Vegetable Productivity, Soil Physicochemical and Biochemical Properties, and Microbiome in Response to Organic Substitution in an Intensive Greenhouse Production System
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region, Experimental Design, and Sample Collection
2.2. Soil Physicochemical and Biochemical Properties
2.3. DNA Extraction, Metagenomic Sequencing, and Bioinformatic Analysis
2.4. Statistical Analysis
3. Results
3.1. Changes in Vegetable Yield and Economic Benefits
3.2. Changes in Soil Physicochemical and Biochemical Properties
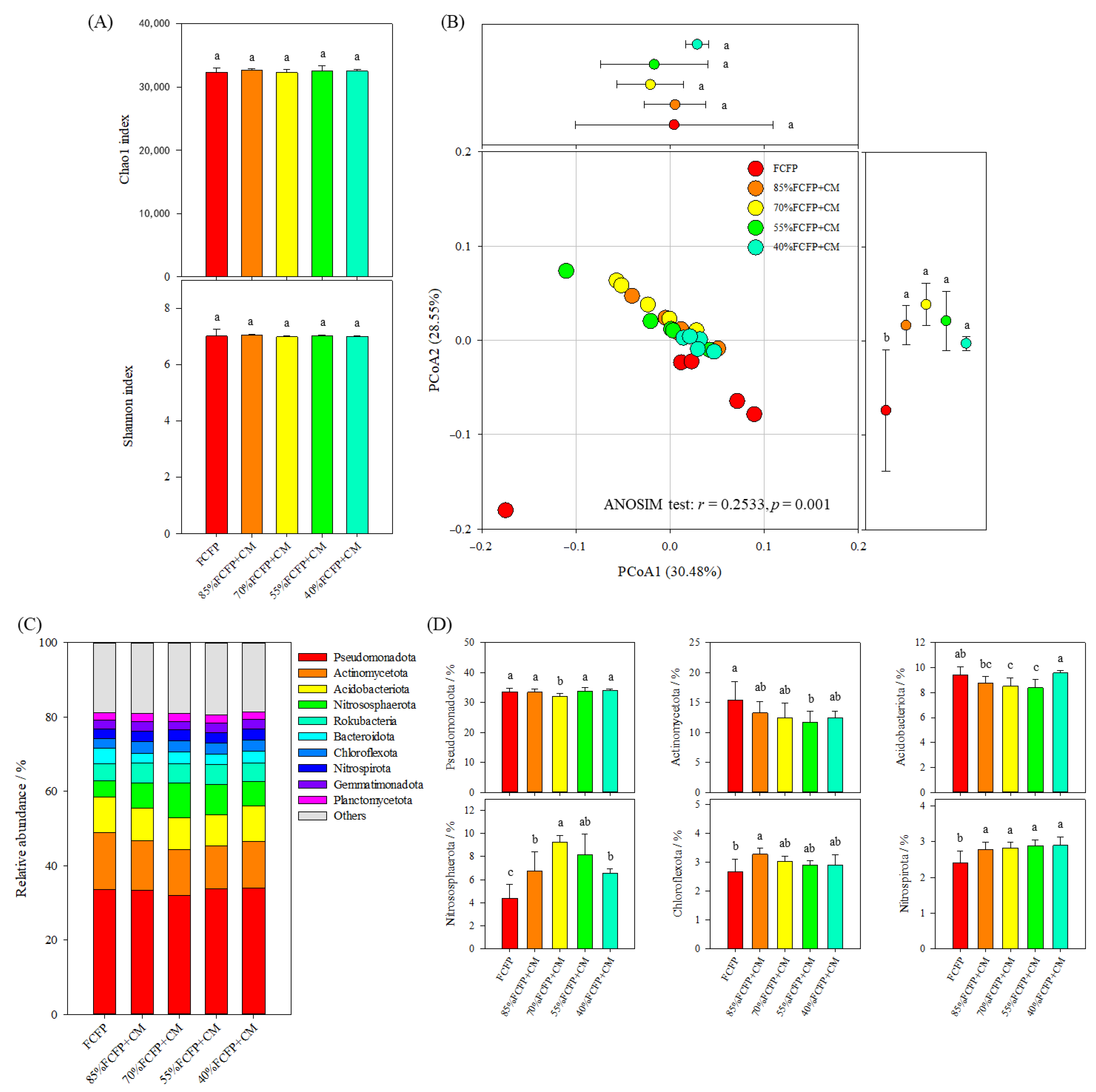
3.3. Species Diversity and Structure of Soil Microbiome
3.4. Functional Diversity and Structure of Soil Microbiome
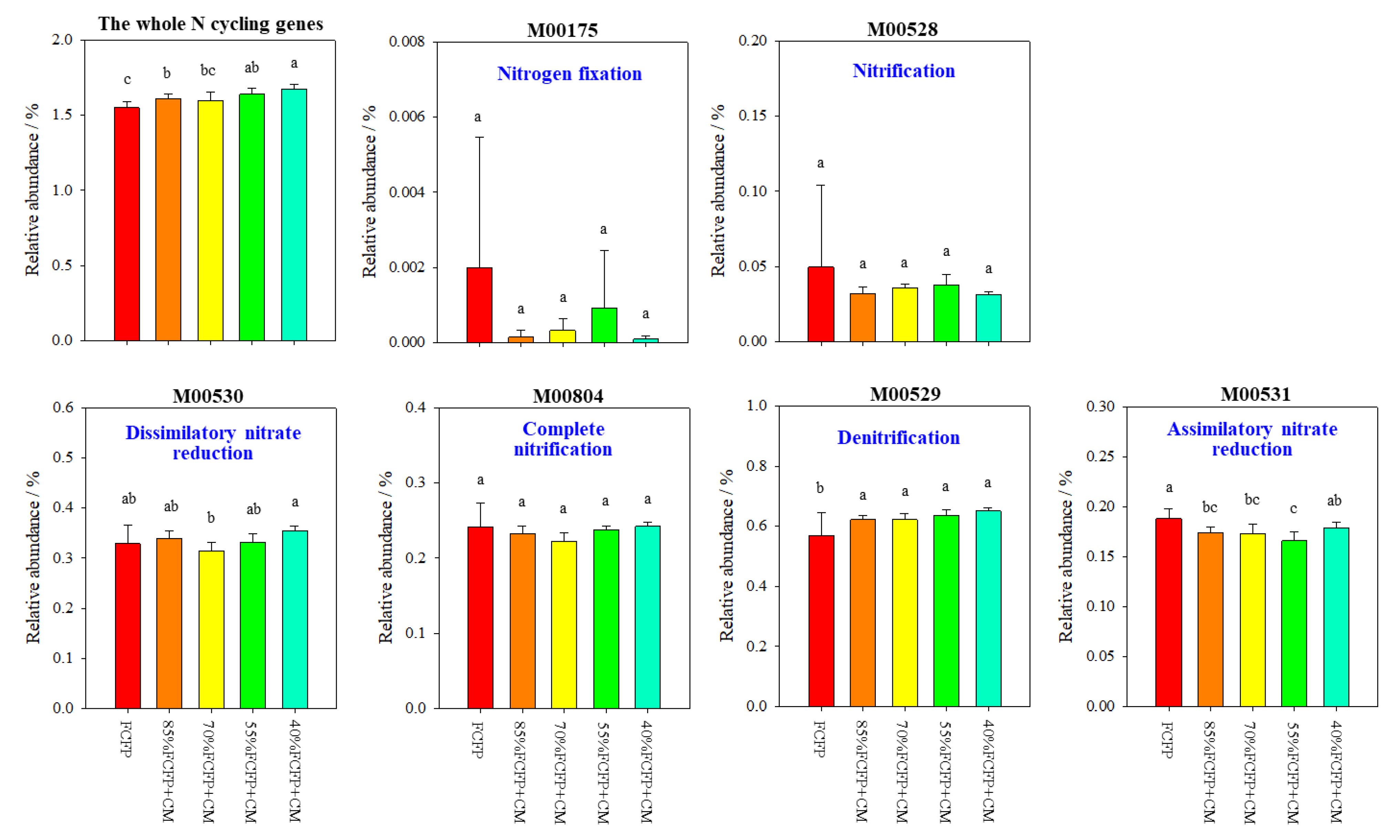
3.5. KEGG Modules and Functional Genes Associated with Soil N Cycling
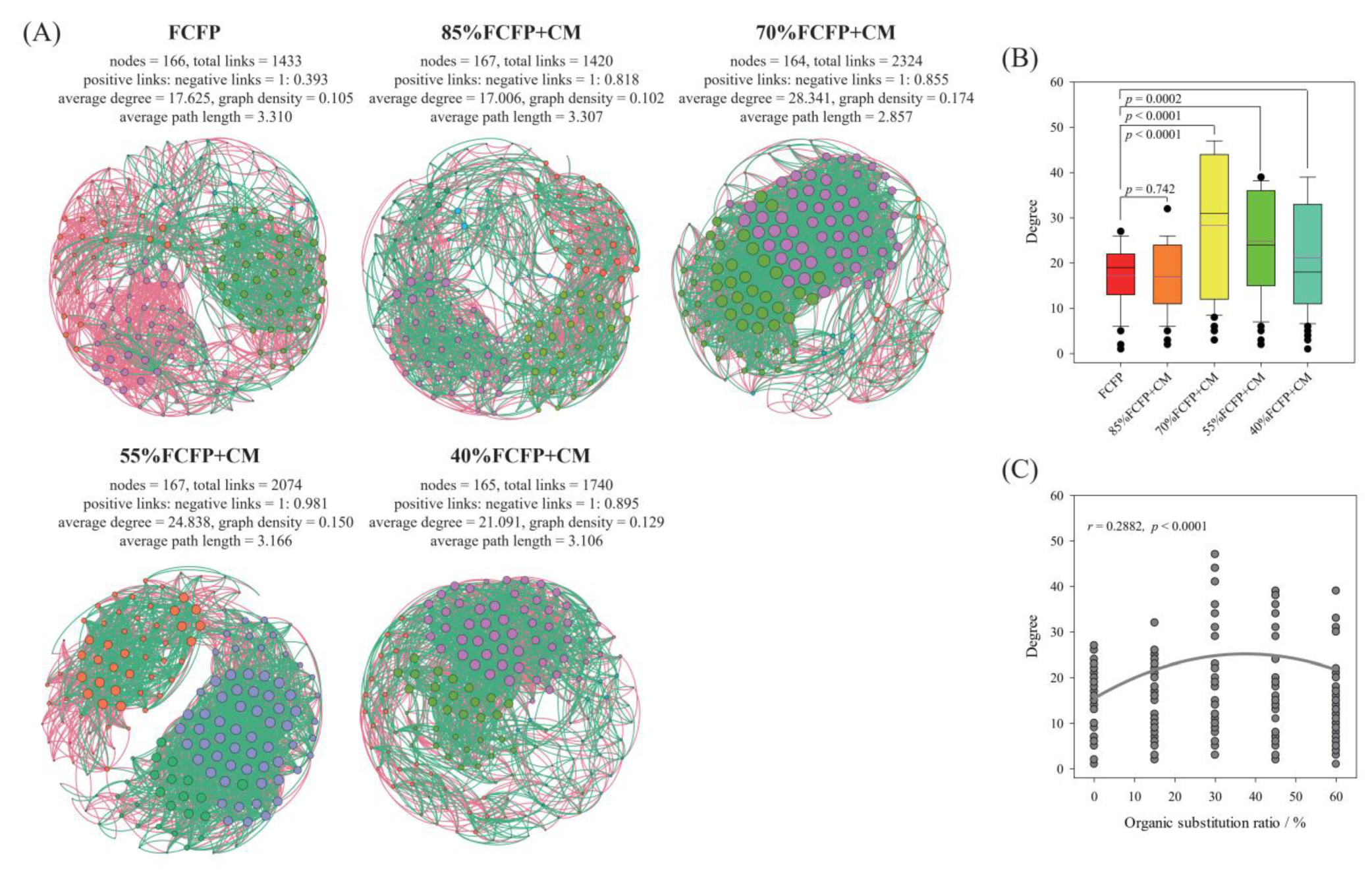
3.6. Co-Occurrence Network of Soil Microbiome
4. Discussion
4.1. Organic Substitution Ratio of 30% Was Suitable for Local Greenhouse Vegetable Cultivation
4.2. Soil Physicochemical and Biochemical Properties Were Altered by Organic Substitution
4.3. Soil Microbiome Did Not Link to the Productivity of Intensive Greenhouse Vegetable System
4.4. Organic Substitution Enriched Denitrification-Associated Functional Gene in Greenhouse Soil
4.5. Complexity of Soil Microbial Network Depends on Organic Substitution Ratio
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Bai, X.; Wang, H.; Yang, M.; Bao, L.; Deng, X.; Chen, Z.; Zhou, J. Optimizing nitrogen and water management for sustainable greenhouse vegetable production with less greenhouse gas emissions. Agric. Ecosyst. Environ. 2023, 352, 108529. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Wang, Q.; Chen, D.; Liang, X. The environmental and socioeconomic benefits of optimized fertilization for greenhouse vegetables. Sci. Total Environ. 2024, 908, 168252. [Google Scholar] [CrossRef]
- Dan, X.; Meng, L.; He, M.; Chen, S.; He, X.; Zhao, C.; Li, X.; Cai, Z.; Zhang, J.; Müller, C. Gross N transformations and plant N use efficiency in intensive vegetable production soils. Soil Biol. Biochem. 2022, 174, 108817. [Google Scholar] [CrossRef]
- Bai, X.; Jiang, Y.; Miao, H.; Xue, S.; Chen, Z.; Zhou, J. Intensive vegetable production results in high nitrate accumulation in deep soil profiles in China. Environ. Pollut. 2021, 287, 117598. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Y.; Hess, F.; Huang, B.; Chen, Z. Nutrient balance and soil changes in plastic greenhouse vegetable production. Nutr. Cycl. Agroecosyst. 2020, 117, 77–92. [Google Scholar] [CrossRef]
- Li, J.; Wan, X.; Liu, X.; Chen, Y.; Slaughter, L.C.; Weindorf, D.C.; Dong, Y. Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Till. Res. 2019, 195, 104366. [Google Scholar] [CrossRef]
- Shen, W.; Lin, X.; Shi, W.; Min, J.; Gao, N.; Zhang, H.; Yin, R.; He, X. Higher rates of nitrogen fertilization decrease soil enzyme activities, microbial functional diversity and nitrification capacity in a Chinese polytunnel greenhouse vegetable land. Plant Soil 2010, 337, 137–150. [Google Scholar] [CrossRef]
- Shen, W.; Hu, M.; Qian, D.; Xue, H.; Gao, N.; Lin, X. Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil 2021, 463, 1–18. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, Q.; Zhan, L.; Xu, X.; Zhang, X.; Dong, Y.; Yan, X.; Xiong, Z. Biochar and organic substitution improved net ecosystem economic benefit in intensive vegetable production. Biochar 2022, 4, 46. [Google Scholar] [CrossRef]
- Liu, L.; Wei, C.; Xu, X.; Min, W.; Li, Y.; Hou, Z.; Zhang, Q.; Anna, G.; Xiong, Z. Bio-manure substitution declines soil N2O and NO emissions and improves nitrogen use efficiency and vegetable quality index. J. Environ. Manag. 2024, 367, 121960. [Google Scholar] [CrossRef]
- Han, Z.; Leng, Y.; Sun, Z.; Li, Z.; Xu, P.; Wu, S.; Liu, S.; Li, S.; Wang, J.; Zou, J. Substitution of organic and bio-organic fertilizers for mineral fertilizers to suppress nitrous oxide emissions from intensive vegetable fields. J. Environ. Manag. 2024, 349, 119390. [Google Scholar] [CrossRef]
- Huang, R.; Liu, J.; He, X.; Xie, D.; Ni, J.; Xu, C.; Zhang, Y.; Ci, E.; Wang, Z.; Gao, M. Reduced mineral fertilization coupled with straw return in field mesocosm vegetable cultivation helps to coordinate greenhouse gas emissions and vegetable production. J. Soils Sediments 2020, 20, 1834–1845. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Wang, Y.; Yan, G.; Janz, B.; Wang, X.; Zhan, Y.; Wang, R.; Zheng, X.; Zhou, M.; et al. Characteristics of annual NH3 emissions from a conventional vegetable field under various nitrogen management strategies. J. Environ. Manag. 2023, 342, 118276. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Liu, K.; Zhou, B.; Gao, H.; Han, X.; Liu, S.; Huang, S.; Zhang, A.; Hua, K.; et al. Minimizing the potential risk of soil nitrogen loss through optimal fertilization practices in intensive agroecosystems. Agron. Sustain. Dev. 2025, 45, 9. [Google Scholar] [CrossRef]
- Xu, X.; Bi, R.; Song, M.; Wang, B.; Dong, Y.; Zhang, Q.; Xiong, Z. Optimizing organic substitution: Balancing carbon sequestration and priming effects of a six-year field experiment for sustainable vegetable production. Sustain. Prod. Consump. 2024, 44, 14–24. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, S.; Li, J.; Liang, B.; Ding, X. Partial substitution of rice husks for manure in greenhouse vegetable fields: Insight from soil carbon stock and aggregate stability. Land Degrad. Dev. 2021, 32, 3962–3972. [Google Scholar] [CrossRef]
