A Seed Vigor Test Through a Biospeckle Laser: A Comparison of Local and Global Analyses
Abstract
1. Introduction
2. Material and Methods
2.1. Seed Preparation
- -
- 436 high-vigor seeds, with 36 used in BSL tests and 400 in the emergence test;
- -
- 436 seeds submitted for the accelerated aging process [24], with 36 used in the BSL test and 400 in the emergence test.
2.2. Emergence Test
2.3. Seed Preparation for BSL Analysis
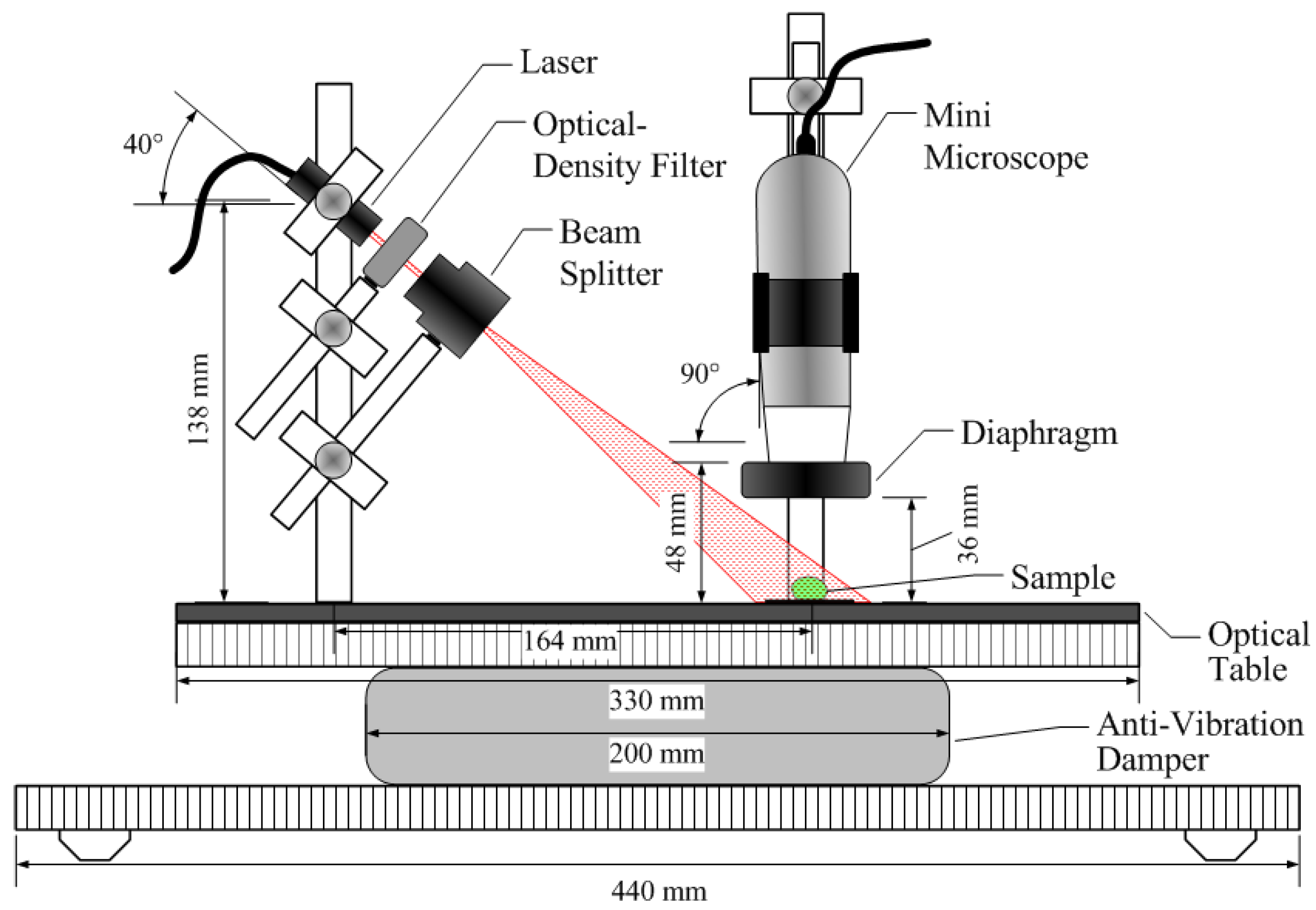
2.4. Hardware for BSL Analysis
2.5. Image Acquisition and Analysis
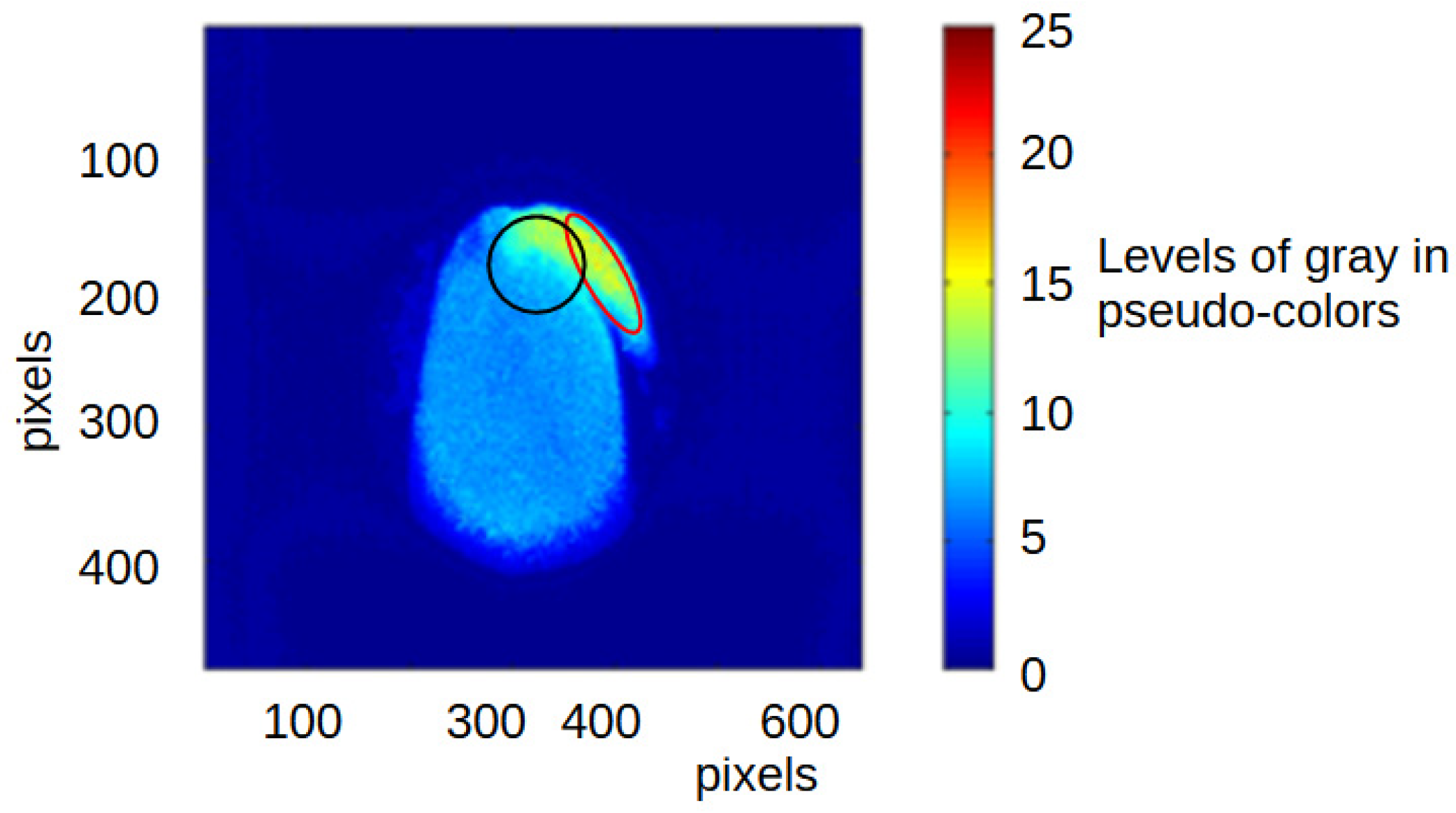
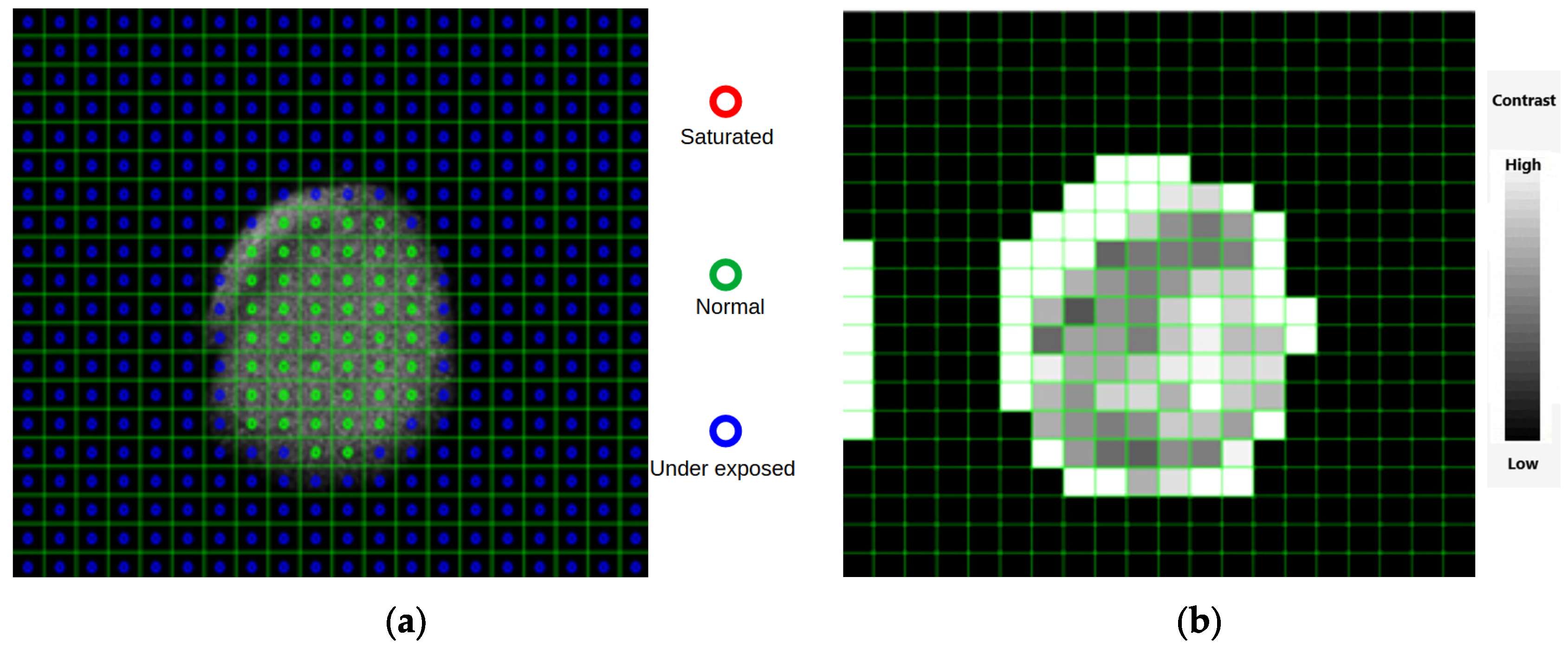
2.5.1. Local Regions of Interest and BSL Analysis
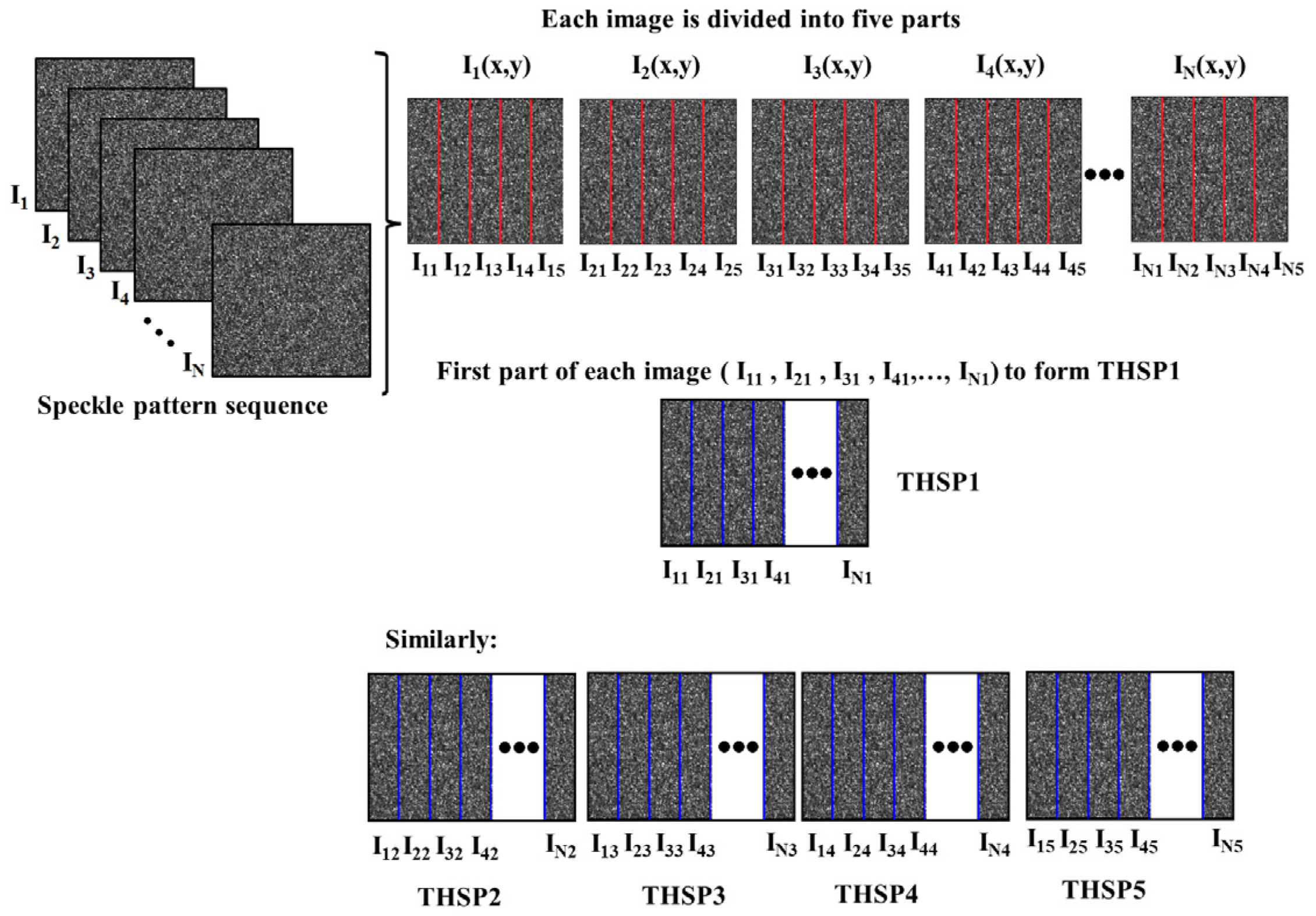
2.5.2. Global Regions of Interest and BSL Analysis
2.6. Statistical Analysis of the BSL Test
3. Results
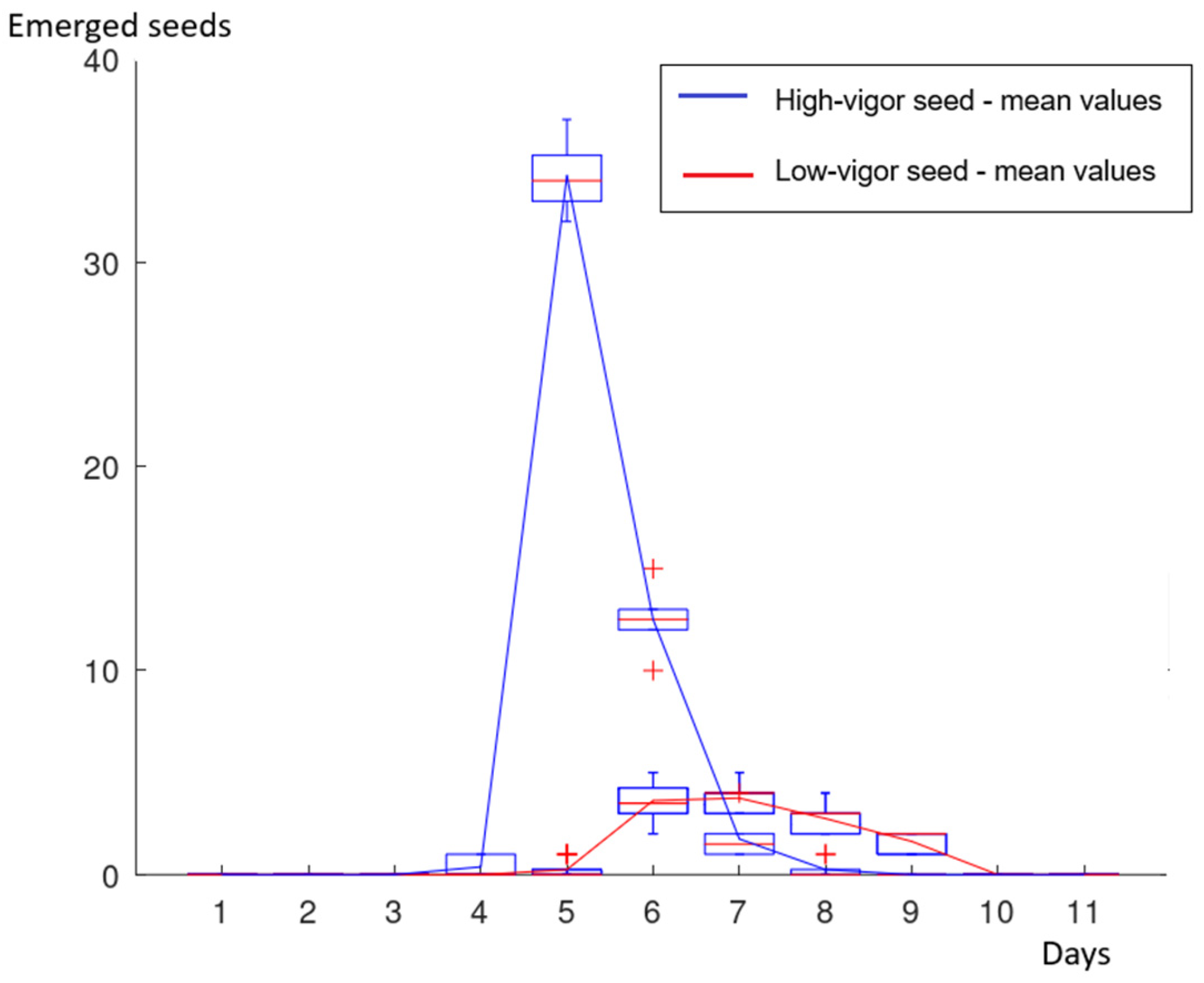
3.1. Emergency Test and Emergence Speed Index (ESI)
3.2. Biospeckle Laser Analysis
3.2.1. Quality Test Protocol
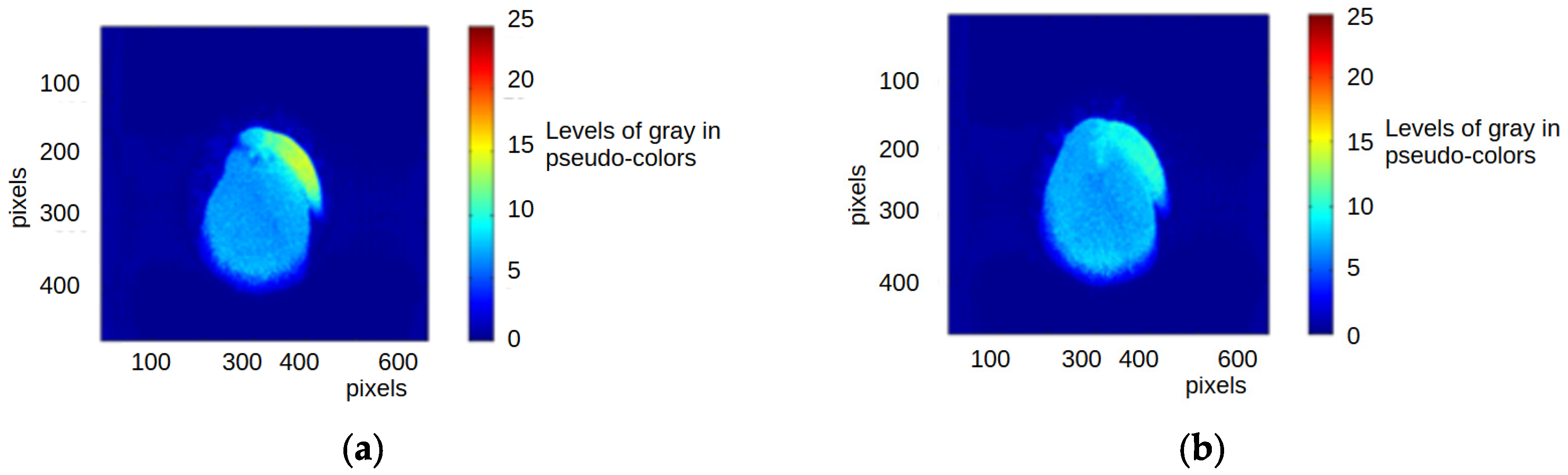
3.2.2. Local Analysis with the BSL Results in the Vascular Region of the Seeds
3.2.3. Local Analysis with the BSL Results in the Embryonic Axis of the Seeds
3.2.4. Global Analysis with the Full THSP
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- ISTA. Seed Vigour Testing. In International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2021; Volume 20, pp. 15–20. [Google Scholar]
