Soil Inoculated with Streptomyces rochei D74 Invokes the Defense Mechanism of Helianthus annuus Against Orobanche cumana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant, Weeds, and Strain
2.2. Sunflower, O. cumana, and S. rochei D74 Co-Culture Experiments
2.3. Pot Experiments Setup
2.4. Plant and Soil Sampling
2.5. Measurement of Endogenous Hormone Levels and Resistance-Related Gene Expression in Sunflower Leaves
2.6. 16S rRNA Gene Sequencing and Bioinformatic Analysis
2.7. Statistical Analysis
3. Results
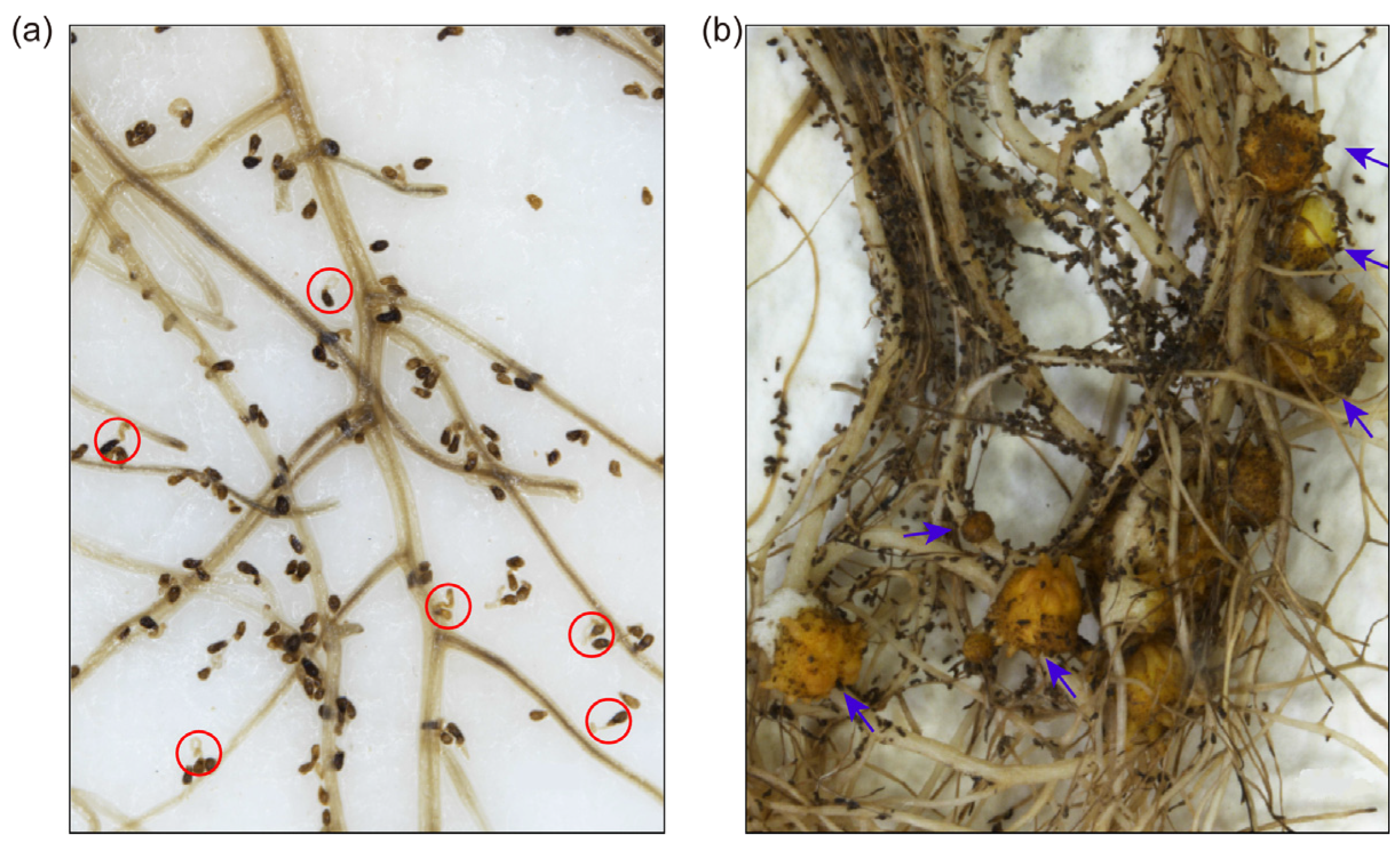
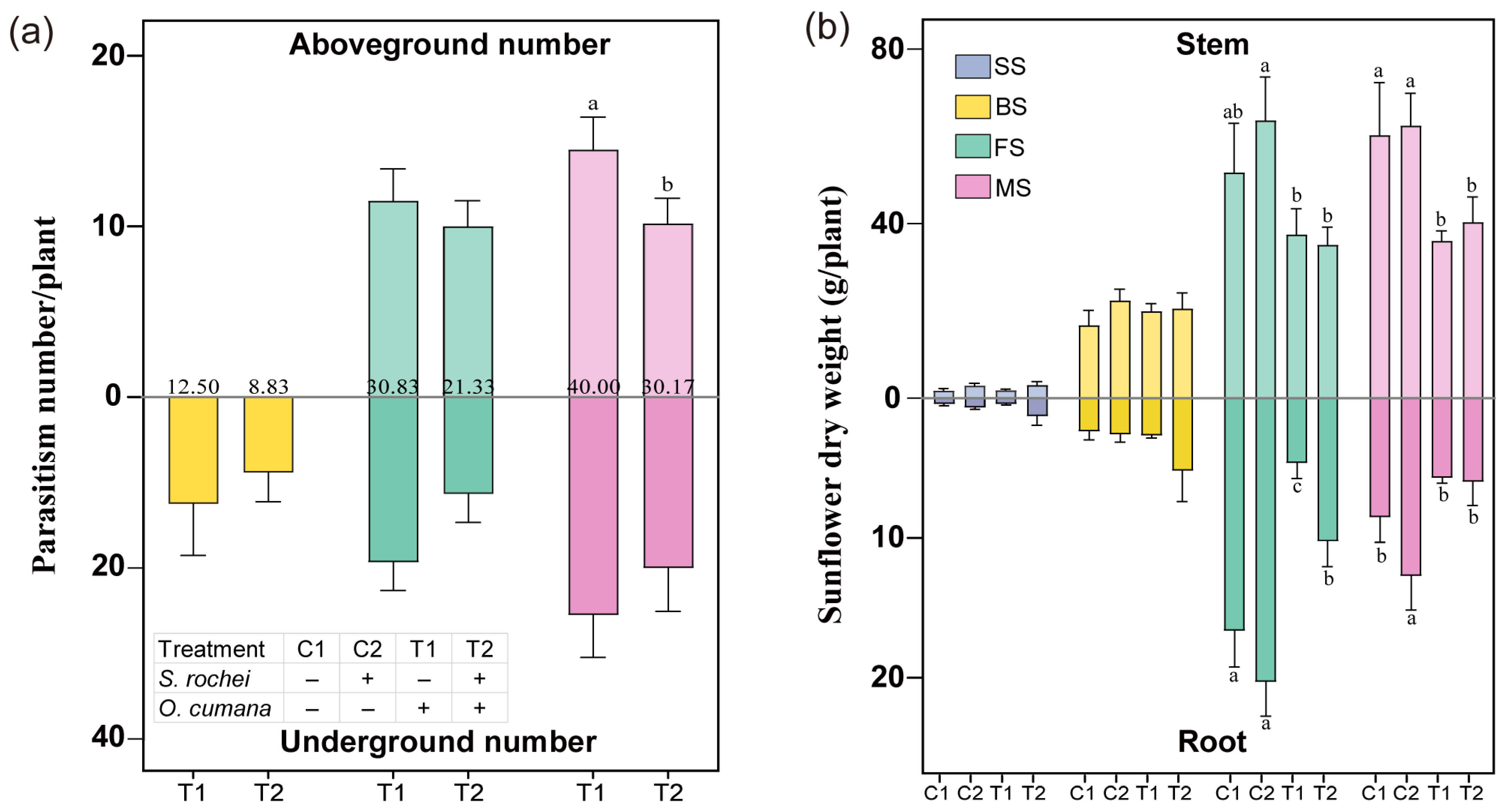
3.1. The S. rochei D74 Strain Inhibited O. cumana Parasite and Facilitated Sunflower Growth
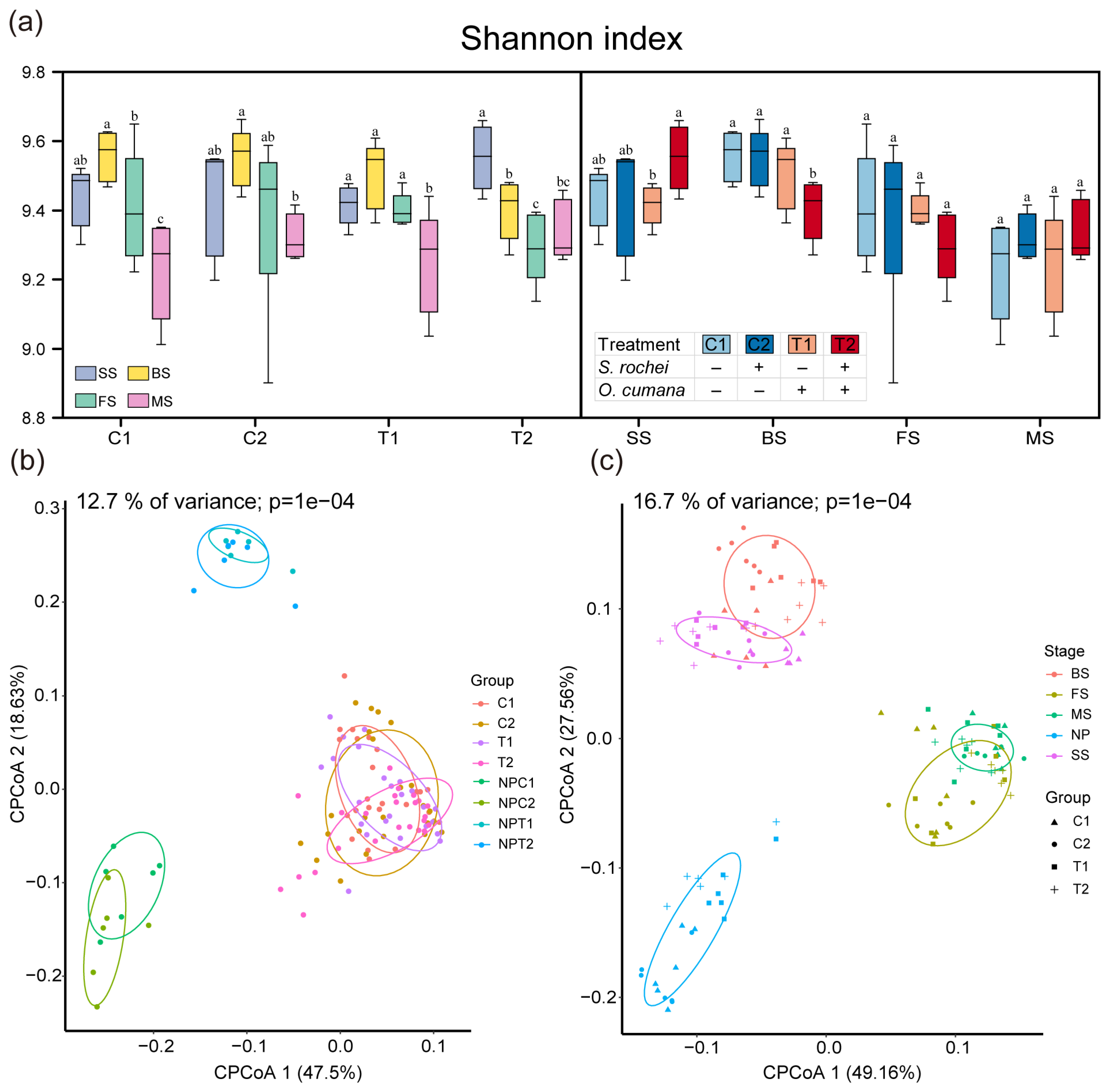
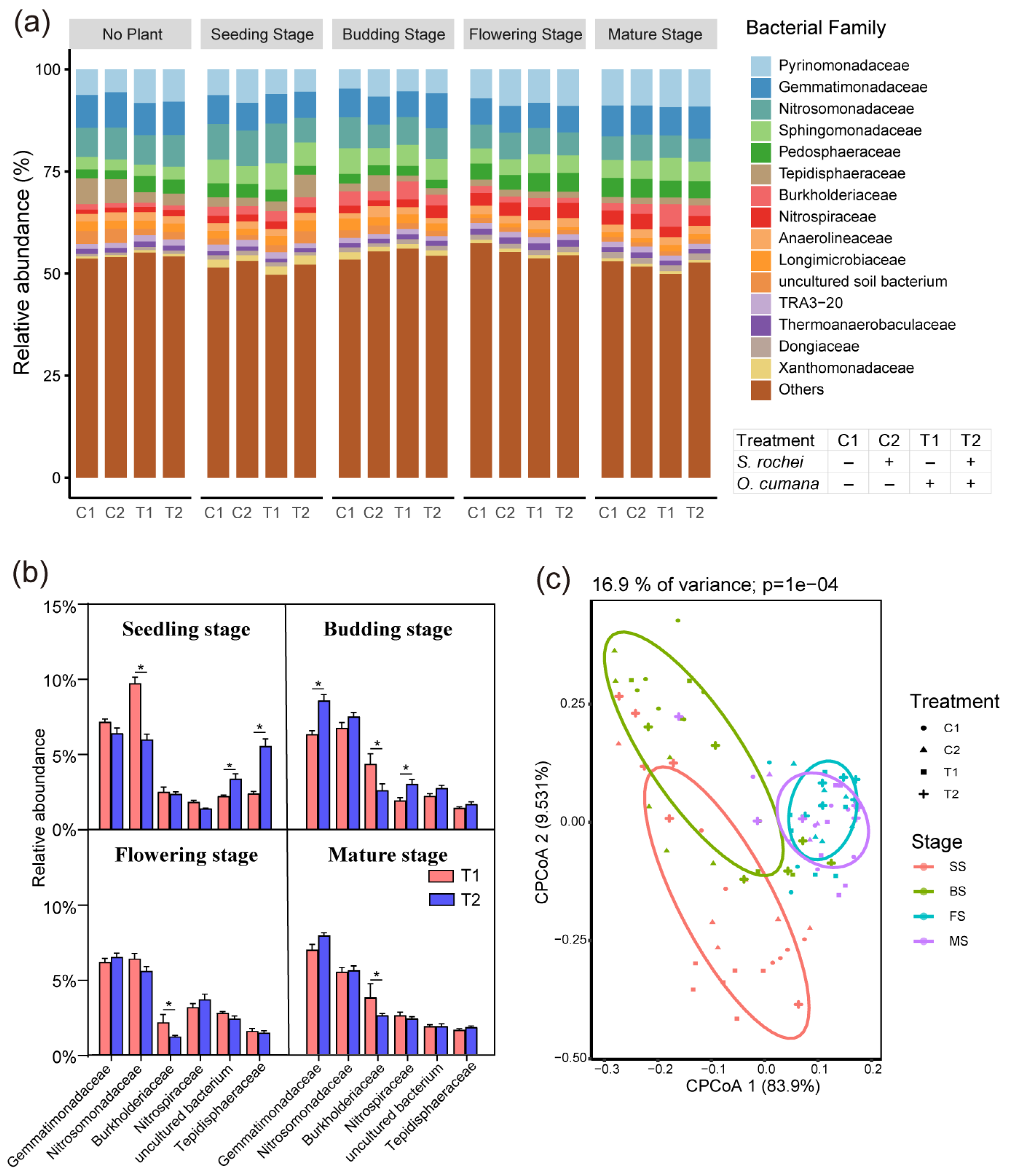
3.2. The O. cumana and S. rochei D74 Strain Effect on Bacterial Microbiota Across Four Developmental Stages of Sunflower
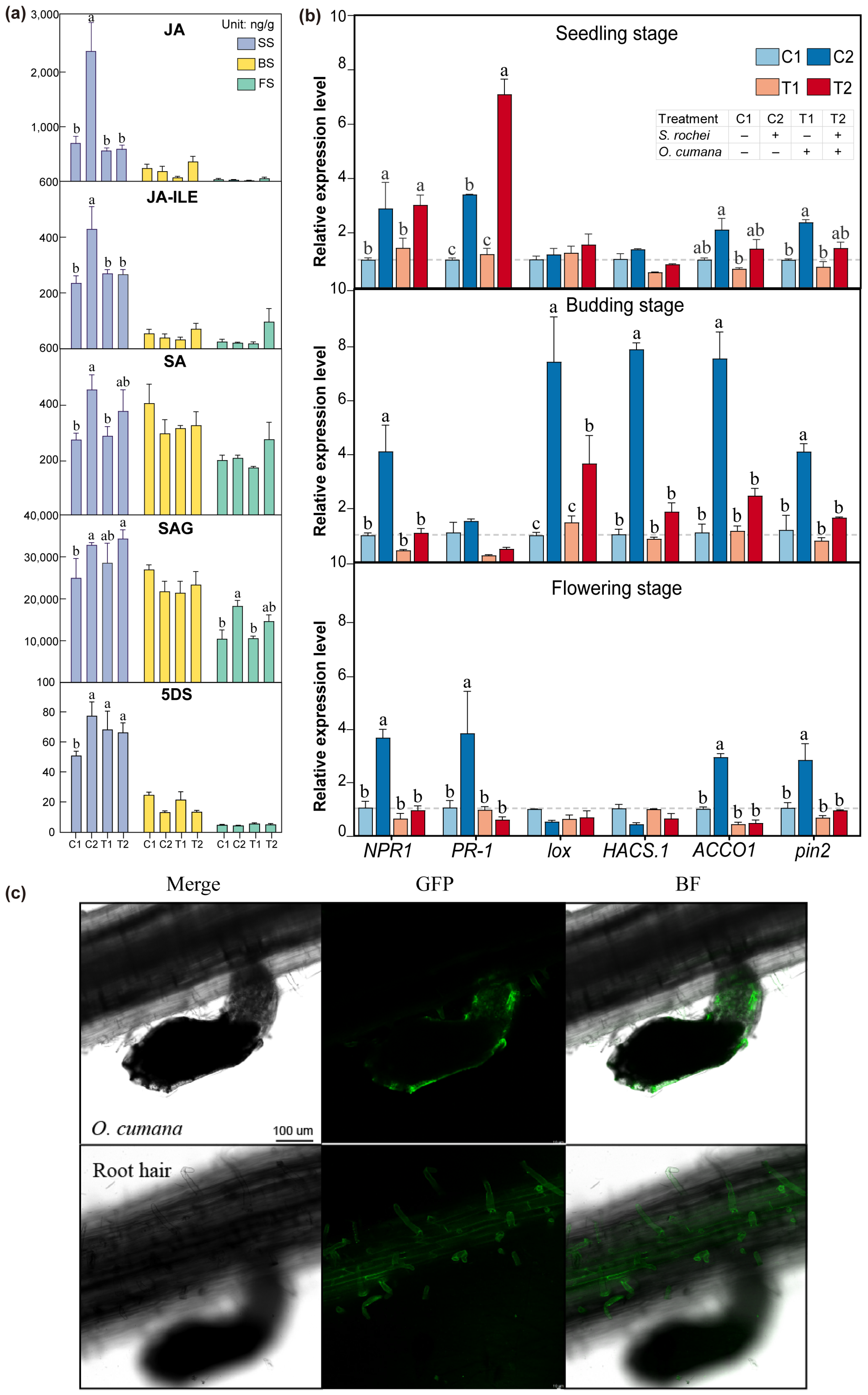
3.3. The S. rochei D74 Strain Putatively Invoked Systemic Resistance of Host to O. cumana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Westwood, J.H.; Yoder, J.I.; Timko, M.P.; Depamphilis, C.W. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Albanova, I.A.; Zagorchev, L.I.; Teofanova, D.R.; Odjakova, M.K.; Kutueva, L.I.; Ashapkin, V.V. Host resistance to parasitic plants—Current knowledge and future perspectives. Plants 2023, 12, 1447. [Google Scholar] [CrossRef] [PubMed]