- Luan, H.; Gao, W.; Huang, S.; Tang, J.; Li, M.; Zhang, H.; Chen, X. Partial substitution of chemical fertilizer with organic amendments affects soil organic carbon composition and stability in a greenhouse vegetable production system. Soil Till. Res. 2019, 191, 185–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Ma, L.; Luan, H.; Tang, J.; Li, R.; Li, M.; Huang, S.; Wang, L. Long-term partial substitution of chemical fertilizer by organic amendments influences soil microbial functional diversity of phosphorus cycling and improves phosphorus availability in greenhouse vegetable production. Agric. Ecosyst. Environ. 2023, 341, 108193. [Google Scholar] [CrossRef]
- Yuan, S.; Li, R.; Zhang, Y.; Luan, H.; Tang, J.; Wang, L.; Ji, H.; Huang, S. Effects of long-term partial substitution of inorganic fertilizer with pig manure and/or straw on nitrogen fractions and microbiological properties in greenhouse vegetable soils. J. Integ. Agric. 2024, 23, 2083–2098. [Google Scholar] [CrossRef]
- Luan, H.; Gao, W.; Huang, S.; Tang, J.; Li, M.; Zhang, H.; Chen, X.; Masiliūnas, D. Organic amendment increases soil respiration in a greenhouse vegetable production system through decreasing soil organic carbon recalcitrance and increasing carbon-degrading microbial activity. J. Soils Sediments 2020, 20, 2877–2892. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Luan, H.; Tang, J.; Li, R.; Li, M.; Zhang, H.; Huang, S. Effects of a decade of organic fertilizer substitution on vegetable yield and soil phosphorus pools, phosphatase activities, and the microbial community in a greenhouse vegetable production system. J. Integ. Agric. 2022, 21, 2119–2133. [Google Scholar] [CrossRef]
- Rong, Q.; Li, R.; Huang, S.; Tang, J.; Zhang, Y.; Wang, L. Soil microbial characteristics and yield response to partial substitution of chemical fertilizer with organic amendments in greenhouse vegetable production. J. Integ. Agric. 2018, 17, 1432–1444. [Google Scholar] [CrossRef]
- Luan, H.; Gao, W.; Huang, S.; Tang, J.; Li, M.; Zhang, H.; Chen, X.; Masiliūnas, D. Substitution of manure for chemical fertilizer affects soil microbial community diversity, structure and function in greenhouse vegetable production systems. PLoS ONE 2020, 15, e0214041. [Google Scholar] [CrossRef]
- Hijbeek, R.; ten Berge, H.F.M.; Whitmore, A.P.; Barkusky, D.; Schröder, J.J.; van Ittersum, M.K. Nitrogen fertiliser replacement values for organic amendments appear to increase with N application rates. Nutr. Cycl. Agroecosyst. 2018, 110, 105–115. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Q.; Zhang, T.; Ma, W.; Velthof, G.L.; Hou, Y.; Oenema, O.; Zhang, F. Benefits and trade-offs of replacing synthetic fertilizers by animal manures in crop production in China: A meta-analysis. Glob. Change Biol. 2020, 26, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chen, Z.; Niu, Z.; Zeng, R.; Ou, J.; Liu, X.; Wang, X. Replacing synthetic nitrogen fertilizer with different types of organic materials improves grain yield in China: A meta-analysis. Agronomy 2021, 11, 2429. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, Q.; Huang, Y.; Yang, N.; Fan, H.; Li, B.; Wang, X.; Yang, L.; Yen, H.; Wu, F.; et al. Determining optimal range of reduction rates for nitrogen fertilization based on responses of vegetable yield and nitrogen losses to reduced nitrogen fertilizer application. Sci. Total Environ. 2024, 924, 171523. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, M.; Liang, X.; Lam, S.K.; Chen, D.; Malik, A.; Li, M.; Lenzen, M.; Zhang, L.; Zhang, R.; et al. Localized nitrogen management strategies can halve fertilizer use in Chinese staple crop production. Nat. Food 2024, 5, 825–835. [Google Scholar] [CrossRef]
- Tu, Q.; Yan, Q.; Deng, Y.; Michaletz, S.T.; Buzzard, V.; Weiser, M.D.; Waide, R.; Ning, D.; Wu, L.; He, Z.; et al. Biogeographic patterns of microbial co-occurrence ecological networks in six American forests. Soil Biol. Biochem. 2020, 148, 107897. [Google Scholar] [CrossRef]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.C.; Xu, J.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef]
- Konopka, A.; Lindemann, S.; Fredrickson, J. Dynamics in microbial communities: Unraveling mechanisms to identify principles. ISME J. 2015, 9, 1488–1495. [Google Scholar] [CrossRef]
- Oña, L.; Shreekar, S.K.; Kost, C. Disentangling microbial interaction networks. Trends Microbiol. 2025, 33, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhou, F.; Ding, S.; Zhu, Z.; Huo, Z.; Chen, Q.; Liu, Z. Addition of high-quality plant residue alters microbial keystone taxa and network complexity and increases soil phosphorus (P) availability. Agronomy 2024, 14, 3036. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gao, T.; Zhu, Q.; Yan, T.; Li, D.; Xue, J.; Wu, Y. Increases in bacterial community network complexity induced by biochar-based fertilizer amendments to karst calcareous soil. Geoderma 2019, 337, 691–700. [Google Scholar] [CrossRef]
- Yu, J.; Deem, L.M.; Crow, S.E.; Deenik, J.L.; Penton, C.R. Biochar application influences microbial assemblage complexity and composition due to soil and bioenergy crop type interactions. Soil Biol. Biochem. 2018, 117, 97–107. [Google Scholar] [CrossRef]
