Soil Nitrification Rate Is Affected by Plant Species and Nitrogen Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Physiological Measures
2.2. Soil Sampling and Nitrification Rate Determination
2.3. Statistical Analysis
3. Results
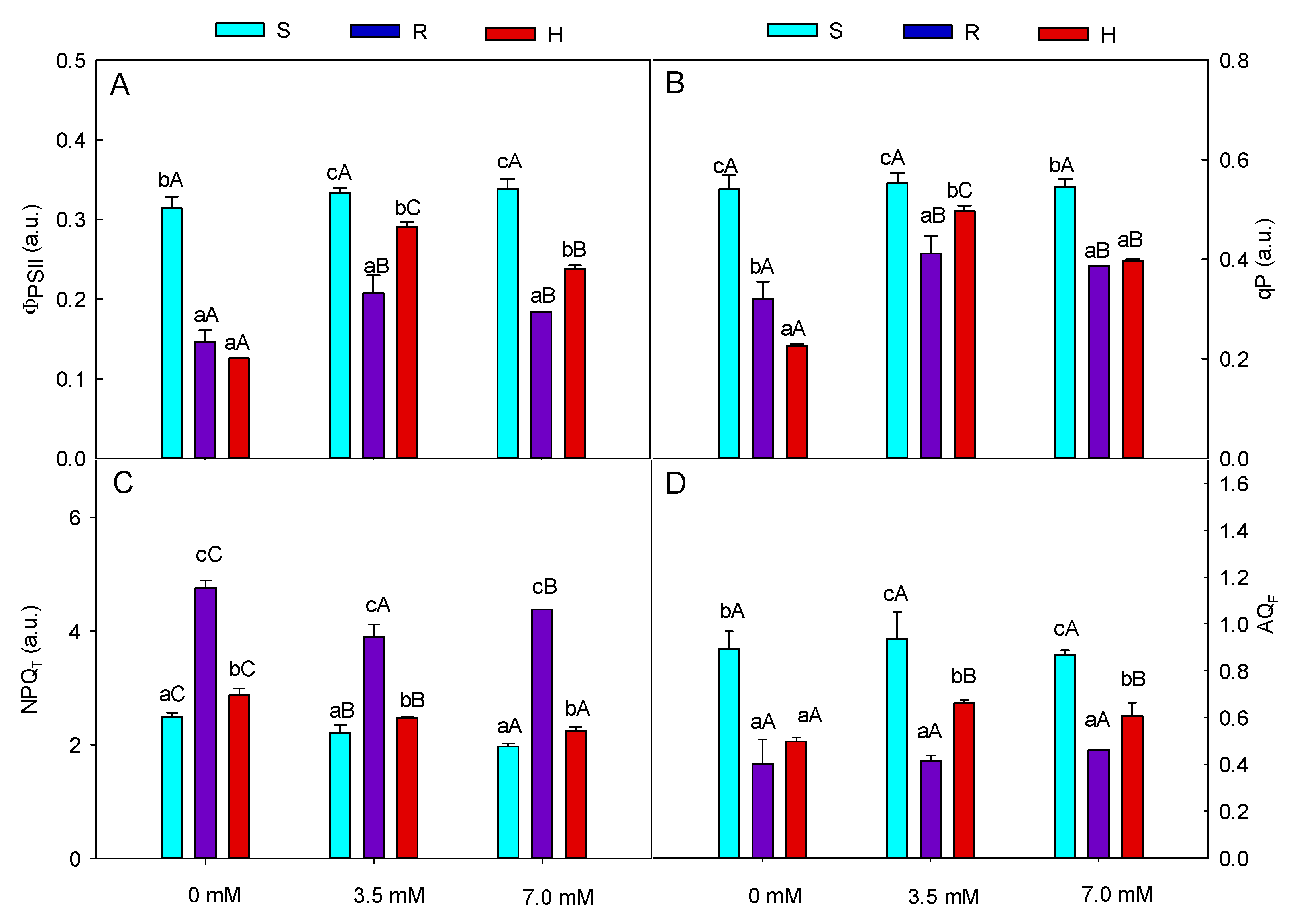
3.1. Plant Physiology
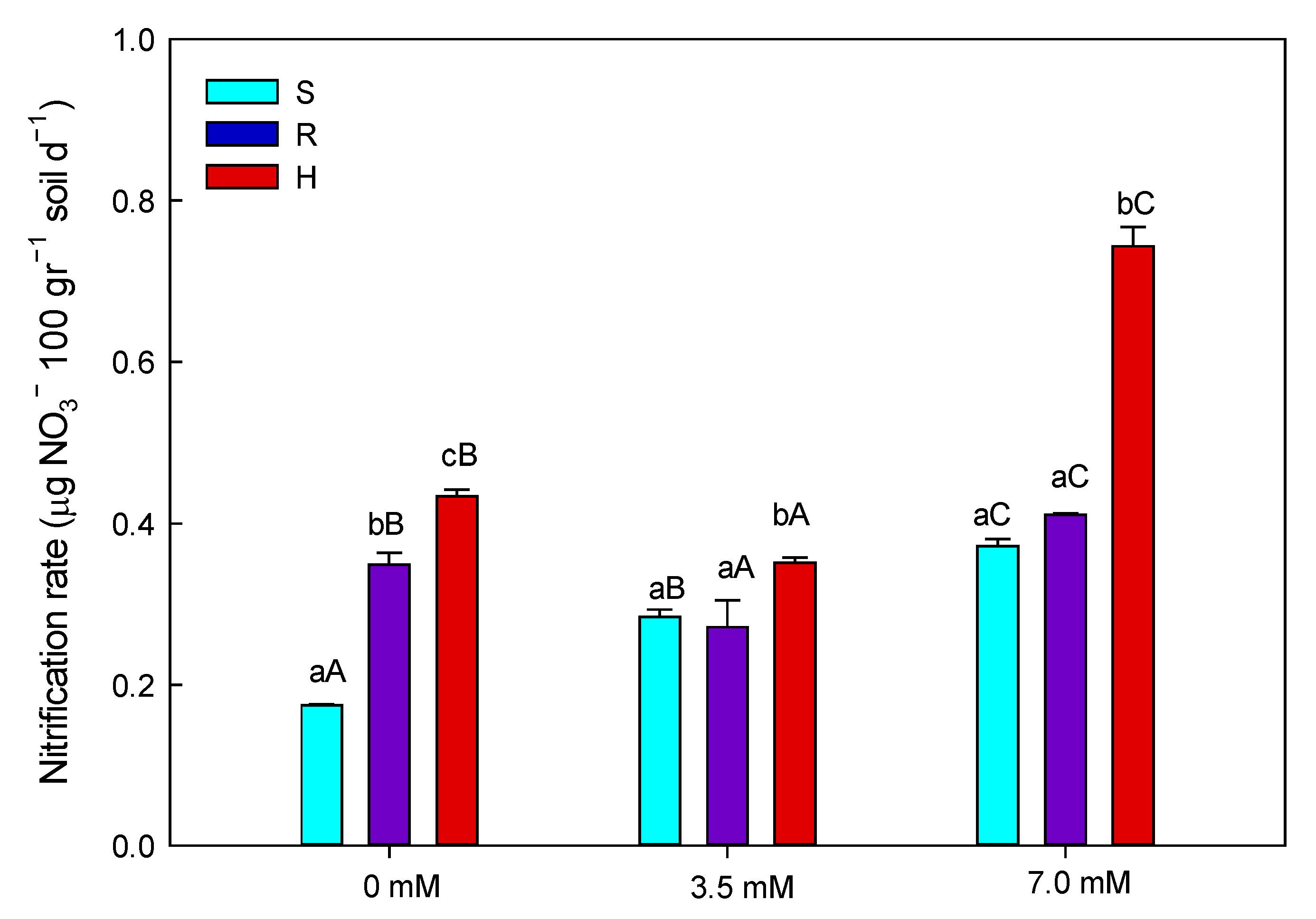
3.2. Nitrification
4. Discussion
4.1. Plant Physiology
4.2. Nitrification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manco, A.; Giaccone, M.; Zenone, T.; Onofri, A.; Tei, F.; Farneselli, M.; Gabbrielli, M.; Allegrezza, M.; Perego, A.; Magliulo, V.; et al. An Overview of N2O Emissions from Cropping Systems and Current Strategies to Improve Nitrogen Use Efficiency. Horticulturae 2024, 10, 754. [Google Scholar] [CrossRef]
- Lata, J.C.; Guillaume, K.; Degrange, V.; Abbadie, L.; Lensi, R. Relationships between root density of the African grass Hyparrhenia diplandra and nitrification at the decimetric scale, an inhibition-stimulation balance hypothesis. Proc. Biol. Sci. 2000, 267, 595–600. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Yoshihashi, T.; Worthington, M.; Nakahara, K.; Ando, Y.; Sahrawat, K.L.; Rao, I.M.; Lata, J.C.; Kishii, M.; Braun, H.J. Suppression of soil nitrification by plants. Plant Sci. 2015, 233, 155–164. [Google Scholar] [CrossRef]
- Campos, J.L.; Garrido-Fernández, J.M.; Méndez, R.; Lema, J.M. Nitrification at high ammonia loading rates in an activated sludge unit. Bioresour. Technol. 1999, 68, 141–148. [Google Scholar]
- Norton, J.M.; Alzerreca, J.J.; Suwa, Y.; Klotz, M.G. Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch. Microbiol. 2002, 177, 139–149. [Google Scholar] [CrossRef]
- Hommes, N.G.; Sayavedra-Soto, L.A.; Arp, D.J. The roles of the three gene copies encoding hydroxylamine oxidoreductase in Nitrosomonas europaea. Arch. Microbiol. 2002, 178, 471–476. [Google Scholar] [CrossRef]
- Qin, F.; Su, H.; Sun, L.; Li, Y. Research Progress Related to Sorghum Biological Nitrification Inhibitors. Agronomy 2024, 14, 1576. [Google Scholar] [CrossRef]