- Abbes, Z.; Kharrat, M.; Delavault, P.; Chaïbi, W.; Simier, P. Nitrogen and carbon relationships between the parasitic weed Orobanche foetida and susceptible and tolerant faba bean lines. Plant Physiol. Biochem. 2009, 47, 153–159. [Google Scholar] [CrossRef]
- Xi, J.; Lei, B.; Liu, Y.X.; Ding, Z.; Liu, J.; Xu, T.; Hou, L.; Han, S.; Qian, X.; Ma, Y. Microbial community roles and chemical mechanisms in the parasitic development of Orobanche cumana. Imeta 2022, 1, e31. [Google Scholar] [CrossRef]
- Tourneur, S.; Combier, J.P.; Plaza, S.; Muños, S.; Delavault, P. microRNA-encoded peptides inhibit seed germination of the root parasitic plant Orobanche cumana. Plants People Planet 2024, 7, 436–447. [Google Scholar] [CrossRef]
- Kumar, P.L.; Cuervo, M.; Kreuze, J.F.; Muller, G.; Kulkarni, G.; Kumari, S.G.; Massart, S.; Mezzalama, M.; Alakonya, A.; Muchugi, A. Phytosanitary interventions for safe global germplasm exchange and the prevention of transboundary pest spread: The role of CGIAR germplasm health units. Plants 2021, 10, 328. [Google Scholar] [CrossRef]
- Duriez, P. Caractérisation Génétique, Moléculaire et Physiologique du Locus Or7 de Résistance à Orobanche cumana Chez le Tournesol. Degree of Doctor, Université Paul Sabatier-Toulouse III, Toulouse, France, 2019. [Google Scholar]