- Machado, C.G.; Silva, G.Z.; Cruz, S.C.S.; Anjos, R.C.L.; Silva, C.L.; Matos, L.F.L.; Smaniotto, A.O. Germination and Vigor of Soybean and Corn Seeds Treated with Mixed Mineral Fertilizers. Plants 2023, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Frota, L.M.; de Oliveira, G.M.; de Oliveira, M.T.R. Seed size improves vigor and emergence of Cenostigma tocantinum seedlings. Aust. J. Crop Sci. 2024, 18, 460–465. [Google Scholar] [CrossRef]
- Singh, P.; Tiwari, B.; Kumar, A.; Gedam, B.; Bhatia, V.; Krejcar, O.; Dobrovolny, M.; Nebhen, J.; Prakash, S. Deep transfer learning based photonics sensor for assessment of seed-quality. Comput. Electron. Agric. 2022, 196, 106891. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, Y.; Li, J.; Zhang, C.; Fan, S. Recent advances in emerging techniques for non-destructive detection of seed viability: A review. Artif. Intell. Agric. 2019, 1, 35–47. [Google Scholar] [CrossRef]
- Xing, M.; Long, Y.; Wang, Q.; Tian, X.; Fan, S.; Zhang, C.; Huang, W. Physiological Alterations and Nondestructive Test Methods of Crop Seed Vigor: A Comprehensive Review. Agriculture 2023, 13, 527. [Google Scholar] [CrossRef]