- Issifu, S.; Acharya, P.; Kaur-Bhambra, J.; Gubry-Rangin, C.; Rasche, F. Biological Nitrification Inhibitors with Antagonistic and Synergistic Effects on Growth of Ammonia Oxidisers and Soil Nitrification. Microb. Ecol. 2024, 87, 143. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ (T): A chlorophyll fluorescence parameter for rapid estimation and imaging of non-photochemical quenching of excitons in photosystem-II-associated antenna complexes. Plant Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef]
- Searles, P.S.; Bloom, A.J. Nitrate photo-assimilation in tomato leaves under short-term exposure to elevated carbon dioxide and low oxygen. Plant Cell Environ. 2003, 26, 1247–1255. [Google Scholar] [CrossRef]
- Bloom, A.J.; Smart, D.R.; Nguyen, D.T.; Searles, P.S. Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc. Natl. Acad. Sci. USA 2002, 99, 1730–1735. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; Virzo De Santo, A. Photosynthetic response of Quercus ilex L. plants grown on compost and exposed to increasing photon flux densities and elevated CO2. Photosynthetica 2005, 43, 615–619. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Duncan, E.G.; Roper, M.M.; Richardson, A.E.; Kirkegaard, J.A.; Peoples, M.P. Root Exudates From Canola Exhibit Biological Nitrification Inhibition And Are Effective In Inhibiting Ammonia Oxidation In Soil. Front. Agr. Sci. Eng. 2022, 9, 177–186. [Google Scholar]
- Makino, Y.; Ueno, O. Structural and physiological responses of the C4 grass Sorghum bicolor to nitrogen limitation. Plant Prod. Sci. 2018, 21, 39–50. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.K.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of nitrogen application rate on the photosynthetic pigment, leaf fluorescence characteristics, and yield of indica hybrid rice and their interrelations. Sci. Rep. 2021, 11, 7485. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Zhang, C.K.; Camberato, J.J.; Jiang, Y.W. Nitrogen and carbon contents, nitrogen use efficiency, and antioxidant responses of perennial ryegrass accessions to nitrogen deficiency. J. Plant Nutr. 2019, 42, 2092–2101. [Google Scholar] [CrossRef]
