Effect of Drying–Rewetting Alternation on Phosphorus Fractions in Restored Wetland
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
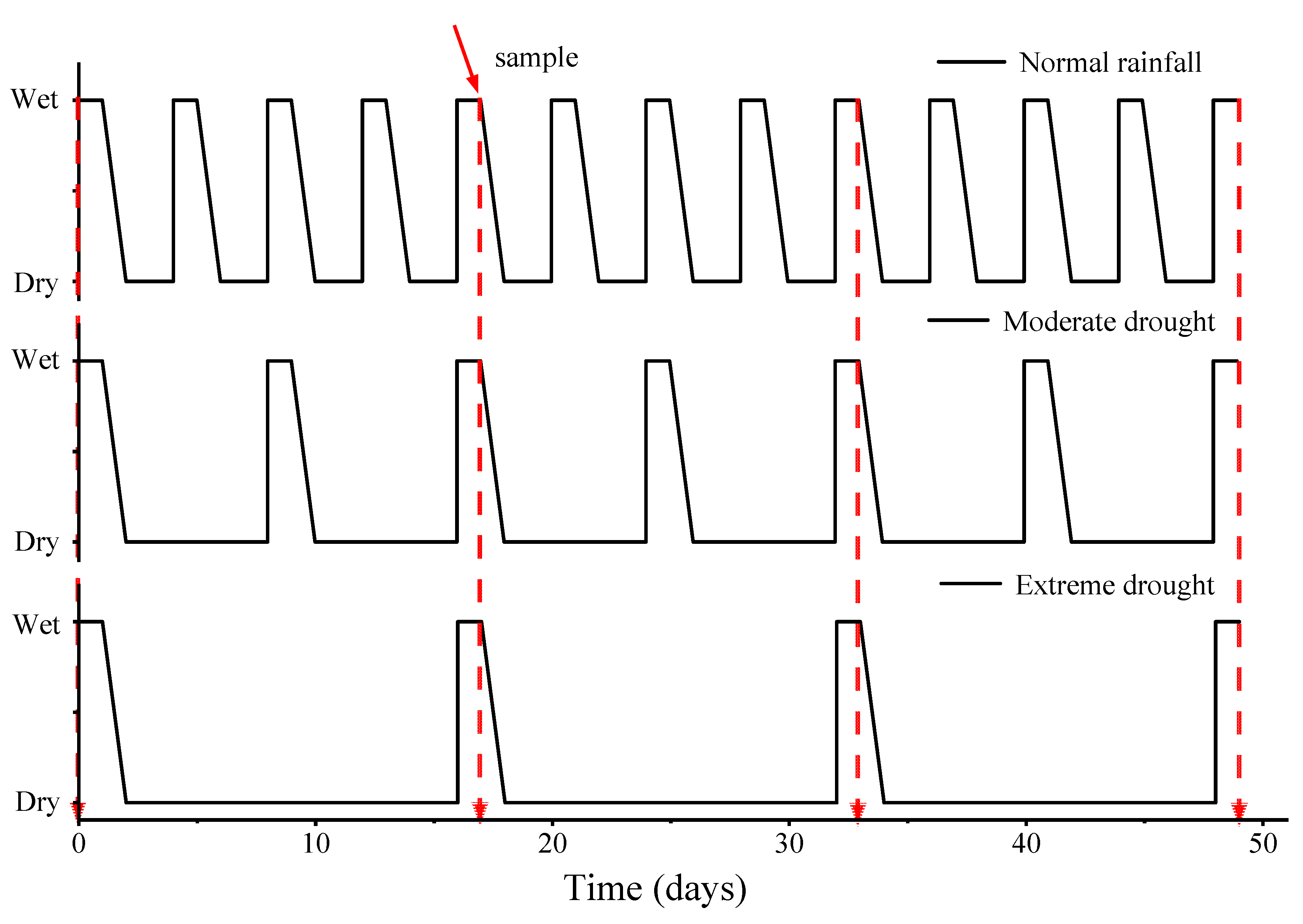
2.2. Experimental Design
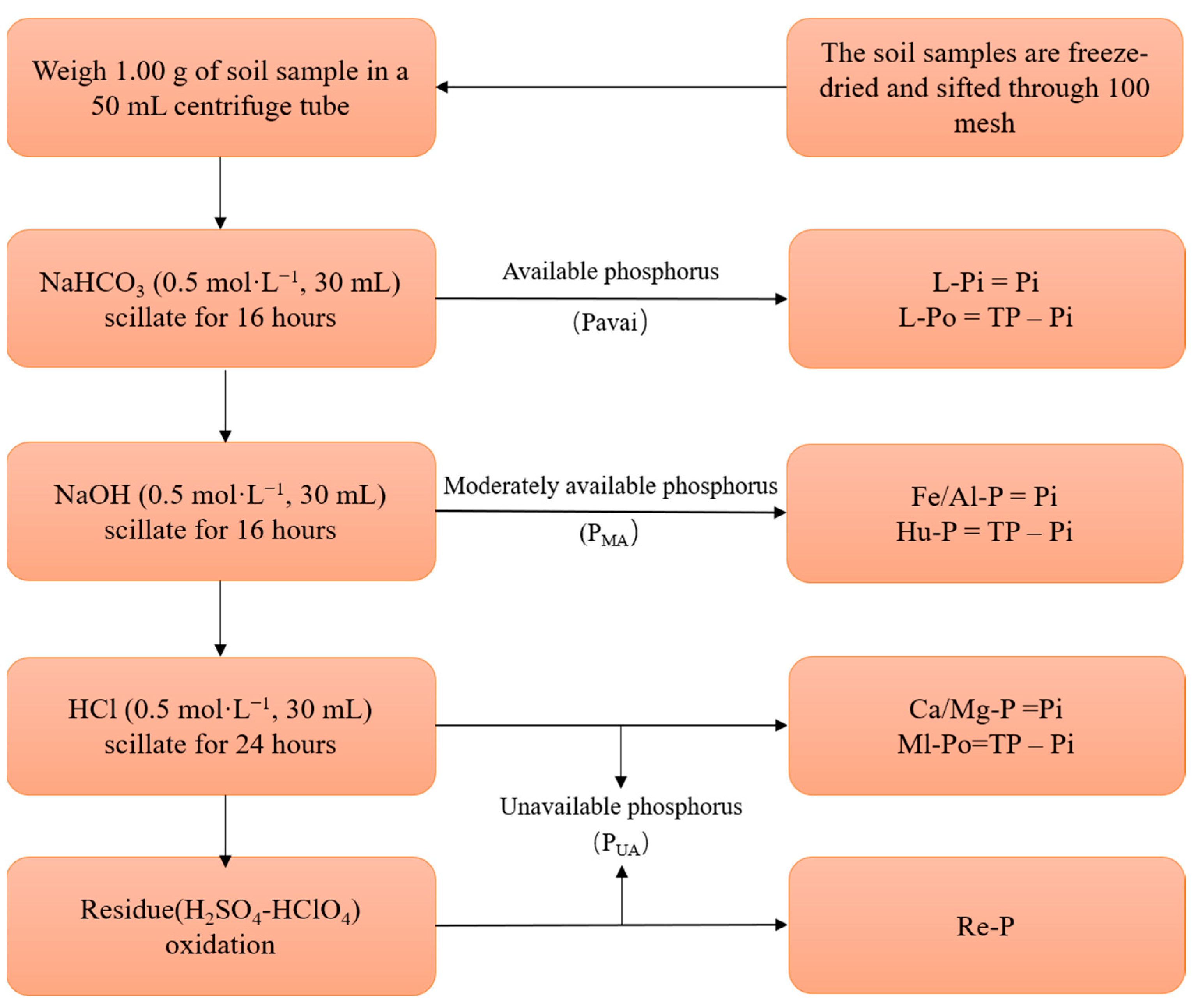
2.3. Physicochemical Analysis of Soil
2.4. Analyses of Microbial
2.5. Statistical Analysis
3. Results
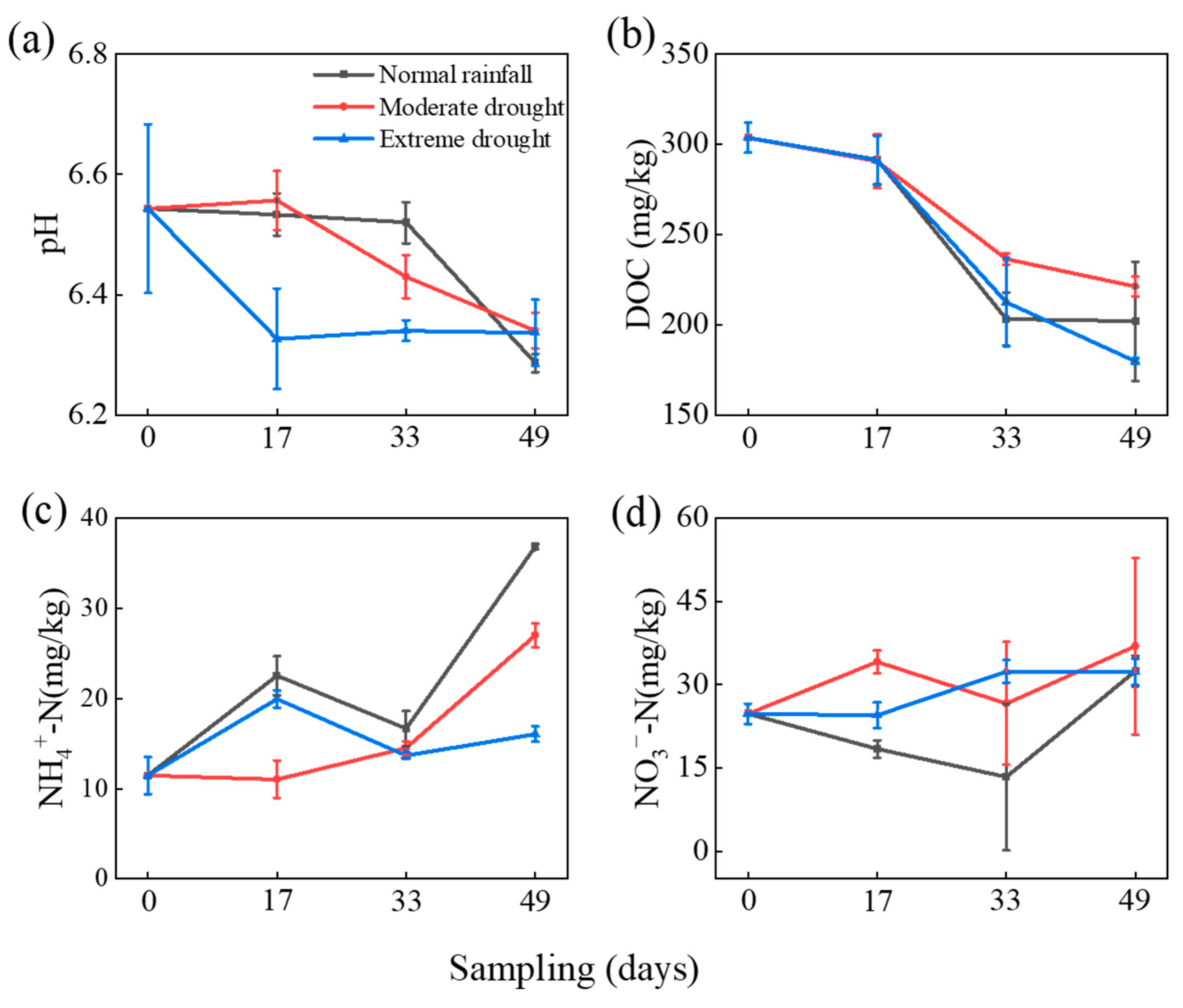
3.1. Effect of DRW on Chemical Properties of Soil
3.2. Effect of DRW on Soil P Fractions
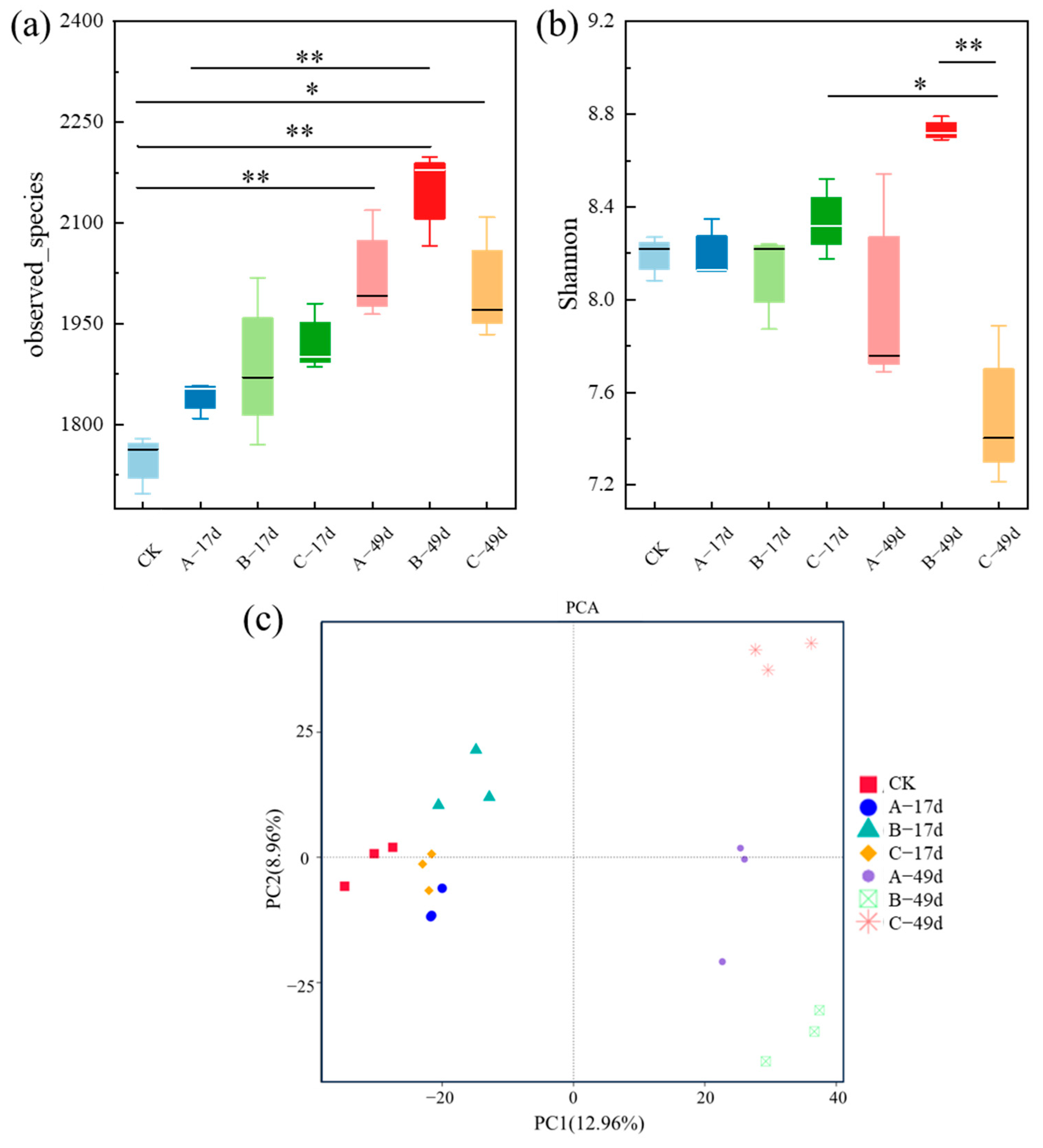
3.3. Effect of DRW on Soil Microbial Community
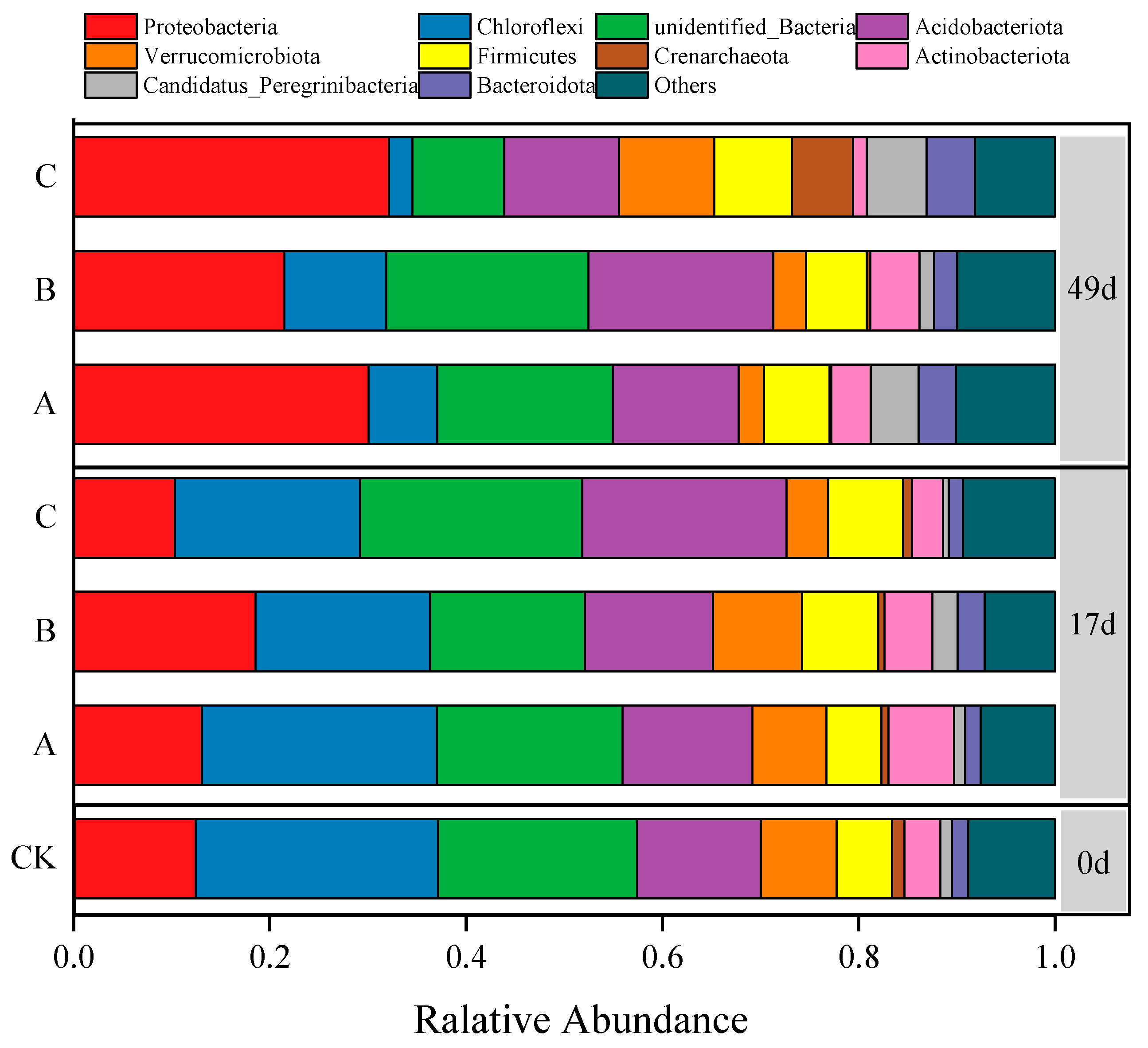
3.3.1. Changes in Soil Bacterial Composition
3.3.2. Changes in the Structure of Soil Bacterial Communities
4. Discussion
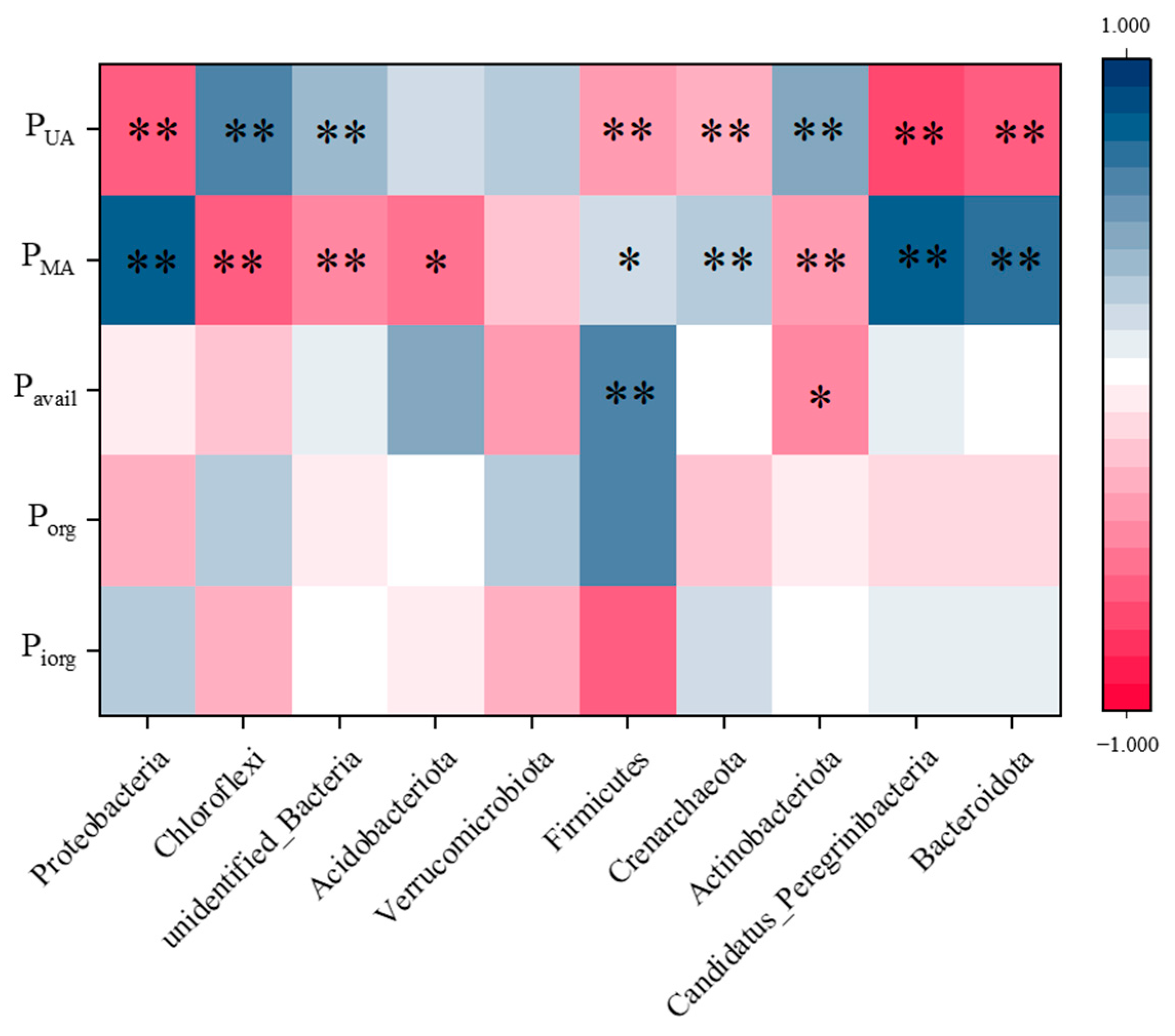
4.1. The Relationship Between Microbial Community Structure and P Fractions
4.2. Effect of DRW on Soil P Mineralization
4.3. Effect of DRW on Soil P Migration
4.4. The Critical Factors of P Fractions in the Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DRW | Drying and rewetting e |
| P | Phosphorus |
| C | Carbon |
| N | Nitrogen |
| DOC | Dissolved organic carbon |
| NH4+-N | Ammonium nitrogen |
| NO3−-N | Nitrate nitrogen |
| TP | Total phosphorus |
| SOC | Soil organic carbon |
| Pavai | Available phosphorus |
| PMA | Moderately available phosphorus |
| PUA | Unavailable phosphorus |
| Porg | Organic phosphorus |
| Piorg | Inorganic phosphorus |
| PVC | Polyvinyl chloride |
References
- Gao, C.; Zhang, M.; Song, K.; Wei, Y.; Zhang, S. Spatiotemporal analysis of anthropogenic phosphorus fluxes in China. Sci. Total Environ. 2020, 721, 137588. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Ou, Y.; Wang, L.; Liang, A.; Yan, B.; Li, Y. Dynamic changes in microbial communities and nutrient stoichiometry associated with soil aggregate structure in restored wetlands. Catena 2021, 197, 104984. [Google Scholar] [CrossRef]
- Wang, C.; Guo, J.; Zhang, W.; Jiang, Y.; Fang, F.; He, W.; Dang, C. Drying-rewetting changes soil phosphorus status and enzymatically hydrolysable organic phosphorus fractions in the water-level fluctuation zone of Three Gorges reservoir. Catena 2021, 204, 105416. [Google Scholar] [CrossRef]
