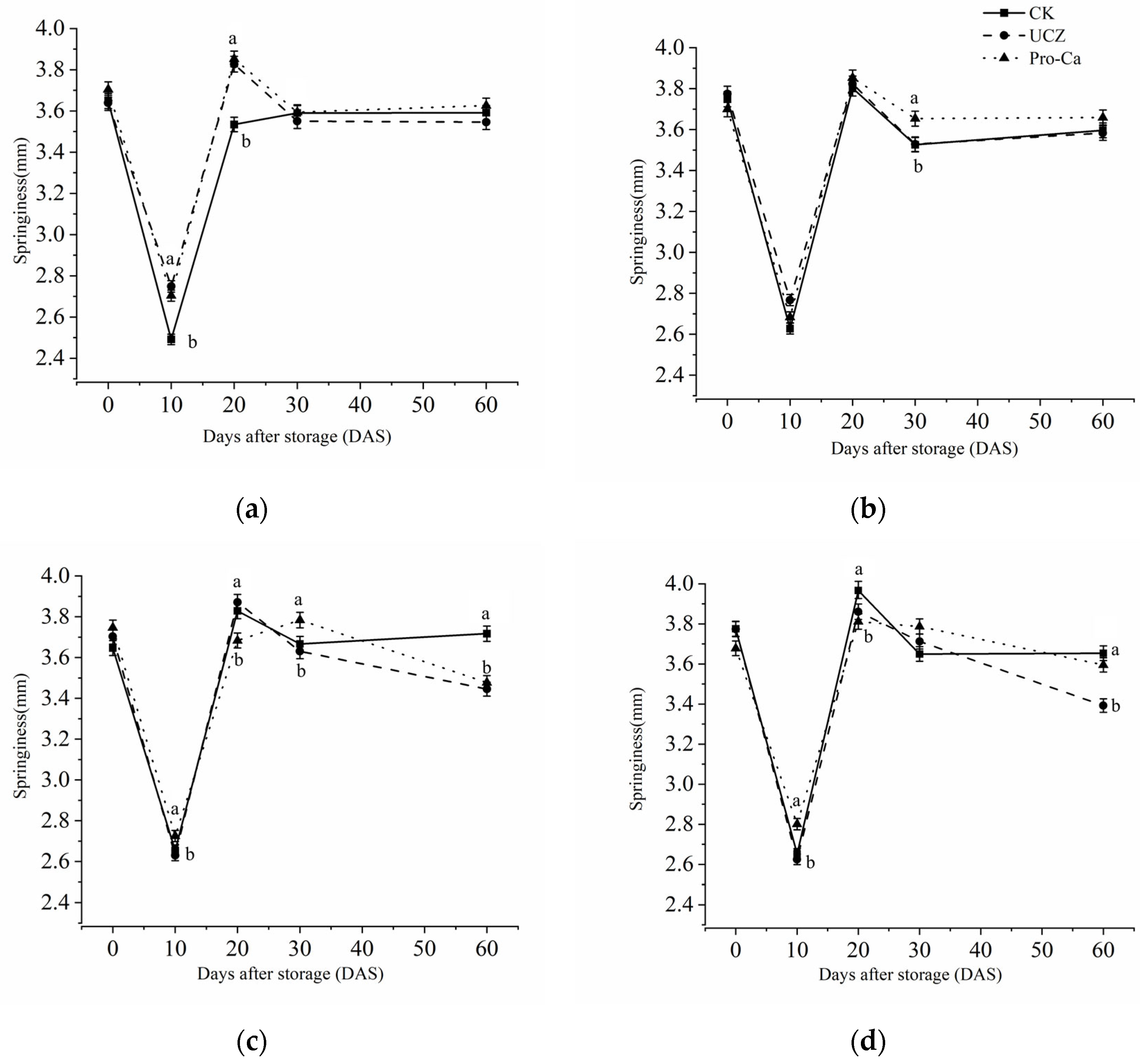
3.7. Springiness
3.8. Chewiness
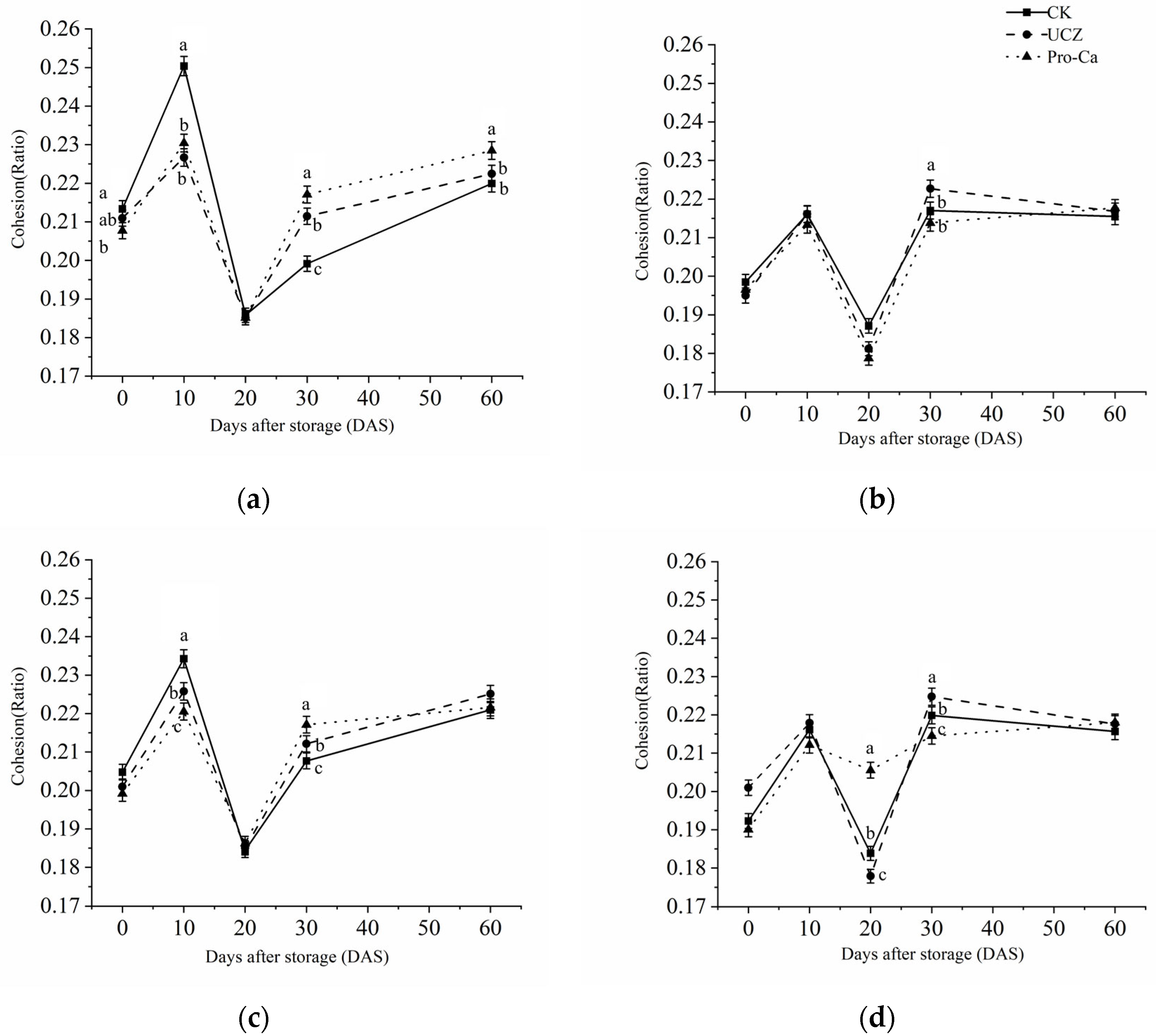
3.9. Cohesion
4. Discussion
4.1. Pro-Ca Enhances Quality and Storage Tolerance Through Sugar Metabolism Regulation
4.2. Pro-Ca Regulation of Carbohydrate Metabolism and Its Effect on Texture
4.3. Field Application of UCZ and Pro-Ca Enhances Stress Resistance and Improves Post-Harvest Preservation of Sweet Potatoes
4.4. Differential and Variety-Specific Effects of PGRs on Sweet Potato Quality Regulation
4.5. Effect of GA on Crop Physiology and Postharvest Management
4.6. Hypotheses About Pro-Ca and UCZ in This Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GA | Gibberellin |
| Pro-Ca | Prohexadione calcium |
| UCZ | Uniconazole |
| PGR | Plant growth retardant |
| TSS | Total soluble solids |
| VDAL | Verticillium dahliae effector |
| W10 | Wanshu 10 |
| Z13 | Zheshu 13 |
References
- Jiang, Z.; Wei, Z.; Zhang, J.; Zheng, C.; Zhu, H.; Zhai, H.; He, S.; Gao, S.; Zhao, N.; Zhang, H.; et al. Source-sink synergy is the key unlocking sweet potato starch yield potential. Nat. Commun. 2024, 15, 7260. [Google Scholar] [CrossRef]
- Saito, S.; Okamoto, M.; Okamoto, M.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Hirai, N.; Todoroki, Y.; Sakata, K.; Nambara, E.; et al. A plant growth retardant, uniconazole, is a potent inhibitor of ABA catabolism in Arabidopsis. Biosci. Biotechnol. Biochem. 2006, 70, 1731–1739. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, H.; Xie, B.; Wang, B.; Hou, F.; Li, A.; Dong, S.; Qin, Z.; Wang, Q.; Zhang, L. Foliar application of uniconazole improves yield through enhancement of photosynthate partitioning and translocation to tuberous roots in sweetpotato. Arch. Agron. Soil Sci. 2020, 66, 316–329. [Google Scholar] [CrossRef]
- Roman, D.L.; Voiculescu, D.I.; Filip, M.; Ostafe, V.; Isvoran, A. Effects of Triazole Fungicides on Soil Microbiota and on the Activities of Enzymes Found in Soil: A Review. Agriculture 2021, 11, 893. [Google Scholar] [CrossRef]
- Si, C.-C.; Li, Y.-J.; Liu, H.-J.; Zhang, H.-Y.; Meng, Y.-Y.; Wang, N.; Shi, C.-Y. Impact of paclobutrazol on storage root number and yield of sweet potato (Ipomoea batatas L.). Field Crops Res. 2023, 300, 109011. [Google Scholar] [CrossRef]
- Davidson, S.E.; Reid, J.B.; Helliwell, C.A. Cytochromes P450 in gibberellin biosynthesis. Phytochem. Rev. 2006, 5, 405–419. [Google Scholar] [CrossRef]