- Rahman, A.; Cho, B. Assessment of seed quality using non-destructive measurement techniques: A review. Seed Sci. Res. 2016, 26, 285–305. [Google Scholar] [CrossRef]
- Braga, R.A., Jr.; Contado, J.L.; Ducatti, K.R.; da Silva, E.A. Analysis of Seed Vigor Using the Biospeckle Laser Technique. AgriEngineering 2025, 7, 3. [Google Scholar] [CrossRef]
- Singh, P.; Chatterjee, A.; Rajput, L.S.; Rana, S.; Kumar, S.; Nataraj, V.; Bhatia, V.; Prakash, S. Development of an intelligent laser biospeckle system for early detection and classification of soybean seeds infected with seed-borne fungal pathogen (Colletotrichum truncatum). Biosyst. Eng. 2021, 212, 442–457. [Google Scholar] [CrossRef]
- Zdunek, A.; Adamiak, A.; Pieczywek, P.M.; Kurenda, A. The biospeckle method for the investigation of agricultural crops: A review. Opt. Lasers Eng. 2014, 4, 20–290. [Google Scholar] [CrossRef]
- Silva, G.M.; Peixoto, L.S.; Fujii, A.K.; Parisi, J.J.D.; Aguiar, R.H.; Fracarolli, J.A. Evaluation of Maize Seeds Treated with Trichodermil® through Biospeckle. J. Agric. Sci. Technol. B 2018, 8, 175–187. [Google Scholar] [CrossRef]
- Braga, R.A.; Dal Fabbro, I.M.; Borem, F.M.; Rabelo, G.F.; Arizaga, R.; Rabal, H.; Trivi, M. Assessment of Seed Viability by Laser Speckle Techniques. Biosyst. Eng. 2003, 86, 287–294. [Google Scholar] [CrossRef]