- Parker, C. Parasitic weeds: A world challenge. Weed Sci. 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Parker, C. Observations on the current status of Orobanche and Striga problems worldwide. Pest. Manage. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef]
- El-Dabaa, M.; Abo-Elwafa, G.; Abd-El-Khair, H. Safe methods as alternative approaches to chemical herbicides for controlling parasitic weeds associated with nutritional crops: A review. Egypt. J. Chem. 2022, 65, 53–65. [Google Scholar]
- Hershenhorn, J.; Eizenberg, H.; Dor, E.; Kapulnik, Y.; Goldwasser, Y. Phelipanche aegyptiaca management in tomato. Weed Res. 2009, 49, 34–47. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Del Moral, L.; Muños, S.; Velasco, L.; Pérez-Vich, B. Genetic and physiological characterization of sunflower resistance provided by the wild-derived Or Deb2 gene against highly virulent races of Orobanche cumana Wallr. Theor. Appl. Genet. 2022, 135, 501–525. [Google Scholar] [CrossRef] [PubMed]
- Duriez, P.; Vautrin, S.; Auriac, M.-C.; Bazerque, J.; Boniface, M.-C.; Callot, C.; Carrère, S.; Cauet, S.; Chabaud, M.; Gentou, F. A receptor-like kinase enhances sunflower resistance to Orobanche cumana. Nat. Plants 2019, 5, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Hegenauer, V.; Fürst, U.; Kaiser, B.; Smoker, M.; Zipfel, C.; Felix, G.; Stahl, M.; Albert, M. Detection of the plant parasite Cuscuta reflexa by a tomato cell surface receptor. Science 2016, 353, 478–481. [Google Scholar] [CrossRef]
- Feys, B.J.; Parker, J.E. Interplay of signaling pathways in plant disease resistance. Trends Genet. 2000, 16, 449–455. [Google Scholar] [CrossRef]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in roles of salicylic acid in plant tolerance responses to biotic and abiotic stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Sugimoto, Y. Molecular responses of sorghum to purple witchweed (Striga hermonthica) parasitism. Weed Sci. 2008, 56, 356–363. [Google Scholar] [CrossRef]
- Grandjean, C.; Veronesi, C.; Rusterucci, C.; Gautier, C.; Maillot, Y.; Leschevin, M.; Fournet, F.; Drouaud, J.; Marcelo, P.; Zabijak, L.; et al. Pectin Remodeling and Involvement of AtPME3 in the Parasitic Plant–Plant Interaction, Phelipanche ramosa–Arabidospis thaliana. Plants 2024, 13, 2168. [Google Scholar] [CrossRef]
- Xi, J.; Ding, Z.; Xu, T.; Qu, W.; Xu, Y.; Ma, Y.; Xue, Q.; Liu, Y.; Lin, Y. Maize rotation combined with Streptomyces rochei D74 to eliminate Orobanche cumana seed bank in the farmland. Agronomy 2022, 12, 3129. [Google Scholar] [CrossRef]
