Soybean Fermentation Broth Value-Added Phosphorus Fertilizer Boosts Crop Growth via Improved Soil Phosphorus Availability and Rhizosphere Microbial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Experimental Design
2.3. Sample Collection and Measurements
2.4. Data Analysis
3. Results and Discussions
3.1. Soil Chemical Attributes
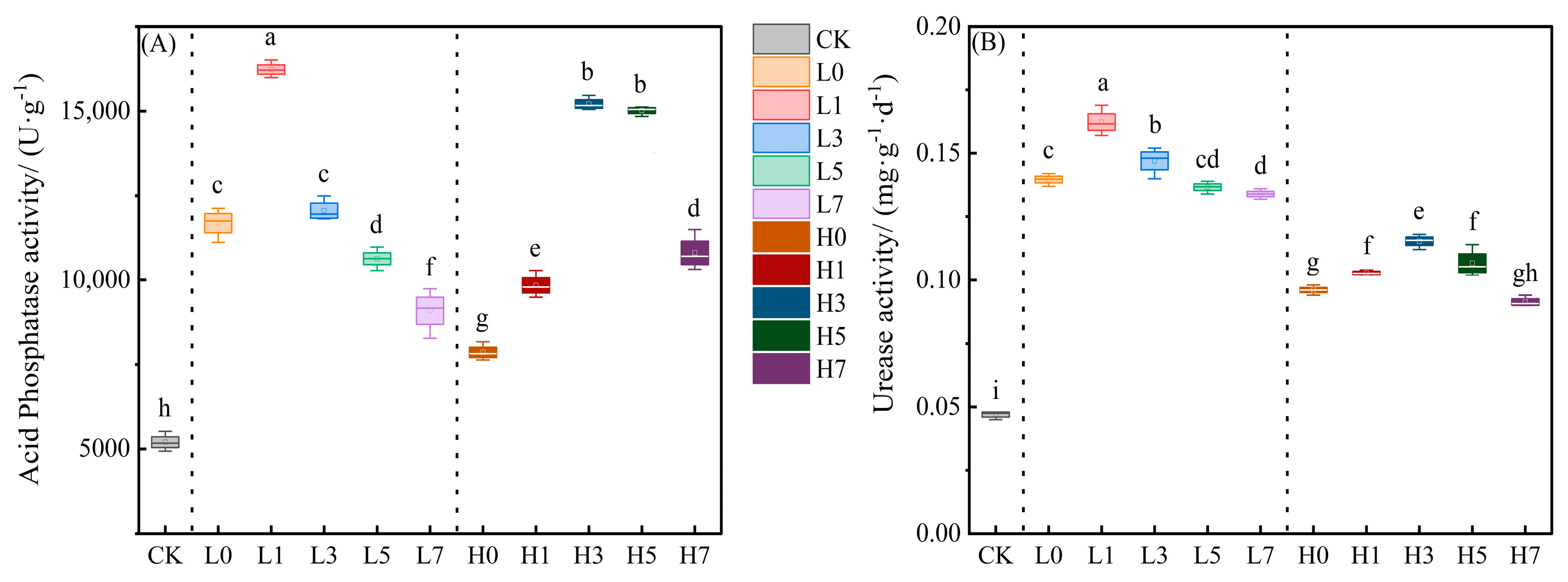
3.2. Soil Acid Phosphatase (ACP) and Urease (URE) Activities
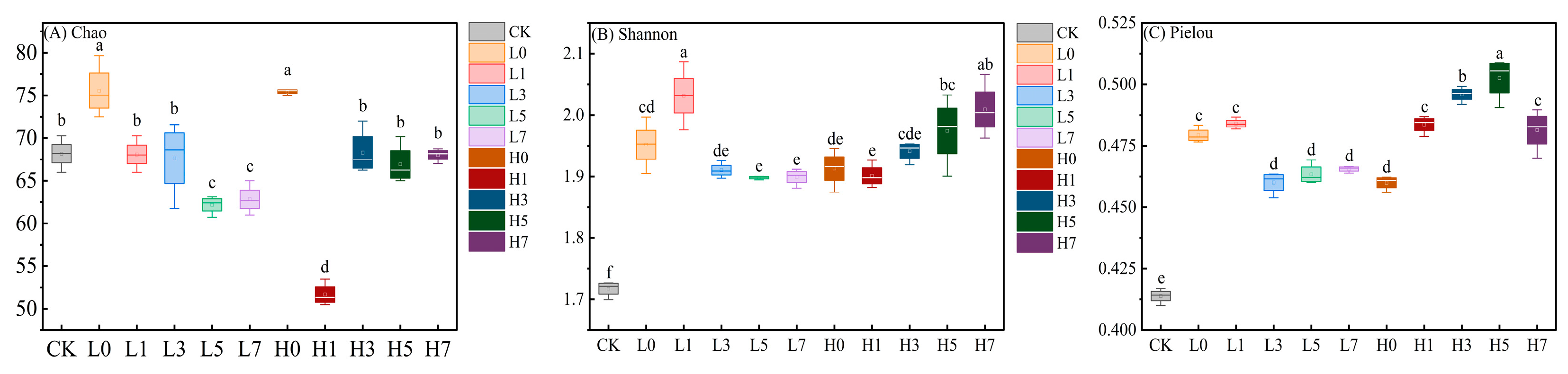
3.3. Soil Microbial Diversity
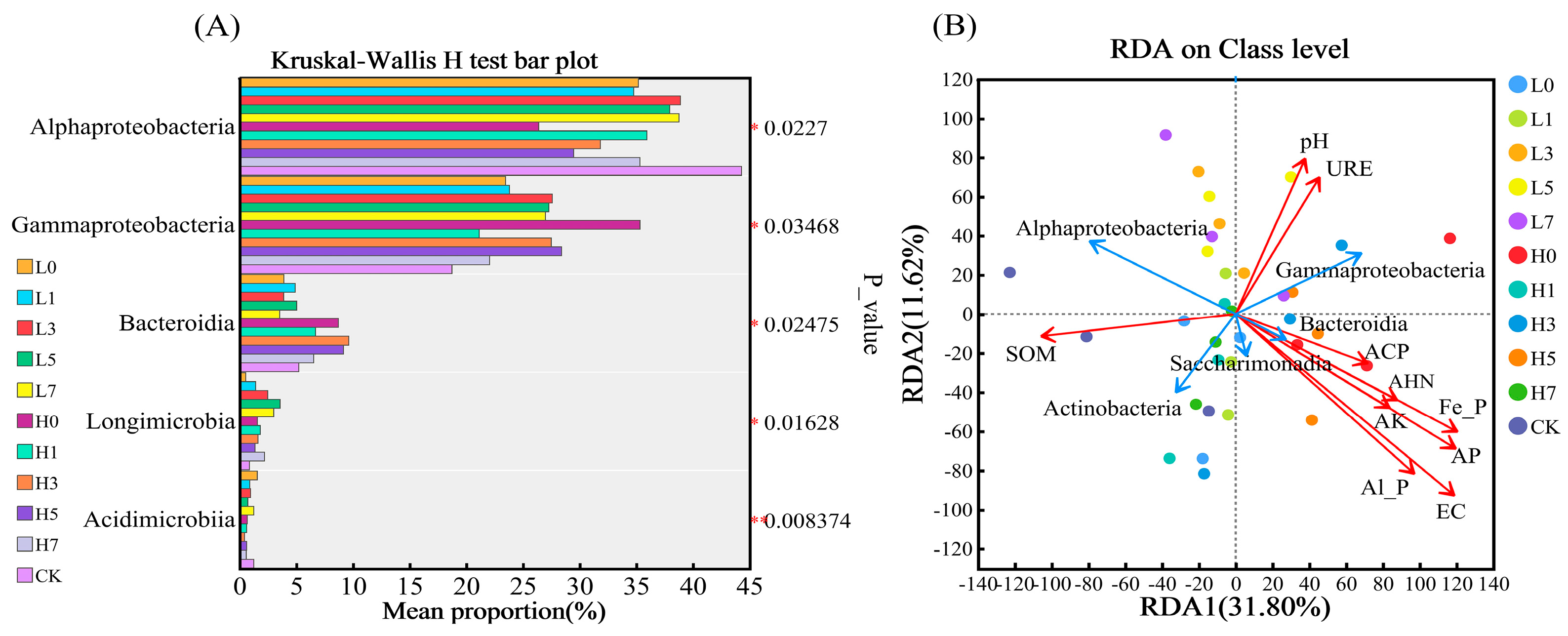
3.4. Soil Microbial Community Structure
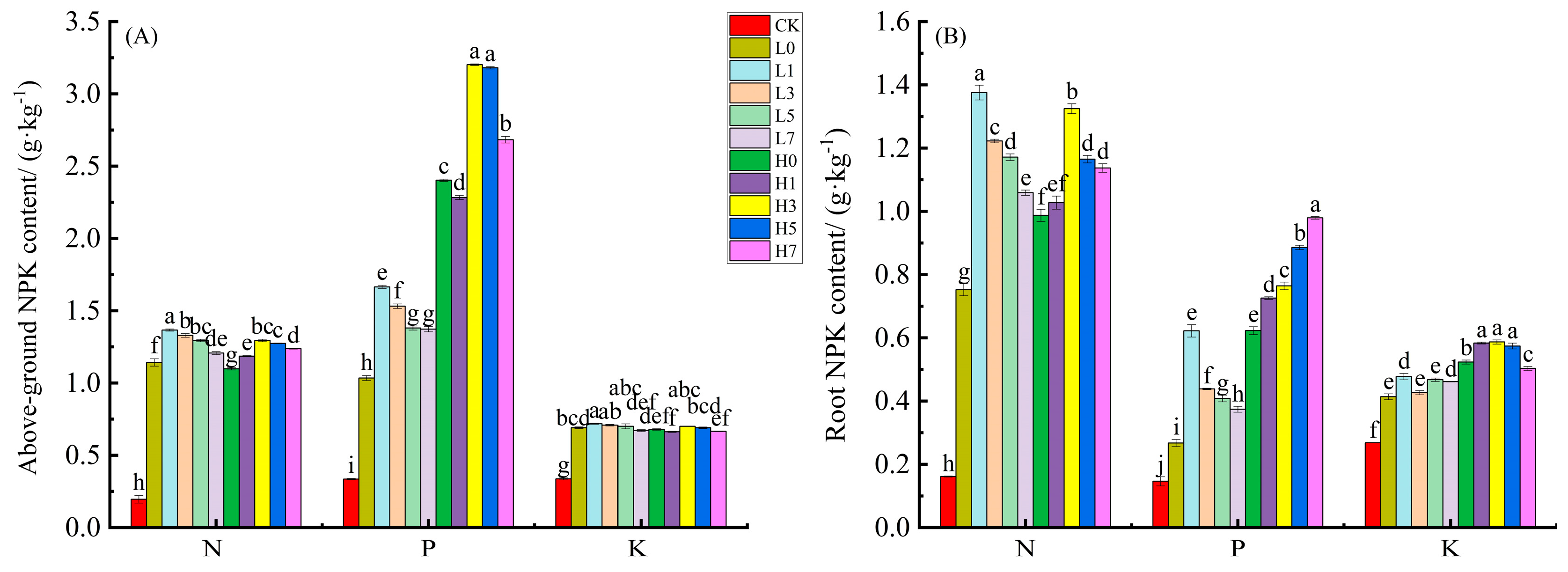
3.5. Seedling Growth and Nutrient Content in Maize
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant Biol. 2014, 56, 192–220. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of Soil Phosphorus and Plant Adaptation Mechanisms to Phosphorus Stress for Sustainable Crop Production: A Review. J. Soil Sci. Plant Nutr. 2012, 12, 547–562. [Google Scholar] [CrossRef]
- Redel, Y.; Cartes, P.; Demanet, R.; Velásquez, G.; Poblete-Grant, P.; Bol, R.; Mora, M.L. Assessment of Phosphorus Status Influenced by Al and Fe Compounds in Volcanic Grassland Soils. J. Soil Sci. Plant Nutr. 2016, 16, 490–506. [Google Scholar] [CrossRef]
- Sun, D.; Yang, X.; Wang, C.; Hao, X.; Hong, J.; Lin, X. Dynamics of Available and Enzymatically Hydrolysable Soil Phosphorus Fractions during Repeated Freeze-Thaw Cycles. Geoderma 2019, 345, 1–4. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus Activators Contribute to Legacy Phosphorus Availability in Agricultural Soils: A Review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, Consequences, Physical, Chemical and Biological Techniques for Mitigation Strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Hofmockel, K.S.; Fierer, N.; Colman, B.P.; Jackson, R.B. Amino Acid Abundance and Proteolytic Potential in North American Soils. Oecologia 2010, 163, 1069–1078. [Google Scholar] [CrossRef]
- Gil, F.; Taf, R.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Moustakas, K.; Chojnacka, K. Advancing Sustainable Agriculture: Converting Dairy Wastes into Amino Acid Fertilizers. Sustain. Chem. Pharm. 2024, 42, 101782. [Google Scholar] [CrossRef]