- Men, S.; Yan, L.; Qian, H. Application of Bio-speckle Activity to Assess Seed Viability. Adv. J. Food Sci. Technol. 2015, 3, 214–218. [Google Scholar] [CrossRef]
- Vivas, P.G.; Resende, L.S.; Braga, R.A., Jr.; Guimarães, R.M.; Azevedo, R.; da Silva, E.A.; Toorop, P.E. Biospeckle activity in coffee seeds is associated non-destructively with seedling quality. Ann. Appl. Biol. 2016, 170, 141–149. [Google Scholar] [CrossRef]
- Braga, R.A.; Azevedo, R.L.; Guimarães, R.M.; Reis, L.V. Magnetic field in coffee seed germination. Sci. Agrotecnol. 2020, 44, e0039020. [Google Scholar] [CrossRef]
- Singh, P.; Chatterjee, A.; Bhatia, V.; Prakash, S. Application of laser biospeckle analysis for assessment of seed priming treatments. Comput. Electron. Agric. 2020, 169, 105212. [Google Scholar] [CrossRef]
- Singh, P.; Chatterjee, A.; Rajput, L.S.; Rana, S.; Bhatia, V.; Prakash, S. Laser biospeckle technique for characterizing the impact of temperature and initial moisture content on seed germination. Opt. Lasers Eng. 2022, 153, 106999. [Google Scholar] [CrossRef]
- Sutton, D.B.; Punja, Z.K. Investigating biospeckle laser analysis as a diagnostic method to assess sprouting damage in wheat seeds. Comput. Electron. Agric. 2017, 141, 238–247. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bhatia, V.; Prakash, S. Anti-spoof touchless 3D fingerprint recognition system using single shot fringe projection and biospeckle analysis. Opt. Lasers Eng. 2017, 95, 1–7. [Google Scholar] [CrossRef]
