Synergistic Effects of Deep Rotary Tillage and Microbial Decomposition Agents on Straw Decomposition, Soil Nutrient Dynamics, and Microbial Communities in Rice Systems
Abstract
1. Introduction
2. Materials and Methods
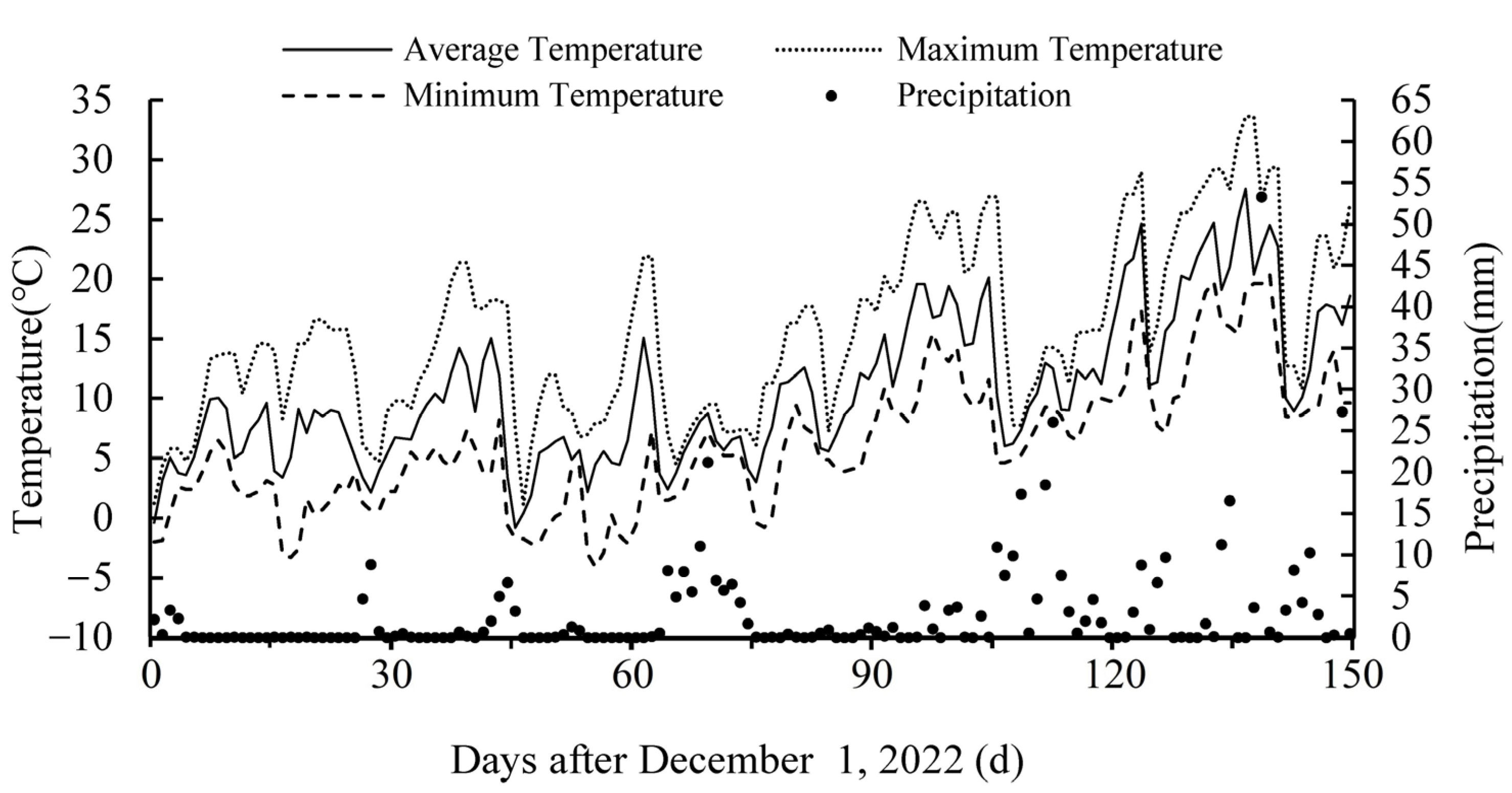
2.1. Experimental Site
2.2. Experimental Design
2.3. Sample Collection and Determination
2.4. Soil Microbial DNA Extraction and Sequencing
2.5. Data Analysis
3. Results
3.1. Straw Decomposition Dynamics
3.2. Soil Fertility Under Different Treatments
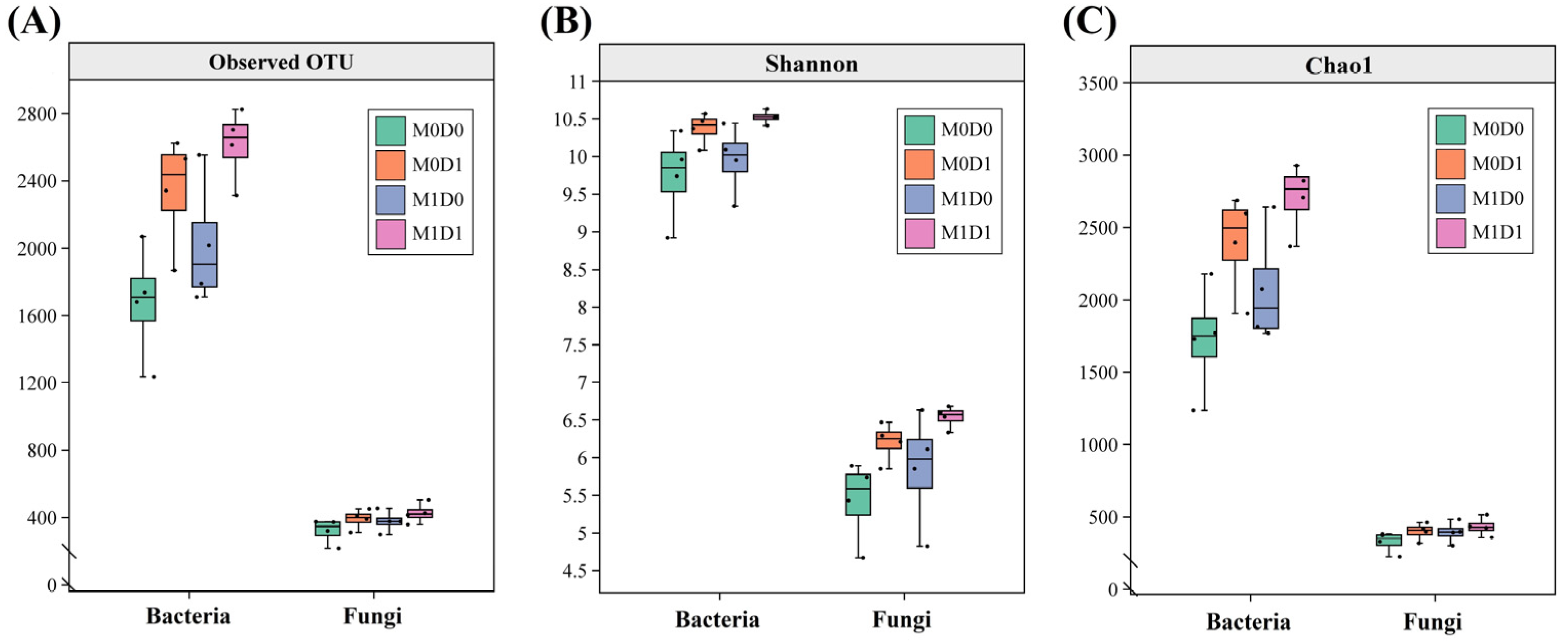
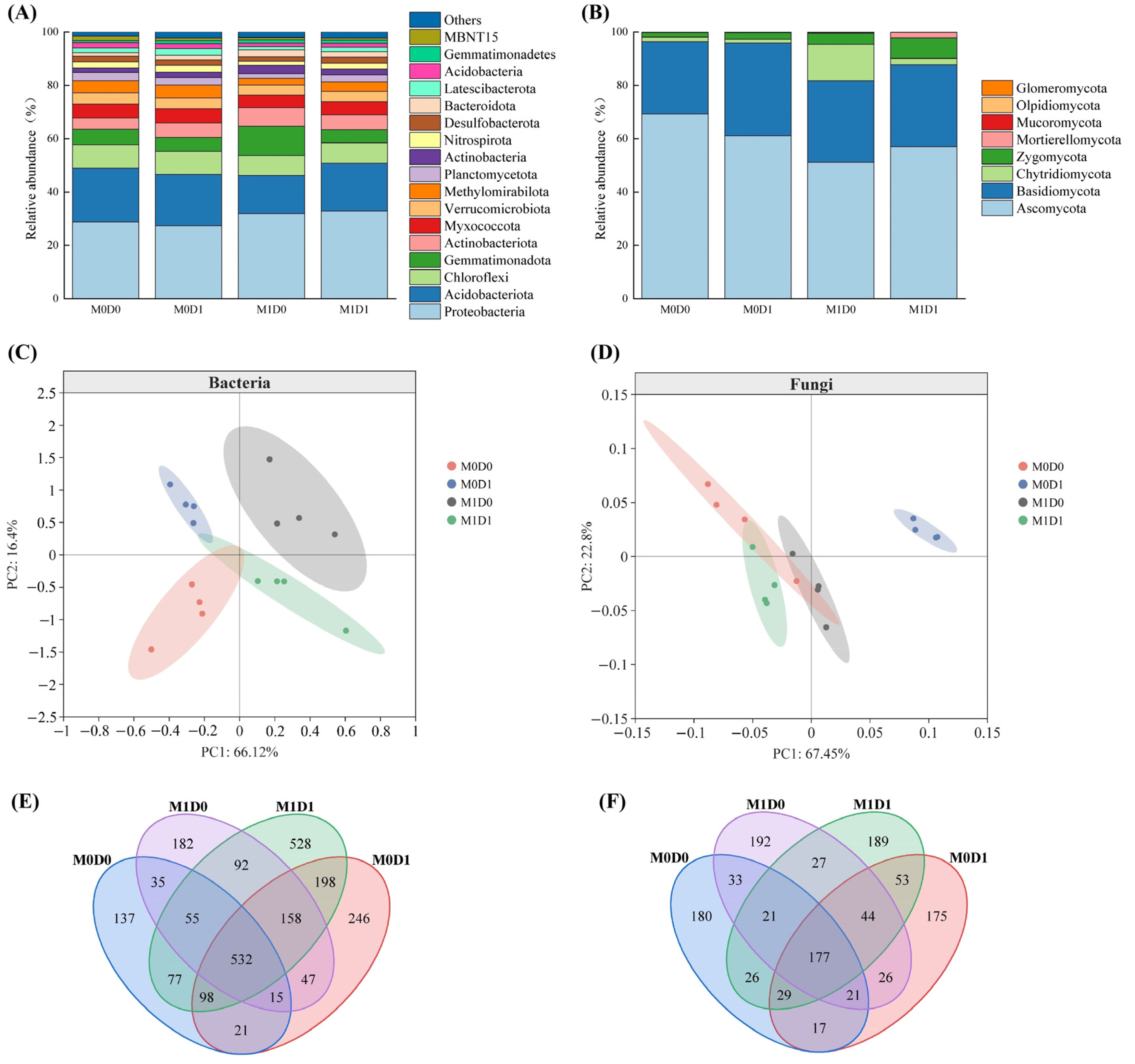
3.3. Soil Microbial Community Diversity Indices Under Different Treatments
3.4. Effects of Different Treatments on Soil Microbial Co-Occurrence Network Structure
3.5. Mechanistic Relationships Among Straw Decomposition, Soil Nutrients, and Microbial Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, J.; Wang, L.; Jia, R.; Zhou, J.; Zang, H.; Wang, J.; Yang, Y.; Jiang, Y.; Wang, Y.; Peixoto, L.; et al. Straw Returning With No-Tillage Alleviates Microbial Metabolic Carbon Limitation and Improves Soil Multifunctionality in the Northeast Plain. Land. Degrad. Dev. 2024, 35, 5149–5161. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Jiao, X.; Jiang, H.; Liu, Y.; Wang, X.; Ma, C. The Fate and Challenges of the Main Nutrients in Returned Straw: A Basic Review. Agronomy 2024, 14, 698. [Google Scholar] [CrossRef]
- Guo, Z.; Ye, W.; Wang, H.; He, W.; Tian, Y.; Hu, G.; Lou, Y.; Pan, H.; Yang, Q.; Zhuge, Y. Straw and Phosphorus Applications Promote Maize (Zea Mays L.) Growth in Saline Soil through Changing Soil Carbon and Phosphorus Fractions. Front. Plant Sci. 2024, 15, 1336300. [Google Scholar] [CrossRef]
- Wang, D.; Chang, Z.; Wang, C.; Zhang, G.; Zhang, S. Regulation and effect of 100% straw return on crop yield and environment. Chin. J. Eco-Agric. 2015, 23, 1073–1082. [Google Scholar]