- Mao, D.; Luo, L.; Wang, Z.; Wilson, M.C.; Zeng, Y.; Wu, B.; Wu, J. Conversions between natural wetlands and farmland in China: A multiscale geospatial analysis. Sci. Total Environ. 2018, 634, 550–560. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, H.; Xing, X.; Xie, T.; Song, C.; Wang, G.; Jing, M. Achievement of Wetland Protection and Restoration and Development Strategies in China. Bull. Chin. Acad. Sci. 2013, 38, 365–375. [Google Scholar]
- Wang, G.; Otte, M.L.; Jiang, M.; Wang, M.; Yuan, Y.; Xue, Z. Does the element composition of soils of restored wetlands resemble natural wetlands? Geoderma 2019, 351, 174–179. [Google Scholar] [CrossRef]
- Brangarí, A.C.; Manzoni, S.; Rousk, J. The mechanisms underpinning microbial resilience to drying and rewetting—A model analysis. Soil Biol. Biochem. 2021, 162, 108400. [Google Scholar] [CrossRef]
- Fan, B.; Wang, H.; Zhai, L.; Li, J.; Fenton, O.; Daly, K.; Liu, H. Leached phosphorus apportionment and future management strategies across the main soil areas and cropping system types in northern China. Sci. Total Environ. 2022, 805, 150441. [Google Scholar] [CrossRef]
- Jalali, M.; Jalali, M. Relation between various soil P extraction methods and sorption parameters in calcareous soils with different texture. Sci. Total Environ. 2016, 566–567, 1080–1093. [Google Scholar] [CrossRef]
- Audet, J.; Zak, D.; Bidstrup, J.; Hoffmann, C. Nitrogen and P retention in Danish restored wetlands. Ambio 2020, 49, 324–336. [Google Scholar] [CrossRef]
- Hou, E.; Chen, C.; Kuang, Y.; Zhang, Y.; Heenan, M.; Wen, D. A structural equation model analysis of P transformations in global unfertilized and uncultivated soils. Glob. Biogeochem. Cycles 2016, 30, 1300–1309. [Google Scholar] [CrossRef]
- Chang, S.C.; Jackson, M.L. Soil P Fractions in Some Representative Soils1. J. Soil Sci. 1958, 9, 109–119. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Eames, J.M.; Klimas, C. A16-year investigation of legacy P discharge from Prairie Wolf Slough: A wetland restored on a former farmed field. Restor. Ecol. 2021, 29, e13340. [Google Scholar] [CrossRef]
