Abstract
With increasing concerns over antibiotic resistance in livestock, there is an urgent need for sustainable alternatives to enhance health and productivity in poultry production. Ginger (Zingiber officinale Roscoe), a phytobiotic recognized for its diverse health benefits, including growth promotion and the improvement of intestinal function, was evaluated for its efficacy. This study investigated the effects of standardized ginger extract on gut morphology, microbiota composition, and growth performance in broiler chickens. A total of 200 day-old (Ross 308) broiler chicks were randomly assigned to four dietary groups: a control group receiving a basal diet and three experimental groups receiving a basal diet supplemented with 2.5 g/kg, 5 g/kg, and 10 g/kg of ginger extract. The performance results demonstrated that dietary ginger supplementation at 5 g/kg significantly improved feed efficiency without adversely affecting final body weight (p < 0.01). Feed intake in broilers was significantly reduced by higher doses of ginger extract (p < 0.01). Broiler chickens supplemented with 5 g/kg of ginger exhibited a significantly higher villous height-to-crypt depth ratio in the duodenum and jejunum (p < 0.05). Groups supplemented with 5 g/kg and 10 g/kg of ginger extract demonstrated a significant decrease in the relative abundance of Proteobacteria and an increase in the proportion of Firmicutes (p < 0.05). In conclusion, the addition of ginger extract at 5 g/kg resulted in improved feed efficiency, intestinal morphology, and microbiota composition.
1. Introduction
Recent trends in food animal production emphasize reducing antibiotic use due to the growing threat of antimicrobial-resistant bacteria [1,2]. Consequently, there is a critical need for sustainable alternatives that promote growth and health while maintaining production efficiency. Phytobiotics are plant-derived compounds that have emerged as promising candidates for this role [3]. Unlike antibiotics, phytobiotics do not contribute to antimicrobial resistance or leave harmful residues that may compromise animal welfare and consumer safety [4].
Among phytobiotics, ginger (Zingiber officinale Roscoe) is widely recognized for its therapeutic properties in traditional medicine and is increasingly studied for its applications in animal nutrition [5]. Ginger rhizomes contain over 400 compounds, with major constituents including carbohydrates (50–70%), lipids (3–8%), and terpenes and phenolic compounds such as gingerols, shogaols, gingerdiol, and gingerdione [6]. These bioactive molecules contribute to various biological activities, including enhanced digestive function through the stimulation of salivary and gastric secretions and increased digestive enzyme activity, which collectively improve nutrient digestibility and feed efficiency [7,8].
Additionally, ginger supplementation has been associated with improved intestinal morphology, enhanced nutrient absorption, and the modulation of gut microbiota. It has demonstrated antimicrobial effects against pathogens such as Escherichia coli, Salmonella spp., and Clostridium perfringens, contributing to intestinal homeostasis and better nutrient utilization [9]. Ginger also exhibits notable antioxidant and anti-inflammatory properties, primarily by upregulating endogenous antioxidant enzymes and suppressing pro-inflammatory cytokines [10].
While most animal studies have utilized ginger root powder, ginger extract is hypothesized to offer greater efficiency and practicality due to its lower inclusion rate [10]. However, variability in active compound concentrations and a lack of standardized formulations pose challenges in ensuring consistent results in animal production. Therefore, the present study employed a standardized ginger extract with a chemically defined composition to address these limitations.
The objective of this study was to evaluate the effects of different concentrations of standardized ginger extract on growth performance, intestinal morphology, and microbial composition in broiler chickens.
2. Materials and Methods
2.1. Animals and Experimental Design
Two hundred day-old (Ross 308) broiler chicks, with an average body weight of 44.5 ± 0.52 g, were obtained from a local hatchery and randomly assigned to four dietary treatment groups. Each group consisted of 5 replicates, with 10 animals per replication. The birds were fed different diets as follows: broilers received a basal diet as the control (CON) group and broilers fed with a basal diet supplemented with 2.5 g/kg of standardized ginger extract (GE1 group), 5 g/kg feed (GE2 group), and 10 g/kg (GE3 group). The experiment was designed as a completely randomized block design, with pens serving as the experimental units. Continuous lighting was provided, with ad libitum feeding and unrestricted access to water. The temperature was maintained at 32 °C during the first 7 days and then decreased by 1 °C every 2 days until reaching 24 °C, which was maintained for the remainder of the experiment. All experimental protocols were approved by the Ethics Committee of the Faculty of Veterinary Medicine, University of Zagreb (approval number: Kl.640-01/19-17/31; ur.br. 251-61-44-19-02), and were conducted in accordance with the European Animal Welfare Act (EP 13/2015).
2.2. Diets and Treatment
The feed was formulated according to specifications to meet the nutrient requirements of Ross 308 broiler chickens. All mixtures were prepared as a single batch. The ginger extract was initially blended with a premix and then combined with other ingredients. Afterward, it was stored in covered containers before being fed to the chickens. Broilers were fed a starter feed mixture (days 1–21) and a grower feed mixture (days 22–42). The formulation of the basal diet, along with the calculated chemical composition, is presented in Table 1. Ginger extract was provided by Tilman S. A. (Somme-Leuze, Belgium). The tested preparation is a standardized dietary supplement containing 10.8% biologically active phenolic compounds.

Table 1.
Formulation and chemical analyses of basal broiler diets (as-fed basis).
2.3. Sample Collection
On day 42, 2 broilers per pen (a total of 40 birds) were randomly selected and euthanized via cervical dislocation for sample collection. Intestinal tissue samples (0.5 cm in length) were taken transversely from each intestinal segment for morphometric measurements. The intestinal segment extending from the gizzard to the pancreatic and bile ducts was designated as the duodenum; the jejunum was defined as the segment from the ducts to Meckel’s diverticulum; and the ileum was identified as the segment extending from the diverticulum to the ileocecal junction [11]. Intestinal segments were gently flushed with phosphate-buffered saline (pH 7.4) to remove the digesta and stored in 10% neutral formaldehyde. At the same time, a 3 cm segment of the ileum with digesta was taken, stored in sterile tubes, and placed in a cooled container with dry ice for transport. The digesta samples were kept at –80 °C before DNA extraction for microbiota profiling using a polygenic microarray targeting 16S rRNA gene sequences.