- Jeon, Y.-W.; Jeon, M.-J. Large-Scale Conversion of Livestock Blood into Amino Acid Liquid Fertilizer and Dry Protein Feedstuff: A Case Study. Processes 2024, 12, 1183. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Simón-Grao, S.; Navarro-Pérez, V.; Alfosea-Simón, M. Scientific Advances in Biostimulation Reported in the 5th Biostimulant World Congress. Horticulturae 2022, 8, 665. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Hu, S.; Li, K.; Zhang, X.; Yang, C.; Zhang, R.; Guo, H. The Impact of the Foliar Application of Amino Acid Aqueous Fertilizer on the Flavor of Potato Tubers. Foods 2023, 12, 3951. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of Nitrogen Utilization by Soil Microorganisms—A Review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Chubukov, V.; Gerosa, L.; Kochanowski, K.; Sauer, U. Coordination of Microbial Metabolism. Nat. Rev. Microbiol. 2014, 12, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.W.; Farrell, M.; Jones, D.L. Bigger May Be Better in Soil N Cycling: Does Rapid Acquisition of Small l-Peptides by Soil Microbes Dominate Fluxes of Protein-Derived N in Soil? Soil Biol. Biochem. 2012, 48, 106–112. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.; Brookes, P.; Evershed, R.; Goulding, K.; Hirsch, P. Measuring the Soil-Microbial Interface: Extraction of Extracellular Polymeric Substances (EPS) from Soil Biofilms. Soil Biol. Biochem. 2014, 72, 163–171. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-Term Nutrient Inputs Shift Soil Microbial Functional Profiles of Phosphorus Cycling in Diverse Agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus Depletion in Forest Soils Shapes Bacterial Communities towards Phosphorus Recycling Systems. Environ. Microbiol. 2016, 18, 1988–2000. [Google Scholar] [CrossRef]
- Elias, M.; Tanaka, M.; Sakai, M.; Toyama, H.; Matsushita, K.; Adachi, O.; Yamada, M. C-Terminal Periplasmic Domain of Escherichia Coli Quinoprotein Glucose Dehydrogenase Transfers Electrons to Ubiquinone. J. Biol. Chem. 2001, 276, 48356–48361. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; He, L.; Xu, X.; Wang, J.; Ren, C.; Guo, Y.; Zhao, F. Different Mechanisms Driving Increasing Abundance of Microbial Phosphorus Cycling Gene Groups along an Elevational Gradient. iScience 2022, 25, 105170. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xu, W.; Cheng, J.; Liu, J.; Pei, W.; Wang, J.; Chuang, S. Amino Acid Fertilizer Strengthens Its Effect on Crop Yield and Quality by Recruiting Beneficial Rhizosphere Microbes. J. Sci. Food Agric. 2023, 103, 5970–5980. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, J.; Ren, Y.; Sun, C.; Deng, X.; Qian, M.; Hu, Z.; Li, R.; Chen, Y.; Shen, Z.; et al. Polyaspartate and Liquid Amino Acid Fertilizer Are Appropriate Alternatives for Promoting the Phytoextraction of Cadmium and Lead in Solanum nigrum L. Chemosphere 2019, 237, 124483. Chemosphere 2019, 237, 124483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Zhao, B.; Li, Y.; Xu, M.; Yan, Y.; Jing, J.; Yuan, L. Glutamic Acid-Enhanced Phosphate Fertilizer Increases Phosphorus Availability in Fluvo-Aquic Soil via Phosphamide (O=P-N) Formation, Decreasing Phosphate Fixation and Increasing Soil Microbial Diversity. J. Soil Sci. Plant Nutr. 2024, 24, 2748–2760. [Google Scholar] [CrossRef]