- Kurenda, A.; Adamiak, A.; Zdunek, A. Temperature effect on apple bio-speckle activity evaluation with different indices. Postharvest Biol. Technol. 2012, 67, 118–123. [Google Scholar] [CrossRef]
- Braga, R.A.; Rivera, F.P.; Moreira, J. A Practical Guide, 1st ed.; Editora UFLA: Lavras, Brazil, 2016; 162p. [Google Scholar]
- Ortiz, T.A.; Gomes, G.R.; Takahashi, L.S.A. Standardization of the accelerated aging test methodology for snap bean seed to differentiate batches by physiological quality. Int. J. Veg. Sci. 2024, 30, 131–137. [Google Scholar] [CrossRef]
- Nakagawa, J. Testes de vigor baseados no desempenho das plântulas. In Vigor de Sementes: Conceitos E Testes, 1st ed.; Krzyzanowski, F.C., Vieira, R.D., França Neto, J.B., Eds.; ABRATES: Londrina, Brazil, 1999; pp. 2.1–2.24. [Google Scholar]
- Pereira, M.D.; Reis, J.A.; Ferrari, C.S.; Florestal, A.M.G.V.-C.; Brasil, S.L. Processamento digital de imagens de plântulas na avaliação do vigor de sementes de Moringa oleifera Lam. Ciênc. Florest. 2020, 30, 291–306. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination-aid selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Moreira, R.R.J.; Cardoso, R.A.; Braga, R.A. Quality test protocol to dynamic laser speckle analysis. Opt. Lasers Eng. 2014, 61, 8–13. [Google Scholar] [CrossRef]