2.4. Performance Analysis
The body weight (BW) of the broilers was measured individually after a 12 h fasting period at the start of the experiment (day 1) and at days 14 and 42 of age. Feed consumption was recorded at a pen level over the same intervals. The feed conversion ratio (FCR) was calculated for the starter feeding (1–21 days), grower feeding (22–42 days), and the entire rearing period (1–42 days).
2.5. Intestinal Morphometry
Fixed samples were processed using the standard technique of paraffin embedding. Cross sections of 5 μm thickness were prepared and subsequently stained with hematoxylin and eosin [12]. Histological preparations of the intestinal tissue samples were examined using a Nikon Microphot-FXA light microscope (Nikon, Tokyo, Japan), and representative fields were photographed with a GXCAM-U3-18 digital camera (GT Vision, Wickhambrook, UK). Images were captured using the GXCapture-T computer program (GT Vision, Wickhambrook, UK). Quantitative morphometric analysis was conducted on the images using the ImageJ software version 1.54a (Bethesda, MD, USA) to measure villus height, crypt depth, and villus surface area. The data was obtained from a mean value of 15 villi per sample (×10). The intestinal villi were measured under a 10x magnification. The height of the villi was determined by measuring the distance from the villus tip to the junction between the villus and the crypt [13]. The surface of the villi was measured by marking the outline of the villi in a computer program. Crypt depth was determined by measuring the distance from the base of the villi to the mucosa [14]. For each bird, measurements were collected from eight villi and crypts per intestinal segment sample and subsequently averaged to represent individual values. Finally, the average heights of intestinal villi, each calculated from eight measurements per bird, were averaged across 10 birds to represent the group mean values. The villi height-to-crypt depth ratio was calculated from the measured values.
2.6. Microbiome Analysis of Ileal Digesta
For DNA extraction from ileal digesta samples, the GenElute™ Kit (Sigma-Aldrich, Burlington, MA, USA) was used. The concentration and purity of genomic DNA, extracted using the GenElute kit, were assessed by spectrophotometric analysis performed on a BioDrop microvolume spectrophotometer (Harvard Bioscience Inc., Holliston, MA, USA).
PCR amplification of the V4 domain of the bacterial 16S rRNA gene was performed using specific primers. The PCR products were extracted using 2% agarose gel electrophoresis, mixed in equal amounts, and purified with the GeneJET Gel Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA). The library was built using the Ion Plus Fragment kit (Thermo Fisher Scientific). After quantification using the Qubit 2.0 fluorometer (Thermo Fisher Scientific) and library testing, the built library was sequenced on the Illumina PE250 sequencing platform at Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). Paired-end reads were merged using FLASH (v1.2.7) [15]. Quality filtering of the raw tags was conducted under specific conditions to obtain high-quality, clean tags [16], following the quality control process of Qiime (v1.7.0) [17]. The tags were compared against the SILVA 138 reference database using the UCHIME algorithm [18] for the detection of chimera sequences. Identified chimeras were removed [19], and the resulting sequences were defined as Effective Tags. These sequences were then clustered into operational taxonomic units (OTUs) based on 97% sequence similarity using the Uparse software (v7.0.1090) [18]. Sequences showing ≥97% similarity were assigned to the same OTU. A representative sequence from each OTU was selected for subsequent annotation. The alpha diversity of the gut microbiota under different dietary treatments was assessed using four indices: observed species, Chao1, Shannon, and Simpson.
The diversity of bacterial species within different groups was assessed through taxonomic analysis of OTUs. Moreover, the composition at the phylum and genus levels was analyzed to explain the structural differences between the various groups. To analyze the diversity in this study, the α-diversity microorganism species wealth index (Chao1) and the microbial community diversity index (Shannon) were used. The higher the Chao1 index, the greater the richness of species in the sample, while a higher Shannon index indicates a greater diversity of microbial communities.
2.7. Statistical Analysis
Data on performance, gut morphology, and microbiota composition and diversity were analyzed using Statistica version 10.0 (StatSoft Inc., Tulsa, OK, USA). All results were consolidated at the treatment and replication levels and presented as mean values with a unified standard deviation. The dependent variables were tested by ANOVA, and Tukey’s test was used to calculate statistical differences between groups. The differences were considered significant at p ≤ 0.05.
3. Results
3.1. Performance Indices
The performance indices of broiler chickens supplemented with ginger extract are summarized in Table 2. Significant differences in body weight were observed between individual treatments on the 21st day of the study. The GE2 group of chickens achieved the highest body weight compared to the other groups. A significant difference (p < 0.02) in body weight was observed between all groups and the GE3 group on day 21 of the study. On the 42nd day of the study, the highest body weight (p < 0.003) was recorded in groups CON and GE2 compared to groups GE1 and GE3. Between days 1 and 21, a significant increase (p < 0.05) in weight gain was observed in group GE2 compared to group GE3. During the period from day 22 to day 42, higher weight gain (p < 0.04) was exhibited by both the CON and GE2 groups compared to GE3. Overall, body weight gain was significantly higher in CON, GE1, and GE2 compared to GE3 (p < 0.03). Higher feed intake (p < 0.02) was recorded in groups CON and GE1 compared to group GE3 during the first three weeks of the trial. Between days 22 and 42, higher feed intake (p < 0.001) was observed in groups CON and GE1 compared to groups GE2 and GE3. Throughout the entire study period (days 1–42), the highest feed intake was recorded in the control group, with groups CON and GE1 exhibiting significantly higher (p < 0.001) feed intake than groups GE2 and GE3. Feed intake showed a near-linear decrease with increasing ginger extract concentration throughout the trial. During the entire study period, the lowest feed conversion ratio was observed in chickens from the GE2 group compared to all other groups (p < 0.01).

Table 2.
Production performance of broiler chickens supplemented with ginger extract.