- Tang, Z.; Zhang, X.; Chen, R.; Ge, C.; Tang, J.; Du, Y.; Jiang, P.; Fang, X.; Zheng, H.; Zhang, C. A Comprehensive Assessment of Rice Straw Returning in China Based on Life Cycle Assessment Method: Implications on Soil, Crops, and Environment. Agriculture 2024, 14, 972. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.X.; Luo, X.M.; Song, J.X.; Huang, B. Effects of Straw Incorporation on Rhizoctonia Solani Inoculum in Paddy Soil and Rice Sheath Blight Severity. J. Agric. Sci. 2014, 152, 741–748. [Google Scholar] [CrossRef]
- Betancur-Corredor, B.; Lang, B.; Russell, D.J. Reducing Tillage Intensity Benefits the Soil Micro- and Mesofauna in a Global Meta-analysis. Eur. J. Soil Sci. 2022, 73, e13321. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Cui, S.; Jagadamma, S.; Zhang, Q. Residue Retention and Minimum Tillage Improve Physical Environment of the Soil in Croplands: A Global Meta-Analysis. Soil Tillage Res. 2019, 194, 104292. [Google Scholar] [CrossRef]
- Ji, B.; Hu, H.; Zhao, Y.; Mu, X.; Liu, K.; Li, C. Effects of Deep Tillage and Straw Returning on Soil Microorganism and Enzyme Activities. Sci. World J. 2014, 2014, 451493. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, M.; Chen, W.; Yuan, D.; Wu, C.; Zhu, J. Simulation Analysis and Experiments for Blade-Soil-Straw Interaction under Deep Ploughing Based on the Discrete Element Method. Agriculture 2023, 13, 136. [Google Scholar] [CrossRef]
- Yang, H.; Fang, C.; Meng, Y.; Dai, Y.; Liu, J. Long-Term Ditch-Buried Straw Return Increases Functionality of Soil Microbial Communities. Catena 2021, 202, 105316. [Google Scholar] [CrossRef]
- Yun, C.; Yan, C.; Xue, Y.; Xu, Z.; Jin, T.; Liu, Q. Effects of Exogenous Microbial Agents on Soil Nutrient and Microbial Community Composition in Greenhouse-Derived Vegetable Straw Composts. Sustainability 2021, 13, 2925. [Google Scholar] [CrossRef]