- Cui, H.; Ou, Y.; Wang, L.; Wu, H.; Yan, B.; Li, Y. Distribution and release of phosphorus fractions associated with soil aggregate structure in restored wetlands. Chemosphere 2019, 223, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Frank Stephano, M.; Geng, Y.; Cao, G.; Wang, L.; Meng, W.; Meiling, Z. Effect of Silicon Fertilizer and Straw Return on the Maize Yield and P Efficiency in Northeast China. Commun. Soil Sci. Plant Anal. 2021, 52, 116–127. [Google Scholar] [CrossRef]
- Sun, D.; Bi, Q.; Xu, H.; Li, K.; Liu, X.; Zhu, J.; Lin, X. Degree of short-term drying before rewetting regulates the bicarbonate-extractable and enzymatically hydrolyzable soil P fractions. Geoderma 2017, 305, 136–143. [Google Scholar] [CrossRef]
- Turner, B.L.; Haygarth, P.M. Phosphorus solubilization in rewetted soils. Nature 2001, 411, 258. [Google Scholar] [CrossRef]
- Wang, C.; Fang, F.; Yuan, Z.; Zhang, R.; Zhang, W.; Guo, J. Spatial variations of soil phosphorus forms and the risks of phosphorus release in the water-level fluctuation zone in a tributary of the Three Gorges Reservoir. Sci. Total Environ. 2020, 699, 134124. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Liu, H.; Zhu, H. Summer Precipitation in Heilongjiang from 1961 to 2017: Climatic Characteristics. Chin. Agric. Sci. Bull. 2019, 35, 115–122. [Google Scholar]
- Fierer, N.; Schimel, J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Hedley, M.J.; White, R.E.; Nye, P.H. Plant-Induced Changes in the Rhizosphere of Rape (Brassica napus Var. Emerald) Seedlings. III. Changes in L Value, Soil Phosphate Fractions and Phosphatase Activity. New Phytol. 1982, 91, 45–56. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, S.; Tian, L.; Ou, Y.; Yan, B.; Liang, A. Freeze-thaw cycles increase the mobility of P fractions based on soil aggregate in restored wetlands. Catena 2022, 209, 105846. [Google Scholar] [CrossRef]