3.2. Quantitative Measurements of Intestinal Tissue
Feeding broiler chickens with ginger extract at different concentrations affected the morphometric parameters of the small intestine, as shown in Table 3. On day 42 of the study, significantly shorter duodenal villi were observed in the GE1 group compared to all other groups (p < 0.01). The greatest crypt depth was recorded in the control group compared to the GE1 and GE3 groups (p < 0.05). A significantly larger duodenal villus surface area (p < 0.05) was measured in the control group than in groups GE1 and GE3. The ratio between villus height and crypt depth was significantly higher (p < 0.03) in groups GE2 and GE3 than in the control and GE1 groups. In the jejunum, significantly longer villi were found in the GE2 group compared to the CON and GE3 groups (p < 0.05). No statistically significant differences were observed in crypt depth or villus surface area among the groups. A significantly higher villus height-to-crypt depth ratio was recorded in the GE2 group compared to all other groups (p < 0.05). In the ileum, significantly greater crypt heights were measured in groups GE1 and GE3 compared to the GE2 group (p < 0.05). A significantly larger jejunal villus surface area was found in the control group compared to groups GE1 and GE2 (p < 0.05).

Table 3.
Histomorphometric parameters of the small intestine.
3.3. Microbiome Composition and Diversity
The composition of the gut microbiota at the phylum and genus levels is presented in Figure 1A. The predominant phyla were identified as Firmicutes, Proteobacteria, Actinobacteria, Campylobacteria, and Bacteroidetes, accounting for 62.4%, 22.9%, 2.65%, 2.03%, and 1.43% of the total sequences, respectively (Figure 2A). In the GE2 and GE3 groups, a significant decrease (p < 0.05) in the relative abundance of Proteobacteria and a significant increase (p < 0.05) in the proportion of Firmicutes were recorded compared to the control group. Additionally, a significant reduction (p < 0.05) in the relative abundance of Campylobacterota and Bacteroidota was observed in these groups. More than 30 genera were identified across all samples; however, only the 10 most abundant are displayed in the histogram in Figure 1B. In the control group, the dominant bacterial communities comprised the genera Pseudomonas, Termoanaerobacterium, Candidatus Arthromitus, and Lactobacillus. In the groups receiving ginger extract, a significant increase (p < 0.05) in the relative abundance of the genera Candidatus Arthromitus and Romboutsia, as well as a significant decrease (p < 0.05) in the genera Pseudomonas and Termoanaerobacterium, was observed. Furthermore, in group GE2, a significantly higher (p < 0.05) representation of bacteria of the genus Escherichia–Shigella was recorded compared to the other groups. In group GE3, significantly higher (p < 0.05) abundances of bacteria of the genera Enterococcus and Lactobacillus were detected compared to all other groups in the study.
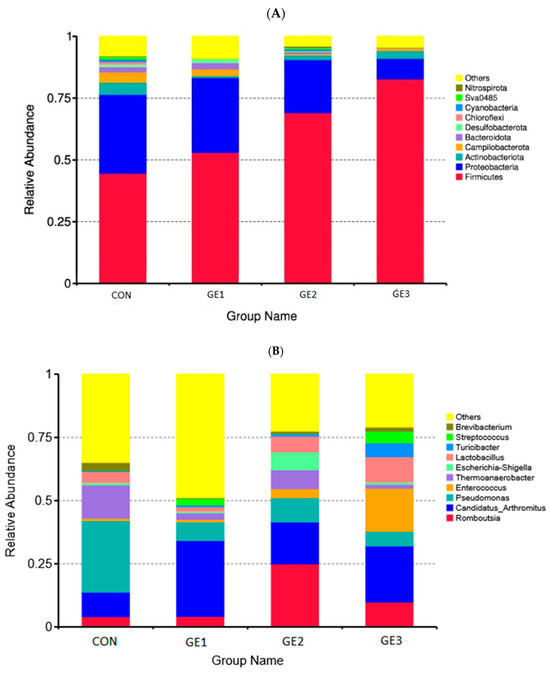
Figure 1.
Composition of the gut microbiota at the phylum level (A) and genus level (B). CON (basal diet), GE1, GE2, and GE3 (ginger extract at 2.5, 5, and 10 g/kg feed).
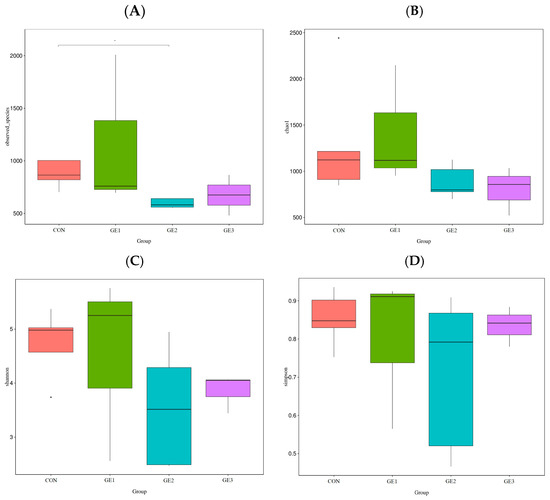
Figure 2.
Impact of ginger extract supplementation on alpha diversity indices of broilers: (A) observed species, (B) Chao 1 index, (C) Shannon index, (D) Simpson index. CON (basal diet), GE1, GE2, and GE3 (ginger extract at 2.5, 5, and 10 g/kg feed).
The structural modulation of the small intestine microbiota is represented by alpha diversity indices (Figure 2). The Shannon index is used as an indicator of bacterial population diversity, while richness is indicated by the Chao1 index. The highest Shannon index value (Figure 2B) and the greatest richness of bacterial OTUs according to the Chao1 index (Figure 2C) were achieved by the GE1 group. In contrast, the lowest diversity was observed in the GE2 group. However, no statistically significant changes (p ≥ 0.05) in the Shannon and Chao1 indices were detected in the ileal microbiome of broilers fed ginger extract compared to the control group. Nevertheless, a significant decrease in observed species was induced by the ginger extract in the GE2 group compared to the control group (Figure 2A).
4. Discussion
4.1. Production Performance
Growth performance in broiler production is a critical parameter for assessing efficiency and economic viability, as it reflects the cumulative effects of numerous internal and external factors, including genetics, feeding strategies, environmental conditions, health status, and farm management practices. In the present study, the effects of ginger extract supplementation at varying concentrations on performance parameters were evaluated. Notable differences in body weight dynamics were observed on days 1, 21, and 42 of the trial.