- Shinde, R.; Shahi, D.K.; Mahapatra, P.; Naik, S.K.; Thombare, N.; Singh, A.K. Potential of Lignocellulose Degrading Microorganisms for Agricultural Residue Decomposition in Soil: A Review. J. Environ. Manag. 2022, 320, 115843. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.; Xing, Z.; Zhang, Q.; Niu, X.; Yu, Y.; Teng, Z.; Chen, J. Enhanced Lignocellulose Degradation and Composts Fertility of Cattle Manure and Wheat Straw Composting by Bacillus Inoculation. J. Environ. Chem. Eng. 2023, 11, 109940. [Google Scholar] [CrossRef]
- Saharan, B.S.; Dhanda, D.; Mandal, N.K.; Kumar, R.; Sharma, D.; Sadh, P.K.; Jabborova, D.; Duhan, J.S. Microbial Contributions to Sustainable Paddy Straw Utilization for Economic Gain and Environmental Conservation. Curr. Res. Microb. Sci. 2024, 7, 100264. [Google Scholar] [CrossRef]
- Guan, X.-K.; Wei, L.; Turner, N.C.; Ma, S.-C.; Yang, M.-D.; Wang, T.-C. Improved Straw Management Practices Promote in Situ Straw Decomposition and Nutrient Release, and Increase Crop Production. J. Clean. Prod. 2020, 250, 119514. [Google Scholar] [CrossRef]
- Li, J.; Gan, G.; Chen, X.; Zou, J. Effects of Long-Term Straw Management and Potassium Fertilization on Crop Yield, Soil Properties, and Microbial Community in a Rice–Oilseed Rape Rotation. Agriculture 2021, 11, 1233. [Google Scholar] [CrossRef]
- Chen, X.; Han, X.; Wang, X.; Guo, Z.; Yan, J.; Lu, X.; Zou, W. Inversion Tillage with Straw Incorporation Affects the Patterns of Soil Microbial Co-Occurrence and Multi-Nutrient Cycling in a Hapli-Udic Cambisol. J. Integr. Agric. 2023, 22, 1546–1559. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, R.; He, T.; Sun, Y.; Wu, M.; Xue, Y.; Meng, F.; Wang, J. Disentangling the Impact of Straw Incorporation on Soil Microbial Communities: Enhanced Network Complexity and Ecological Stochasticity. Sci. Total Environ. 2023, 863, 160918. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis. China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Methods of Soil Analysis. Part 3: Chemical Methods; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Logue, J.B.; Stedmon, C.A.; Kellerman, A.M.; Nielsen, N.J.; Andersson, A.F.; Laudon, H.; Lindström, E.S.; Kritzberg, E.S. Experimental Insights into the Importance of Aquatic Bacterial Community Composition to the Degradation of Dissolved Organic Matter. ISME J. 2016, 10, 533–545. [Google Scholar] [CrossRef]
- Karlsson, I.; Friberg, H.; Steinberg, C.; Persson, P. Fungicide Effects on Fungal Community Composition in the Wheat Phyllosphere. PLoS ONE 2014, 9, e111786. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- He, J. Decomposition Characteristics and Nutrient Release of Straw Returned under a Rice–Wheat Rotation System. Soils Fertil. 2022, 8, 221–230. [Google Scholar]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Murayama, S. Decomposition Kinetics of Straw Saccharides and Synthesis of Microbial Saccharides under Field Conditions. J. Soil Sci. 1984, 35, 231–242. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Wang, C.; Wang, Y. Comparison of the Effect of NaOH Pretreatment and Microbial Agents on Rice Straw Decomposition. Agronomy 2023, 13, 816. [Google Scholar] [CrossRef]
- Weisskopf, P.; Reiser, R.; Rek, J.; Oberholzer, H.-R. Effect of Different Compaction Impacts and Varying Subsequent Management Practices on Soil Structure, Air Regime and Microbiological Parameters. Soil Tillage Res. 2010, 111, 65–74. [Google Scholar] [CrossRef]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. A Review on Bacterial Contribution to Lignocellulose Breakdown into Useful Bio-Products. Int. J. Environ. Res. Public Health 2021, 18, 6001. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. Effects of Microbial Inoculants on Phosphorus and Potassium Availability, Bacterial Community Composition, and Chili Pepper Growth in a Calcareous Soil: A Greenhouse Study. J. Soils Sediments 2019, 19, 3597–3607. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, W.; Li, F.; Li, S.; Han, Y.; Li, P. Effects of Tillage Depth and Lime Application on Acidification Reduction and Nutrient Availability in Vertisol Soil. Agriculture 2024, 14, 1728. [Google Scholar] [CrossRef]
- Tian, J.; Xing, Z.; Guo, B.; Hu, Y.; Wei, H.; Gao, H.; Zhang, H.; Li, M.; Zhang, H. Wheat Straw Incorporation Coupled With Direct Seeding Method Influence Nitrogen Uptake and Translocation in Rice. Food Energy Secur. 2024, 13, e70018. [Google Scholar] [CrossRef]
- Malik, M.A.; Khan, K.S.; Marschner, P.; Fayyaz-ul-Hassan. Microbial Biomass, Nutrient Availability and Nutrient Uptake by Wheat in Two Soils with Organic Amendments. J. Soil Sci. Plant Nutr. 2013, 13, 955–966. [Google Scholar] [CrossRef]
- Jayaramaiah, R.H.; Egidi, E.; Macdonald, C.A.; Wang, J.; Jeffries, T.C.; Megharaj, M.; Singh, B.K. Soil Initial Bacterial Diversity and Nutrient Availability Determine the Rate of Xenobiotic Biodegradation. Microb. Biotechnol. 2022, 15, 318–336. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a PH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Xiong, R.; He, X.; Gao, N.; Li, Q.; Qiu, Z.; Hou, Y.; Shen, W. Soil PH Amendment Alters the Abundance, Diversity, and Composition of Microbial Communities in Two Contrasting Agricultural Soils. Microbiol. Spectr. 2024, 12, e0416523. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Wu, M.; Jiang, C.; Chen, X.; Cai, Z.; Wang, B.; Zhang, J.; Zhang, T.; Li, Z. Soil PH Rather than Nutrients Drive Changes in Microbial Community Following Long-Term Fertilization in Acidic Ultisols of Southern China. J. Soils Sediments 2018, 18, 1853–1864. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; de Quadros, P.D.; Davis-Richardson, A.; Camargo, F.A.O.; Clark, I.M.; McGrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil PH Determines Microbial Diversity and Composition in the Park Grass Experiment. Microb. Ecol. 2015, 69, 395–406. [Google Scholar] [CrossRef]
