Diversity and Spatial Distribution of Phytopathogenic Fungi as Biological Control Agents for Goosegrass (Eleusine indica)
Abstract
1. Introduction
2. Materials and Methods
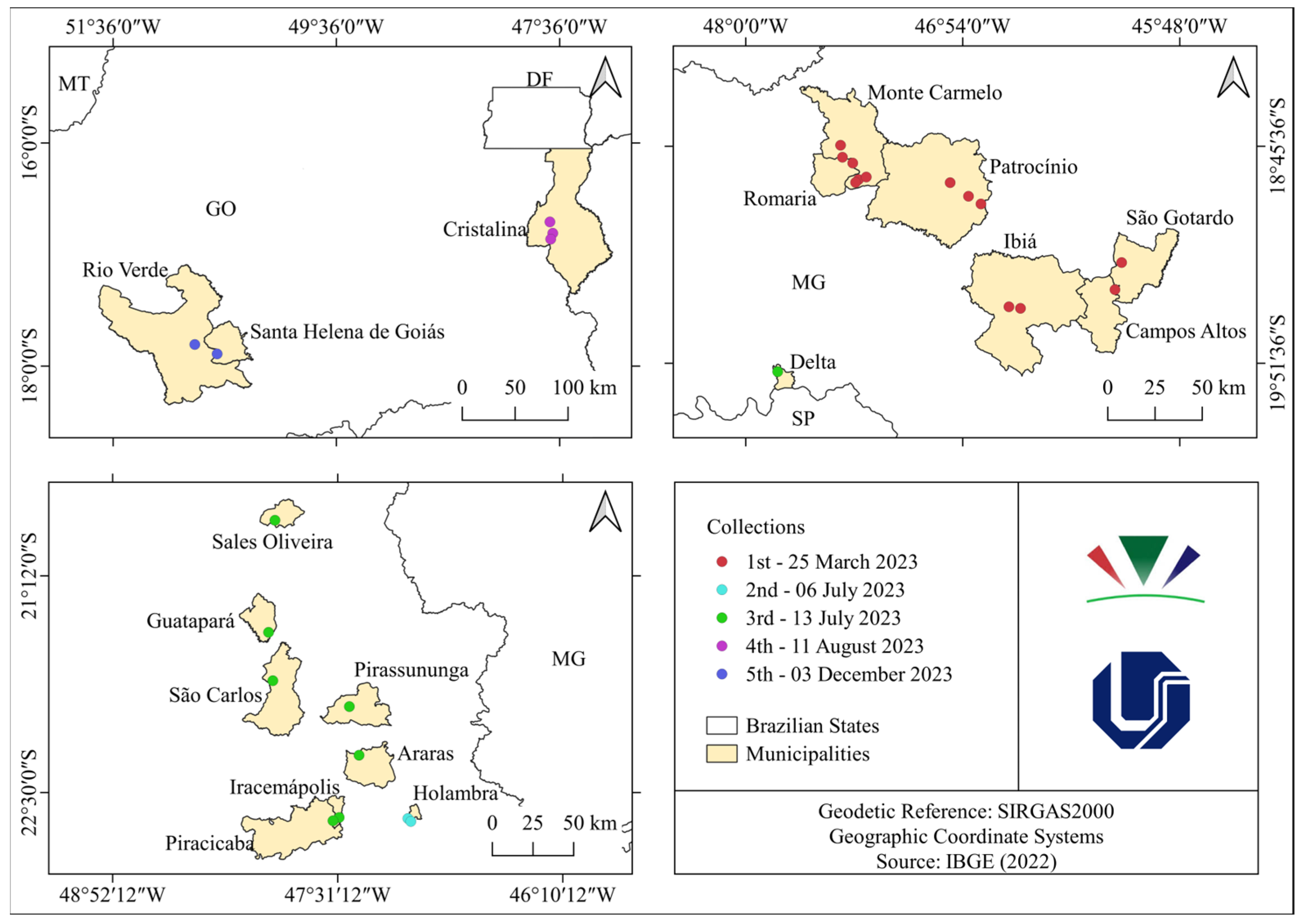
2.1. Surveys
2.2. Obtaining and Identifying the Isolates
2.3. Pathogenicity Test
2.4. DNA Extraction
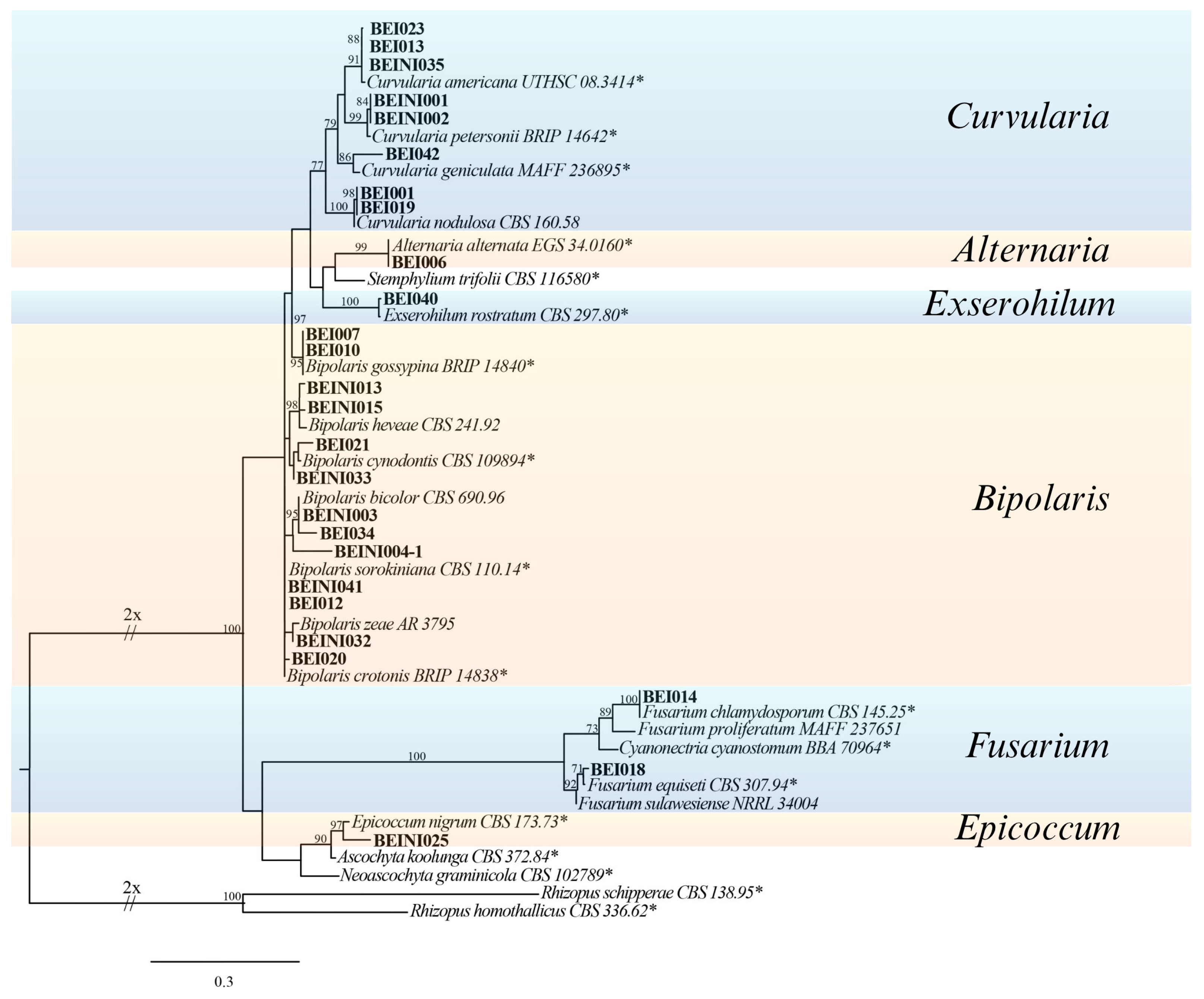
2.5. Phylogenetic Analysis
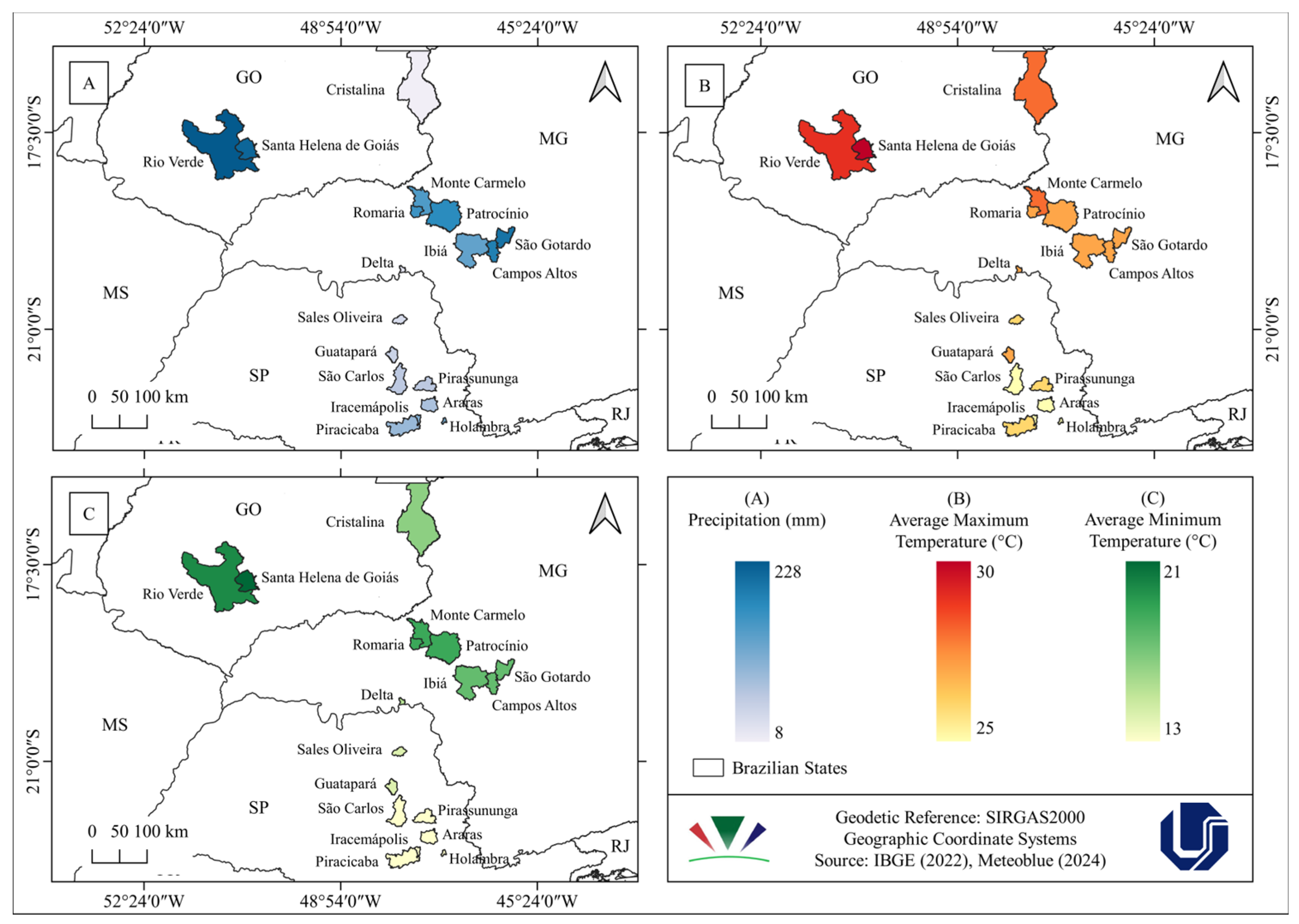