- Kaler, N.; Bhatia, V.; Mishra, A.K. Deep Learning-Based Robust Analysis of Laser Bio-Speckle Data for Detection of Fungal-Infected Soybean Seeds. IEEE Access 2023, 11, 89331–89348. [Google Scholar] [CrossRef]
- Oulamara, A.; Tribillon, G.; Duvernoy, J. Biological Activity Measurement on Botanical Specimen Surfaces Using a Temporal Decorrelation Effect of Laser Speckle. J. Mod. Opt. 1989, 36, 165–179. [Google Scholar] [CrossRef]
- Xu, Z.; Joenathan, C.; Khorana, B.M. Temporal and spatial properties of the time-varying speckles of botanical specimens. Opt. Eng. 1995, 34, 1487–1502. [Google Scholar] [CrossRef]
- Arizaga, R.; Trivi, M.; Rabal, H. Speckle time evolution characterization by the co-occurrence matrix analysis. Opt. Laser Technol. 1999, 31, 163–169. [Google Scholar] [CrossRef]
- Carvalho, N.M.; Nakagawa, J. (Eds.) Sementes: Ciência, Tecnologia e Produção, 5th ed.; Funep: Jaboticabal, Brazil, 2012; 590p. [Google Scholar]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hao, Y.; Teng, C.; Fan, X.; Yang, X.; Liu, M.; Ren, G.; Tan, C. Effects of Salt Stimulation on Lunasin Accumulation and Activity during Soybean Germination. Foods 2020, 9, 118. [Google Scholar] [CrossRef] [PubMed]