- Gordon, W.B.; Pierzynski, G.M. Corn Hybrid Response to Starter Fertilizer Combinations. J. Plant Nutr. 2006, 29, 1287–1299. [Google Scholar] [CrossRef]
- Barry, D.A.J.; Miller, M.H. Phosphorus Nutritional Requirement of Maize Seedlings for Maximum Yield. Agron. J. 1989, 81, 95–99. [Google Scholar] [CrossRef]
- Chang, S.C.; Jackson, M.L. Fractionation of Soil Phosphorus. Soil Sci. 1957, 84, 133–144. [Google Scholar] [CrossRef]
- Ho, G.-H.; Ho, T.-I.; Hsieh, K.-H.; Su, Y.-C.; Lin, P.-Y.; Yang, J.; Yang, K.-H.; Yang, S.-C. γ-Polyglutamic Acid Produced by Bacillus Subtilis (Natto): Structural Characteristics, Chemical Properties and Biological Functionalities. J. Chin. Chem. Soc. 2006, 53, 1363–1384. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Gao, D.; Wang, L.; Li, J.; Wei, Z.; Shi, Y. Effects of Poly-γ-Glutamic Acid (γ-PGA) on Plant Growth and Its Distribution in a Controlled Plant-Soil System. Sci. Rep. 2017, 7, 6090. [Google Scholar] [CrossRef]
- Friedman, S. Soil Properties Influencing Apparent Electrical Conductivity: A Review. Comput. Electron. Agri. 2005, 46, 45–70. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, C.; Xu, X.; Feng, X.; Xu, H. Effect of Poly (γ-Glutamic Acid) on Wheat Productivity, Nitrogen Use Efficiency and Soil Microbes. J. Soil Sci. Plant Nutr. 2013, 13, 744–755. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Y. Soil and Fertilizer Science; Agricultural University Press: Beijing, China, 2011; 350p, ISBN 978-7-5655-0298-9. [Google Scholar]
- Chen, X.; Yan, X.; Wang, M.; Cai, Y.; Weng, X.; Su, D.; Guo, J.; Wang, W.; Hou, Y.; Ye, D.; et al. Long-Term Excessive Phosphorus Fertilization Alters Soil Phosphorus Fractions in the Acidic Soil of Pomelo Orchards. Soil Tillage Res. 2022, 215, 105214. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, X.; Wang, L.; Xiang, W.; Alharbi, H.A.; Lei, P.; Kuzyakov, Y. Regulation of Soil Phosphorus Availability and Composition during Forest Succession in Subtropics. For. Ecol. Manag. 2021, 502, 119706. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Z.; Zhao, B.; Yuan, L.; Li, Y.; Wen, Y. Effects of value-added phosphate fertilizers on transformation of inorganic phosphorus in calcareous soils. J. Plant Nutr. Fertil. 2013, 19, 1183–1191. [Google Scholar] [CrossRef]