- Bao, Y.; Feng, Y.; Stegen, J.C.; Wu, M.; Chen, R.; Liu, W.; Zhang, J.; Li, Z.; Lin, X. Straw Chemistry Links the Assembly of Bacterial Communities to Decomposition in Paddy Soils. Soil Biol. Biochem. 2020, 148, 107866. [Google Scholar] [CrossRef]
- Ding, X.L.; He, H.-B.; Bai, Z.; Xie, H.-T.; Zhang, B.; Zhang, X.-D. Effects of Nitrogen Supply Level on Microbial Transformation of Amino Sugar in a Mollisol Amended with Maize Straw. Chin. J. Appl. Ecol. 2009, 20, 2207–2213. [Google Scholar]
- Xia, W.; Ren, X.; Chen, Y. Deep Vertical Rotary Tillage Increases the Diversity of Bacterial Communities and Alters the Bacterial Network Structure in Soil Planted to Corn. Can. J. Soil Sci. 2022, 102, 946–958. [Google Scholar] [CrossRef]
- Xia, W.; Chen, Y. Effects of Fenlong Tillage on Soil Bacterial Community Diversity and Microbial Network Structure in Arable Land. Environ. Sci. 2023, 44, 1095–1103. [Google Scholar] [CrossRef]
- Rasche, F.; Cadisch, G. The Molecular Microbial Perspective of Organic Matter Turnover and Nutrient Cycling in Tropical Agroecosystems—What Do We Know? Biol. Fertil. Soils 2013, 49, 251–262. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Jiang, J.; Meng, X.; Huang, Z.; Wu, H.; He, L.; Xiong, F.; Liu, J.; Zhong, R.; et al. Sugarcane/Peanut Intercropping System Improves Physicochemical Properties by Changing N and P Cycling and Organic Matter Turnover in Root Zone Soil. PeerJ 2021, 9, e10880. [Google Scholar] [CrossRef]
- Marinho, E.B.; de Oliveira, A.L.; Zandonadi, D.B.; Benedito, L.E.C.; de Souza, R.B.; de Figueiredo, C.C.; Busato, J.G. Organic Matter Pools and Nutrient Cycling in Different Coffee Production Systems in the Brazilian Cerrado. Agrofor. Syst. 2014, 88, 767–778. [Google Scholar] [CrossRef]