- Ma, Y.; Jia, J.; An, Y.; Wang, Z.; Mao, J. Potential of Some Hybrid Maize Lines to Induce Germination of Sunflower Broomrape. Crop Sci. 2013, 53, 260–270. [Google Scholar] [CrossRef]
- Sousa, G.V.; Teles, V.L.; Pereira, E.G.; Modolo, L.V.; Costa, L.M. Interactions between As and Se upon long exposure time and effects on nutrients translocation in golden flaxseed seedlings. J. Hazard. Mater. 2021, 402, 123565. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Q.; He, F.; Li, Y.; Xue, Q.; Lai, H. Biocontrol of root diseases and growth promotion of the tuberous plant Aconitum carmichaelii induced by Actinomycetes are related to shifts in the rhizosphere microbiota. Microb. Ecol. 2020, 79, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.; Zhou, X.; Wang, M.-M.; Zhang, A.-M.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Eevers, N.; Gielen, M.; Sánchez-López, A.; Jaspers, S.; White, J.; Vangronsveld, J.; Weyens, N. Optimization of isolation and cultivation of bacterial endophytes through addition of plant extract to nutrient media. Microb. Biotechnol. 2015, 8, 707–715. [Google Scholar] [CrossRef]
- Lv, X.; Lv, J.; Gao, S.; Xiang, G.; Yao, Y. Acetic acid enhances the tolerance of grapevines to NaHCO3 stress by increasing SA production. Sci. Hortic. 2021, 288, 110338. [Google Scholar] [CrossRef]