- Ding, K.; Shan, Y.; Wang, L.; Tian, G.; Li, F.; Wang, H.; Pang, Z.; Pan, Y.; Jiang, H. Physiological response of potato leaves to uniconazole under drought stress during the tuber expansion period. Hortic. Environ. Biotechnol. 2024, 65, 847–866. [Google Scholar] [CrossRef]
- Guo, D.; He, R.; Luo, L.; Zhang, W.; Fan, J. Enantioselective acute toxicity, oxidative stress effects, neurotoxicity, and thyroid disruption of uniconazole in zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2022, 29, 40157–40168. [Google Scholar] [CrossRef]
- Subramanian, S.; Tehrani, R.; Van Aken, B. Transcriptomic response of Arabidopsis thaliana exposed to hydroxylated polychlorinated biphenyls (OH-PCBs). Int. J. Phytoremediat. 2019, 21, 52–59. [Google Scholar] [CrossRef]
- Zhang, Z. Prohexadione calcium regulates wheat tolerance to drought stress by maintaining water balance and promoting antioxidant metabolism and photosynthesis. Plant Soil Environ. 2024, 70, 673–681. [Google Scholar] [CrossRef]
- Evans, J.R.; Evans, R.R.; Regusci, C.; Rademacher, W. Mode of Action, Metabolism, and Uptake of BAS-125W, Prohexadione Calcium. Hortscience 1997, 34, 1200–1201. [Google Scholar] [CrossRef]
- Liu, M.; Feng, N.; Zheng, D.; Zhang, R. Prohexadione Calcium and Gibberellin Improve Osmoregulation, Antioxidant Response and Ion Homeostasis to Alleviate NaCl Stress in Rice Seedlings. Agronomy 2024, 14, 1318. [Google Scholar] [CrossRef]
- Deng, R.; Li, Y.; Feng, N.-J.; Zheng, D.-F.; Du, Y.-W.; Khan, A.; Xue, Y.-B.; Zhang, J.-Q.; Feng, Y.-N. Integrative Analyses Reveal the Physiological and Molecular Role of Prohexadione Calcium in Regulating Salt Tolerance in Rice. Int. J. Mol. Sci. 2024, 25, 9124. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Dai, Z.; Chen, Y.; Shao, Z.; Wang, C.; Jin, X.; Wang, Y.; Feng, L. Prohexadione-calcium improves grape quality by regulating endogenous hormones, sugar and acid metabolism and related enzyme activities in grape berries. BMC Plant Biol. 2024, 24, 122. [Google Scholar] [CrossRef]
- Hawerroth, F.J.; Petri, J.L.; Leite, G.B.; Yoshikawa, E.R. Application timing of prohexadione calcium on vegetative growth control of ‘imperial gala’ apples. Rev. Bras. Frutic. 2012, 34, 957–963. [Google Scholar] [CrossRef]