Although body weights were uniform across groups on day 1, a significantly lower body weight was observed in the GE3 group on day 21. This trend persisted through today 42, resulting in the lowest final body weight among all treatment groups. These findings align with previous reports indicating a negative impact of ginger on weight gain at inclusion levels ranging from 6 to 60 g/kg [8,20,21]. When body weight was analyzed in relation to feed intake, a significant reduction in feed consumption was noted in the GE3 group by day 21, continuing into the second feeding phase, suggesting that the lower final body weight may be due to reduced feed intake.
The situation observed in the GE1 group is somewhat more complex to explain. This group, which was administered the lowest dose of the tested preparation, was nevertheless found to have a significantly lower final body weight. No difference in body weight was observed on day 21; however, during the second phase of fattening, weight gain was reduced compared to the control and GE2 groups, despite feed intake not being diminished. Conversely, a final body weight comparable to that of the control group was achieved by the GE2 group, despite significantly lower feed intake during both phases of fattening. These results are consistent with findings reported by several authors, where no correlation was found between ginger supplementation and increased body weight in broilers [22,23,24,25,26]. However, some studies have reported positive effects of ginger supplementation on broiler body weight [27,28,29]. According to these reports, gingerol, one of the active components, is believed to stimulate the secretion of digestive enzymes, thereby enhancing the digestive process and promoting the utilization of ingested nutrients. The pancreas is stimulated to produce greater numbers of digestive enzymes, resulting in increased nutrient digestibility and absorption, which supports higher growth rates [30].
Regarding broiler feed intake, it was immediately observed that a significant adverse effect on consumption was caused by the tested preparation, particularly as the dosage increased. Lower feed intake was recorded in all experimental groups compared to the control group, although the difference in the GE1 group was not statistically significant. These results are consistent with those reported by Incharoen et al. [13], where a similar pattern was observed in a study using dry fermented ginger, which caused a dose-dependent decrease in feed consumption; however, unlike in our study, no reduction in finalbody weight was reported. A possible explanation for this may be attributed to the rduced villous height-to-crypt depth ratio observed across all intestinal segments of the GE1 group, which leads to a decrease in nutrient absorption. The negative impact of high doses of ginger on feed intake was also noted by Herawati et al. [24], where a significant reduction in feed intake was reported at 20 g/kg, although no adverse effect on final broiler weight was observed. A similar trend was observed in a study by Al-Khalaifah et al. [7], in which increasing doses of ginger (5, 10, and 15 g/kg) were associated with reduced feed intake in broiler chickens but did not negatively affect body weight gain. Contrary to these findings, significantly lower feed intake and final body weight were reported by Zomrawi et al. [31] in broilers receiving the lowest ginger dose (5 g/kg), while no significant differences were observed at higher doses (10 and 15 g/kg). In general, it has been concluded by most studies that no significant effect on feed consumption is exerted by ginger supplementation [13,22,23,26,32,33], although a smaller number of studies have reported a positive effect on feed consumption [27,34,35,36].
Feed utilization is considered one of the key parameters that best describe the efficiency of animal production. Since feed accounts for the largest single cost for producers, production costs can be significantly reduced by improving feed utilization, especially during periods of substantial raw material price fluctuations. In this study, a significant improvement in feed conversion was observed in broilers from the GE2 group compared to all other groups. This finding is consistent with results reported by several authors [13,28,34,35,37], where lower feed conversion ratios were also observed in broilers administered similar doses of ginger (5 g/kg). The improvement in feed efficiency is thought to be attributable to the stimulation of gastric secretions and salivary gland activity. This stimulation is believed to lead to a reduction in pathogenic microorganisms and an overall decrease in microbial fermentation in the gut, resulting in enhanced digestive and absorptive capacity [13,32,38,39]. Conversely, a negative effect on feed utilization was observed only when the supplement was administered at extremely high concentrations (30 g/kg) [40]. In contrast, no significant effect of ginger supplementation on feed utilization has been reported in other studies [22,23,26,33].
4.2. Gut Morphometry
To explain the influence of ginger supplementation on the morphometric parameters of the chicken small intestine, it is essential to consider that the chemical composition of the diet is a key factor affecting the intestinal structure and, consequently, its absorptive capacity. This, in turn, ultimately influences the growth performance of broilers [41]. Intestinal villi are known to adapt rapidly to changes in the luminal environment, which is largely shaped by dietary composition. Accordingly, increased villus height is associated with an expanded absorptive surface area and enhanced nutrient absorption capacity [42]. The crypt region is responsible for producing enterocytes that migrate from the base to the tip of the villi, replacing senescent cells that are shed into the lumen [43]. These epithelial cells are continuously and rapidly renewed, a process critical for maintaining normal intestinal function [44]. Villus height (VH) and the villus height-to-crypt depth ratio (VH:CD) are widely used as positive indicators of intestinal mucosal integrity, whereas crypt depth (CD) alone is often considered a negative indicator of gut health. Longer villi are linked to greater mucosal digestive enzyme activity [45], and a higher VH:CD ratio is typically associated with improved nutrient absorption. In contrast, deeper crypts suggest poorer absorptive function, as they reflect increased epithelial turnover. A deeper crypt may also indicate accelerated regeneration of the intestinal lining [46]. Furthermore, shorter villi coupled with deeper crypts have been associated with a reduced number of absorptive cells and an increased proportion of secretory cells, which leads to elevated mucin secretion. Alterations in the quantity or composition of mucin on the intestinal mucosal surface can impair nutrient absorption and may increase the energy required to sustain intestinal function [41].
In the present study, the effects of ginger supplementation on histological parameters were observed in multiple segments of the small intestine. In the duodenum, a significant decrease in villus height was recorded in the GE1 group compared to the control group, accompanied by a reduction in crypt depth. A significant decrease in villus surface area was also noted in all treated groups relative to the control. However, a significantly higher villus height-to-crypt depth (VH:CD) ratio was observed in the GE2 and GE3 groups compared to the control.
In the jejunum, villus height was significantly increased in the GE2 group, while crypt depth remained unchanged compared to the control group. Similarly, the VH:CD ratio was significantly higher in the GE2 group. In the ileum, no significant differences in villus height were observed among the groups. However, a smaller villus surface area was recorded in the GE1 and GE2 groups, with no significant differences in the VH:CD ratio.