- Gabasawa, A.I. Phosphorus Cycle Enzymes to Remedy Soil Phosphorus Deficiency. In Sustainable Agriculture Reviews 58: Phosphorus Use Efficiency for Sustainable Agriculture; Iqbal, A., Iqbal, M., Alamzeb, M., Meizhen, S., Xiling, Z., Arif, M., Du, X., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 177–205. ISBN 978-3-031-16155-1. [Google Scholar] [CrossRef]
- Wu, M.; Wei, Q.; Xu, L.; Li, H.; Oelmüller, R.; Zhang, W. Piriformospora Indica Enhances Phosphorus Absorption by Stimulating Acid Phosphatase Activities and Organic Acid Accumulation in Brassica Napus. Plant Soil 2018, 432, 333–344. [Google Scholar] [CrossRef]
- Tian, J.; Wei, K.; Condron, L.M.; Chen, Z.; Xu, Z.; Chen, L. Impact of Land Use and Nutrient Addition on Phosphatase Activities and Their Relationships with Organic Phosphorus Turnover in Semi-Arid Grassland Soils. Biol. Fertil. Soils 2016, 52, 675–683. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–243. ISBN 978-3-642-15271-9. [Google Scholar] [CrossRef]
- Gou, X.; Ren, Y.; Qin, X.; Wei, X.; Wang, J. Global Patterns of Soil Phosphatase Responses to Nitrogen and Phosphorus Fertilization. Pedosphere 2024, 34, 200–210. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing Soil Microbial Diversity Is Associated with Decreasing Microbial Biomass under Nitrogen Addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- de Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/Bacterial Ratios in Grasslands with Contrasting Nitrogen Management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef]
- Li, Y.; Pan, F.; Yao, H. Response of Symbiotic and Asymbiotic Nitrogen-Fixing Microorganisms to Nitrogen Fertilizer Application. J. Soils Sediments 2019, 19, 1948–1958. [Google Scholar] [CrossRef]
- Gupta, R.S. The Phylogeny of Proteobacteria: Relationships to Other Eubacterial Phyla and Eukaryotes. FEMS Microbiol. Rev. 2000, 24, 367–402. [Google Scholar] [CrossRef]
- Sharma, V.; Vashishtha, A.; Jos, A.L.M.; Khosla, A.; Basu, N.; Yadav, R.; Bhatt, A.; Gulani, A.; Singh, P.; Lakhera, S.; et al. Phylogenomics of the Phylum Proteobacteria: Resolving the Complex Relationships. Curr. Microbiol. 2022, 79, 224. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, M.A.; Lamont, B.B. Competition and Facilitation between Australian and Spanish Legumes in Seven Australian Soils. Plant Species Biol. 2016, 31, 256–271. [Google Scholar] [CrossRef]