- Banning, N.C.; Grant, C.D.; Jones, D.L.; Murphy, D.V. Recovery of Soil Organic Matter, Organic Matter Turnover and Nitrogen Cycling in a Post-Mining Forest Rehabilitation Chronosequence. Soil Biol. Biochem. 2008, 40, 2021–2031. [Google Scholar] [CrossRef]
- Hicks, L.C.; Lajtha, K.; Rousk, J. Nutrient Limitation May Induce Microbial Mining for Resources from Persistent Soil Organic Matter. Ecology 2021, 102, e03328. [Google Scholar] [CrossRef]
- Alessi, A.M.; Bird, S.M.; Oates, N.C.; Li, Y.; Dowle, A.A.; Novotny, E.H.; deAzevedo, E.R.; Bennett, J.P.; Polikarpov, I.; Young, J.P.W.; et al. Defining Functional Diversity for Lignocellulose Degradation in a Microbial Community Using Multi-Omics Studies. Biotechnol. Biofuels 2018, 11, 166. [Google Scholar] [CrossRef]
- Shen, Q.; Tang, J.; Sun, H.; Yao, X.; Wu, Y.; Wang, X.; Ye, S. Straw Waste Promotes Microbial Functional Diversity and Lignocellulose Degradation during the Aerobic Process of Pig Manure in an Ectopic Fermentation System via Metagenomic Analysis. Sci. Total Environ. 2022, 838, 155637. [Google Scholar] [CrossRef]
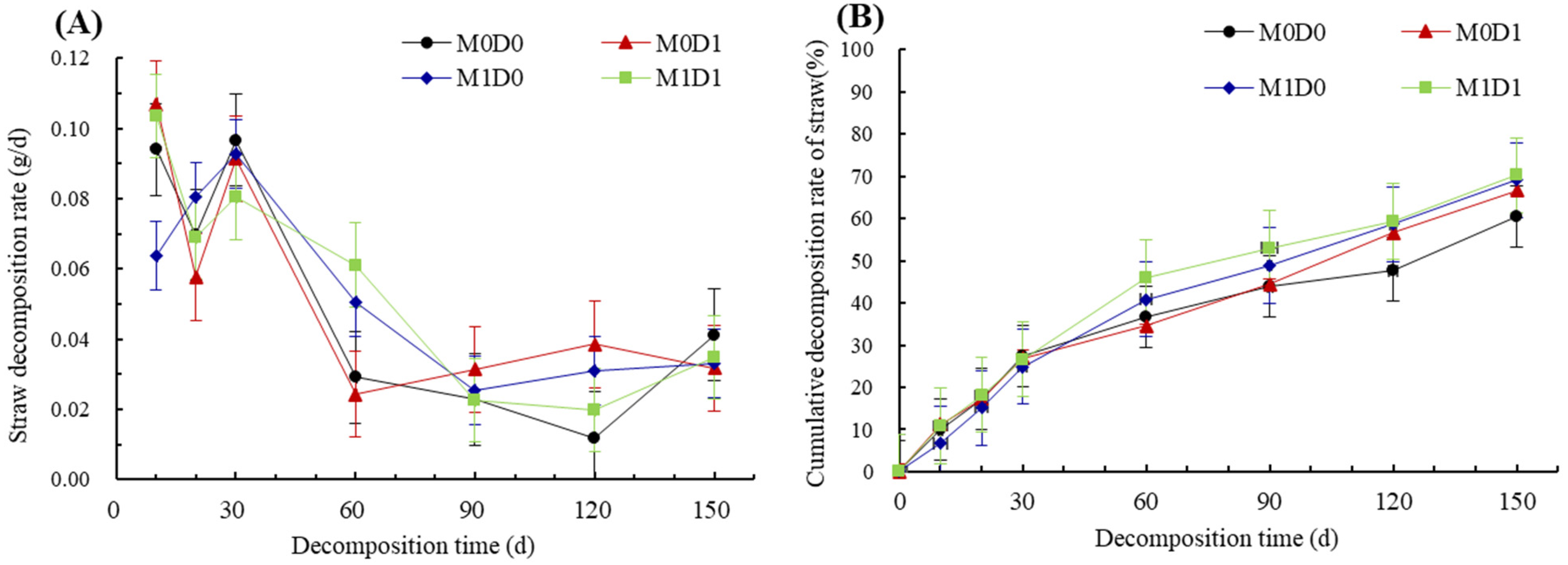
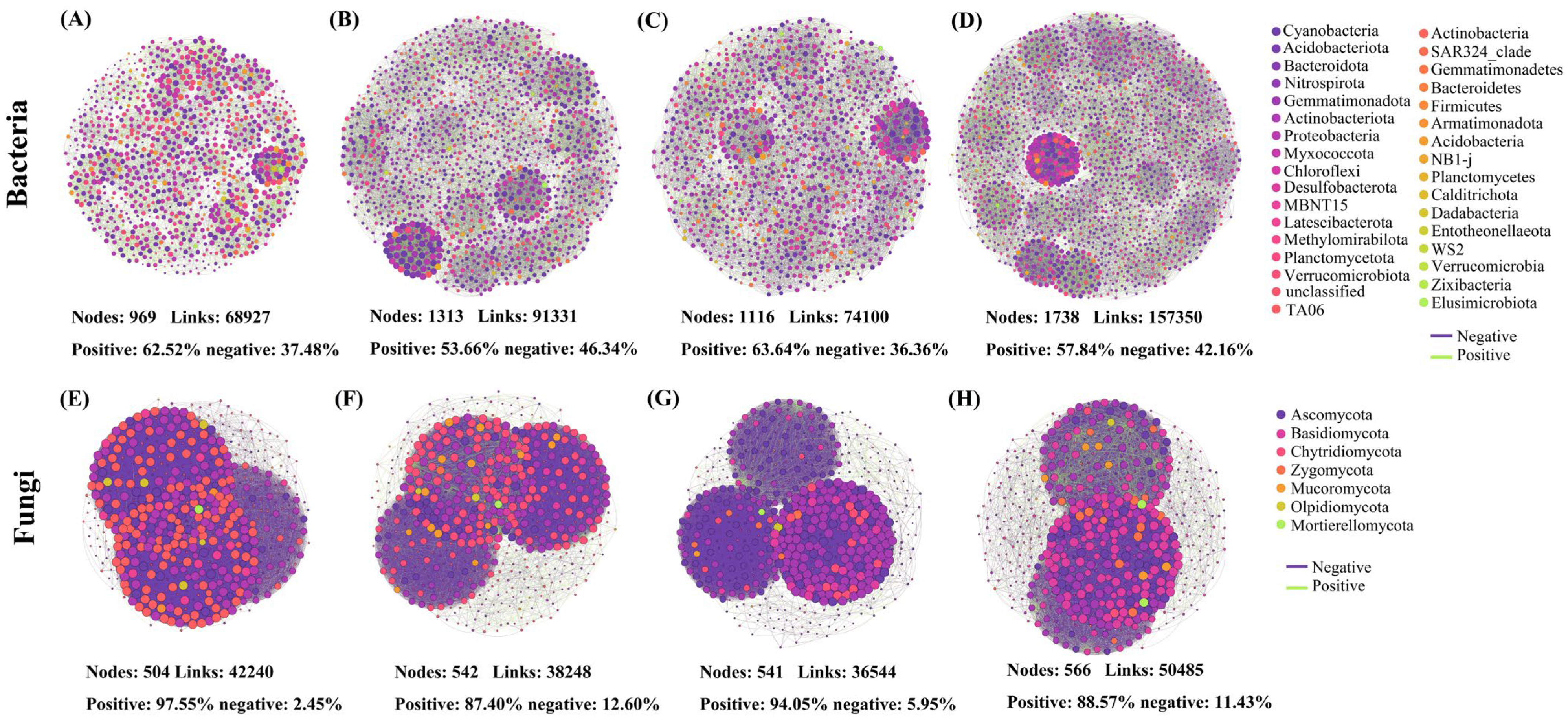
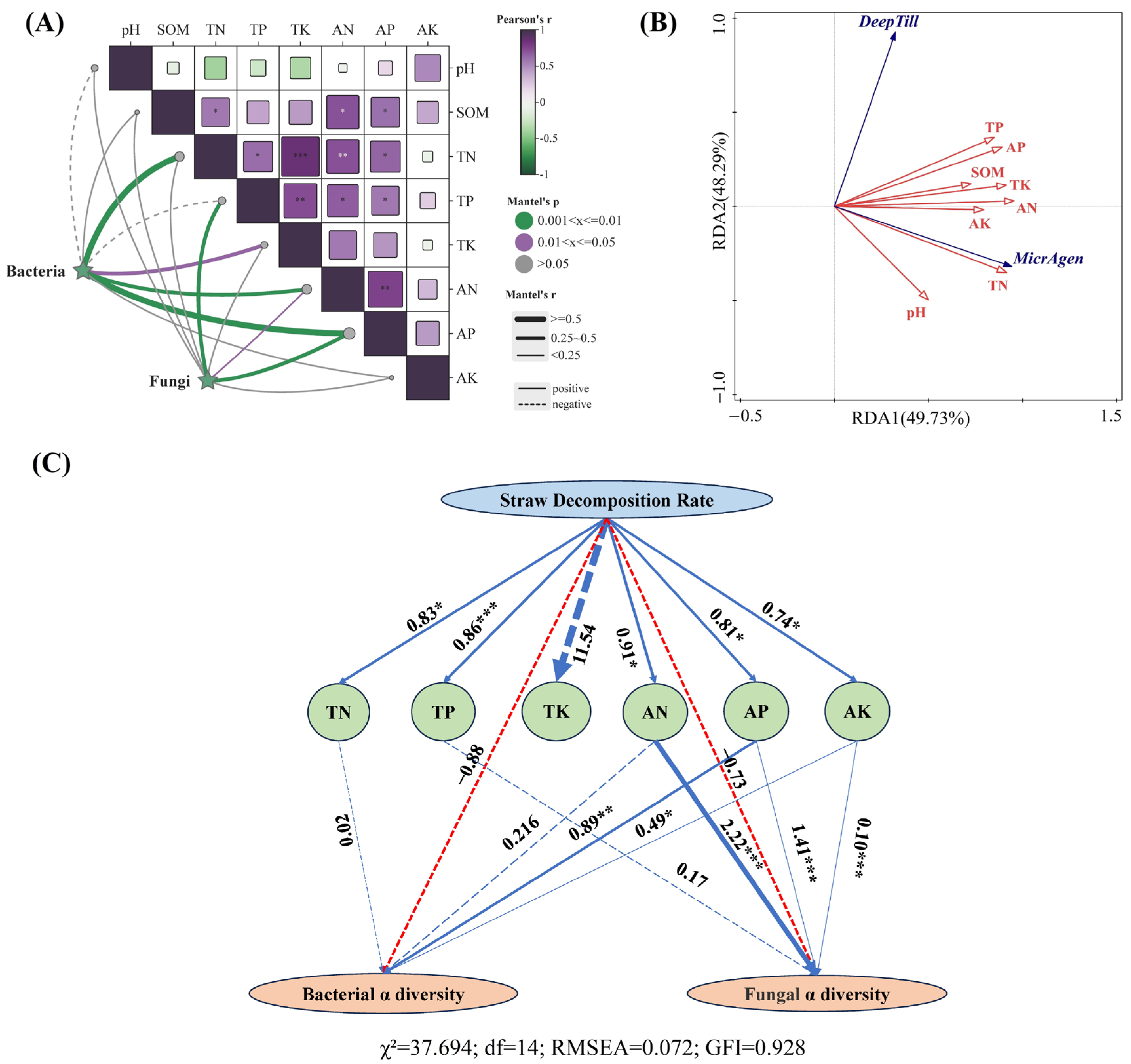
| Treatment | Fitted Equation | RMSE | k | T50 (d) | T95 (d) |
|---|---|---|---|---|---|
| M0D0 | y = 0.9447e−0.0058t | 0.0385 | 0.0058 | 109 | 506 |
| M0D1 | y = 0.96e−0.0067t | 0.0378 | 0.0067 | 97 | 438 |
| M1D0 | y = 0.9885e−0.0077t | 0.0204 | 0.0077 | 88 | 387 |
| M1D1 | y = 0.9665e−0.0081t | 0.0284 | 0.0081 | 81 | 364 |
| Treatment | pH | SOM(g∙kg−1) | TN(g∙kg−1) | TP(g∙kg−1) | TK(g∙kg−1) | AN (mg∙kg−1) | AP (mg∙kg−1) | AK (mg∙kg−1) |
|---|---|---|---|---|---|---|---|---|
| M0D0 | 5.90 ± 0.02 ab | 29.60 ± 0.35 b | 1.22 ± 0.01 d | 1.40 ± 0.01 c | 4.40 ± 0.30 c | 134.32 ± 2.81 c | 9.14 ± 0.23 c | 228.65 ± 7.29 c |