2.6. Analysis of Spatial Distribution of Phytopathogenic Fungi on Eleusine indica and Associated Climatic Conditions
3. Results
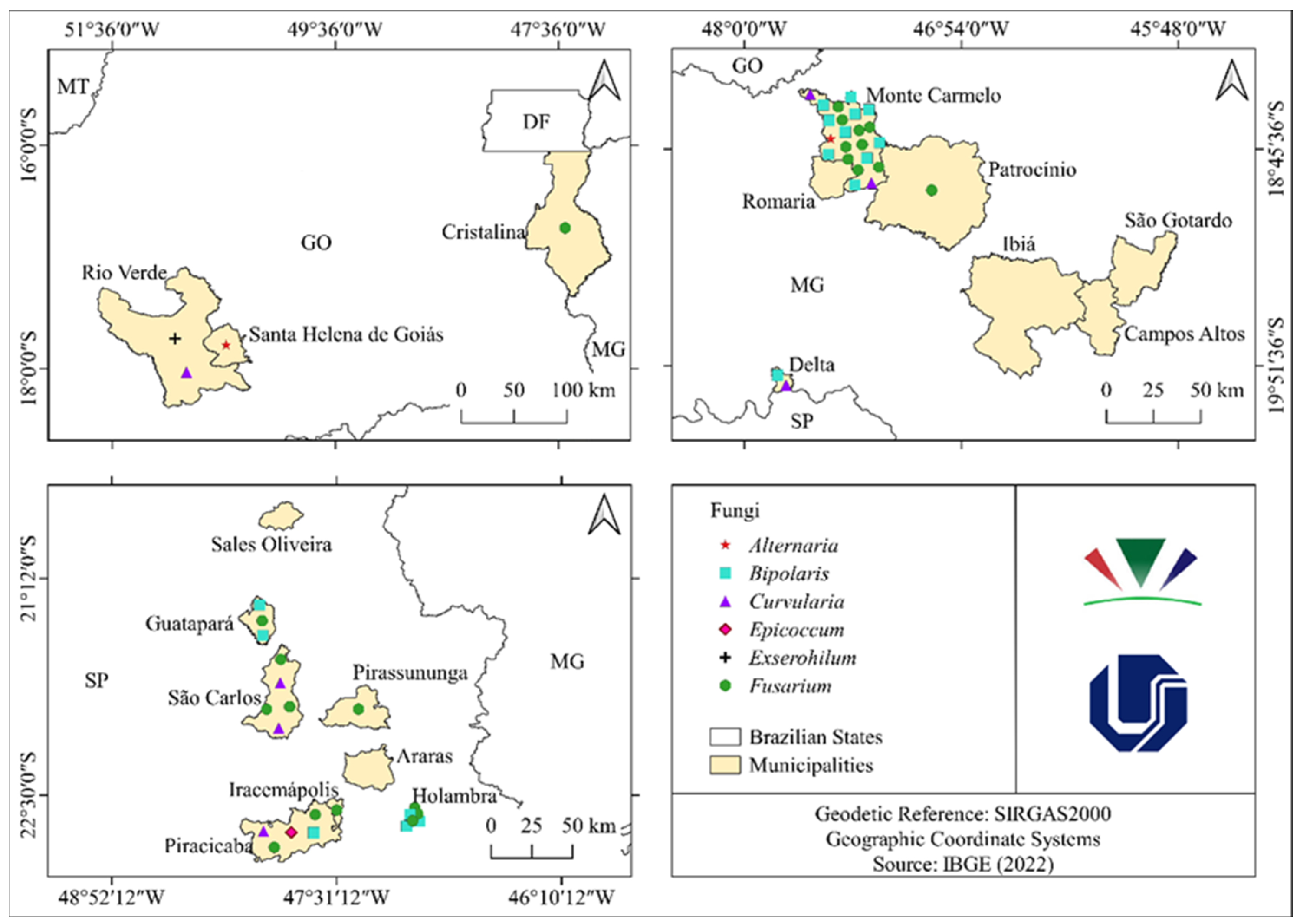
3.1. Surveys and Pathogenicity Test
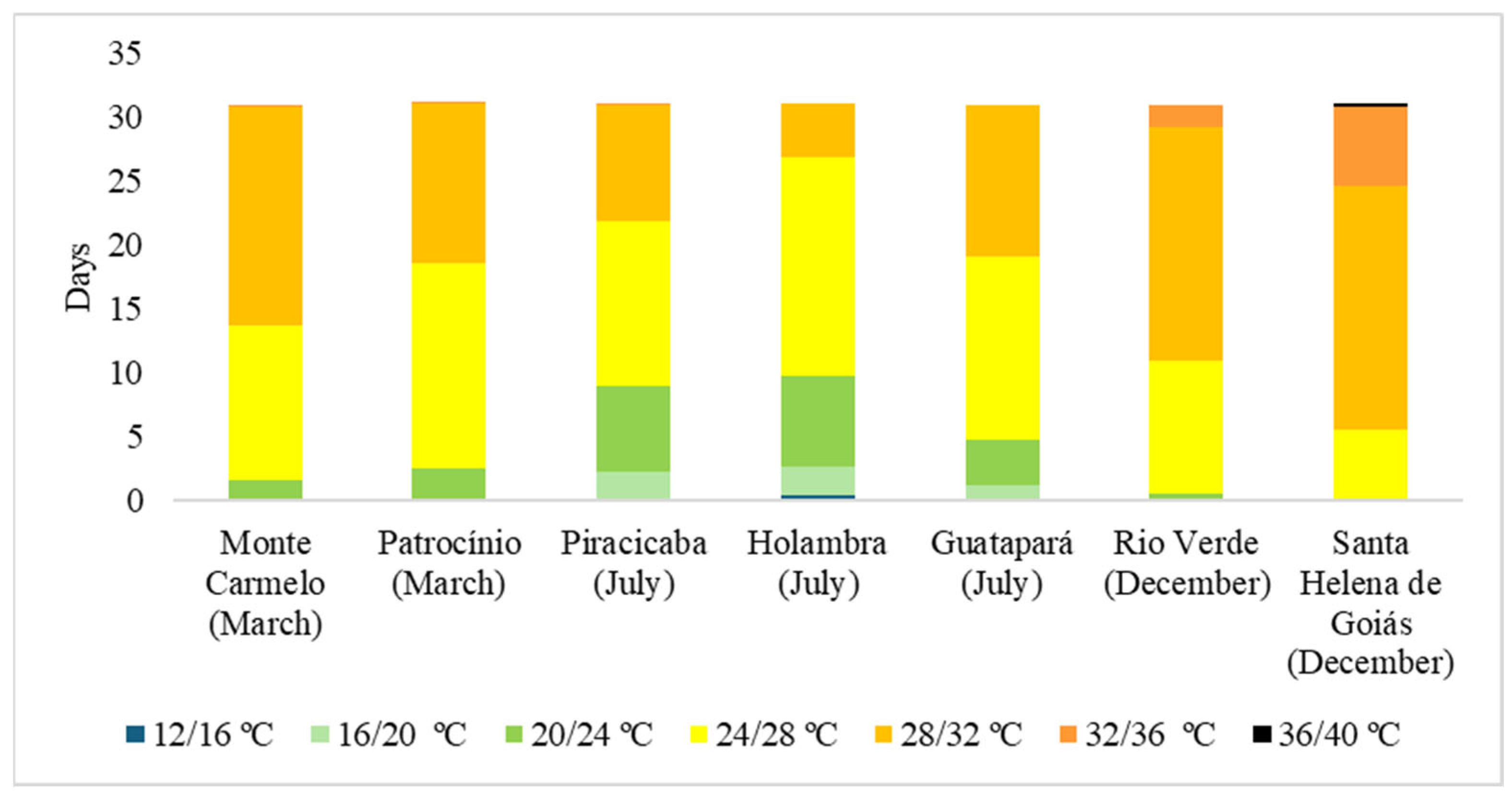
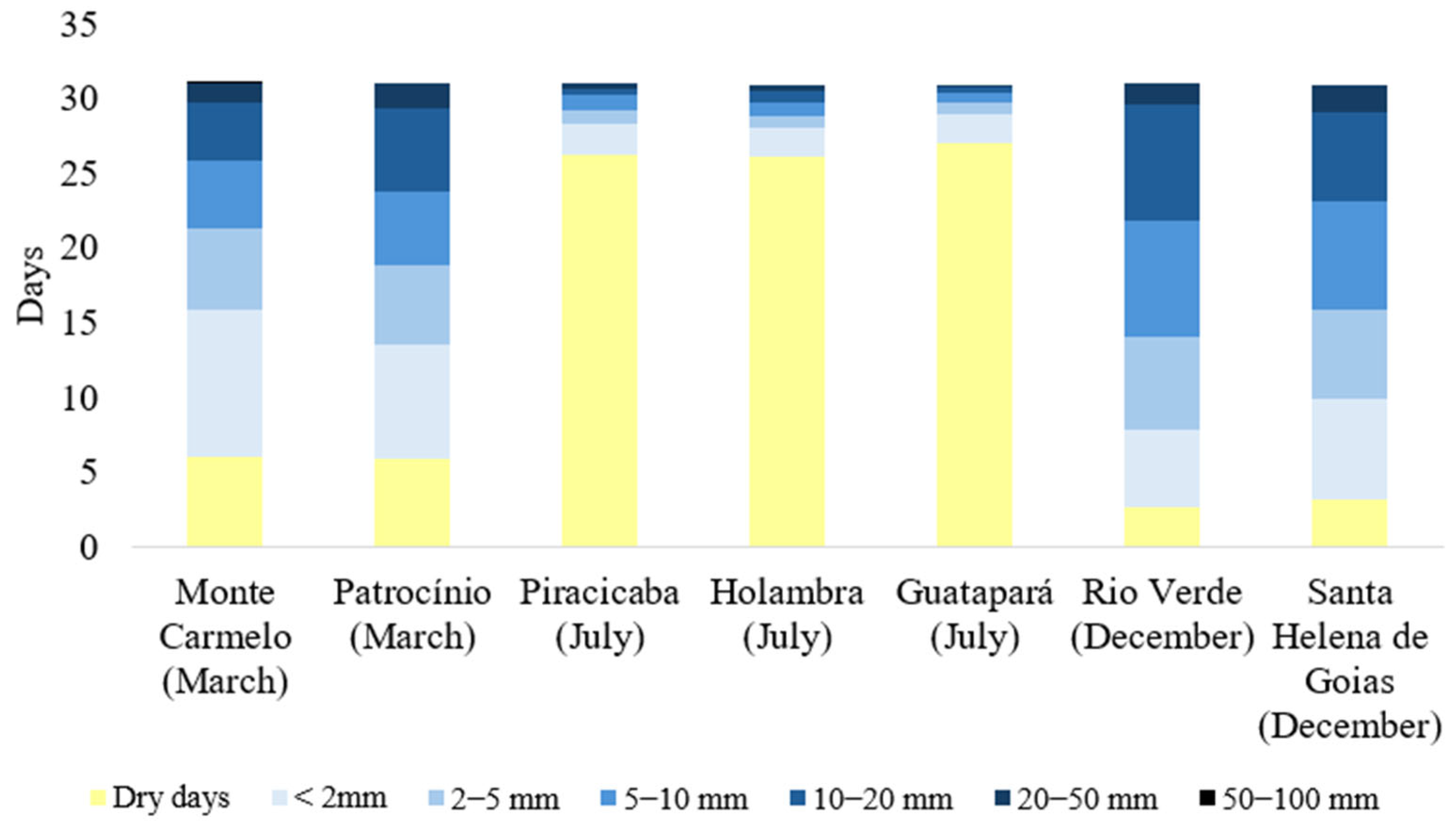
3.2. Analysis of Spatial Distribution of Phytopathogenic Fungi on Eleusine indica and Associated Climatic Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fontes, J.R.A.; Shiratsuchi, L.S.; Neves, J.L.; Júlio, L.D.; Sodré Filho, J. Manejo Integrado de Plantas Daninhas; Embrapa Cerrados: Planaltina, Brazil, 2003. [Google Scholar]
- Dias, A.; Carvalho, S.; Marcolini, L.; Melo, M.; Christoffoleti, P. Competitiveness of alexandergrass or Bengal dayflower with soybean. Planta Daninha 2010, 28, 515–522. [Google Scholar] [CrossRef]
- Dalazen, G.; Curioletti, L.; Cagliari, D.; Stacke, R.; Guedes, J. Hairy fleabane as a source of major insect pests of soybean. Planta Daninha 2016, 34, 403–409. [Google Scholar] [CrossRef]
- Zanatta, J.F.; Figueiredo, S.; Fontana, L.C.; Procópio, S.O. Interferência de plantas daninhas em culturas olerícolas. Rev. da FZVA 2006, 13, 39–57. [Google Scholar]
- IBAMA. Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. Relatórios de Comercialização de Agrotóxicos—Boletim Anual de Produção, Importação, Exportação e Vendas de Agrotóxicos. Boletim 2023. Available online: https://www.gov.br/ibama/pt-br (accessed on 15 February 2025).