- Chen, K.; Yu, H.; Wang, H.; Li, P.; Cheng, K.; Wu, T.; Zhu, L. The interaction strength of keystone module in cross-kingdom network determines microbial carbon metabolic stability under temperature stress. Appl. Soil Ecol. 2025, 206, 105906. [Google Scholar] [CrossRef]
- Zhang, Z.; Furman, A. Soil redox dynamics under dynamic hydrologic regimes—A review. Sci. Total Environ. 2021, 763, 143026. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Zhou, J.; Li, H.; He, Z.; Van Nostrand, J.D.; Xu, X. Redox potential and microbial functional gene diversity in wetland sediments under simulated warming conditions: Implications for phosphorus mobilization. Hydrobiologia. 2015, 743, 221–235. [Google Scholar] [CrossRef]
- Lang, M.; Li, H.; Lakshmanan, P.; Chen, Y.; Chen, X. phoD-harboring bacterial community composition dominates organic P mineralization under long-term P fertilization in acid purple soil. Front. Microbiol. 2022, 13, 1045919. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Yan, D.; Zhu, Y.G.; Zhang, Y.; Zhang, Z. Effects of wet-dry alternation on organic phosphorus dynamics and sediment characteristics in the intertidal zone of Nansi Lake. Ecotoxicol. Environ. Saf. 2024, 281, 116668. [Google Scholar] [CrossRef]
- Berkelmann, D.; Suhl, L.L.; Schneider, D.; Meryandini, A.; Daniel, R. Soil bacterial community composition of different tropical land use systems in Jambi province, Indonesia. Microbiol. Resour. Announc. 2015, 14, e01018–e01024. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Flooding and prolonged drought have differential legacy impacts on soil nitrogen cycling, microbial communities and plant productivity. Plant Soil 2018, 431, 371–387. [Google Scholar] [CrossRef]
- Lynch, M.D.; Neufeld, J.D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 2015, 13, 217–229. [Google Scholar] [CrossRef]
- Yevdokimov, I.; Larionova, A.; Blagodatskaya, E. Microbial immobilization of phosphorus in soils exposed to drying-rewetting and freeze-thawing cycles. Biol. Fertil. Soils 2016, 52, 685–696. [Google Scholar] [CrossRef]