- Roux, C.; Lemarquand, A.; Orain, G.; Campion, C.; Simoneau, P.; Poupard, P. Effects of the plant growth regulator prohexadione-calcium and the SAR-inducer acibenzolar-S-methyl on the quality of apples at harvest. J. Hortic. Sci. Biotechnol. 2006, 81, 139–145. [Google Scholar] [CrossRef]
- Malachowska, M.; Majak, T.; Krupa, T.; Tomala, K. Increasing Productivity and Fruit Quality of ‘Mutsu’ Apple Orchard by Dwarfing Treatments. Agriculture 2024, 14, 1838. [Google Scholar] [CrossRef]
- Xu, X.; Pan, X.; Zhang, H.; Lv, Z.; Xia, J.; Cheng, P.; George, M.S.; Chen, Y.; Pang, L.; Lu, G. Effects of Foliar Application of Uniconazole on the Storage Quality of Tuberous Roots in Sweetpotato. Agronomy 2022, 12, 2983. [Google Scholar] [CrossRef]
- Fairbairn, N. A modified anthrone reagent. Chem. Ind. 1953, 4, 86. [Google Scholar]
- Katayama, K.; Nishinaka, M.; Nakamura, Y.; Kuranouchi, T.; Ohara-Takada, A.; Fujita, K.; Kitahara, K. New Sweetpotato Lines have High Amylose and Resistant Starch Contents. Starch-Starke 2019, 71, 1800180. [Google Scholar] [CrossRef]
- Xu, X.; Waters, D.; Blanchard, C.; Tan, S.H. A study on Australian sorghum grain fermentation performance and the changes in Zaopei major composition during solid-state fermentation. J. Cereal Sci. 2021, 98, 103160. [Google Scholar] [CrossRef]
- Yu, Y.F.; Kleuter, M.; Dinani, S.T.; Trindade, L.M.; Goot, A.J.V. The role of plant age and leaf position on protein extraction and phenolic compounds removal from tomato (Solanum lycopersicum) leaves using food-grade solvents. Food Chem. 2023, 406, 10. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Chen, K.; Zhang, H.; Zhou, S.; Lv, Z.; Chen, Y.; Cui, P.; Cui, Z.; Lu, G. Comprehensive Evaluation of Raw Eating Quality in 81 Sweet Potato (Ipomoea batatas (L.) Lam) Varieties. Foods 2023, 12, 261. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Feng, N.J.; Zheng, D.F.; Ma, G.H.; Feng, S.J.; Liu, M.L.; Yu, M.L.; Huang, X.X.; Huang, A.Q. Physiological and transcriptome analysis reveals that prohexadione-calcium promotes rice seedling’s development under salt stress by regulating antioxidant processes and photosynthesis. PLoS ONE 2023, 18, e0286505. [Google Scholar] [CrossRef]
- Deng, R.; Zheng, D.F.; Feng, N.J.; Khan, A.; Zhang, J.Q.; Sun, Z.Y.; Li, J.H.; Xiong, J.; Ding, L.C.; Yang, X.H.; et al. Prohexadione Calcium Improves Rice Yield Under Salt Stress by Regulating Source-Sink Relationships During the Filling Period. Plants 2025, 14, 211. [Google Scholar] [CrossRef]