- Xiao, H.-M.; Cai, W.-J.; Ye, T.-T.; Ding, J.; Feng, Y.-Q. Spatio-temporal profiling of abscisic acid, indoleacetic acid and jasmonic acid in single rice seed during seed germination. Anal. Chim. Acta 2018, 1031, 119–127. [Google Scholar] [CrossRef]
- Yang, C.; Hu, L.; Ali, B.; Islam, F.; Bai, Q.; Yun, X.; Yoneyama, K.; Zhou, W. Seed treatment with salicylic acid invokes defence mechanism of Helianthus annuus against Orobanche cumana. Ann. Appl. Biol. 2016, 169, 408–422. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Li, Y.; Sun, Y.; Xue, Q.; Lai, H. Streptomyces pactum Act12 controls tomato yellow leaf curl virus disease and alters rhizosphere microbial communities. Biol. Fertil. Soils 2019, 55, 149–169. [Google Scholar] [CrossRef]
- Kawazu, K.; Mochizuki, A.; Sato, Y.; Sugeno, W.; Murata, M.; Seo, S.; Mitsuhara, I. Different expression profiles of jasmonic acid and salicylic acid inducible genes in the tomato plant against herbivores with various feeding modes. Arthropod-Plant Interact. 2012, 6, 221–230. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Berkman, S.J.; Roscoe, E.M.; Bourret, J.C. Comparing self-directed methods for training staff to create graphs using Graphpad Prism. J. Appl. Behav. Anal. 2019, 52, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. Msystems 2016, 1, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.