- Gaiero, J.R.; Tosi, M.; Bent, E.; Boitt, G.; Khosla, K.; Turner, B.L.; Dunfield, K.E. Soil microbial communities influencing organic phosphorus mineralization in a coastal dune chronosequence in New Zealand. FEMS Microbiol. Ecol. 2021, 97, fiab034. [Google Scholar] [CrossRef]
- Pistocchi, C.; Mészáros, É.; Tamburini, F.; Frossard, E.; Bünemann, E.K. Biological processes dominate phosphorus dynamics under low phosphorus availability in organic horizons of temperate forest soils. Soil Biol. Biochem. 2018, 126, 64–75. [Google Scholar] [CrossRef]
- George, T.S.; Giles, C.D.; Menezes-Blackburn, D.; Condron, L.M.; Gama-Rodrigues, A.C.; Jaisi, D.; Haygarth, P.M. Organic phosphorus in the terrestrial environment: A perspective on the state of the art and future priorities. Plant Soil 2018, 427, 191–208. [Google Scholar] [CrossRef]
- Novair, S.B.; Hosseini, H.M.; Etesami, H.; Razavipour, T. Impact of drying–rewetting cycles and organic amendments on phosphorus speciation of paddy soil. Soil Res. 2021, 59, 472–487. [Google Scholar] [CrossRef]
- Blackwell, M.S.A.; Brookes, P.C.; de La Fuente-Martinez, N.; Gordon, H.; Murray, P.J.; Snars, K.E.; Haygarth, P.M. Phosphorus Solubilization and Potential Transfer to Surface Waters from the Soil Microbial Biomass Following Drying-Rewetting and Freezing-Thawing. Adv. Agron. 2010, 106, 1–35. [Google Scholar] [CrossRef]
- Blackwell, M.S.A.; Brookes, P.C.; de la Fuente-Martinez, N.; Murray, P.J.; Snars, K.E.; Williams, J.K.; Haygarth, P.M. Effects of soil drying and rate of re-wetting on concentrations and fractions of P in leachate. Biol. Fertil. Soils 2009, 45, 635–643. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Helfenstein, J.; Frossard, E.; Pistocchi, C.; Chadwick, O.; Vitousek, P.; Tamburini, F. Soil phosphorus exchange as affected by drying-rewetting of three soils from a Hawaiian climatic gradient. Front. Soil Sci. 2021, 1, 738464. [Google Scholar] [CrossRef]
- Gu, S.; Gruau, G.; Malique, F.; Dupas, R.; Petitjean, P.; Gascuel-Odoux, C. Drying/rewetting cycles stimulate release of colloidal-bound phosphorus in riparian soils. Geoderma 2018, 321, 32–41. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, Y.; Huang, S.; Li, X.; Wang, Z.; Li, D. Sediment P release in response to flood event across different land covers in a restored wetland. Environ. Sci. Pollut. Res. 2019, 26, 9113–9122. [Google Scholar] [CrossRef]