- Zhu, M. The unique importance of sweetpotato: Insights focusing on genetic improvements of salt and drought tolerance. Sci. Hortic. 2025, 339, 113848. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Steffens, C.A.; de Freitas, S.T.; Silveira, J.P.G.; Denardi, V.; Katsurayama, J.M. Post bloom spraying apple trees with prohexadione-calcium and gibberellic acid affects vegetative growth, fruit mineral content and bitter pit incidence. In Proceedings of the 30th International Horticultural Congress (IHC)—Bridging the World through Horticulture/International Symposium on Strategies and Technologies to Maintain Quality and Reduce Postharvest Losses, Turkish Soc Hort Sci, Istanbul, Turkey, 12–16 August 2018; pp. 193–199. [Google Scholar]
- Mei, W.; Yang, S.; Xiong, J.; Khan, A.; Zhao, L.; Du, X.; Huo, J.; Zhou, H.; Sun, Z.; Yang, X.; et al. Prohexadione-Calcium Reduced Stem and Tiller Damage and Maintained Yield by Improving the Photosynthetic and Antioxidant Capacity of Rice (Oryza sativa L.) Under NaCl Stress. Plants 2025, 14, 188. [Google Scholar] [CrossRef]
- Liu, M.; Feng, N.; Zheng, D.; Meng, F. Comparative Study of the Mechanisms Underlying the Effects of Prohexadione-Calcium and Gibberellin on the Morphogenesis and Carbon Metabolism of Rice Seedlings Under NaCl Stress. Plants 2025, 14, 1240. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, Y.; Sang, Z.; Ge, Y.; Bai, C.; Fu, A.; Wang, Q.; Watkins, C.B.; Zuo, J. Multi-omics analysis reveals the mechanism of calcium-reduced quality deterioration in mechanically injured green pepper fruit. Postharvest Biol. Technol. 2023, 204, 112437. [Google Scholar] [CrossRef]
- Haleema, B.; Rab, A.; Hussain, S.A.; Sajid, M.; Arif, M.; Shah, S.T.; Ullah, I.; Miraj, G.; Humaira; Basit, A. Influence of calcium concentrations and sources on the fruit quality of tomato (Lycopersicon esculentum) at different storage conditions. Fresenius Environ. Bull. 2020, 29, 1866–1877. [Google Scholar]
- Paiva, E.A.S. Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol. 2019, 223, 1707–1711. [Google Scholar] [CrossRef]
- Griffith, C.; Einhorn, T.C. The effect of plant growth regulators on xylem differentiation, water and nutrient transport, and bitter pit susceptibility of apple. Sci. Hortic. 2023, 310, 111709. [Google Scholar] [CrossRef]
- Reitz, N.F.; Mitcham, E.J. Impact of postharvest dips with abscisic acid, prohexadione, calcium, or water on bitter pit incidence and apple physiology. Postharvest Biol. Technol. 2025, 219, 113202. [Google Scholar] [CrossRef]