| SV | DF | SQ | MS | F Value | p |
|---|---|---|---|---|---|
| Seed | 1 | 3.578927 | 3.578927 | 2.671 | 0.1067 |
| Error | 70 | 93.802268 | 1.340032 | ||
| Corrected Total | 71 | 97.381195 | |||
| CV (%) = | 17.24 | ||||
| Overall Mean: 6.7135208 | Number of observations: 72 | ||||
| Seed | ||||
|---|---|---|---|---|
| High Vigor | 6.936472 a | 80.211417 a | 30.855500 a | 1013.004444 a |
| Low vigor | 6.490569 a | 70.684417 a | 27.191083 a | 932.284722 a |
| CV (%) | 17.24 | 33.75 | 32.40 | 24.38 |
| SV | DF | SQ | MS | F Statistic | p-Value |
|---|---|---|---|---|---|
| Seed | 1 | 222.472441 | 222.472441 | 44.252 | 0.0000 |
| Error | 70 | 351.920212 | 5.027432 | ||
| Corrected Total | 71 | 574.392653 | |||
| CV (%) = | 23.53 | ||||
| Overall Mean: 9.5286875 | Number of observations: 72 | ||||
| Seed | ||||
|---|---|---|---|---|
| High Vigor | 11.286497 a | 219.023028 a | 84.338694 a | 1884.731389 a |
| Low vigor | 7.770878 b | 101.746917 b | 38.956444 b | 1152.799167 b |
| CV (%) | 23.53 | 44.15 | 42.65 | 29.02 |
| SV | DF | SQ | MS | F Statistic | p-Value |
|---|---|---|---|---|---|
| Seed | 1 | 1.190425 | 1.190425 | 19.484 | 0.0000 |
| Error | 70 | 4.276794 | 0.061097 | ||
| Corrected Total | 71 | 5.467219 | |||