- Qu, F.; Shao, H.; Meng, L.; Yu, J.; Xia, J.; Sun, J.; Li, Y. Forms and vertical distributions of soil phosphorus in newly formed coastal wetlands in the Yellow River Delta estuary. Land Degrad. Dev. 2018, 29, 4219–4226. [Google Scholar] [CrossRef]
- Helfenstein, J.; Jegminat, J.; McLaren, T.I.; Frossard, E. Soil solution phosphorus turnover: Derivation, interpretation, and insights from a global compilation of isotope exchange kinetic studies. Biogeosciences 2018, 15, 105–114. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.; Lu, H.; Shao, Y.; Liu, S.; Fu, S. Drought promotes soil phosphorus transformation and reduces phosphorus bioavailability in a temperate forest. Sci. Total Environ. 2020, 732, 139295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, S.; Li, D. Decomposition of cyanobacterial bloom contributes to the formation and distribution of iron-bound phosphorus (Fe-P): Insight for cycling mechanism of internal phosphorus loading. Sci. Total Environ. 2019, 652, 696–708. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, B.; Cheng, W. Decadally cycling soil carbon is more sensitive to warming than faster-cycling soil carbon. Glob. Change Biol. 2015, 21, 4602–4612. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, H.; Cui, R.; Hu, W.; Wang, C.; Chen, A.; Zhang, D. Shallow groundwater table fluctuations: A driving force for accelerating the migration and transformation of phosphorus in cropland soil. Water Res. 2025, 275, 123209. [Google Scholar] [CrossRef]
- Mabilde, L.; De Neve, S.; Sleutel, S. Regional analysis of groundwater phosphate concentrations under acidic sandy soils: Edaphic factors and water table strongly mediate the soil P-groundwater P relation. J. Environ. Manag. 2017, 203, 429–438. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, Y.; Liu, R.; Liu, X.; Xue, Y. Impact of varying dissolved organic carbon load on sediment phosphorus release and its periodic mechanisms. Environ. Res. 2024, 259, 119558. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Tiecher, T.; Barrón, V. Iron oxides and organic matter on soil P availability. Ciência E Agrotecnol. 2016, 40, 369–379. [Google Scholar] [CrossRef]