- Chen, S.; Qi, G.; Luo, T.; Zhang, H.; Jiang, Q.; Wang, R.; Zhao, X. Continuous-cropping tobacco caused variance of chemical properties and structure of bacterial network in soils. Land Degrad. Dev. 2018, 29, 4106–4120. [Google Scholar] [CrossRef]
- Riddley, M.; Hepp, S.; Hardeep, F.N.U.; Nayak, A.; Liu, M.; Xing, X.; Zhang, H.; Liao, J. Differential roles of deterministic and stochastic processes in structuring soil bacterial ecotypes across terrestrial ecosystems. Nat. Commun. 2025, 16, 2337. [Google Scholar] [CrossRef]
- Wang, K.; Xue, K.; Liu, W.; Zhang, B.; Wu, W.; Zhao, R.; Cui, L.; Wang, Z.; Zhou, S.; Tang, L.; et al. Warming decouples associations between microbial network complexity and ecosystem multifunctionality in alpine grasslands. Agric. Ecosyst. Environ. 2024, 374, 109189. [Google Scholar] [CrossRef]
- Yang, X.; Song, W.; Yang, X.; Yang, T.; Bao, W.; Wang, C.; Li, J.; Zhong, S.; Jiang, Q.; Li, L.; et al. Microbial network structure, not plant and microbial community diversity, regulates multifunctionality under increased precipitation in a cold steppe. Front. Microbiol. 2024, 14, 1349747. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Li, Y.; Zhao, T.; Zheng, J.; Qiao, J.; Zhang, F.; Han, G.; Bisseling, T.; Zhao, M. Destabilized bacterial and fungal network weakens soil multifunctionality under increasing grazing stress. Appl. Soil Ecol. 2025, 206, 105827. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Chen, X.; Meng, Z.; Xu, R.; Duoji, D.; Zhang, J.; He, J.; Wang, Z.; Chen, J.; et al. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Chen, X.; Tian, J.; Liu, S.; Wei, Z.; Wang, Y.; Song, X.; Zhang, X.; Bai, Y. The complexity of the bacterial community in response to fertilization determines forage production in a semiarid grassland. Ecol. Indic. 2022, 139, 108918. [Google Scholar] [CrossRef]
- Luo, W.; Wang, P.; Liu, J.; Tao, J. Microbial keystone taxa and network complexity, rather than diversity, sustain soil multifunctionality along an elevational gradient in a subtropical karst mountain. CATENA 2025, 256, 109115. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, J.; Wang, B.; Fan, B.; Zhou, G. Soil microbial network complexity predicts soil multifunctionality better than soil microbial diversity during grassland-farmland-shrubland conversion on the Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2025, 379, 109356. [Google Scholar] [CrossRef]
- Tao, J.; Meng, D.; Qin, C.; Liu, X.; Liang, Y.; Xiao, Y.; Liu, Z.; Gu, Y.; Li, J.; Yin, H. Integrated network analysis reveals the importance of microbial interactions for maize growth. Appl. Microbiol. Biotechnol. 2018, 102, 3805–3818. [Google Scholar] [CrossRef]
- Biggs, C.R.; Yeager, L.A.; Bolser, D.G.; Bonsell, C.; Dichiera, A.M.; Hou, Z.; Keyser, S.R.; Khursigara, A.J.; Lu, K.; Muth, A.F.; et al. Does functional redundancy affect ecological stability and resilience? A review and meta-analysis. Ecosphere 2020, 11, e03184. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, F. Effect of reduced subsequent N supply and organic amendment on soil bacterial community in a wheat-maize rotation system with over-fertilization. Appl. Soil Ecol. 2023, 181, 104659. [Google Scholar] [CrossRef]
- Singh, B.K.; Jiang, G.; Wei, Z.; Sáez-Sandino, T.; Gao, M.; Liu, H.; Xiong, C. Plant pathogens, microbiomes, and soil health. Trends Microbiol. 2025. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, M.; Petropoulos, E.; Zhang, J.; Nie, J.; Liao, Y.; Li, Z.; Lin, X.; Feng, Y. Responses of paddy soil bacterial community assembly to different long-term fertilizations in southeast China. Sci. Total Environ. 2019, 656, 625–633. [Google Scholar] [CrossRef]
- Xue, C.; Ryan Penton, C.; Zhu, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Ling, N.; Shen, Q. Alterations in soil fungal community composition and network assemblage structure by different long-term fertilization regimes are correlated to the soil ionome. Biol. Fert. Soils 2018, 54, 95–106. [Google Scholar] [CrossRef]
- Ma, B.; Lv, X.; Cai, Y.; Chang, S.X.; Dyck, M.F. Liming does not counteract the influence of long-term fertilization on soil bacterial community structure and its co-occurrence pattern. Soil Biol. Biochem. 2018, 123, 45–53. [Google Scholar] [CrossRef]
- Li, B.; Roley, S.S.; Duncan, D.S.; Guo, J.; Quensen, J.F.; Yu, H.; Tiedje, J.M. Long-term excess nitrogen fertilizer increases sensitivity of soil microbial community to seasonal change revealed by ecological network and metagenome analyses. Soil Biol. Biochem. 2021, 160, 108349. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, D.; Li, Q.; Liu, H.; Wang, Y.; Xu, J.; Du, B.; Guo, D.; Liu, Y.; Fu, H.; et al. Nonlinear response of soil microbial network complexity to long-term nitrogen addition in a semiarid grassland: Implications for soil carbon processes. Agric. Ecosyst. Environ. 2025, 380, 109407. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Zhang, Y.; Li, Y.; Wang, F.; Shen, C. Soil microbiome response to reduced nitrogen supply in an over-fertilized wheat-maize system. Agronomy 2024, 14, 2631. [Google Scholar] [CrossRef]
- Wang, C.; Shi, Z.; Li, A.; Geng, T.; Liu, L.; Liu, W. Long-term nitrogen input reduces soil bacterial network complexity by shifts in life history strategy in temperate grassland. iMeta 2024, 3, e194. [Google Scholar] [CrossRef]
- Ma, L.; Niu, W.; Li, G.; Du, Y.; Sun, J.; Zhang, Q.; Siddique, K.H.M. Crucial role of rare taxa in preserving bacterial community stability. Land Degrad. Dev. 2024, 35, 1397–1410. [Google Scholar] [CrossRef]