- Heap, I. The International Survey of Herbicide Resistant Weeds. Online. Internet. Available online: www.weedscience.org (accessed on 10 April 2025).
- Riechers, D.E.; Soltani, N.; Chauhan, B.S.; Concepcion, J.C.T.; Geddes, C.M.; Jugulam, M.; Kaundun, S.S.; Preston, C.; Wuerrfel, R.J.; Sikkema, P.H. Herbicide resistance is complex: A global review of cross resistance in weeds within herbicide groups. Weed Sci. 2024, 72, 465–486. [Google Scholar] [CrossRef]
- Gerwick, B.C. Thirty Years of Herbicide Discovery: Surveying the Past and Contemplating the Future; Informa: London, UK, 2010. [Google Scholar]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef]
- IM, H. International Survey of Herbicide Resistant Weeds. Acessed em 20 December de 2023; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Davis, A.S.; Frisvold, G.B. Are herbicides a once in a century method of weed control? Pest Manag. Sci. 2017, 73, 2209–2220. [Google Scholar] [CrossRef]
- Copping, L.G. The evolution of crop protection companies. Outlooks Pest Manag. 2018, 29, 25–37. [Google Scholar] [CrossRef]
- Lorenzi, H. Manual de Identificação e Controle de Plantas Daninhas: Plantio Direto e Convencional; Instituto Plantarum: Nova Odessa, Brazil, 2014. [Google Scholar]
- Vidal, R.A.; Lamego, F.P.; Portes, E.D.S.; Trezzi, M.M. Resistência de Eleusine indica aos inibidores de ACCase. Planta Daninha 2006, 24, 163–171. [Google Scholar] [CrossRef][Green Version]
- Lorenzi, H. Plantas daninhas do Brasil: Terrestres, aquáticas, parasitas e tóxicas; Instituto Plantarum: Nova Odessa, Brazil, 2008. [Google Scholar]
- Silva, W.L. Herbicidas Residuais no Controle de Eleusine indica e na Seletividade da Cultura da Soja. Dissertação (Master’s thesis), Instituto Federal Goiano, Goias, Brazil, 2020. [Google Scholar]
- Carvalho, L.B. Plantas Daninhas; Editado Pelo Autor: Lages, Brazil, 2013. [Google Scholar]
- Andrade Junior, E.R.D. Capim pé-de-galinha (Eleusine indica) resistente a herbicidas inibidores da ACCase em áreas algodoeiras de mato grosso. Ph.D. Thesis, Universidade Federal de Mato Grosso, Cuiabá, Brazil, 2018. [Google Scholar]
- Kumar, V.; Aggarwal, N.K.; Malik, A. Bioherbicidal Concept: A novel strategy to control weeds. In Microbial Bioprospecting for Sustainable Development; Springer: Singapore, 2018; pp. 29–40. [Google Scholar] [CrossRef]
- Alfenas, A.C.; Mafia, R.G. Métodos em Fitopatologia; Editora UFV: Viçosa, Brazil, 2016. [Google Scholar]
- Castellani, A. Viability of some pathogenic fungi in distilled water. J. Trp. Med. Hyg. 1939, 42, 225. [Google Scholar]
- White, T.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: Enabling high-impact science for phylogenetics researchers with limited resources. In Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the Extreme to the Campus and Beyond, Anais, NY, USA, 16–20 July 2012; pp. 1–8. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree 2014, v1.4.4: A Graphical Viewer of Phylogenetic Trees. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 23 January 2025).
- Leach, M.D.; Cowen, L.E.; Heitman, J. Surviving the Heat of the Moment: A fungal pathogens perspective. PLoS Pathog. 2013, 8, e1003163. [Google Scholar] [CrossRef] [PubMed]
- Almiman, B. Identifying the optimal temperature and water activity conditions of phytopathogenic fungi recovered from Al-Baha province. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 640–651. [Google Scholar] [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef] [PubMed]
- Ezziyyani, M.; Hamdache, A.; Asraoui, M.; Requena, M.E.; Egea-Gilabert, C.; Castillo, M.E.C. Effect of Climate Change on Growth, Development and Pathogenicity of Phytopathogenic Telluric Fungi. Adv. Intell. Syst. Comput. 2019, 911, 14–21. [Google Scholar] [CrossRef]
- Khan, N.A.; Asaf, S.; Ahmad, W.; Jan, R.; Bilal, S.; Khan, I.; Khan, A.L.; Kim, K.-M.; Al-Harrasi, A. Diversity, Lifestyle, Genomics, and Their Functional Role of Cochliobolus, Bipolaris, and Curvularia Species in Environmental Remediation and Plant Growth Promotion under Biotic and Abiotic Stressors. J. Fungi 2023, 9, 254. [Google Scholar] [CrossRef]
- Muniz, P.H.P.C.; de Oliveira, T.A.S.; Duarte, E.A.A.; Rodrigues, F.; Carvalho, D.D.C. Characterization of Bipolaris bicolor germination: Effects of a physical factor on fungal adaptability. Braz. J. Microbiol. 2024, 55, 3521–3528. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Saluci, J.C.G.; Vivas, M.; Santos, J.S.; de Andrade Junior, M.S.; Vivas, J.M.S.; Ramos, G.K.S.; Graviana, G.A. Characterization of the Bipolaris maydis: Symptoms and pathogenicity in popcorn genotypes (Zea mays L.). Braz. J. Biol. 2024, 84, e256799. [Google Scholar] [CrossRef] [PubMed]
- Kleczewski, N.M.; Flory, S.L.; Clay, K. Variation in Pathogenicity and Host Range of Bipolaris sp. Causing Leaf Blight Disease on the Invasive GrassMicrostegium vimineum. Weed Sci. 2012, 60, 486–493. [Google Scholar] [CrossRef]