- Zheng, Y. The Effects of Alternating Wet and Dry Conditions on Soil Nitrogen Transformation and Biological Characteristics. Master’s Thesis, Donghua University, Shanghai, China, 2013. [Google Scholar]
- Yang, K.; Li, S.; Sun, Y.; Cartmill, A.D.; López, I.F.; Ma, C.; Zhang, Q. Effects of combined nitrogen and phosphorus application on soil phosphorus fractions in alfalfa (Medicago sativa L.) production in China. Front. Plant Sci. 2024, 15, 1380738. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Liu, H.; Wang, X. Anaerobic ammonium oxidation coupled to Fe (III) reduction: Discovery, mechanism and application prospects in wastewater treatment. Sci. Total Environ. 2022, 818, 151687. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Kadlec, R.H.; Flaig, E.; Gale, P.M. Phosphorus Retention in Streams and Wetlands: A Review. Crit. Rev. Environ. Sci. Technol. 2019, 29, 83–146. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil P Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Gao, D.C.; Bai, E.; Li, M.H.; Zhao, C.H.; Yu, K.L.; Hagedorn, F. Responses of soil nitrogen and phosphorus cycling to drying and rewetting cycles: A meta-analysis. Soil Biol. Biochem. 2020, 148, 107896. [Google Scholar] [CrossRef]
- Zhu, H.; Bing, H.; Wu, Y.; Sun, H.; Zhou, J. Low molecular weight organic acids regulate soil P availability in the soils of subalpine forests, eastern Tibetan Plateau. Catena 2021, 203, 105328. [Google Scholar] [CrossRef]
- Matthews, K.E.; Facelli, J.M.; Cavagnaro, T.R. Response of soil microbial community structure, carbon and nitrogen cycling to drying and rewetting. Appl. Soil Ecol. 2023, 192, 105099. [Google Scholar] [CrossRef]
- Deslippe, J.R.; Bentley, S.B. The role of wetland restoration in mediating phosphorus ecosystem services in agricultural landscapes. Curr. Opin. Biotechnol. 2025, 91, 103227. [Google Scholar] [CrossRef]