- Ling, N.; Zhu, C.; Xue, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Shen, Q. Insight into how organic amendments can shape the soil microbiome in long-term field experiments as revealed by network analysis. Soil Biol. Biochem. 2016, 99, 137–149. [Google Scholar] [CrossRef]
- Schmid, C.A.O.; Schröder, P.; Armbruster, M.; Schloter, M. Organic amendments in a long-term field trial—Consequences for the bulk soil bacterial community as revealed by network analysis. Microb. Ecol. 2018, 76, 226–239. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Q.; Feng, Z.; Liu, Z.; Li, H.; Sun, Y.; Liu, C.; Lai, H. Long-term organic fertilization improves the productivity of kiwifruit (Actinidia chinensis Planch.) through increasing rhizosphere microbial diversity and network complexity. Appl. Soil Ecol. 2020, 147, 103426. [Google Scholar] [CrossRef]
- Yang, L.; Sun, R.; Li, J.; Zhai, L.; Cui, H.; Fan, B.; Wang, H.; Liu, H. Combined organic-inorganic fertilization builds higher stability of soil and root microbial networks than exclusive mineral or organic fertilization. Soil Ecol. Lett. 2022, 5, 220142. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Tan, W.; Di, H.; Xu, J.; Li, Y. High manure load reduces bacterial diversity and network complexity in a paddy soil under crop rotations. Soil Ecol. Lett. 2020, 2, 104–119. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Zhang, Y.; Wang, F.; Li, Y.; Shen, C.; Chen, B. Exploring suitable nitrification inhibitor in an intensively cultivated greenhouse soil and its effect on the abundance and community of soil ammonia oxidizers. Agronomy 2025, 15, 255. [Google Scholar] [CrossRef]
- Bao, S. Analytical Methods in Soil and Agricultural Chemistry; China Agricultural Press: Beijing, China, 2008. [Google Scholar]
- Li, Z.; Luo, Y.; Teng, Y. Analytical Methods in Soil and Environmental Microbiology; Science Press: Beijing, China, 2008. [Google Scholar]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Han, C.; Shi, C.; Liu, L.; Han, J.; Yang, Q.; Wang, Y.; Li, X.; Fu, W.; Gao, H.; Huang, H.; et al. Majorbio Cloud 2024: Update single-cell and multiomics workflows. iMeta 2024, 3, e217. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.; Zhou, X.; Zhang, A.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Mansoldo, F.R.P.; An, J.; Kou, Y.; Zhang, X.; Zeng, J.; Vermelho, A.; Wang, J.; Yao, M. Microbial habitat specificity largely affects microbial co-occurrence patterns and functional profiles in wetland soils. Geoderma 2022, 418, 115866. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Zhang, Y.; Shen, C. N fertilizer reduction combined with organic amendment enhances soil multifunctionality by altering nutrient availability and fungal community structure in an intensive grain production system. J. Soil Sci. Plant Nutr. 2024, 24, 3969–3985. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, Z.; Zhang, W. Managing nutrient for both food security and environmental sustainability in China: An experiment for the world. Front. Agric. Sci. Eng. 2014, 1, 53–61. [Google Scholar]
- Wang, X.; Zou, C.; Gao, X.; Guan, X.; Zhang, W.; Zhang, Y.; Shi, X.; Chen, X. Nitrous oxide emissions in Chinese vegetable systems: A meta-analysis. Environ. Pollut. 2018, 239, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Qasim, W.; Xia, L.; Lin, S.; Wan, L.; Zhao, Y.; Butterbach-Bahl, K. Global greenhouse vegetable production systems are hotspots of soil N2O emissions and nitrogen leaching: A meta-analysis. Environ. Pollut. 2021, 272, 116372. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Lei, H.; Xu, C.; Huadong, K.; Hu, L. Characteristics and spatial distribution of apparent nitrogen balance in the greenhouse vegetable cropping system in China. J. Agric. Resour. Environ. 2020, 37, 353–360. [Google Scholar]
- Dong, J.; Xu, Y.; Quan, Z.; Yin, Y.; Zhao, Y.; Xu, Q.; Tian, K.; Huang, B.; Cai, Z.; Ma, Y.; et al. The obstacles and countermeasures of soil sustainability in protected horticulture in China. Acta Pedol. Sinica. 2024, 61, 1467–1480. [Google Scholar]
- Du, L.; Zhang, Z.; Chen, Y.; Wang, Y.; Zhou, C.; Yang, H.; Zhang, W. Heterogeneous impact of soil acidification on crop yield reduction and its regulatory variables: A global meta-analysis. Field Crop Res. 2024, 319, 109643. [Google Scholar] [CrossRef]
- Guo, H.; Ye, C.; Zhang, H.; Pan, S.; Ji, Y.; Li, Z.; Liu, M.; Zhou, X.; Du, G.; Hu, F.; et al. Long-term nitrogen & phosphorus additions reduce soil microbial respiration but increase its temperature sensitivity in a Tibetan alpine meadow. Soil Biol. Biochem. 2017, 113, 26–34. [Google Scholar]
- Xu, P.; Wu, J.; Wang, H.; Han, S.; Zhu, J.; Fu, Q.; Geng, M.; Hu, H.; Huang, Q. Long-term partial substitution of chemical fertilizer with green manure regulated organic matter mineralization in paddy soil dominantly by modulating organic carbon quality. Plant Soil 2021, 468, 459–473. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, D.; Gherardi, L.A.; Liu, Y.; Wang, Y.; Elser, J.J.; Fu, H. Linkages of stoichiometric imbalances to soil microbial respiration with increasing nitrogen addition: Evidence from a long-term grassland experiment. Soil Biol. Biochem. 2019, 138, 107580. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fert. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, G.; Wang, Y.; Fei, J.; Xiangmin, R.; Peng, J.; Tian, C.; Zhang, Y. Crop rotation-driven change in physicochemical properties regulates microbial diversity, dominant components, and community complexity in paddy soils. Agric. Ecosyst. Environ. 2023, 343, 108278. [Google Scholar] [CrossRef]
