Combined Metagenomic and Metabolomic Analysis to Evaluate the Comprehensive Effects of Trichoderma and 6PP on Vineyard Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Microbial Metabolites
2.2. Experimental Trial
2.3. Nematode Communities Extraction and Identification
2.4. Microbial Communities Isolation and Identification
2.5. Metagenomic Analysis
2.6. Metabolomic Analysis
2.7. Statistical Analysis
3. Results
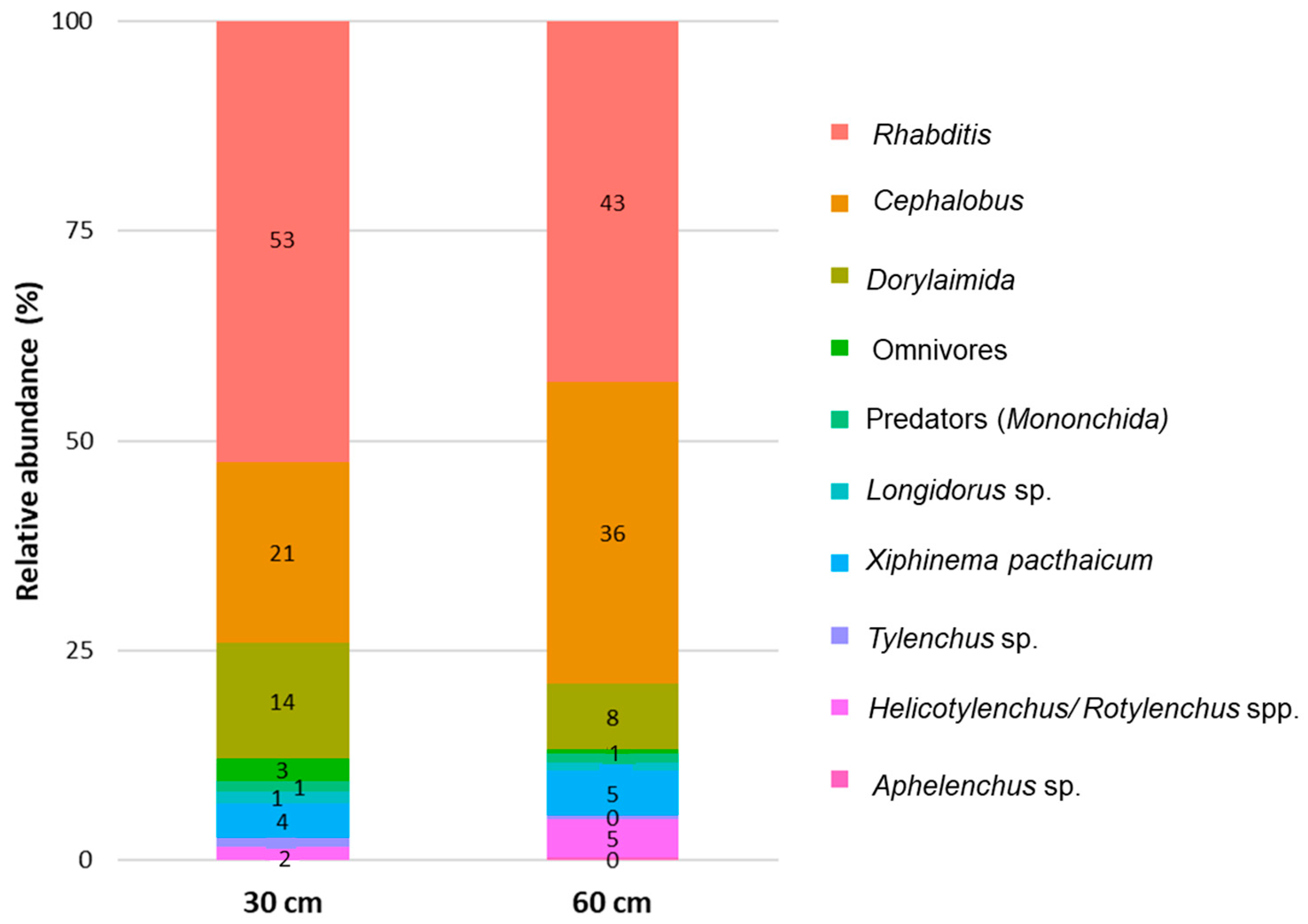
3.1. Nematodes and Microbial Communities Characterization
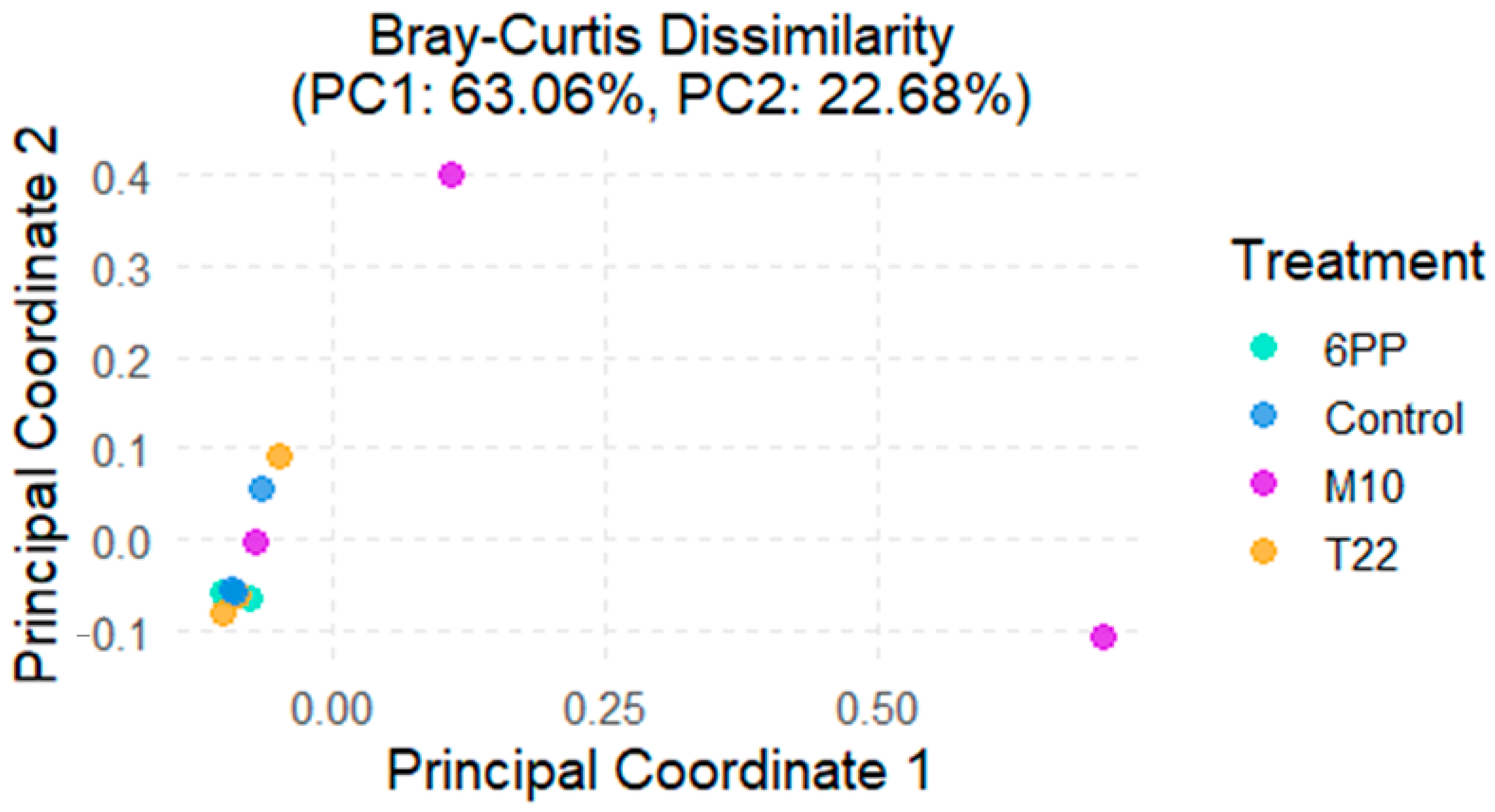
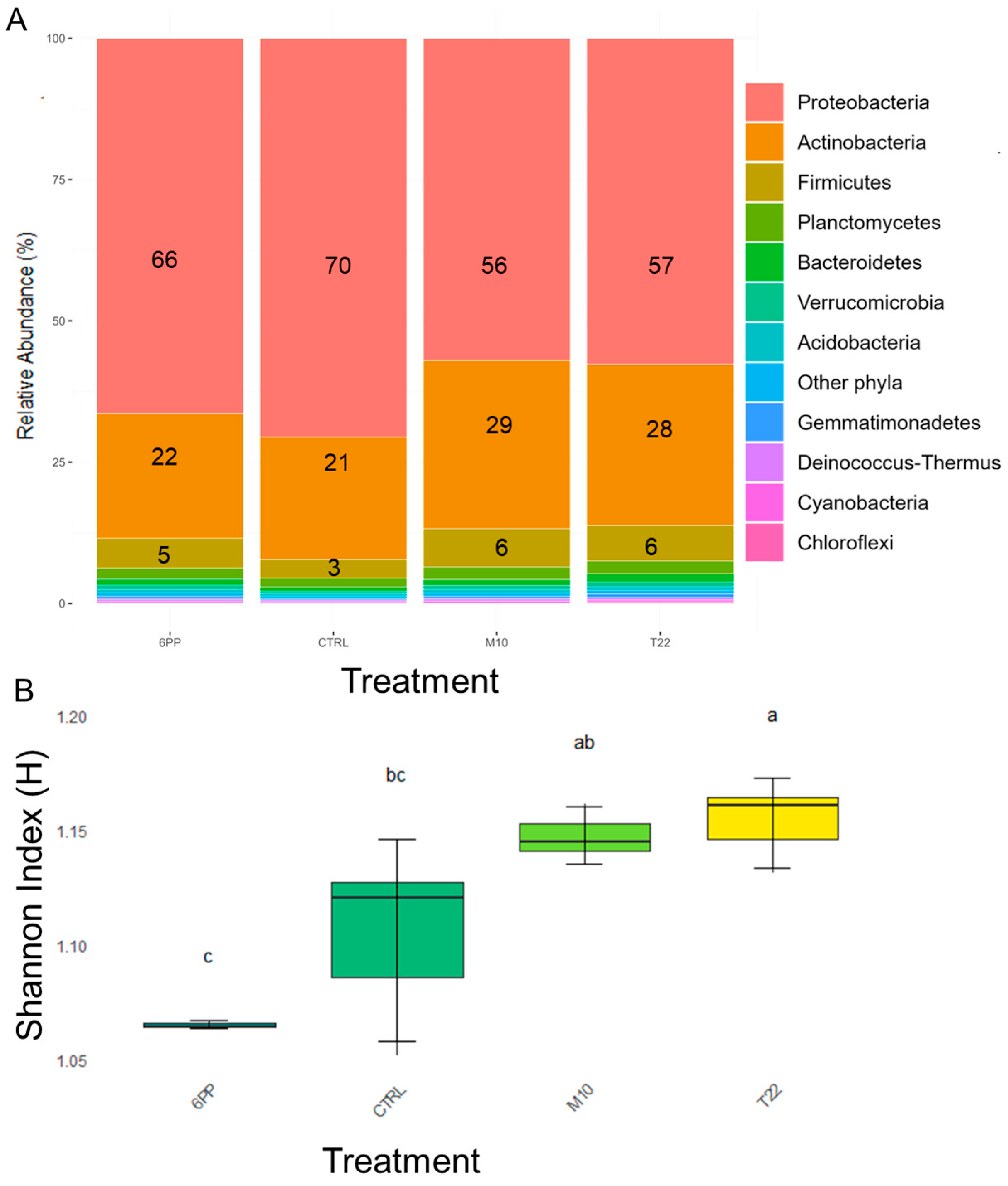