- Zhang, Z.; Poulter, B.; Feldman, A.F.; Ying, Q.; Ciais, P.; Peng, S.; Li, X. Recent intensification of wetland methane feedback. Nat. Clim. Change 2023, 13, 430–433. [Google Scholar] [CrossRef]
- Chen, H.; Jarosch, K.A.; Meszaros, E.; Frossard, E.; Zhao, X.R.; Oberson, A. Repeated drying and rewetting differently affect abiotic and biotic soil phosphorus (P) dynamics in a sandy soil: A 33P soil incubation study. Soil Biol. Biochem. 2021, 153, 108079. [Google Scholar] [CrossRef]

| Index | Value |
|---|---|
| pH | 6.54 ± 0.14 |
| Dissolved organic carbona (DOC) | 303.75 ± 8.40 mg/kg |
| Soil organic carbon (SOC) | 73.94 ± 0.51 g/kg |
| Total nitrogen (TN) | 7.70 ± 0.12 g/kg |
| Total phosphorus (TP) | 1.12 ± 0.003 g/kg |
| Ammonia nitrogen (NH4+-N) | 11.45 ± 2.07 mg/kg |
| Nitrate nitrogen (NO3−-N) | 24.73 ± 1.80 mg/kg |
| Name | Explains % | Total Explanation % | p |
|---|---|---|---|
| SOC | 67.7 ** | 67.7 | 0.002 ** |
| Shannon index | 18.4 * | 86.1 | 0.034 * |
| pH | 11.3 | 97.4 | 0.230 |
| NH4+−N | 1.5 | 98.9 | 0.588 |
| DOC | 1.1 | 100 | 0.614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Li, Y.; Wang, L.; Hussain, N.; Bai, B.; Zhou, J.; Ren, Y. Effect of Drying–Rewetting Alternation on Phosphorus Fractions in Restored Wetland. Agriculture 2025, 15, 1720. https://doi.org/10.3390/agriculture15161720
Ren M, Li Y, Wang L, Hussain N, Bai B, Zhou J, Ren Y. Effect of Drying–Rewetting Alternation on Phosphorus Fractions in Restored Wetland. Agriculture. 2025; 15(16):1720. https://doi.org/10.3390/agriculture15161720
Chicago/Turabian StyleRen, Mingyue, Yingxin Li, Lixia Wang, Naseer Hussain, Bing Bai, Jie Zhou, and Yongxing Ren. 2025. "Effect of Drying–Rewetting Alternation on Phosphorus Fractions in Restored Wetland" Agriculture 15, no. 16: 1720. https://doi.org/10.3390/agriculture15161720
APA StyleRen, M., Li, Y., Wang, L., Hussain, N., Bai, B., Zhou, J., & Ren, Y. (2025). Effect of Drying–Rewetting Alternation on Phosphorus Fractions in Restored Wetland. Agriculture, 15(16), 1720. https://doi.org/10.3390/agriculture15161720