- Bergstrand, K. Organic fertilizers in greenhouse production systems—A review. Sci. Hortic. 2022, 295, 110855. [Google Scholar] [CrossRef]
- Gutser, R.; Ebertseder, T.; Weber, A.; Schraml, M.; Schmidhalter, U. Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J. Plant Nutr. Soil Sci. 2005, 168, 439–446. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Deng, T.; Shi, L. Towards sustainable productivity of greenhouse vegetable soils: Limiting factors and mitigation strategies. Plants 2024, 13, 2885. [Google Scholar] [CrossRef] [PubMed]
- De Graaff, M.; Hornslein, N.; Throop, H.L.; Kardol, P.; van Diepen, L.T.A. Effects of agricultural intensification on soil biodiversity and implications for ecosystem functioning: A meta-analysis. Adv. Agron. 2019, 155, 1–44. [Google Scholar]
- Chakraborty, D.; Jousset, A.; Wei, Z.; Banerjee, S. Rare taxa in the core microbiome. Trends Microbiol. 2025, 33, 727–737. [Google Scholar] [CrossRef]
- Xu, Q.; Vandenkoornhuyse, P.; Li, L.; Guo, J.; Zhu, C.; Guo, S.; Ling, N.; Shen, Q. Microbial generalists and specialists differently contribute to the community diversity in farmland soils. J. Adv. Res. 2022, 40, 17–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Ran, W.; Xu, W.; Song, Y. ITS amplicon sequencing revealed that rare taxa of tea rhizosphere fungi are closely related to the environment and provide feedback on tea tree diseases. Microbiol. Spectr. 2024, 13, e01889-24. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, C.; Li, L.; Li, H.; Li, W.; Huang, F. Rare bacterial and fungal taxa respond strongly to combined inorganic and organic fertilization under short-term conditions. Appl. Soil Ecol. 2024, 203, 105639. [Google Scholar] [CrossRef]
- Kong, D.; Jin, Y.; Yu, K.; Swaney, D.P.; Liu, S.; Zou, J. Low N2O emissions from wheat in a wheat-rice double cropping system due to manure substitution are associated with changes in the abundance of functional microbes. Agric. Ecosyst. Environ. 2021, 311, 107318. [Google Scholar] [CrossRef]
- Yang, G.; Ma, Y.; Xu, W.; Ma, X.; Lu, C. Spent mushroom substrate as a substitute for chemical fertilizer changes N-cycling genes and reduces N2O emission in different textured soils. Biol. Fert. Soils 2024, 60, 87–99. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, Q.; Zhu, S.; Duan, P.; Zhang, X.; Wu, Z.; Xiong, Z. Organic substitutions aggravated microbial nitrogen limitation and decreased nitrogen-cycling gene abundances in a three-year greenhouse vegetable field. J. Environ. Manag. 2021, 288, 112379. [Google Scholar] [CrossRef]
- Hou, H.; Liu, X.; Zhou, W.; Ji, J.; Lan, X.; Lv, Z.; Liu, Y.; Zhang, J.; Müller, C. N transformation mechanisms and N dynamics of organic fertilisers as partial substitutes for chemical fertilisers in paddy soils. J. Soils Sediments 2022, 22, 2516–2529. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Shangguan, Z. Soil carbon and nitrogen fractions in the soil profile and their response to long-term nitrogen fertilization in a wheat field. CATENA 2015, 135, 38–46. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Luo, S.; Xia, Y.; Xu, Q.; Yang, L.; Yue, K.; Fan, Y. Influence of nitrogen fertilization on soil organic nitrogen components in agroecosystems: A meta-analysis. Acta Ecol. Sin. 2025, 45, 1328–1338. [Google Scholar]
- Chen, Y.; Li, Y.; Qiu, T.; He, H.; Liu, J.; Duan, C.; Cui, Y.; Huang, M.; Wu, C.; Fang, L. High nitrogen fertilizer input enhanced the microbial network complexity in the paddy soil. Soil Ecol. Lett. 2023, 6, 230205. [Google Scholar] [CrossRef]
- Rohe, L.; Apelt, B.; Vogel, H.J.; Well, R.; Wu, G.M.; Schlüter, S. Denitrification in soil as a function of oxygen availability at the microscale. Biogeosciences 2021, 18, 1185–1201. [Google Scholar] [CrossRef]
- Ju, S.; Min, J.; Li, Z.; Hao, Y.; Wu, X.; Kronzucker, H.J.; Shi, W. Manure-nitrogen substitution for urea leads to higher yield but increases N2O emission in vegetable production on nitrate-rich soils. Agric. Ecosyst. Environ. 2025, 383, 109541. [Google Scholar] [CrossRef]
- Xiao, X.; Xie, G.; Yang, Z.; He, N.; Yang, D.; Liu, M. Variation in abundance, diversity, and composition of nirK and nirS containing denitrifying bacterial communities in a red paddy soil as affected by combined organic-chemical fertilization. Appl. Soil Ecol. 2021, 166, 104001. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, D.; Ma, Y.; Yang, G.; Ambus, P.L.; Liu, X.; Luo, J. Effects of long-term organic fertilizer substitutions on soil nitrous oxide emissions and nitrogen cycling gene abundance in a greenhouse vegetable field. Appl. Soil Ecol. 2023, 188, 104877. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, X.; Yu, Q.; Jin, Y.; Jiang, P.; Wu, S.; Liu, S.; Zou, J. Mitigation of N2O emissions in water-saving paddy fields: Evaluating organic fertilizer substitution and microbial mechanisms. J. Integr. Agric. 2024, 23, 3159–3173. [Google Scholar] [CrossRef]