| CV (%) = | 13.20 | ||||
| Overall Mean: 1.8728917 | Number of observations: 72 | ||||
| Seed | |
|---|---|
| High Vigor | 2.001475 a |
| Low vigor | 1.744308 b |
| CV (%) | 13.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicentini, B.; Braga, R.A.; Contado, J.L.; Gomes, J.E.d.S.; Gonzalez-Peña, R.d.J. A Seed Vigor Test Through a Biospeckle Laser: A Comparison of Local and Global Analyses. Agriculture 2025, 15, 1553. https://doi.org/10.3390/agriculture15141553
Vicentini B, Braga RA, Contado JL, Gomes JEdS, Gonzalez-Peña RdJ. A Seed Vigor Test Through a Biospeckle Laser: A Comparison of Local and Global Analyses. Agriculture. 2025; 15(14):1553. https://doi.org/10.3390/agriculture15141553
Chicago/Turabian StyleVicentini, Bruno, Roberto Alves Braga, José Luís Contado, José Eduardo da Silva Gomes, and Rolando de Jesus Gonzalez-Peña. 2025. "A Seed Vigor Test Through a Biospeckle Laser: A Comparison of Local and Global Analyses" Agriculture 15, no. 14: 1553. https://doi.org/10.3390/agriculture15141553
APA StyleVicentini, B., Braga, R. A., Contado, J. L., Gomes, J. E. d. S., & Gonzalez-Peña, R. d. J. (2025). A Seed Vigor Test Through a Biospeckle Laser: A Comparison of Local and Global Analyses. Agriculture, 15(14), 1553. https://doi.org/10.3390/agriculture15141553