The present findings are in partial agreement with those reported by Karangiya et al. [47], where increases in villus height and crypt depth were observed following ginger administration. Similarly, Shewita and Taha [8] reported longer villi and a higher villus height-to-crypt depth (VH:CD) ratio in all broiler groups fed ginger at inclusion levels ranging from 2 to 6 g/kg. In the current study, positive morphological changes were observed in the duodenum and jejunum of the GE2 group. Specifically, the duodenum exhibited elongated villi and significantly reduced crypt depth, while the jejunum displayed increased villus height and an improved VH:CD ratio, findings that are consistent with enhanced feed efficiency and improved small intestinal function.
In contrast, other studies have reported improved performance in broilers without significant changes in villus height or surface area. However, the increased surface area of duodenal epithelial cells and enhanced mitotic activity within the crypts were attributed to ginger supplementation [13]. Conversely, no beneficial effects on intestinal morphometry were observed in broiler chickens exposed to heat stress, as reported by other authors [25].
4.3. Microbiome
The gut microbiome, which comprises billions of microorganisms, is recognized as a complex ecosystem mediating the interaction between the host and its environment [48]. The gut microbiome plays an important role in maintaining the health status of animals [49]. It is closely related to digestion, absorption, and metabolism, as well as immunity and susceptibility to disease [50]. In addition, the microbiome influences the fermentation of carbohydrates, especially polysaccharides, and thus promotes the absorption of nutrients and energy supply in the animal organism [51]. Although the therapeutic use of antibiotics is effective in combating bacterial infections, it can lead to an imbalance in the intestinal microbiome. This can result in the destruction of gut bacteria and trigger an inflammatory response, leading to increased levels of anti-inflammatory cytokines [52]. As phytobiotics generally do not induce bacterial resistance and do not trigger an inflammatory response, their use is particularly important to maintain the structural stability and diversity of the intestinal microbiota.
It has been suggested by previous research that gut health benefits from a high diversity of bacterial populations, as competition for resources and colonization between rich microbial communities reduces the likelihood of pathogen proliferation and subsequent infection [53]. In the present study, there was no statistically significant difference in the diversity or richness of bacterial genera in the ileum of chickens fed ginger extract. This stability in microbiome diversity suggests that ginger extract does not disrupt microbial equilibrium. It is believed by some authors that the dynamics of the gut microbiota are altered in response to changes in diet and age [54], whereas it is argued by others [55] that the microbiome is influenced by age rather than dietary treatment.
The structure of the gut microbiota in broiler chickens, analyzed at the phylum level, revealed nearly linear changes across all treatments. An increase in the relative abundance of Firmicutes was observed, which showed a positive correlation with the administered ginger dose, while the proportion of Proteobacteria simultaneously decreased. Based on previous studies, Firmicutes have been identified as key contributors to butyric acid production, which serves as an energy source for the growth and maintenance of intestinal epithelial cells [56]. The ratio of Firmicutes to Bacteroidetes has been shown to play an essential role in nutrient absorption and intestinal homeostasis in poultry [57]. An elevated proportion of Firmicutes has been associated with the suppression of pathogenic bacteria, the restoration of intestinal homeostasis, and improved nutrient absorption. Conversely, an increased abundance of Bacteroidetes has been linked to impaired nutrient uptake and microbial dysbiosis [58]. Previous research has indicated that a high relative abundance of Proteobacteria is a marker of intestinal imbalance, with elevated levels reflecting stunted growth or an unstable microbiota composition [59]. Furthermore, Proteobacteria include several zoonotic pathogens, such as Escherichia, Salmonella, and Campylobacter, along with other clinically significant genera [60,61]. In the present study, the inclusion of ginger extract significantly reduced the relative abundance of harmful Proteobacteria in the GE2 and GE3 groups, contributing to the maintenance of a more balanced gut microbiota at the phylum level.
The addition of ginger extract also changed the structure of the microbiota at the genus level. In chickens fed with ginger extract, we observed an increase in the relative abundance of bacteria of the genera Candidatus Arthromitus and Romboutsia and a decrease in the genera Pseudomonas and Termoanaerobacterium. In the GE3 group, a higher representation of bacteria of the genera Enterococcus and Lactobacillus was observed compared to all other groups in the study. An increase in bacteria of the genus Candidatus Arthromitus was observed in all test groups, but particularly in the GE1 group. Candidatus Arthromitus belongs to a group of segmented filamentous bacteria that are a unique group of commensals within the Lachnospiraceae family [62]. These bacteria are characterized by their ability to adhere to the intestinal epithelium of the ileum and modulate the host’s immune system [63]. A decrease in bacteria of this genus has been observed in various pathological conditions of broilers, such as necrotic enteritis caused by E. maxima and C. perfringens [64]. A significant increase in bacteria from the genus Romboutsia was observed in groups GE2 and GE3. These are commensal bacteria [65] that are involved in the metabolic reactions of the host, including the utilization of carbohydrates, fermentation of individual amino acids, anaerobic respiration, and the production of end products from the metabolic process [66]. These bacteria serve as valuable intestinal biomarkers, as they play a crucial role in maintaining the health status of the host [67].
Our results are in agreement with those of the authors who reported an increase in this bacterial genus in broilers fed with the probiotic Bacillus Subtilis [68]. In all groups of broilers receiving the tested preparation, a significant reduction in bacteria of the genus Pseudomonas was observed compared to the control group. In previous studies, an increased proportion of Pseudomonas bacteria was observed in the intestines of immunocompromised animals due to bacterial infection [57]. In the GE2 group, an increased proportion of bacteria of the genus Escherichia–Shigella was observed. This is somewhat surprising considering that this group achieved the best production results and recorded no mortality during the trial. An increased level of bacteria of this genus can lead to colibacillosis in poultry and has been observed in the microbiome of broilers where E. coli infection was caused [69]. Similar results were obtained by Qorbanpour et al. [23] in the ileal contents of broilers. A significantly higher proportion of bacteria of the genus Lactobacillus was found in the GE3 group compared to the other groups. This is consistent with authors who have tested ginger preparations in broilers [70,71]. The increase in the proportion of bacteria of the genus Lactobacillus is considered favorable due to its positive effect on the intestinal immune system [72] and the stimulation of bacteria that use lactate and produce butyrate [73]. In addition, a significantly higher proportion of bacteria of the genus Enterococcus belonging to probiotic species such as Lactobacillus, Bacillus, Bifidobacterium, Streptococcus, and Faecalibacterium was observed in the same group, which has a positive effect on the health and performance of broilers [74].