- Barbosa ASV. Locais de Produção na Qualidade Fisiológica e Sanitária de Sementes de Urochloa decumbens. Dissertação, Universidade Estadual Paulista, São Paulo, Brazil, 2023. [Google Scholar]
- Sun, X.; Qi, X.; Wang, W.; Liu, X.; Zhao, H.; Wu, C.; Chang, X.; Zhang, M.; Chen, H.; Gong, G. Etiology and Symptoms of Maize Leaf Spot Caused by Bipolaris spp. in Sichuan, China. Pathogens 2020, 9, 229. [Google Scholar] [CrossRef]
- Adhikari, A. Identification, Epidemiology, Genomics and Biocontrol of Bipolaris Species, Pathogens of Crops and Invasive Grasses. Dissertação, University of Florida, Gainesville, FL, USA, 2022. [Google Scholar]
- Moraes, C. Produção Massal e Influência de Fatores Físicos no Cultivo e Viabilidade de Bipolaris Euphorbiae. Dissertação, Universidade Estadual Paulista, São Paulo, Brazil, 2009. [Google Scholar]
- Muniz PHPC. Fotomorfogênese e Patogenicidade de Bipolaris bicolor em Trigo, Milho e Sorgo. Dissertação (Master’s thesis), Universidade Estadual de Goias, Goias, Brazil, 2022. [Google Scholar]
- Mshelia, L.P.; Selamat, J.; Samsudin, N.I.P.; Rafii, M.Y.; Abdul Mutalib, N.-A.; Nordin, N.; Berthiller, F. Effect of temperature, water activity and carbon dioxide on fungal growth and mycotoxin production of acclimatised isolates of Fusarium verticillioides and F. graminearum. Toxins 2020, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Gerling, M.; Petry, L.; Barkusky, D.; Büttner, C.; Müller, M.E.H. Infected grasses as inoculum for Fusarium infestation and mycotoxin accumulation in wheat with and without irrigation. Mycotoxin Res. 2023, 39, 19–31. [Google Scholar] [CrossRef]
- Bencheikh, A.; Belabed, I.; Rouag, N. Fusarium head blight of wheat: Current knowledge on associated species and their mycotoxins, pathogenicity diversity, and management strategies. Australas. Plant Pathol. 2024, 53, 457–471. [Google Scholar] [CrossRef]
- Sousa, R.R. Incidência de Fusarium verticillioides em Sementes de Milho e Métodos de Inoculação em Diferentes Genótipos e Estágios Fenológicos. Dissertação, Universidade Federal do Tocantins, Palmas, Brazil, 2017. [Google Scholar]
- Viana, F.M.P.; dos SANTOS, A.A.; Freire, F.; Cardoso, J.E.; Vidal, J.C. Recomendações para o Controle das Principais Doenças que Afetam a Cultura do Melão na Região Nordeste; Embrapa: Fortaleza, Brazil, 2001. [Google Scholar]
- Desai, S.; Dubey, S.C.; Prasad, R.D. Impacts of climate change on Fusarium species vis-à-vis adaptation strategies. Indian Phytopathol. 2020, 73, 593–603. [Google Scholar] [CrossRef]
- Gutiérrez-Pozo, M.; Verheecke-Vaessen, C.; Kourmpetli, S.; Terry, L.A.; Medina, A. Effect of Temperature, Relative Humidity, and Incubation Time on the Mycotoxin Production by Fusarium spp. Responsible for Dry Rot in Potato Tubers. Toxins 2024, 16, 414. [Google Scholar] [CrossRef]
- Beccari, G.; Hao, G.; Liu, H. Fusarium pathogenesis: Infection mechanisms and disease progression in host plants. Front. Plant Sci. 2022, 13, 1020404. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Sha, Y. Distribution, pathogenicity and disease control of Fusarium tricinctum. Front. Microbiol. 2022, 13, 939927. [Google Scholar] [CrossRef]
- Rocha, A.B.O. Efeito da luz fluorescente e da radiação UV-C no controle da podridão seca e rhizoctoniose em batata-semente após a colheita. Rev. Científica Multidiscip. Núcleo Do Conhecimento 2019, 4, 155–169. [Google Scholar]
- Meepagala, K.M.; Johnson, R.D.; Duke, S.O. Curvularin and Dehydrocurvularin as Phytotoxic Constituents from Curvularia intermedia Infecting Pandanus amaryllifolius. J. Agric. Chem. Env. 2016, 5, 12–22. [Google Scholar] [CrossRef]
- Torres, D.P. Etiologia da Mancha de Curvularia em Gladíolo e Aspectos da Interação Planta-Patógeno. Dissertação, Universidade Federal de Viçosa, Viçosa, Brazil, 2013. [Google Scholar]
- Vaz-de-Melo, A.; Afferri, F.S.; Dotto, M.A.; Peluzio, J.M.; dos Santos, G.R.; de Carvalho, E.V. Reação de híbridos de milho à Curvularia spp., sob dois níveis de adubação com nitrogênio, no sul do Tocantins. Sci. Agrar. 2010, 11, 149–154. [Google Scholar] [CrossRef][Green Version]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Crous, P.W. Multi-locus phylogeny of the genus Curvularia and description of ten new species. Mycol. Prog. 2020, 19, 559–588. [Google Scholar] [CrossRef]
- dos Santos, P.R.R.; Leão, E.U.; Aguiar, R.W.d.S.; de Melo, M.P.; dos Santos, G.R. Morphological and molecular characterization of Curvularia lunata pathogenic to andropogon grass. Bragantia 2018, 77, 326–332. [Google Scholar] [CrossRef]
- Mehta, T.; Meena, M.; Nagda, A. Bioactive compounds of Curvularia species as a source of various biological activities and biotechnological applications. Front. Microbiol. 2022, 13, 1069095. [Google Scholar] [CrossRef]
- Suehara, M.B.; da Silva, M.C.P. Prevalência de fungos anemófilos no Brasil e a correlação com doenças respiratórias e infecções fúngicas. Cienc. Saude Coletiva 2023, 28, 3289–3300. [Google Scholar] [CrossRef]