- Xie, L.; Li, L.; Xie, J.; Wang, J.; Mumtaz, M.Z.; Effah, Z.; Fudjoe, S.K.; Khaskheli, M.A.; Luo, Z.; Li, L. Optimal substitution of inorganic fertilizer with organic amendment sustains rainfed maize production and decreases soil N2O emissions by modifying denitrifying bacterial communities in Northern China. Eur. J. Agron. 2024, 160, 127287. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Y.; Liu, J.; Gao, J.; Zhang, Y.; Ni, J.; Xie, D.; Wang, Z.; Gao, M. Partial substitution of chemical fertilizer by organic materials changed the abundance, diversity, and activity of nirS-type denitrifying bacterial communities in a vegetable soil. Appl. Soil Ecol. 2020, 152, 103589. [Google Scholar] [CrossRef]
- Tang, Q.; Xia, Y.; Ti, C.; Shan, J.; Zhou, W.; Li, C.; Yan, X.; Yan, X. Partial organic fertilizer substitution promotes soil multifunctionality by increasing microbial community diversity and complexity. Pedosphere 2023, 33, 407–420. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Ge, Y.; Lei, Y.; Wei, X.; Xu, Y.; Zheng, X. Partial substitution of nitrogen fertilizers by organic products of rural waste co-composting impacts on farmland soil quality. Environ. Technol. Innov. 2024, 33, 103470. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Xu, X.; Liu, M.; Jiang, R.; Zhang, J.; Duan, Y.; He, P.; Zhou, W. Response of soil microbial properties in the life cycle of potatoes to organic substitution regimes in North China. Soil Till. Res. 2024, 237, 106000. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Y.; Jin, D.; Zhou, Y.; Mukhamed, B.; Liu, D.; Feng, B. The application of biochar and organic fertilizer substitution regulates the diversities of habitat specialist bacterial communities within soil aggregates in proso millet farmland. Biochar 2025, 7, 6. [Google Scholar] [CrossRef]
- He, H.; Peng, M.; Hou, Z.; Li, J. Organic substitution contrasting direct fertilizer reduction increases wheat productivity, soil quality, microbial diversity and network complexity. Environ. Technol. Innov. 2024, 36, 103784. [Google Scholar] [CrossRef]
- Che, J.; Wu, Y.; Yang, H.; Chang, Y.; Wu, W.; Lyu, L.; Wang, X.; Cao, F.; Li, W. Enhanced complexity of interkingdom co-occurrence networks in blueberry rhizosphere microbial communities under soil pH stress. Appl. Soil Ecol. 2025, 212, 106191. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Fang, J.; Chu, H.; Adams, J.M. Soil microbial network complexity varies with pH as a continuum, not a threshold, across the North China Plain. Front. Microbiol. 2022, 13, 895687. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Liu, J.; Pan, X.; Huang, Y.; Cui, X.; Wang, Y.; Jin, Y.; Sheng, J. Neutral pH induces complex and stable soil microbial networks in agricultural ecosystems. Plant Soil 2025. [Google Scholar] [CrossRef]
- Guo, Y.; Song, B.; Li, A.; Wu, Q.; Huang, H.; Li, N.; Yang, Y.; Adams, J.M.; Yang, L. Higher pH is associated with enhanced co-occurrence network complexity, stability and nutrient cycling functions in the rice rhizosphere microbiome. Environ. Microbiol. 2022, 24, 6200–6219. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Cucumber Season | Tomato Season | The Whole Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Chicken Manure | N | P | K | Chicken Manure | N | P | K | Chicken Manure | |
| FCFP | 1235 | 168 | 1794 | 0 | 925 | 126 | 1343 | 0 | 2160 | 294 | 3137 | 0 |
| 85%FCFP+CM | 1050 | 168 | 1794 | 14,660 | 786 | 126 | 1343 | 0 | 1836 | 294 | 3137 | 14,660 |
| 70%FCFP+CM | 865 | 168 | 1794 | 29,321 | 647 | 126 | 1343 | 0 | 1512 | 294 | 3137 | 29,321 |
| 55%FCFP+CM | 680 | 168 | 1794 | 43,981 | 508 | 126 | 1343 | 0 | 1188 | 294 | 3137 | 43,981 |
| 40%FCFP+CM | 494 | 168 | 1794 | 58,642 | 370 | 126 | 1343 | 0 | 864 | 294 | 3137 | 58,642 |
| Treatment | Annual Input (USD hm−2) | Annual Output (USD hm−2) | Net Economic Benefits (USD hm−2) | ||
|---|---|---|---|---|---|
| Chemical Fertilizer | Chicken Manure | Cucumber | Tomato | ||
| FCFP | 9507 | 0 | 53014 ± 2493 a | 17483 ± 897 a | 60990 ± 2580 a |
| 85%FCFP+CM | 8081 | 537 | 54887 ± 4808 a | 18087 ± 1672 a | 64357 ± 4261 a |
| 70%FCFP+CM | 6655 | 1074 | 51933 ± 4435 a | 17111 ± 1340 a | 61316 ± 4740 a |
| 55%FCFP+CM | 5229 | 1611 | 41285 ± 3079 b | 13165 ± 1585 b | 47611 ± 5128 b |
| 40%FCFP+CM | 3803 | 2147 | 34465 ± 5038 c | 12208 ± 1567 b | 40722 ± 6038 c |
| FCFP | 85%FCFP+CM | 70%FCFP+CM | 55%FCFP+CM | 40%FCFP+CM | |
|---|---|---|---|---|---|
| TOC/g kg−1 | 13.79 ± 0.99 c | 16.54 ± 0.92 a | 15.92 ± 0.56 ab | 15.47 ± 1.23 ab | 14.91 ± 0.83 bc |
| TN/g kg−1 | 1.62 ± 0.64 a | 1.84 ± 0.44 a | 1.93 ± 0.49 a | 1.77 ± 0.48 a | 1.67 ± 0.29 a |
| pH | 7.65 ± 0.09 c | 7.54 ± 0.16 c | 7.88 ± 0.10 a | 7.83 ± 0.07 ab | 7.70 ± 0.15 bc |
| AN/mg kg−1 | 161.50 ± 2.88 a | 124.55 ± 21.08 bc | 111.04 ± 5.29 c | 134.20 ± 12.68 b | 114.64 ± 2.67 c |
| AP/mg kg−1 | 213.53 ± 16.89 a | 196.23 ± 6.40 ab | 184.25 ± 13.54 b | 206.19 ± 14.64 a | 211.00 ± 4.59 a |
| AK/mg kg−1 | 338.00 ± 13.29 d | 483.00 ± 63.65 a | 410.60 ± 7.40 bc | 422.00 ± 31.57 b | 371.40 ± 12.76 cd |
| CAT/mL KMnO4 g−1 h−1 | 8.37 ± 0.42 b | 7.93 ± 0.18 c | 8.62 ± 0.27 ab | 8.81 ± 0.09 a | 8.59 ± 0.10 ab |
| URE/µg NH4+-N g−1 h−1 | 77.66 ± 2.78 a | 77.48 ± 2.54 a | 74.15 ± 4.31 ab | 71.13 ± 1.49 b | 71.79 ± 3.64 b |
| GLU/µg glucose g−1 h−1 | 45.91 ± 10.04 ab | 46.98 ± 8.70 a | 35.59 ± 7.16 b | 32.79 ± 7.99 b | 42.43 ± 11.61 ab |
| SUR/µg glucose g−1 h−1 | 154.94 ± 13.04 d | 198.09 ± 6.12 ab | 201.66 ± 8.99 a | 190.06 ± 2.68 bc | 186.05 ± 1.79 c |
| ALP/µg phenol g−1 h−1 | 6.07 ± 1.12 a | 4.40 ± 0.59 b | 6.90 ± 1.20 a | 5.63 ± 0.76 ab | 4.44 ± 1.60 b |
| ARY/µg PNP g−1 h−1 | 0.11 ± 0.04 abc | 0.18 ± 0.05 a | 0.16 ± 0.06 ab | 0.09 ± 0.06 bc | 0.06 ± 0.02 c |