5. Conclusions
The incorporation of ginger extract at a concentration of 5 g/kg was found to improve feed efficiency and the morphometric characteristics of the small intestine. Importantly, the evaluated additive did not disrupt microbial homeostasis and was shown to significantly support the maintenance of a favorable intestinal microbiota. Based on these findings, this inclusion rate can be considered effective and potentially advantageous in broiler nutrition.
Author Contributions
Conceptualization and design, M.Đ.J. and H.V.; data curation and formal analysis, M.B. and S.V.; funding acquisition, M.S., investigation, Ž.G. and M.Đ.J.; methodology, I.Ž.Ž.; supervision, H.V.; writing, M.Đ.J. and H.V.; writing—review and editing, D.Đ. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Zagreb, Short-term scientific support No. 576/2023.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Veterinary Faculty, University of Zagreb (No. Kl.640-01/19-17/31; ur.br. 251-61-44-19-02).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to patent protection.
Conflicts of Interest
Author Martina Đuric Jaric was employed by the company Krka-Farma d.o.o. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ME | Metabolic Energy |
| BW | Body Weight |
| FCR | Feed Conversion Ratio |
| BWG | Body Weight Gain |
| FI | Feed Intake |
| OTU | Operational Taxonomic Unit |
| VH:CD | Villous Height-to-Crypt Depth Ratio |
References
- Bava, R.; Castagna, F.; Lupia, C.; Poerio, G.; Liguori, G.; Lombardi, R.; Naturale, M.D.; Mercuri, C.; Bulotta, R.M.; Britti, D.; et al. Antimicrobial Resistance in Livestock: A Serious Threat to Public Health. Antibiotics 2024, 13, 551. [Google Scholar] [CrossRef]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Vet. J. 2020, 73, 2. [Google Scholar] [CrossRef]
- Obianwuna, C.X.; Oleforuh-Okoleh, V.U.; Onu, P.N.; Zhang, H.; Qiu, K.; Wu, S. Phytobiotics in poultry: Revolutionizing broiler chicken nutrition with plant-derived gut health enhancers. J. Anim. Sci. Biotechnol. 2024, 15, 169. [Google Scholar] [CrossRef]
- Suresh, G.; Das, R.K.; Kaur Brar, S.; Rouissi, T.; Avalos Ramirez, A.; Chorfi, Y.; Godbout, S. Alternatives to antibiotics in poultry feed: Molecular perspectives. Crit. Rev. Microbiol. 2018, 4, 318–335. [Google Scholar] [CrossRef]
- Kizhakkayil, J.; Sasikumar, B. Diversity, characterization and utilization of ginger: A review. Plant Genet. Resour. 2011, 9, 464–477. [Google Scholar] [CrossRef]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.; Al-Nasser, A.; Al-Surrayai, T.; Sultan, H.; Al-Attal, D.; Al-Kandari, R.; Al-Saleem, H.; Al-Holi, A.; Dashti, F. Effect of Ginger Powder on Production Performance, Antioxidant Status, Hematological Parameters, Digestibility, and Plasma Cholesterol Content in Broiler Chickens. Animals 2022, 12, 901. [Google Scholar] [CrossRef]
- Shewita, R.S.; Taha, A.E. Influence of dietary supplementation of ginger powder at different levels on growth performance, haematological profiles, slaughter traits and gut morphometry of broiler chickens. S. Afr. J. Anim. Sci. 2018, 48, 997–1008. [Google Scholar] [CrossRef]
- Dosu, G.; Obanla, T.O.; Zhang, S.; Sang, S.; Adetunji, A.O.; Fahrenholz, A.C.; Ferket, P.R.; Nagabhushanam, K.; Fasina, Y.O. Supplementation of ginger root extract into broiler chicken diet: Effects on growth performance and immunocompetence. Poult. Sci. 2023, 102, 10. [Google Scholar] [CrossRef]
- An, S.; Liu, G.; Guo, X.; An, Y.; Wang, R. Ginger extract enhances antioxidant ability and immunity of layers. Anim. Nutr. 2019, 5, 407–409. [Google Scholar] [CrossRef]
- Yamauchi, K.; Buwjoom, T.; Koge, K.; Ebashi, T. Histological alterations of the intestinal villi and epithelial cells in chickens fed dietary sugar cane extract. Br. Poult. Sci. 2006, 47, 544–553. [Google Scholar]
- Bancroft, J.; Steven, A.; Turner, D.R. Theory and Practice of Histological Techniques, 4th ed.; Churchill Livingstone: Edinburgh, UK, 1996. [Google Scholar]
- Incharoen, T.; Yamauchi, K.; Thongwittaya, N. Intestinal villus histological alterations in broilers fed dietary dried fermented ginger. J. Anim. Physiol. Anim. Nutr. 2010, 94, 130–137. [Google Scholar] [CrossRef]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar]
- Caporaso, J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Ademola, S.; Farinu, G.; Babatunde, G. Serum lipid, growth and haematological parameters of broilers fed garlic, ginger and their mixtures. World J. Agric. Sci. 2009, 5, 99–104. [Google Scholar]
- Al-Homidan, A.A. Efficacy of using different sources and levels of Allium sativum and Zingiber officinale on broiler chicks performance. Saudi J. Biol. Sci. 2005, 12, 96–102. [Google Scholar]
- Barazesh, H.; Boujar Pour, M.; Salari, S.; Abadi, T.M. The effect of ginger powder on performance, carcass characteristics and blood parameters of broilers. Int. J. Adv. Biomed. Res. 2013, 1, 1645–1651. [Google Scholar]
- Qorbanpour, M.; Fahim, T.; Javandel, F.; Nosrati, M.; Paz, E.; Seidavi, A.; Ragni, M.; Laudadio, V.; Tufarelli, V. Effect of Dietary Ginger (Zingiber officinale Roscoe) and Multi-Strain Probiotic on Growth and Carcass Traits, Blood Biochemistry, Immune Responses and Intestinal Microflora in Broiler Chickens. Animals 2018, 8, 117. [Google Scholar] [CrossRef]