- Ma, A.; Zhang, D.; Wang, G.; Wang, K.; Li, Z.; Gao, Y.; Li, H.; Bian, C.; Cheng, J.; Han, Y.; et al. Verticillium dahliae effector VDAL protects MYB6 from degradation by interacting with PUB25 and PUB26 E3 ligases to enhance Verticillium wilt resistance. Plant Cell 2021, 33, 3675–3699. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, F.; Yang, F.; Zhong, X.Y.; Li, Q.P.; Ren, W.J. Changes in chemical composition and starch structure in rice noodle cultivar influence Rapid Visco analysis and texture analysis profiles under shading. Food Chem. X 2022, 14, 100360. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, C.C.; Jiang, B.Z.; Mo, X.Y.; Wang, Z.Y. Optimization of HS-SPME for GC-MS Analysis and Its Application in Characterization of Volatile Compounds in Sweet Potato. Molecules 2021, 26, 5808. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, X.; Liang, H.; Ji, Y.; Liu, M. Effects of Comparative Metabolism on Tomato Fruit Quality under Different Levels of Root Restriction. HortScience 2023, 58, 885–892. [Google Scholar] [CrossRef]
- Rashedy, A.A.; Abd-ElNafea, M.H.; Khedr, E.H. Co-application of proline or calcium and humic acid enhances productivity of salt stressed pomegranate by improving nutritional status and osmoregulation mechanisms. Sci. Rep. 2022, 12, 14285. [Google Scholar] [CrossRef]
- Shehata, R.S. Proline in action: Enhancing fruit quality. DYSONA Appl. Sci. 2025, 6, 8–15. [Google Scholar] [CrossRef]
- Gohari, G.; Molaei, S.; Kheiry, A.; Ghafouri, M.; Razavi, F.; Lorenzo, J.M.; Juárez-Maldonado, A. Exogenous Application of Proline and L-Cysteine Alleviates Internal Browning and Maintains Eating Quality of Cold Stored Flat ‘Maleki’ Peach Fruits. Horticulturae 2021, 7, 469. [Google Scholar] [CrossRef]
- Njiti, V.N.; Xia, Q.; Tyler, L.S.; Stewart, L.D.; Tenner, A.T.; Zhang, C.; Alipoe, D.; Chukwuma, F.; Gao, M. Influence of Prohexadione Calcium on Sweetpotato Growth and Storage Root Yield. HortScience 2013, 48, 73–76. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, H.R.; Kang, J.H.; Hwang, S.J. Prohexadione-Calcium Application during Vegetative Growth Affects Growth of Mother Plants, Runners, and Runner Plants of Maehyang Strawberry. Agronomy 2019, 9, 155. [Google Scholar] [CrossRef]