- Valle, T.L. Influência da Qualidade da luz no Crescimento e Esporulação do Metarhizium Anisopliae (Metch.) Sorokin. Dissertação, Universidade de São Paulo, São Paulo, Brazil, 1984. [Google Scholar]
- Liu, B.; Xiao, X.; Zhou, X.; Zhou, J.; Lan, L.; Long, X.; Pan, Y.; Du, M.; Zhao, X. Effects of Lactobacillus plantarum CQPC01-fermented soybean milk on activated carbon-induced constipation through its antioxidant activity in mice. Food Sci. Nutr. 2019, 7, 2068–2082. [Google Scholar] [CrossRef]
- Camera, J.N.; Deuner, C.C. Limiar térmico para a germinação de conídios de Exserohilum turcicum. Rev. Ceres 2017, 67, 478–487. [Google Scholar] [CrossRef][Green Version]
- Machado, M.R.F. Interactions Between Exserohilum Turcicum and Maize Seeds: Detection, Effects and Transmission of the Pathogen. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2021. [Google Scholar]
- Casela, C.R.; Ferreira, A.D.S.; Fernandes, F.; Pinto, N.D.A. Cultivo do Milho: Doenças Foliares; Embrapa: Sete Lagoas, Brazil, 2002. [Google Scholar]
- Leach, C.M.; Fullerton, R.A.; Young, K. Northern leaf blight of maize in New Zealand: Release and dispersal of conidia of Drechslera turcica. Phytopathology 1977, 67, 380–387. [Google Scholar] [CrossRef]
- White, D.G. Compendium of Corn Diseases; The American Phytopathological Society: Saint Paul, MN, USA, 1999. [Google Scholar]
- Bhowmik, T.P.; Prasada, R. Physiologic specialization in Helminthosporium turcicum Pass. From India. Phytopathol. Zeits-Chrift 1970, 68, 84–87. [Google Scholar] [CrossRef]
- Carrascal-Hernández, D.C.; Flórez-López, E.; Peralta-Ruiz, Y.; Chaves-López, C.; Grande-Tovar, C.D. Eco-Friendly Biocontrol Strategies of Alternaria Phytopathogen Fungus: A focus on gene-editing techniques. Agriculture 2022, 12, 1722. [Google Scholar] [CrossRef]
- Fernandes, C.; Casadevall, A.; Gonçalves, T. Mechanisms of Alternaria pathogenesis in animals and plants. Fems Microbiol. Rev. 2023, 47, fuad061. [Google Scholar] [CrossRef]
- Giorni, P.; Bulla, G.; Bellotti, G.; Antinori, M.E.; Guerrieri, M.C.; Fiorini, A.; Bertuzzi, T.; Puglisi, E. In planta evaluation of different bacterial consortia for the protection of tomato plants against Alternaria spp. infection and Alternaria toxins presence in fruits. Front. Hortic. 2024, 3, 1447425. [Google Scholar] [CrossRef]
- Balodi, R.; Raghavendra, K.V.; Singh, P.K.; Hussain, Z.; Suroshe, S.S.; Kumar, P.; Chander, S. Alternaria alternata species complex impairing solanaceous vegetables in Northern parts of India: An emerging problem in Solanum lycopersicum L. 3 Biotech 2024, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Amabile, R.F.; Vasconcelos, C.M.; Gomes, A.C. Severidade da mancha-de-alternária em cultivares de girassol na região do Cerrado do Distrito Federal. Pesqui. Agropecu. Bras. 2002, 37, 251–257. [Google Scholar] [CrossRef]
- Siqueira IF Girassol; Fundação Instituto Agronômico do Paraná: Londrina, Brazil, 1980.
- Coelho, F.S.; Alvarenga, M.A.R.; Leão, A.B.; Rodrigues, L. Controle de Alternaria solani com fungicidas na cultura do tomateiro. Rev. Fac. Nac. Agron. Medellín 2011, 64, 5845–5851. [Google Scholar]
- Liu, Y.; Ahmed, A.; Munir, S.; Chen, L.; He, P.; He, Y.; Tang, P.; Kong, B.; Wu, Y.; He, P. Alternaria alternata Pathogen from Cuscuta japonica Could Serve as a Potential Bioherbicide. J. Fungi 2024, 10, 494. [Google Scholar] [CrossRef]
- Pyrri, I.; Bruffaerts, N.; Radovic, M.; D’hOoge, E.; Janjusevic, L.; Sikoparija, B. Variability in Alternaria alternata spore characteristics under different culture conditions: Implications for automatic detection using air flow cytometry. Aerobiologia 2024, 40, 437–446. [Google Scholar] [CrossRef]
- Walker, H.L. Control of Sicklepod, Showy Crotalaria and Coffee Senna with Fungal Pathogen. U.S. Patent 4,390,360, 28 June 1983. [Google Scholar]
- DE Ávila, Z.R.; DE Mello, S.C.M.; Ribeiro, Z.M.D.A.; Fontes, E.M.G. Produção de inóculo de Alternaria cassie. Pesqui. Agropecu. Bras. 2000, 35, 533–541. [Google Scholar] [CrossRef][Green Version]
- Rajarammohan, S.; Paritosh, K.; Pental, D.; Kaur, J. Comparative genomics of Alternaria species provides insights into the pathogenic lifestyle of Alternaria brassicae—A pathogen of the Brassicaceae family. BMC Genom. 2019, 20, 1036. [Google Scholar] [CrossRef]
- Cao, C.; Gong, S.; Li, Y.; Tang, J.; Li, T.; Zhang, Q. Pathogenic Factors and Mechanisms of the Alternaria Leaf Spot Pathogen in Apple. Horticulturae 2024, 10, 212. [Google Scholar] [CrossRef]
- The World Bank Group. Explore Historical and Projected Climate Data, Climate Data by Sector, Impacts, Key Vulnerabilities and What Adaptation Measures Are Being Taken. Explore the Overview for a General Context of How Climate Change Is Affecting Brazil. 2021. Available online: https://climateknowledgeportal.worldbank.org/country/brazil/climate-data-historical (accessed on 13 October 2024).