- Herawati, O. The effect of red ginger as phytobiotic on body weight gain, feed conversion and internal organs condition of broiler. Int. J. Poult. Sci. 2010, 9, 963–967. [Google Scholar]
- Khonyoung, D.; Yamauchi, K.; Buwjoom, T.; Maneewan, B.; Thongwittaya, N. Effects of dietary dried fermented ginger on growth performance, carcass quality, and intestinal histology of heat-stressed broilers. Can. J. Anim. Sci. 2012, 92, 307–317. [Google Scholar] [CrossRef]
- Zhang, G.F.; Yang, Z.B.; Wang, Y.; Yang, W.R.; Jiang, S.Z.; Gai, G.S. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 2009, 88, 2159–2166. [Google Scholar] [CrossRef]
- Zidan, D.E.; Kahilo, K.A.; El-Far, A.H.; Sadek, K.M. Ginger (Zingiber officinale) and thymol dietary supplementation improve the growth performance, immunity and antioxidant status in broilers. Global Vet. 2016, 16, 530–538. [Google Scholar]
- Rio, T.; Vidyarthi, V.K.; Zuyie, R. Effect of dietary supplementation of ginger powder (Zingiber officinale) on performance of broiler chicken. Livest. Res. Int. 2019, 7, 125–131. [Google Scholar]
- Javid, M.A.; Waqas, Y.; Akhtar, M.S. Evaluation of the Comparative Effect of Feed Additive of Allium Sativum and Zingiber officinale on Bird Growth and Histomorphometric Characteristics of Small Intestine in Broilers. Braz. J. Poult. Sci. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Risdianto, D.; Suthama, N.; Suprijatna, E.; Sunarso, S. Inclusion effect of ginger and turmeric mixture combined with Lactobacillus spp. isolated from rumen fluid of cattle on health status and growth of broiler. J. Indones. Trop. Anim. Agric. 2019, 44, 423. [Google Scholar] [CrossRef]
- Zomrawi, W.B.; Abdelatti, K.H.A.; Dousa, B.M.; Mahala, A.G. The effect of ginger root powder (Zingiber officinale) supplementation on broiler chick performance, blood and serum constituents. J. Anim. Feed Res. 2012, 1, 457–460. [Google Scholar]
- Onu, P.N. Evaluation of two herbal spices as feed additives for finisher broilers. Biotechnol. Anim. Husb. 2010, 26, 383–392. [Google Scholar] [CrossRef]
- Habibi, R.; Sadeghi, G.H.; Karimi, A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br. Poult. Sci. 2014, 55, 228–237. [Google Scholar] [CrossRef]
- George, O.S.; Kaegon, S.G.; Igbokwe, A.A. Effects of graded levels of ginger (Zingiber officinale) meal as feed additive on growth performance characteristics of broiler chicks. Int. J. Sci. Res. 2015, 4, 805–808. [Google Scholar]
- Asghar, M.U.; Rahman, A.; Hayat, Z.; Rafique, M.K.; Badar, I.H.; Yar, M.K.; Ijaz, M. Exploration of Zingiber officinale effects on growth performance, immunity and gut morphology in broilers. Braz. J. Biol. 2021, 83, e250296. [Google Scholar] [CrossRef]
- Tekeli, A.; Kutlu, H.R.; Celik, L. Effect of Z. offincinale and propolis extracts on the performance, carcass and some blood parameters of broiler chicks. Cur. Res. Poult. Sci. 2011, 1, 12–23. [Google Scholar] [CrossRef]
- Ebrahimnezhad, Y.; Azarakhsh, V.; Salmanzadeh, M. The effects of ginger root (Zingiber officiale) processed to different levels on growth performance, carcass characteristics and blood biochemistry parameters in broiler chickens. Bull. Environ. Pharm. Life Sci. 2014, 3, 203–208. [Google Scholar]
- Greathead, H. Plants and plant extracts for improving animal productivity. Proc. Nutr. Soc. 2003, 62, 279–290. [Google Scholar] [CrossRef]
- Incharoen, T.; Yamauchi, K. Production performance, egg quality and intestinal histology in laying hens fed dietary dried fermented ginger. Int. J. Poult. Sci. 2009, 8, 1078–1085. [Google Scholar] [CrossRef]
- Huthail Najib, H.; Al-Homidan, I.; Fathi, M.M.; Al-Suhim, A.A. Black seeds (Nigella sativa) and ginger powder (Zingiber officinale) effect on growth performance and immune response of broiler chickens. Asian J. Anim. Sci. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Hamedi, S.; Rezaian, M.; Shomali, T. Histological changes of small intestinal mucosa of cocks due to sunflower meal single feeding. Am. J. Anim. Vet. Sci. 2011, 6, 171–175. [Google Scholar]
- Izadi, H.; Arshami, J.; Golian, A.; Reza Raji, M. Effects of chicory root powder on growth performance and histomorphometry of jejunum in broiler chicks. Vet. Res. Forum 2013, 4, 169–174. [Google Scholar]
- Uni, Z.; Gal-Garber, O.; Geyra, A.; Sklan, D.; Yahav, S. Changes in growth and function of chick small intestine epithelium due to early thermal conditioning. Poult. Sci. 2001, 80, 438–445. [Google Scholar] [CrossRef]
- Thomson, A.B.R.; Keelan, M.; Thiesen, A.; Clandinin, M.T.; Ropeleski, M.; Wild, G.E. Small bowel review diseases of the small intestine. Dig. Dis. Sci. 2001, 46, 2555–2566. [Google Scholar] [CrossRef]
- Heak, C.; Sukon, P.; Kongpechr, S.; Tengjaroenkul, B.; Chuachan, K. Effect of Direct-fed Microbials on Intestinal Villus Height in Broiler Chickens: A Systematic Review and Meta-Analysis of Controlled Trials. Int. J. Poult. Sci. 2017, 16, 403–414. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar]
- Karangiya, V.K.; Savsani, H.H.; Patil, S.S.; Garg, D.D.; Murthy, K.S.; Ribadiya, N.K.; Vekariya, S.J. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World 2016, 9, 245–250. [Google Scholar] [CrossRef]
- Blum, H.E. The human microbiome. Adv. Med. Sci. 2017, 62, 414–420. [Google Scholar] [CrossRef]