- Lauber, C.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Gupta, R.S. Protein Phylogenies and Signature Sequences: A Reappraisal of Evolutionary Relationships among Archaebacteria, Eubacteria, and Eukaryotes. Microbiol. Mol. Biol. Rev. 1998, 62, 1435–1491. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Mukhtar, T.; Singh, B. Evolutionary Relationships among Photosynthetic Prokaryotes (Heliobacterium chlorum, Chloroflexus aurantiacus, Cyanobacteria, Chlorobium tepidum and Proteobacteria): Implications Regarding the Origin of Photosynthesis. Mol. Microbiol. 1999, 32, 893–906. [Google Scholar] [CrossRef]
- Williams, K.P.; Gillespie, J.J.; Sobral, B.W.S.; Nordberg, E.K.; Snyder, E.E.; Shallom, J.M.; Dickerman, A.W. Phylogeny of Gammaproteobacteria. J. Bacteriol. 2010, 192, 2305–2314. [Google Scholar] [CrossRef]
- Niepceron, M.; Martin-Laurent, F.; Crampon, M.; Portet-Koltalo, F.; Akpa-Vinceslas, M.; Legras, M.; Bru, D.; Bureau, F.; Bodilis, J. GammaProteobacteria as a Potential Bioindicator of a Multiple Contamination by Polycyclic Aromatic Hydrocarbons (PAHs) in Agricultural Soils. Environ. Pollut. 2013, 180, 199–205. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, K.L.; Zhao, X.Q.; Zhang, H.Q.; Li, D.; Li, J.J.; Shen, R.F. Balanced Fertilization over Four Decades Has Sustained Soil Microbial Communities and Improved Soil Fertility and Rice Productivity in Red Paddy Soil. Sci. Total Environ. 2021, 793, 148664. [Google Scholar] [CrossRef] [PubMed]
- Gypser, S.; Freese, D. Phosphorus Release from Vivianite and Hydroxyapatite by Organic and Inorganic Compounds. Pedosphere 2020, 30, 190–200. [Google Scholar] [CrossRef]
- Li, H.-Z.; Bi, Q.; Yang, K.; Zheng, B.-X.; Pu, Q.; Cui, L. D2O-Isotope-Labeling Approach to Probing Phosphate-Solubilizing Bacteria in Complex Soil Communities by Single-Cell Raman Spectroscopy. Anal. Chem. 2019, 91, 2239–2246. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of Bacterial Non-Specific Acid (phoC) and Alkaline (phoD) Phosphatase Genes in Bulk and Rhizosphere Soil from Organically Managed Soybean Fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, C.; Li, W.X.; Song, W.F.; Shen, R.F. Soil Nutrients Drive Function and Composition of phoC-Harboring Bacterial Community in Acidic Soils of Southern China. Front. Microbiol. 2019, 10, 2654. [Google Scholar] [CrossRef]
- Garrity, G.M.; Bell, J.A.; Liburn, T. Class III. Gammaproteobacteria Class. Nov. In Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: London, UK, 1984; pp. 1–59. ISBN 0683041088. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of Phosphorus and Nitrogen in the Rhizosphere and Plant Growth Promotion by Microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Assuero, S.G.; Mollier, A.; Pellerin, S. The Decrease in Growth of Phosphorus-Deficient Maize Leaves Is Related to a Lower Cell Production. Plant Cell Environ. 2004, 27, 887–895. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. ISBN 978-981-10-9044-8. [Google Scholar] [CrossRef]