- Zhang, C.; Tanabe, K.; Tamura, F.; Itai, A.; Yoshida, M. Roles of gibberellins in increasing sink demand in Japanese pear fruit during rapid fruit growth. Plant Growth Regul. 2007, 52, 161–172. [Google Scholar] [CrossRef]
- Rüscher, D.; Corral, J.M.; Carluccio, A.V.; Klemens, P.A.W.; Gisel, A.; Stavolone, L.; Neuhaus, H.E.; Ludewig, F.; Sonnewald, U.; Zierer, W. Auxin signaling and vascular cambium formation enable storage metabolism in cassava tuberous roots. J. Exp. Bot. 2021, 72, 3688–3703. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Lan, Z.; Huang, R.; Tan, Y.; Huang, D.; Gu, J.; Pan, C. Hormonal and transcriptional analyses provides new insights into the molecular mechanisms underlying root thickening and isoflavonoid biosynthesis in Callerya speciosa (Champ. ex Benth.) Schot. Sci. Rep. 2021, 11, 9. [Google Scholar] [CrossRef]
- Si, C.C.; Liang, Q.G.; Liu, H.J.; Wang, N.; Kumar, S.; Chen, Y.L.; Zhu, G.P. Response Mechanism of Endogenous Hormones of Potential Storage Root to Phosphorus and Its Relationship with Yield and Appearance Quality of Sweetpotato. Front. Plant Sci. 2022, 13, 872422. [Google Scholar] [CrossRef]
- Haider, M.W.; Nafees, M.; Ahmad, I.; Ali, B.; Maryam; Iqbal, R.; Vodnar, D.C.; Marc, R.A.; Kamran, M.; Saleem, M.H.; et al. Postharvest dormancy-related changes of endogenous hormones in relation to different dormancy-breaking methods of potato (Solanum tuberosum L.) tubers. Front. Plant Sci. 2022, 13, 2022. [Google Scholar] [CrossRef]
- Xie, Y.; Onik, J.C.; Hu, X.; Duan, Y.; Lin, Q. Effects of (S)-Carvone and Gibberellin on Sugar Accumulation in Potatoes during Low Temperature Storage. Molecules 2018, 23, 3118. [Google Scholar] [CrossRef]
- Zhu, T.; Pei, H.; Li, Z.; Zhang, M.; Chen, C.; Li, S. The Postharvest Application of Carvone, Abscisic Acid, Gibberellin, and Variable Temperature for Regulating the Dormancy Release and Sprouting Commencement of Mini-Tuber Potato Seeds Produced under Aeroponics. Plants 2023, 12, 3952. [Google Scholar] [CrossRef]
- Fadillah, R.; Wildani, R.; Salsabila, V.P.Z. Role of Gibberellic Acid (GA3) in enhancing growth and yield of hydroponically grown lettuce (Lactuca sativa L.). Ilmu Pertan. Agric. Sci. 2024, 9, 85–93. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, X.; Su, T.; Wang, W.; Xin, X.; Zhang, B.; Zhang, D.; Yu, Y.; Wang, Z.; Zhang, F.; et al. Exogenous Gibberellin Delays Postharvest Leaf Senescence in Pak Choi by Modulating Transcriptomic and Metabolomic Profiles. Foods 2025, 14, 981. [Google Scholar] [CrossRef]