- Grond, K.; Sandercock, B.; Jumpponen, A.; Zeglin, L.H. The avian gut microbiota: Community, physiology and function in wild birds. J. Avian Biol. 2018, 49, e1788. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Temmerman, R.; Pelligand, L.; Schelstraete, W.; Antonissen, G.; Garmyn, A.; Devreese, M. Enrofloxacin Dose Optimization for the Treatment of Colibacillosis in Broiler Chickens Using a Drinking Behaviour Pharmacokinetic Model. Antibiotics 2021, 10, 604. [Google Scholar] [CrossRef]
- Huyben, D.; Vidakovic, A.; Hallgren, S.W.; Langeland, M. High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens). Aquaculture 2019, 500, 485–491. [Google Scholar] [CrossRef]
- Isaacson, R.; Kim, H.B. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012, 13, 100–109. [Google Scholar] [CrossRef]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef]
- Peng, L.; Shi, H.; Gong, Z.; Yi, P.; Tang, B.; Shen, H.; Fu, B. Protective effects of gut microbiota and gut microbiota-derived acetate on chicken colibacillosis induced by avian pathogenic Escherichia coli. Vet. Microbiol. 2021, 261, 109187. [Google Scholar] [CrossRef]
- Oh, J.K.; Pajarillo, E.A.B.; Chae, J.P.; Kim, I.H.; Kang, D.K. Protective effects of Bacillus subtilis against Salmonella infection in the microbiome of Hy-Line Brown layers. Asian-Australasian. J. Anim. Sci. 2017, 30, 1332–1339. [Google Scholar] [CrossRef]
- Adalsteinsdottir, S.A.; Magnusdottir, O.K.; Halldorsson, T.I.; Birgisdottir, B.E. Towards an individualized nutrition treatment: Role of the gastrointestinal microbiome in the interplay between diet and obesity. Curr. Obes. Rep. 2018, 7, 289–293. [Google Scholar] [CrossRef]
- Pedroso, A.A.; Menten, J.F.M.; Lambais, M.R. The Structure of Bacterial Community in the Intestines of Newly Hatched Chicks. J. Appl. Poult. Res. 2005, 14, 232–237. [Google Scholar] [CrossRef]
- Salaheen, S.; Kim, S.W.; Haley, B.J.; Van Kessel, J.A.S.; Biswas, D. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front. Microbiol. 2017, 8, 2088. [Google Scholar] [CrossRef]
- Clavijo, V.; Florez, M. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Thompson, C.L.; Vier, R.; Mikaelyan, A.; Wienemann, T.; Brune, A. Candidatus Arthromitus revised: Segmented filamentous bacteria in arthropod guts are members of Lachnospiraceae. Environ. Microbiol. 2012, 14, 1454–1465. [Google Scholar] [CrossRef]
- Thompson, C.L.; Mikaelyan, A.; Brune, A. Immune-modulating gut symbionts are not “Candidatus Arthromitus”. Mucosal Immunol. 2013, 6, 200–201. [Google Scholar] [CrossRef]
- Kim, J.E.; Lillehoj, H.S.; Hong, Y.H.; Kim, G.B.; Lee, S.H.; Lillehoj, E.P.; Bravo, D.M. Dietary Capsicum and Curcuma longa oleoresins increase intestinal microbiome and necrotic enteritis in three commercial broiler breeds. Res. Vet. Sci. 2015, 102, 150–158. [Google Scholar] [CrossRef]
- Magruder, M.; Edusei, E.; Zhang, L.; Albakry, S.; Satlin, M.J.; Westblade, L.F.; Malha, L.; Sze, C.; Lubetzky, M.; Dadhania, D.M. Gut commensal microbiota and decreased risk for Enterobacteriaceae bacteriuria and urinary tract infection. Gut Microbes 2020, 12, 1805281. [Google Scholar] [CrossRef]
- Gerritsen, J.; Hornung, B.; Ritari, J.; Paulin, L.; Rijkers, G.T.; Schaap, P.J.; De Vos, W.M.; Smidt, H. A comparative and functional genomics analysis of the genus Romboutsia provides insight into adaptation to an intestinal lifestyle. bioRxiv 2019, bioRxiv 845511. [Google Scholar] [CrossRef]
- Mangifesta, M.; Mancabelli, L.; Milani, C.; Gaiani, F.; De’Angelis, N.; De’Angelis, G.; Van Sinderen, D.; Ventura, M.; Turroni, F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018, 8, 13974. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, G.; Shao, D.; Wang, Q.; Hu, Y.; Wu, T.; Ji, C.; Shi, S. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult. Sci. 2021, 100, 100935. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Zhang, M.; Gu, C.; Wang, H.; Feng, J.; Bao, L.; Wu, Y.; Chen, S.; Zhang, X. Influence of Huangqin Decoction on the immune function and fecal microbiome of chicks after experimental infection with Escherichia coli O78. Sci. Rep. 2022, 5, 12. [Google Scholar]
- Saleem, M.U.; Javid, M.A.; Akthar, S.; Kiani, F.A.; Naseer, O.; Waqas, M.Y. Comparative effects of different concentrations of garlic (Allium sativum) and ginger (Zingiber Officinale) on growth performance, goblet cell histochemistry and gut microbiota of broilers. Indian J. Anim. Res. 2020, 54, 874–878. [Google Scholar]
- Tekeli, A. Potential use of Plant Extracts and Propolis to be Natural Growth Promoter in Broiler Chicks Diets. Ph.D. Thesis, Çukurova University, Graduate School of Natural and Applied Sciences, Adana, Türkiye, 2007; pp. 1–164. [Google Scholar]
- Sengupta, R.; Altermann, E.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediat. Inflamm. 2013, 23, 237921. [Google Scholar] [CrossRef]
- De Maesschalck, C.; Eeckhaut, V.; Maertens, L.; De Lange, L.; Marchal, L.; Nezer, C.; De Baere, S.; Croubels, S.; Daube, G.; Dewulf, J.; et al. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microbiol. 2015, 81, 5880–5888. [Google Scholar] [CrossRef]
- Kabir, S.M.L. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009, 10, 3531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).