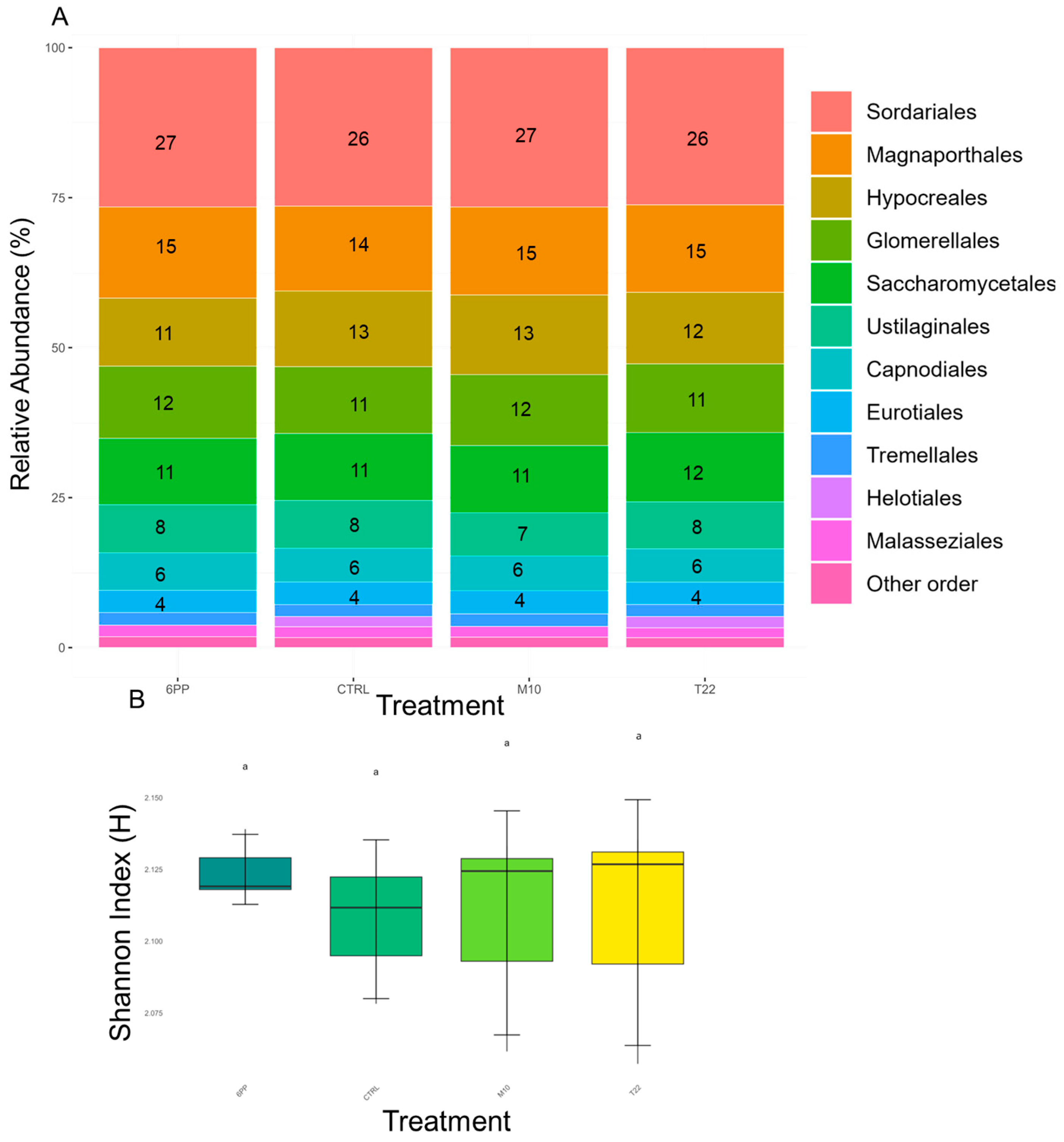
3.2. Soil Microbiome
3.3. Plant Growth and Grape Maturity
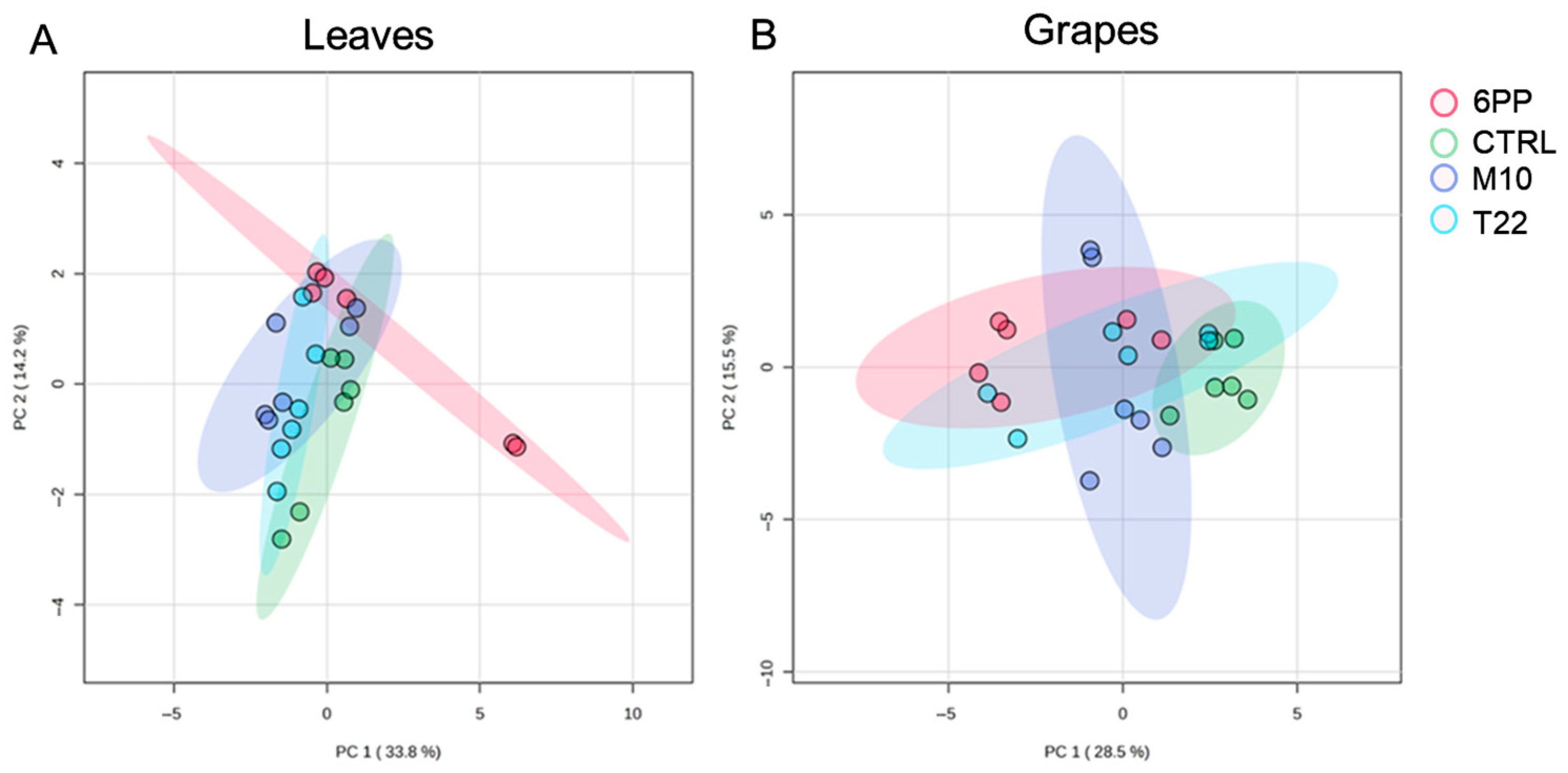
3.4. Untargeted Metabolomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- UIV. Italy Wine Report. 2023. Available online: https://www.tridge.com/it/market-reports/italy-wine-report-2023-challenging-season-for-italian-producers (accessed on 20 April 2024).
- Ribalta-Pizarro, C.; Muñoz, P.; Munné-Bosch, S. Tissue-specific hormonal variations in grapes of irrigated and non-irrigated grapevines (Vitis vinifera cv. “Merlot”) growing under mediterranean field conditions. Front. Plant Sci. 2021, 12, 621587. [Google Scholar] [CrossRef]