- Krämer, K.; Brock, J.; Heyr, A.G. Interaction of Nitrate Assimilation and Photorespiration at Elevated CO2. Front. Plant Sci. 2022, 13, 897924. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) to nitrogen supply under long photoperiod. Front. Plant Sci. 2020, 11, 572293. [Google Scholar] [CrossRef]
- Yang, Y.; Zha, W.; Tang, K.; Deng, G.; Du, G.; Liu, F. Effect of nitrogen supply on growth and nitrogen utilization in hemp (Cannabis sativa L.). Agronomy 2021, 11, 2310. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Ono, H.; Yoshida, M.; Yoshihashi, T.; Zhu, Y.; Zakir, H.A.K.M.; Deshpande, S.P.; Hash, C.T.; et al. Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil. 2013, 366, 243–259. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, H.; Shen, Q.; Ishikawa, T.; Subbarao, G.V. Interplay among NH uptake, rhizosphere pH and plasma membrane H + 4+-ATPase determine the release of BNIs in sorghum roots-possible mechanisms and underling hypothesis. Plant Soil. 2012, 358, 131–141. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Soil type | Sandy loam |
| Texture (%): | |
| clay | 15 |
| silt | 25 |
| sand | 60 |
| Bulk density (g cm−3) | 1.10 |
| pHH2O | 7.4 |
| CaCO3 (%) | 2.1 |
| C/N | 8.0 |
| Organic matter (%) | 2.8 |
| Organic carbon (%) | 1.6 |
| P (g kg−1) | 0.088 |
| K (g kg−1) | 1.4 |
| Total N (%) | 0.2 |
| N-NO3 (g kg−1) | 0.011 |
| N-NH4 (g kg−1) | 0.012 |
| Species | N Levels | Replicates | ||
|---|---|---|---|---|
| Sorghum | 0 mM | 3.5 mM | 7.0 mM | 4 |
| Ryegrass | 0 mM | 3.5 mM | 7.0 mM | 4 |
| Hemp | 0 mM | 3.5 mM | 7.0 mM | 4 |
| Sorghum | Ryegrass | Hemp | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 mM | 3.5 mM | 7.0 mM | 0 mM | 3.5 mM | 7.0 mM | 0 mM | 3.5 mM | 7.0 mM | |