| GME | 11.94 ± 1.05 ab | 12.71 ± 1.00 a | 12.75 ± 1.38 a | 10.26 ± 2.68 b | 10.31 ± 1.36 b |
| Genus | FCFP | 85%FCFP+CM | 70%FCFP+CM | 55%FCFP+CM | 40%FCFP+CM |
|---|---|---|---|---|---|
| Acidobacterium | 0.28 ± 0.05 d | 0.34 ± 0.01 b | 0.38 ± 0.03 a | 0.29 ± 0.01 cd | 0.33 ± 0.02 bc |
| Rhodospirillum | 0.33 ± 0.08 a | 0.22 ± 0.02 b | 0.20 ± 0.03 b | 0.31 ± 0.02 a | 0.21 ± 0.02 b |
| Microvirga | 0.25 ± 0.02 c | 0.34 ± 0.04 ab | 0.37 ± 0.05 a | 0.33 ± 0.02 b | 0.30 ± 0.01 b |
| Hypericibacter | 0.23 ± 0.03 b | 0.22 ± 0.01 b | 0.19 ± 0.03 c | 0.27 ± 0.01 a | 0.23 ± 0.02 b |
| Nitrosopumilus | 0.08 ± 0.02 c | 0.11 ± 0.03 b | 0.15 ± 0.01 a | 0.14 ± 0.02 a | 0.12 ± 0.01 ab |
| Methyloceanibacter | 0.26 ± 0.05 b | 0.37 ± 0.09 a | 0.27 ± 0.03 b | 0.29 ± 0.04 b | 0.36 ± 0.02 a |
| Promineifilum | 0.17 ± 0.03 c | 0.46 ± 0.03 a | 0.47 ± 0.07 a | 0.35 ± 0.08 b | 0.36 ± 0.03 b |
| unclassified Nitrososphaeraceae | 1.59 ± 0.65 c | 2.63 ± 0.81 b | 4.02 ± 0.10 a | 3.36 ± 0.48 ab | 2.95 ± 0.33 b |
| Aldersonia | 0.31 ± 0.08 a | 0.12 ± 0.01 b | 0.10 ± 0.03 b | 0.10 ± 0.03 b | 0.09 ± 0.01 b |
| Steroidobacter | 1.36 ± 0.21 a | 1.13 ± 0.15 ab | 0.97 ± 0.11 b | 1.08 ± 0.27 b | 1.34 ± 0.03 a |
| Rubrobacter | 0.19 ± 0.02 b | 0.23 ± 0.01 a | 0.23 ± 0.01 a | 0.20 ± 0.01 b | 0.20 ± 0.01 b |
| unclassified Dormibacteraeota | 0.14 ± 0.02 b | 0.18 ± 0.02 a | 0.17 ± 0.01 a | 0.15 ± 0.01 b | 0.14 ± 0.01 b |
| Dongia | 0.56 ± 0.13 a | 0.41 ± 0.05 b | 0.36 ± 0.05 b | 0.53 ± 0.05 a | 0.37 ± 0.06 b |
| Chloracidobacterium | 1.58 ± 0.96 a | 0.94 ± 0.11 b | 0.88 ± 0.12 b | 0.90 ± 0.07 b | 1.32 ± 0.16 ab |
| Phenylobacterium | 0.14 ± 0.01 a | 0.12 ± 0.01 ab | 0.12 ± 0.00 c | 0.12 ± 0.01 bc | 0.13 ± 0.00 ab |
| Reyranella | 0.20 ± 0.07 a | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.19 ± 0.00 a | 0.17 ± 0.00 a |
| Rhodococcus | 0.45 ± 0.09 a | 0.21 ± 0.02 b | 0.19 ± 0.06 b | 0.19 ± 0.04 b | 0.18 ± 0.02 b |
| Sphingomonas | 3.05 ± 1.05 ab | 2.47 ± 0.36 ab | 2.12 ± 0.54 b | 2.87 ± 0.78 ab | 3.30 ± 0.44 a |
| Parvularcula | 0.19 ± 0.07 a | 0.08 ± 0.02 bc | 0.04 ± 0.02 c | 0.11 ± 0.04 abc | 0.15 ± 0.10 ab |
| Nocardia | 0.24 ± 0.03 a | 0.12 ± 0.01 b | 0.11 ± 0.02 b | 0.11 ± 0.02 b | 0.11 ± 0.01 b |
| Gaiellasilicea | 0.27 ± 0.05 c | 0.46 ± 0.07 a | 0.46 ± 0.06 a | 0.37 ± 0.05 b | 0.33 ± 0.04 bc |
| Methylobacterium | 0.11 ± 0.01 c | 0.14 ± 0.01 ab | 0.14 ± 0.01 a | 0.14 ± 0.01 ab | 0.13 ± 0.00 b |
| Pelagibius | 0.13 ± 0.02 b | 0.18 ± 0.02 a | 0.18 ± 0.03 a | 0.18 ± 0.01 a | 0.14 ± 0.01 b |
| Actinomadura | 0.14 ± 0.06 a | 0.13 ± 0.01 a | 0.12 ± 0.01 a | 0.11 ± 0.01 a | 0.11 ± 0.01 a |
| Defluviicoccus | 0.11 ± 0.01 c | 0.15 ± 0.02 ab | 0.16 ± 0.02 a | 0.15 ± 0.02 ab | 0.13 ± 0.01 bc |
| Inquilinus | 0.10 ± 0.01 c | 0.14 ± 0.02 ab | 0.14 ± 0.02 a | 0.14 ± 0.01 ab | 0.12 ± 0.02 bc |
| Limnobacter | 0.19 ± 0.05 b | 0.30 ± 0.14 ab | 0.26 ± 0.08 b | 0.30 ± 0.09 ab | 0.42 ± 0.08 a |
| Leptolyngbya | 0.18 ± 0.03 b | 0.20 ± 0.01 b | 0.19 ± 0.01 b | 0.20 ± 0.02 b | 0.23 ± 0.02 a |
| Ilumatobacter | 0.67 ± 0.18 ab | 0.62 ± 0.12 ab | 0.67 ± 0.16 ab | 0.50 ± 0.09 b | 0.71 ± 0.06 a |
| unclassified Nitrososphaerota | 0.54 ± 0.15 b | 0.76 ± 0.25 ab | 0.97 ± 0.15 ab | 1.13 ± 0.61 a | 0.67 ± 0.04 b |
| KEGG Module | Function | FCFP | 85%FCFP+CM | 70%FCFP+CM | 55%FCFP+CM | 40%FCFP+CM |
|---|---|---|---|---|---|---|
| M00786 | Fumitremorgin alkaloid biosynthesis | 0.008 ± 0.004 a | 0.003 ± 0.001 b | 0.003 ± 0.000 b | 0.004 ± 0.001 b | 0.004 ± 0.001 b |
| M00378 | F420 biosynthesis | 0.106 ± 0.018 c | 0.134 ± 0.012 ab | 0.149 ± 0.009 a | 0.143 ± 0.021 a | 0.120 ± 0.005 bc |
| M00538 | Toluene degradation | 0.027 ± 0.002 a | 0.023 ± 0.002 b | 0.018 ± 0.002 c | 0.024 ± 0.002 b | 0.022 ± 0.002 b |
| M00094 | Ceramide biosynthesis | 0.008 ± 0.002 a | 0.008 ± 0.001 a | 0.007 ± 0.001 a | 0.008 ± 0.001 a | 0.008 ± 0.001 a |
| M00736 | Nocardicin A biosynthesis | 0.009 ± 0.005 a | 0.004 ± 0.001 b | 0.004 ± 0.001 b | 0.005 ± 0.001 b | 0.004 ± 0.001 b |
| M00023 | Tryptophan biosynthesis | 0.546 ± 0.015 b | 0.555 ± 0.010 ab | 0.565 ± 0.003 a | 0.563 ± 0.011 a | 0.554 ± 0.004 ab |
| M00960 | Lysine degradation | 0.111 ± 0.017 a | 0.106 ± 0.004 ab | 0.098 ± 0.006 b | 0.109 ± 0.003 ab | 0.109 ± 0.004 ab |
| M00144 | NADH: quinone oxidoreductase | 1.799 ± 0.009 bc | 1.828 ± 0.020 a | 1.813 ± 0.030 ab | 1.781 ± 0.012 cd | 1.770 ± 0.023 d |
| M00358 | Coenzyme M biosynthesis | 0.093 ± 0.009 c | 0.106 ± 0.006 b | 0.117 ± 0.010 a | 0.109 ± 0.005 ab | 0.101 ± 0.005 bc |
| M00096 | C5 isoprenoid biosynthesis | 0.558 ± 0.003 a | 0.543 ± 0.007 b | 0.527 ± 0.012 c | 0.540 ± 0.011 b | 0.556 ± 0.007 a |
| M00763 | Ornithine biosynthesis | 0.050 ± 0.005 c | 0.061 ± 0.012 bc | 0.075 ± 0.007 a | 0.073 ± 0.015 ab | 0.063 ± 0.003 abc |
| M00529 | Denitrification | 0.569 ± 0.078 b | 0.621 ± 0.013 a | 0.622 ± 0.019 a | 0.636 ± 0.020 a | 0.651 ± 0.011 a |
| M00883 | Lipoic acid biosynthesis | 0.096 ± 0.003 a | 0.100 ± 0.003 a | 0.100 ± 0.005 a | 0.097 ± 0.003 a | 0.098 ± 0.005 a |
| M00959 | Guanine ribonucleotide degradation | 0.587 ± 0.022 b | 0.614 ± 0.008 a | 0.608 ± 0.012 a | 0.590 ± 0.003 b | 0.597 ± 0.005 ab |
| M00847 | Heme biosynthesis | 0.101 ± 0.008 c | 0.113 ± 0.009 bc | 0.132 ± 0.007 a | 0.121 ± 0.014 ab | 0.113 ± 0.004 bc |
| M00159 | V/A-type ATPase | 0.092 ± 0.016 c | 0.125 ± 0.027 ab | 0.153 ± 0.013 a | 0.146 ± 0.030 ab | 0.119 ± 0.006 b |
| M00884 | Lipoic acid biosynthesis | 0.085 ± 0.004 a | 0.089 ± 0.002 a | 0.089 ± 0.005 a | 0.086 ± 0.002 a | 0.087 ± 0.003 a |