- Hu, L.; Zheng, Q.; Chen, Z.; Qin, Y.; Si, H.; Ji, J.; Li, Q.; Yang, Z.; Wu, Y. Pre-harvest treatment with gibberellin (GA3) and nitric oxide donor (SNP) enhances post-harvest firmness of grape berries. Food Chem. Mol. Sci. 2025, 10, 100235. [Google Scholar] [CrossRef]
- Ozturk, B.; Erdal, A.; Onur, S.; Orhan, K.; Gun, S. Effects of GA3, CACl2 and Modified Atmosphere Packaging (MAP) Applications on Fruit Quality of Sweet Cherry at Cold Storage. Int. J. Fruit Sci. 2022, 22, 696–710. [Google Scholar] [CrossRef]
- Milović, M.; Kevrešan, Ž.; Mastilović, J.; Kovač, R.; Kalajdžić, J.; Magazin, N.; Bajić, A.; Milić, B.; Barać, G.; Keserović, Z. Could an Early Treatment with GA and BA Impact Prolonged Cold Storage and Shelf Life of Apricot? Horticulturae 2022, 8, 1220. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Li, X.; Wu, R.; Zhang, X.; Fan, X.; Li, G.; Gong, H.; Yin, X.; Zhang, A. The mechanism of gibberellins treatment suppressing kiwifruit postharvest ripening processes by transcriptome analysis. Postharvest Biol. Technol. 2023, 198, 112223. [Google Scholar] [CrossRef]
- Qiao, H.; Wu, W.; Zhang, Y.; Kong, Q.; Chen, H.; Wang, L.; Fang, X.; Gao, H. Impact of abscisic acid treatment on postharvest storage quality and volatile flavor substances in blueberries. eFood 2024, 5, e148. [Google Scholar] [CrossRef]
- Singh, V.; Sergeeva, L.; Ligterink, W.; Aloni, R.; Zemach, H.; Doron-Faigenboim, A.; Yang, J.; Zhang, P.; Shabtai, S.; Firon, N. Gibberellin Promotes Sweetpotato Root Vascular Lignification and Reduces Storage-Root Formation. Front. Plant Sci. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.; Hu, H.; Wang, S. Study on the Activity of Cell Wall Degradation Enzyme of Sweet Potato in Its Storage. Food Ferment. Ind. 2012, 38, 186–189. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Akoumianakis, K.A.; Vemmos, S.N.; Passam, H.C. The effect of postharvest application of gibberellic acid and benzyl adenine on the duration of dormancy of potatoes produced by plants grown from TPS. Postharvest Biol. Technol. 2007, 46, 54–62. [Google Scholar] [CrossRef]




| Year | Cultivar | Treatments | Soluble Sugar Content g·100 g−1 FW | Starch Content g·100 g−1 FW | Apparent Amylose Content g·100 g−1 FW |
|---|---|---|---|---|---|
| 2023 | Z13 | CK | 8.6 ± 0.5 b | 69.8 ± 0.3 b | 26.2 ± 0.2 c |