| Chlorophylls | 0.60 ± 0.03 aA | 0.61 ± 0.05 aA | 0.59 ± 0.05 aA | 0.84 ± 0.13 bA | 0.87 ± 0.15 bA | 1.10 ± 0.13 bA | 0.39 ± 0.02 cA | 0.52 ± 0.01 aB | 0.55 ± 0.02 aB |
| Flavonols | 0.25 ± 0.02 aA | 0.13 ± 0.03 aA | 0.27 ± 0.05 aA | 0.36 ± 0.03 bA | 0.13 ± 0.03 aB | 0.10 ± 0.02 bB | 0.28 ± 0.03 cA | 0.31 ± 0.03 bA | 0.25 ± 0.02 aA |
| Anthocyanins | 0.022 ± 0.0045 aA | 0.027 ± 0.0046 aA | 0.031 ± 0.0068 aA | 0.062 ± 0.0011 bA | 0.031 ± 0.0079 aA | 0.037 ± 0.010 aA | 0.022 ± 0.0051 aA | 0.0044 ± 0.00015 bB | 0.016 ± 0.0029 bA |
| NFI | 2.86 ± 0.51 aA | 3.86 ± 0.7 6aA | 2.92 ± 0.60 aA | 2.45 ± 0.38 aA | 7.02 ± 1.29 bB | 10.58 ± 2.12 bB | 1.55 ± 0.16 bA | 1.85 ± 0.20 cA | 2.28 ± 0.18 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, L.; Maglione, G.; Garcia-Sanchez, F.; Yabor, L.; Riccardi, M.; Ottaiano, L.; Di Matteo, B.; Nocerino, R.; Manco, A.; Tedeschi, A. Soil Nitrification Rate Is Affected by Plant Species and Nitrogen Levels. Agriculture 2025, 15, 1740. https://doi.org/10.3390/agriculture15161740
Vitale L, Maglione G, Garcia-Sanchez F, Yabor L, Riccardi M, Ottaiano L, Di Matteo B, Nocerino R, Manco A, Tedeschi A. Soil Nitrification Rate Is Affected by Plant Species and Nitrogen Levels. Agriculture. 2025; 15(16):1740. https://doi.org/10.3390/agriculture15161740
Chicago/Turabian StyleVitale, Luca, Giuseppe Maglione, Francsico Garcia-Sanchez, Lourdes Yabor, Maria Riccardi, Lucia Ottaiano, Bruno Di Matteo, Rosario Nocerino, Antonio Manco, and Anna Tedeschi. 2025. "Soil Nitrification Rate Is Affected by Plant Species and Nitrogen Levels" Agriculture 15, no. 16: 1740. https://doi.org/10.3390/agriculture15161740
APA StyleVitale, L., Maglione, G., Garcia-Sanchez, F., Yabor, L., Riccardi, M., Ottaiano, L., Di Matteo, B., Nocerino, R., Manco, A., & Tedeschi, A. (2025). Soil Nitrification Rate Is Affected by Plant Species and Nitrogen Levels. Agriculture, 15(16), 1740. https://doi.org/10.3390/agriculture15161740