- Barber, S.A.; Mackay, A.D. Root Growth and Phosphorus and Potassium Uptake by Two Corn Genotypes in the Field. Fertil. Res. 1986, 10, 217–230. [Google Scholar] [CrossRef]
- Jungk, A. Root Hairs and the Acquisition of Plant Nutrients from Soil. J. Plant Nutr. Soil Sci. 2001, 164, 121–129. [Google Scholar] [CrossRef]
- Foehse, D.; Claassen, N.; Jungk, A.; Foehse, D.; Claassen, N.; Jungk, A. Phosphorus Efficiency of Plants II. Significance of Root Radius Root Hairs and Cation Anion Balance for Phosphorus Influx in Seven Plant Species. Plant Soil 1991, 132, 261–272. [Google Scholar] [CrossRef]
- Bruns, H.; Ebelhar, M. Nutrient Uptake of Maize Affected by Nitrogen and Potassium Fertility in a Humid Subtropical Environment. Commun. Soil Sci. Plan 2006, 37, 275–293. [Google Scholar] [CrossRef]
- Bennett, W.F.; Pesek, J.; Hanway, J. Effect of Nitrogen on Phosphorus Absorption by Corn. Agron. J. 1962, 54, 437–442. [Google Scholar] [CrossRef]
- Bélanger, G.; Richards, J.E. Relationship between P and N Concentrations in Timothy. Can. J. Plant Sci. 1999, 79, 65–70. [Google Scholar] [CrossRef]
- Singh, R.; Sawatzky, S.K.; Thomas, M.; Akin, S.; Zhang, H.; Raun, W.; Arnall, D.B. Nitrogen, Phosphorus, and Potassium Uptake in Rain-Fed Corn as Affected by NPK Fertilization. Agronomy 2023, 13, 1913. [Google Scholar] [CrossRef]
- Tayyab, M.; Islam, W.; Noman, A.; Pang, Z.; Li, S.; Lin, S.; Lin, W.; Hua, Z. Sugarcane Cultivars Manipulate Rhizosphere Bacterial Communities’ Structure and Composition of Agriculturally Important Keystone Taxa. 3 Biotech 2022, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Bragazza, L.; Parisod, J.; Buttler, A.; Bardgett, R.D. Biogeochemical Plant-Soil Microbe Feedback in Response to Climate Warming in Peatlands. Nat. Clim. Change 2013, 3, 273–277. [Google Scholar] [CrossRef]
- Li, X.; Zeng, R.; Liao, H. Improving Crop Nutrient Efficiency through Root Architecture Modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-Soil-Microbes: A Tripartite Interaction for Nutrient Acquisition and Better Plant Growth for Sustainable Agricultural Practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]


| Treatment | Phosphorus Levels (g·kg−1) | Soybean Fermentation Broth Dilution Factor and Treatment Number | Fertilizer Application Rates | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 100- Fold | 300- Fold | 500- Fold | 700- Fold | KH2PO4 (g·kg−1) | CO(NH2)2 (g·kg−1) | KCl (g·kg−1) | ||