| UCZ | 9.3 ± 0.2 ab | 68.8 ± 0.2 c | 28.5 ± 0.2 b | ||
| Pro-Ca | 11.6 ± 0.9 a | 70.6 ± 0.1 a | 29.6 ± 0.1 a | ||
| W10 | CK | 9.0 ± 0.4 a | 67.4 ± 0.1 a | 32.9 ± 0.7 a | |
| UCZ | 9.1 ± 0.1 a | 67.2 ± 0.3 a | 33.6 ± 0.5 a | ||
| Pro-Ca | 9.0 ± 0.3 a | 66.3 ± 0.2 b | 28.8 ± 0.1 b | ||
| 2024 | Z13 | CK | 9.6 ± 0.1 a | 70.4 ± 0.4 a | 26.7 ± 0.4 b |
| UCZ | 8.1 ± 0.3 b | 69.9 ± 0.2 ab | 28.8 ± 0.6 a | ||
| Pro-Ca | 9.6 ± 0.1 a | 69.3 ± 0.6 b | 28.9 ± 0.9 a | ||
| W10 | CK | 8.8 ± 0.3 b | 67.9 ± 0.1 a | 32.1 ± 0.9 a | |
| UCZ | 8.8 ± 0.1 b | 66.5 ± 0.2 c | 32.5 ± 1.8 a | ||
| Pro-Ca | 11.0 ± 0.6 a | 67.4 ± 0.1 b | 25.3 ± 0.1 b |
| Year | Cultivar | Treatments | Dry Matter Weight g·100 g−1 (1 Week) | Dry Matter Weight g·100 g−1 (1 Month) |
|---|---|---|---|---|
| 2023 | Z13 | CK | 37.5 ± 0.6 a | 38.7 ± 2.4 a |
| UCZ | 37.7 ± 1.7 a | 38.4 ± 1.1 a | ||
| Pro-Ca | 36.6 ± 2.2 a | 38.2 ± 0.2 a | ||
| W10 | CK | 31.7 ± 1.1 a | 33.3 ± 1.5 a | |
| UCZ | 32.1 ± 1.6 a | 34.1 ± 1.2 a | ||
| Pro-Ca | 32.0 ± 0.2 a | 34.1 ± 0.1 a | ||
| 2024 | Z13 | CK | 37.2 ± 0.2 a | 39.0 ± 3.9 a |
| UCZ | 37.2 ± 2.3 a | 35.6 ± 0.3 a | ||
| Pro-Ca | 36.4 ± 0.6 a | 37.0 ± 0.3 a | ||
| W10 | CK | 31.8 ± 1.0 a | 33.3 ± 0.3 a | |
| UCZ | 32.0 ± 1.7 a | 35.0 ± 1.0 a | ||
| Pro-Ca | 31.5 ± 1.2 a | 33.7 ± 1.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xia, J.; Xu, X.; Shen, T.; Gao, K.; Zhu, Y.; Lu, G.; Lv, Z. Comparison of the Effects of Prohexadione Calcium and Uniconazole on Sweet Potato Storage and Texture Quality. Agriculture 2025, 15, 2005. https://doi.org/10.3390/agriculture15192005
Li J, Xia J, Xu X, Shen T, Gao K, Zhu Y, Lu G, Lv Z. Comparison of the Effects of Prohexadione Calcium and Uniconazole on Sweet Potato Storage and Texture Quality. Agriculture. 2025; 15(19):2005. https://doi.org/10.3390/agriculture15192005
Chicago/Turabian StyleLi, Jiayi, Jiaping Xia, Ximing Xu, Tiechen Shen, Kanghao Gao, Yueming Zhu, Guoquan Lu, and Zunfu Lv. 2025. "Comparison of the Effects of Prohexadione Calcium and Uniconazole on Sweet Potato Storage and Texture Quality" Agriculture 15, no. 19: 2005. https://doi.org/10.3390/agriculture15192005
APA StyleLi, J., Xia, J., Xu, X., Shen, T., Gao, K., Zhu, Y., Lu, G., & Lv, Z. (2025). Comparison of the Effects of Prohexadione Calcium and Uniconazole on Sweet Potato Storage and Texture Quality. Agriculture, 15(19), 2005. https://doi.org/10.3390/agriculture15192005