| M00967 | Flavone degradation | 0.0008 ± 0.0003 b | 0.0009 ± 0.0004 b | 0.0007 ± 0.0002 b | 0.001 ± 0.0003 ab | 0.0014 ± 0.0006 a |
| M00914 | Coenzyme A biosynthesis | 0.295 ± 0.009 c | 0.320 ± 0.014 ab | 0.333 ± 0.011 a | 0.330 ± 0.019 ab | 0.315 ± 0.006 b |
| M00128 | Ubiquinone biosynthesis | 0.024 ± 0.007 a | 0.017 ± 0.001 b | 0.017 ± 0.002 b | 0.019 ± 0.001 b | 0.019 ± 0.002 ab |
| M00072 | N-glycosylation by oligosaccharyltransferase | 0.011 ± 0.002 b | 0.015 ± 0.005 ab | 0.017 ± 0.005 ab | 0.018 ± 0.007 a | 0.012 ± 0.001 ab |
| M00911 | Riboflavin biosynthesis | 0.175 ± 0.010 b | 0.183 ± 0.012 ab | 0.190 ± 0.006 a | 0.194 ± 0.007 a | 0.183 ± 0.006 ab |
| M00031 | Lysine biosynthesis | 0.070 ± 0.010 c | 0.086 ± 0.018 bc | 0.108 ± 0.010 a | 0.105 ± 0.024 ab | 0.088 ± 0.005 abc |
| M00083 | Fatty acid biosynthesis | 1.216 ± 0.046 a | 1.194 ± 0.008 ab | 1.170 ± 0.032 b | 1.178 ± 0.021 ab | 1.189 ± 0.019 ab |
| M00861 | beta-Oxidation | 0.099 ± 0.070 a | 0.059 ± 0.005 a | 0.058 ± 0.003 a | 0.060 ± 0.001 a | 0.063 ± 0.002 a |
| M00882 | Lipoic acid biosynthesis | 0.085 ± 0.004 a | 0.090 ± 0.002 a | 0.089 ± 0.006 a | 0.086 ± 0.002 a | 0.087 ± 0.003 a |
| M00055 | N-glycan precursor biosynthesis | 0.043 ± 0.003 c | 0.051 ± 0.006 b | 0.060 ± 0.004 a | 0.051 ± 0.005 b | 0.050 ± 0.003 b |
| M00664 | Nodulation | 0.012 ± 0.001 b | 0.015 ± 0.001 a | 0.016 ± 0.001 a | 0.015 ± 0.002 a | 0.015 ± 0.001 a |
| M00146 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex | 0.027 ± 0.005 b | 0.028 ± 0.002 b | 0.028 ± 0.002 b | 0.034 ± 0.005 a | 0.032 ± 0.003 ab |
| M00545 | Trans-cinnamate degradation | 0.378 ± 0.021 d | 0.405 ± 0.016 ab | 0.418 ± 0.003 a | 0.398 ± 0.015 bc | 0.384 ± 0.009 cd |
| KEGG Module | Gene | FCFP | 85%FCFP+CM | 70%FCFP+CM | 55%FCFP+CM | 40%FCFP+CM |
|---|---|---|---|---|---|---|
| Denitrification | narG | 0.0505 ± 0.0081 a | 0.0545 ± 0.0035 a | 0.0501 ± 0.0040 a | 0.0540 ± 0.0029 a | 0.0567 ± 0.0020 a |
| Denitrification | napA | 0.0152 ± 0.0032 a | 0.0157 ± 0.0010 a | 0.0153 ± 0.0007 a | 0.0158 ± 0.0013 a | 0.0163 ± 0.0023 a |
| Denitrification | napB | 0.0037 ± 0.0010 a | 0.0043 ± 0.0005 a | 0.0040 ± 0.0006 a | 0.0041 ± 0.0008 a | 0.0040 ± 0.0007 a |
| Denitrification | nirK | 0.0624 ± 0.0087 b | 0.0784 ± 0.0065 a | 0.0859 ± 0.0022 a | 0.0857 ± 0.0046 a | 0.0788 ± 0.0026 a |
| Denitrification | nirS | 0.0064 ± 0.0009 b | 0.0080 ± 0.0007 a | 0.0083 ± 0.0002 a | 0.0078 ± 0.0009 a | 0.0087 ± 0.0009 a |
| Denitrification | norB | 0.0259 ± 0.0059 a | 0.0253 ± 0.0013 a | 0.0238 ± 0.0017 a | 0.0239 ± 0.0029 a | 0.0275 ± 0.0009 a |
| Denitrification | norC | 0.0057 ± 0.0015 a | 0.0062 ± 0.0004 a | 0.0059 ± 0.0005 a | 0.0062 ± 0.0008 a | 0.0065 ± 0.0005 a |
| Denitrification | nosZ | 0.0232 ± 0.0037 b | 0.0246 ± 0.0009 ab | 0.0262 ± 0.0012 ab | 0.0254 ± 0.0024 ab | 0.0274 ± 0.0014 a |
| Assimilatory nitrate reduction | narB | 0.0061 ± 0.0019 a | 0.0046 ± 0.0005 b | 0.0053 ± 0.0004 ab | 0.0044 ± 0.0008 b | 0.0044 ± 0.0005 b |
| Assimilatory nitrate reduction | nasA | 0.0406 ± 0.0031 ab | 0.0394 ± 0.0026 ab | 0.0368 ± 0.0033 b | 0.0367 ± 0.0037 b | 0.0427 ± 0.0011 a |
| Assimilatory nitrate reduction | nasB | 0.0012 ± 0.0003 a | 0.0010 ± 0.0001 a | 0.0009 ± 0.0002 a | 0.0010 ± 0.0003 a | 0.0010 ± 0.0001 a |
| Assimilatory nitrate reduction | nirA | 0.0070 ± 0.0006 bc | 0.0081 ± 0.0010 a | 0.0084 ± 0.0005 a | 0.0078 ± 0.0001 ab | 0.0068 ± 0.0006 c |
| Assimilatory nitrate reduction | nasE | 0.0086 ± 0.0047 a | 0.0070 ± 0.0008 a | 0.0078 ± 0.0006 a | 0.0075 ± 0.0013 a | 0.0064 ± 0.0006 a |
| Assimilatory nitrate reduction | nasD | 0.0119 ± 0.0019 a | 0.0100 ± 0.0005 b | 0.0098 ± 0.0005 b | 0.0091 ± 0.0005 b | 0.0104 ± 0.0008 b |
| Dependent Variable | Independent Variable | Contribution of Independent Variable | Significance of Independent Variable | Coefficient of Determination of Full Model | Significance of Full Model |
|---|---|---|---|---|---|
| AD | pH | 68.90% | p < 0.001 | R2 = 0.592 | p < 0.001 |
| AP | 33.00% | p = 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, H.; Cheng, Y.; Zhang, Y.; Li, Y.; Wang, F.; Shen, C.; Chen, B. Vegetable Productivity, Soil Physicochemical and Biochemical Properties, and Microbiome in Response to Organic Substitution in an Intensive Greenhouse Production System. Agriculture 2025, 15, 1493. https://doi.org/10.3390/agriculture15141493
Liu X, Xu H, Cheng Y, Zhang Y, Li Y, Wang F, Shen C, Chen B. Vegetable Productivity, Soil Physicochemical and Biochemical Properties, and Microbiome in Response to Organic Substitution in an Intensive Greenhouse Production System. Agriculture. 2025; 15(14):1493. https://doi.org/10.3390/agriculture15141493
Chicago/Turabian StyleLiu, Xing, Haohui Xu, Yanan Cheng, Ying Zhang, Yonggang Li, Fei Wang, Changwei Shen, and Bihua Chen. 2025. "Vegetable Productivity, Soil Physicochemical and Biochemical Properties, and Microbiome in Response to Organic Substitution in an Intensive Greenhouse Production System" Agriculture 15, no. 14: 1493. https://doi.org/10.3390/agriculture15141493
APA StyleLiu, X., Xu, H., Cheng, Y., Zhang, Y., Li, Y., Wang, F., Shen, C., & Chen, B. (2025). Vegetable Productivity, Soil Physicochemical and Biochemical Properties, and Microbiome in Response to Organic Substitution in an Intensive Greenhouse Production System. Agriculture, 15(14), 1493. https://doi.org/10.3390/agriculture15141493