| L group | 0.05 | L0 | L1 | L3 | L5 | L7 | 0.0957 | 0.3269 | 0.19 |
| H group | 0.15 | H0 | H1 | H3 | H5 | H7 | 0.287 | 0.3269 | 0.084 |
| CK | 0 | / | / | / | / | / | / | 0.3269 | 0.25 |
| Treatment | Chemical attributes | |||||
|---|---|---|---|---|---|---|
| pH | EC (mS·cm−1) | Soil Organic Matter (SOM) (g·kg−1) | Alkaline Hydrolyzable Nitrogen (AHN) (mg·kg−1) | Available Potassium (AK) (mg·kg−1) | ||
| CK | 5.69 ± 0.01 h | 0.69 ± 0.01 f | 8.26 ± 0.24 g | 32.20 ± 0.81 j | 19.04 ± 0.52 h | |
| L group | L0 | 6.04 ± 0.01 c | 0.66 ± 0.00 g | 10.24 ± 0.09 f | 46.43 ± 0.23 i | 65.92 ± 0.06 g |
| L1 | 6.22 ± 0.01 a | 0.74 ± 0.01 e | 14.92 ± 0.13 a | 62.77 ± 0.84 f | 67.47 ± 0.07 ef | |
| L3 | 6.12 ± 0.02 b | 0.55 ± 0.01 i | 13.95 ± 0.06 b | 60.43 ± 0.62 g | 67.05 ± 0.11 f | |
| L5 | 6.13 ± 0.01 b | 0.51 ± 0.01 j | 12.78 ± 0.10 c | 54.60 ± 0.00 h | 67.03 ± 0.10 f | |
| L7 | 6.12 ± 0.02 b | 0.62 ± 0.01 h | 10.99 ± 0.06 e | 53.20 ± 0.81 h | 66.60 ± 0.01 f | |
| H group | H0 | 5.77 ± 0.01 f | 1.06 ± 0.01 a | 11.13 ± 0.17 e | 79.10 ± 0.40 c | 68.19 ± 0.53 de |
| H1 | 5.70 ± 0.01 g | 0.89 ± 0.01 b | 12.62 ± 0.10 c | 99.40 ± 0.70 a | 68.73 ± 0.11 d | |
| H3 | 6.00 ± 0.01 d | 0.89 ± 0.01 c | 14.13 ± 0.08 b | 82.13 ± 0.7 b | 70.75 ± 0.49 a | |
| H5 | 6.15 ± 0.01 b | 0.79 ± 0.01 d | 14.35 ± 0.19 b | 73.03 ± 0.62 d | 69.71 ± 0.48 abc | |
| H7 | 5.92 ± 0.01 e | 0.84 ± 0.00 f | 12.08 ± 0.06 d | 68.37 ± 0.93 e | 68.60 ± 0.41 cd | |
| Treatment | Chemical attributes | |||||
| Available Phosphorus (AP) (mg·kg−1) | Al-P (mg·kg−1) | Fe-P (mg·kg−1) | ||||
| CK | 0.98 ± 0.14 j | 2.17 ± 0.07 k | 7.93 ± 0.09 j | |||
| L group | L0 | 6.6 ± 0.08 i | 25.27 ± 0.07 h | 32.90 ± 0.11 i | ||
| L1 | 21.73 ± 0.22 e | 66.33 ± 0.07 d | 77.62 ± 0.29 c | |||
| L3 | 19.24 ± 0.13 f | 50.00 ± 0.12 f | 46.45 ± 0.19 g | |||
| L5 | 12.21 ± 0.29 g | 11.18 ± 0.127 i | 40.38 ± 0.11 h | |||
| L7 | 10.11 ± 0.17 h | 9.62 ± 0.07 j | 40.76 ± 0.09 h | |||
| H group | H0 | 39.26 ± 0.26 d | 46.82 ± 0.24 g | 61.14 ± 0.20 f | ||
| H1 | 41.02 ± 0.29 c | 62.94 ± 0.18 e | 75.83 ± 0.14 d | |||
| H3 | 50.95 ± 0.28 a | 111.63 ± 3.68 a | 85.23 ± 0.20 a | |||
| H5 | 50.71 ± 0.18 a | 91.87 ± 0.12 b | 79.30 ± 0.19 b | |||
| H7 | 42.59 ± 0.20 b | 71.82 ± 0.14 c | 64.23 ± 0.11 e | |||
| Treatment | Above-Ground Growth | Biomass | Root Growth | |||||
|---|---|---|---|---|---|---|---|---|
| Plant Height (cm) | Stem Diameter (mm) | SPAD | Fresh Weight (g) | Total Length (cm) | Surface Area (cm2) | |||
| Above-Ground | Root | |||||||
| CK | 78.51 ± 0.74 h | 9.5 ± 0.18 i | 20.73 ± 0.33 i | 17.46 ± 0.47 j | 8.91 ± 0.67 i | 1070.90 ± 2.90 i | 366.35 ± 2.10 f | |