-I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.J.; Purahong, W.; Wubet, T.; Hyde, K.D.; Zhang, W.; Xu, H.; Zhang, G.; Fu, C.; Liu, M.; Xing, Q.; et al. Direct comparison of culture-dependent and culture-independent molecular approaches reveal the diversity of fungal endophytic communities in stems of grapevine (Vitis vinifera). Fungal Divers. 2018, 90, 85–107. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Qin, Y.; Chen, T.; Lu, M.; Qian, X.; Guo, X.; Bai, Y. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell 2020, 12, 315–330. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open–Source, Platform–Independent, Community–Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Gómez-Rubio, V. ggplot2-elegant graphics for data analysis (2nd Edition). J. Stat. Softw. 2017, 77, 1–3. [Google Scholar] [CrossRef]
- Xi, J.; Xu, T.-q.; Liu, Y.-t.; Ma, Y.-q.; Xue, Q.-h.; Lin, Y.-b. Effect of Streptomyces rochei D74 on sunflower, Orobanche cumana, and their rhizosphere microorganisms. Acta Microbiol. Sin. 2023, 2, 745–759. [Google Scholar]
- Yoneyama, K.; Xie, X.; Yoneyama, K. Strigolactones and biological activity. In Natural Products; Sicker, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3583–3604. [Google Scholar]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Kusumoto, D.; Goldwasser, Y.; Xie, X.; Yoneyama, K.; Takeuchi, Y.; Yoneyama, K. Resistance of red clover (Trifolium pratense) to the root parasitic plant Orobanche minor is activated by salicylate but not by jasmonate. Ann. Bot. 2007, 100, 537–544. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, J.; Xu, T.; Ding, Z.; Li, C.; Han, S.; Liang, R.; Ma, Y.; Xue, Q.; Lin, Y. Soil Inoculated with Streptomyces rochei D74 Invokes the Defense Mechanism of Helianthus annuus Against Orobanche cumana. Agriculture 2025, 15, 1492. https://doi.org/10.3390/agriculture15141492
Xi J, Xu T, Ding Z, Li C, Han S, Liang R, Ma Y, Xue Q, Lin Y. Soil Inoculated with Streptomyces rochei D74 Invokes the Defense Mechanism of Helianthus annuus Against Orobanche cumana. Agriculture. 2025; 15(14):1492. https://doi.org/10.3390/agriculture15141492
Chicago/Turabian StyleXi, Jiao, Tengqi Xu, Zanbo Ding, Chongsen Li, Siqi Han, Ruina Liang, Yongqing Ma, Quanhong Xue, and Yanbing Lin. 2025. "Soil Inoculated with Streptomyces rochei D74 Invokes the Defense Mechanism of Helianthus annuus Against Orobanche cumana" Agriculture 15, no. 14: 1492. https://doi.org/10.3390/agriculture15141492
APA StyleXi, J., Xu, T., Ding, Z., Li, C., Han, S., Liang, R., Ma, Y., Xue, Q., & Lin, Y. (2025). Soil Inoculated with Streptomyces rochei D74 Invokes the Defense Mechanism of Helianthus annuus Against Orobanche cumana. Agriculture, 15(14), 1492. https://doi.org/10.3390/agriculture15141492