- Ballarin, A.S.; Sone, J.S.; Gesualdo, G.C.; Schwamback, D.; Reis, A.; Almagro, A.; Wendland, E.C. CLIMBra-climate change dataset for Brazil. Sci. Data 2023, 10, 47. [Google Scholar] [CrossRef]
- Marengo, J.; Espinoza, J.C.; Bettolli, L.; Cunha, A.P.; Molina-Carpio, J.; Skansi, M.; Correa, K.; Ramos, A.M.; Salinas, R.; Sierra, J.-P. A cold wave of winter 2021 in central South America: Characteristics and impacts. Clim. Dyn. 2023, 61, 2599–2621. [Google Scholar] [CrossRef]
- Yadav, U.; Singh, P.C. Biotic Stress to Plants: Fungal pathogen as a major biotic stress. In Plant-Microbe Interaction and Stress Management; Chauhan, P.S., Tewari, S.K., Misra, S., Eds.; Springer: New York, NY, USA, 2024; pp. 1–305. [Google Scholar] [CrossRef]
- Flores-Nunez, V.M.; Stukenbrock, E.H. The impact of filamentous plant pathogens on the host microbiota. BMC Biol. 2024, 22, 175. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shameem, N.; Jatav, H.S.; Sathyanarayana, E.; Parray, J.A.; Poczai, P.; Sayyed, R.Z. Fungal endophytes to combat biotic and abiotic stresses for climate-smart and sustainable agriculture. Front. Plant Sci. 2022, 13, 953836. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Hema, P.; Murali, M.; Shilpa, N.; Nataraj, K.; Basavaraj, G.L.; Singh, S.B.; Aiyaz, M.; Udayashankar, A.C.; Amruthesh, K.N. Fungal Endophytes as Mitigators against Biotic and Abiotic Stresses in Crop Plants. J. Fungi 2024, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Dietzel, K.; Valle, D.; Fierer, N.; U’Ren, J.M.; Barberán, A. Geographical Distribution of Fungal Plant Pathogens in Dust Across the United States. Front. Ecol. Evol. 2019, 7, 304. [Google Scholar] [CrossRef]
- Chen, M.; Yang, J.; Xue, C.; Tu, T.; Su, Z.; Feng, H.; Shi, M.; Zeng, G.; Zhang, D.; Qian, X. Community composition of phytopathogenic fungi significantly influences ectomycorrhizal fungal communities during subtropical forest succession. Appl. Microbiol. Biotechnol. 2024, 108, 99. [Google Scholar] [CrossRef]


| Selected Isolates | Score | Selected Isolates | Score |
|---|---|---|---|
| BEI001 | 4 | BEI040 | 2 |
| BEI006 | 2 | BEI042 | 1 |
| BEI007 | 2 | BEINI001 | 3 |
| BEI010 | 3 | BEINI002 | 3 |
| BEI012 | 3 | BEINI003 | 1 |
| BEI013 | 3 | BEINI004-1 | 3 |
| BEI014 | 3 | BEINI013 | 3 |
| BEI018 | 2 | BEINI015 | 3 |
| BEI019 | 2 | BEINI025 | 1 |
| BEI020 | 4 | BEINI032 | 1 |
| BEI021 | 1 | BEINI033 | 3 |
| BEI023 | 1 | BEINI035 | 1 |
| BEI034 | 2 | BEINI041 | 3 |
| Collection | City | Quantity of Phytopathogenic Isolates |
|---|---|---|
| 1 | Monte Carmelo (MG) | 22 |
| Patrocínio (MG) | 1 | |
| 2 | Holambra (SP) | 6 |
| 3 | Delta (MG) | 5 |
| Guatapará (SP) | 5 | |
| Iracemápolis (SP) | 3 | |
| Piracicaba (SP) | 1 | |
| Pirassununga (SP) | 2 | |
| São Carlos (SP) | 1 | |
| 4 | Cristalina (GO) | 1 |
| 5 | Rio Verde (GO) | 2 |
| Santa Helena de Goiás (GO) | 1 |
| Genus | City | Collection | Quantity |
|---|---|---|---|
| Bipolaris | Monte Carmelo (MG) | 1 (March) | 8 |
| Holambra (SP) | 2 (July) | 3 | |
| Piracicaba (SP) | 3 (July) | 1 | |
| Guatapará (SP) | 3 (July) | 2 | |
| Fusarium | Monte Carmelo (MG) | 1 (March) | 3 |
| Patrocínio (MG) | 1 (March) | 1 | |
| Holambra (SP) | 2 (July) | 1 | |
| Piracicaba (SP) | 3 (July) | 2 | |
| Curvularia | Monte Carmelo (MG) | 1 (March) | 1 |
| Piracicaba (SP) | 3 (July) | 1 | |
| Exserohilum | Rio Verde (GO) | 5 (December) | 1 |
| Alternaria | Monte Carmelo (MG) | 1 (March) | 1 |
| Santa Helena de Goiás (GO) | 5 (December) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbris, C.; Silva, M.N.; da Silva, L.A.; de Oliveira, V.H.R.; Queiroz, M.F.; Inokuti, E.M.; Vieira, B.S.; Firmino, A.L. Diversity and Spatial Distribution of Phytopathogenic Fungi as Biological Control Agents for Goosegrass (Eleusine indica). Agriculture 2025, 15, 1721. https://doi.org/10.3390/agriculture15161721
Fabbris C, Silva MN, da Silva LA, de Oliveira VHR, Queiroz MF, Inokuti EM, Vieira BS, Firmino AL. Diversity and Spatial Distribution of Phytopathogenic Fungi as Biological Control Agents for Goosegrass (Eleusine indica). Agriculture. 2025; 15(16):1721. https://doi.org/10.3390/agriculture15161721
Chicago/Turabian StyleFabbris, Claudia, Monara Nogueira Silva, Leticia Alves da Silva, Victor Humberto Ribeiro de Oliveira, Marcia Ferreira Queiroz, Eliane Mayumi Inokuti, Bruno Sérgio Vieira, and André Luiz Firmino. 2025. "Diversity and Spatial Distribution of Phytopathogenic Fungi as Biological Control Agents for Goosegrass (Eleusine indica)" Agriculture 15, no. 16: 1721. https://doi.org/10.3390/agriculture15161721
APA StyleFabbris, C., Silva, M. N., da Silva, L. A., de Oliveira, V. H. R., Queiroz, M. F., Inokuti, E. M., Vieira, B. S., & Firmino, A. L. (2025). Diversity and Spatial Distribution of Phytopathogenic Fungi as Biological Control Agents for Goosegrass (Eleusine indica). Agriculture, 15(16), 1721. https://doi.org/10.3390/agriculture15161721