| L group | L0 | 137.80 ± 0.67 e | 22.98 ± 0.24 fg | 40.2 ± 0.55 d | 210.97 ± 0.56 e | 36.97 ± 1.24 de | 1657.59 ± 23.8 g | 1048.52 ± 15.2 c |
| L1 | 150.57 ± 0.81 a | 26.82 ± 0.38 a | 46.83 ± 0.18 a | 273.28 ± 3.91 a | 56.94 ± 0.45 a | 2128.40 ± 13.16 d | 1182.70 ±5.03 b | |
| L3 | 148.16 ± 1.81 a | 24.45 ± 0.15 cd | 44.03 ± 0.47 b | 225.86 ± 1.11 c | 49.09 ± 0.58 b | 2268.14 ± 23.56 c | 1213.30 ± 22.07 b | |
| L5 | 143.69 ± 1.47 bc | 23.49 ± 0.19 def | 42.2 ± 0.31 c | 224.93 ± 3.45 c | 48.58 ± 1.13 b | 2041.74 ± 18.77 e | 1068.16 ±2.18 c | |
| L7 | 141.17 ± 1.60 cd | 23.25 ± 0.18 ef | 41.4 ± 0.23 cd | 222.14 ± 2.81 d | 43.18 ± 1.36 c | 1536.40 ± 23.63 h | 1058.46 ± 6.12 c | |
| H group | H0 | 127.61 ± 1.27 fg | 22.02 ± 0.40 gh | 27.17 ± 0.56 h | 191.49 ± 3.81 h | 34.29 ± 0.72 fg | 2027.39 ± 4.55 e | 744.00 ± 6.52 e |
| H1 | 135.86 ± 0.81 e | 21.94 ± 0.14 h | 38.2 ± 0.49 e | 194.41 ± 1.10 g | 37.97 ± 0.67 d | 1951.69 ± 5.73 f | 885.89 ± 3.76 d | |
| H3 | 141.35 ± 1.42 bc | 25.49 ± 0.22 b | 38.6 ± 0.53 e | 243.89 ± 3.81 b | 49.39 ± 1.42 b | 2557.45 ± 16.44 b | 1318.60 ± 6.48 a | |
| H5 | 138.43 ± 1.41 de | 24.59 ± 0.36 c | 36.8 ± 0.50 f | 199.40 ± 0.92 f | 35.66 ± 0.47 def | 2852.01 ± 16.92 a | 1041.22 ± 9.00 c | |
| H7 | 130.04 ± 0.82 f | 22.20 ± 0.2 gh | 34.17 ± 0.19 g | 170.83 ± 2.31 i | 28.34 ± 1.17 h | 2165.95 ± 6.77 d | 882.18 ± 7.53 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; He, D.; Huang, W.; Wang, T.; Deng, L. Soybean Fermentation Broth Value-Added Phosphorus Fertilizer Boosts Crop Growth via Improved Soil Phosphorus Availability and Rhizosphere Microbial Activity. Agriculture 2025, 15, 1440. https://doi.org/10.3390/agriculture15131440
Zhang X, He D, Huang W, Wang T, Deng L. Soybean Fermentation Broth Value-Added Phosphorus Fertilizer Boosts Crop Growth via Improved Soil Phosphorus Availability and Rhizosphere Microbial Activity. Agriculture. 2025; 15(13):1440. https://doi.org/10.3390/agriculture15131440
Chicago/Turabian StyleZhang, Xinyi, Danyi He, Wuzhihui Huang, Tingyi Wang, and Lansheng Deng. 2025. "Soybean Fermentation Broth Value-Added Phosphorus Fertilizer Boosts Crop Growth via Improved Soil Phosphorus Availability and Rhizosphere Microbial Activity" Agriculture 15, no. 13: 1440. https://doi.org/10.3390/agriculture15131440
APA StyleZhang, X., He, D., Huang, W., Wang, T., & Deng, L. (2025). Soybean Fermentation Broth Value-Added Phosphorus Fertilizer Boosts Crop Growth via Improved Soil Phosphorus Availability and Rhizosphere Microbial Activity. Agriculture, 15(13), 1440. https://doi.org/10.3390/agriculture15131440