| M0D1 | 5.86 ± 0.05 b | 30.33 ± 0.45 ab | 1.25 ± 0.01 c | 1.42 ± 0.01 bc | 4.97 ± 0.08 b | 137.41 ± 1.34 c | 9.76 ± 0.11 b | 234.52 ± 8.79 bc |
| M1D0 | 6.06 ± 0.13 a | 30.88 ± 0.67 ab | 1.40 ± 0.01 b | 1.44 ± 0.01 b | 5.38 ± 0.07 a | 144.65 ± 1.87 b | 9.90 ± 0.06 b | 247.80 ± 2.69 ab |
| M1D1 | 5.97 ± 0.04 ab | 31.43 ± 0.88 a | 1.37 ± 0.01 a | 1.52 ± 0.03 a | 5.58 ± 0.08 a | 150.39 ± 0.94 a | 10.28 ± 0.08 a | 255.65 ± 6.74 a |
| M | * | * | ** | ** | ** | ns | ** | ** |
| D | ns | ns | ns | ** | * | ns | ** | ns |
| M × D | ns | ns | ** | * | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Huang, J.; Tan, Y.; Yang, L.; Li, Y.; Xia, B.; Li, H.; Deng, X. Synergistic Effects of Deep Rotary Tillage and Microbial Decomposition Agents on Straw Decomposition, Soil Nutrient Dynamics, and Microbial Communities in Rice Systems. Agriculture 2025, 15, 1447. https://doi.org/10.3390/agriculture15131447
Wang X, Huang J, Tan Y, Yang L, Li Y, Xia B, Li H, Deng X. Synergistic Effects of Deep Rotary Tillage and Microbial Decomposition Agents on Straw Decomposition, Soil Nutrient Dynamics, and Microbial Communities in Rice Systems. Agriculture. 2025; 15(13):1447. https://doi.org/10.3390/agriculture15131447
Chicago/Turabian StyleWang, Xinyue, Jie Huang, Yanting Tan, Lili Yang, Yuanhuan Li, Bing Xia, Hailin Li, and Xiaohua Deng. 2025. "Synergistic Effects of Deep Rotary Tillage and Microbial Decomposition Agents on Straw Decomposition, Soil Nutrient Dynamics, and Microbial Communities in Rice Systems" Agriculture 15, no. 13: 1447. https://doi.org/10.3390/agriculture15131447
APA StyleWang, X., Huang, J., Tan, Y., Yang, L., Li, Y., Xia, B., Li, H., & Deng, X. (2025). Synergistic Effects of Deep Rotary Tillage and Microbial Decomposition Agents on Straw Decomposition, Soil Nutrient Dynamics, and Microbial Communities in Rice Systems. Agriculture, 15(13), 1447. https://doi.org/10.3390/agriculture15131447