- Smith, H.M.; Clarke, C.W.; Smith, B.P.; Carmody, B.M.; Thomas, M.R.; Clingeleffer, P.R.; Powell, K.S. Genetic identification of SNP markers linked to a new grape phylloxera resistant locus in Vitis cinerea for marker-assisted selection. BMC Plant Biol. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Askary, T.H.; Khalil, A.; Nazir, N.; Khan, A.A.; Banday, S.A. Nematode parasites of grapevines. In Sustainable Agriculture Reviews 31; Lichtfouse, E., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 389–423. [Google Scholar]
- Malossini, U.; Ioriatti, C.; Mescalchin, E.; Varner, M.; Giacometti, R.; D’Errico, F.P. The nematofauna associated to grapevine in Trentino (Italia Settentrionale). Redia 2008, 91, 147–150. [Google Scholar]
- M’rabet Samaali, B.; Loulou, A.; MougouHamdane, A.; Kallel, S. Acquisition and transmission of Grapevine fanleaf virus (GFLV) by Xiphinema index and Xiphinema italiae (Longidoridae). J. Helminthol. 2024, 98, e26. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Villate, L.; Gutierrez-Gutierrez, C.; Castillo, P.; Van Ghelder, C.; Plantard, O.; Esmenjaud, D. Phylogeography of the soil-borne vector nematode Xiphinema index highly suggests Eastern origin and dissemination with domesticated grapevine. Sci. Rep. 2019, 9, 7313. [Google Scholar] [CrossRef]
- Dutta, T.K.; Phani, V. The pervasive impact of global climate change on plant-nematode interaction continuum. Front. Plant Sci. 2023, 14, 1143889. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Bonanomi, G.; Motti, R.; Idbella, M. Trick of the Trade: Unveiling the Importance of Feedstock Chemistry in Trichoderma-Organic Amendments-Based Bio-Stimulants. Horticulturae 2023, 9, 957. [Google Scholar] [CrossRef]
- Illescas, M.; Rubio, M.B.; Hernández-Ruiz, V.; Morán-Diez, M.E.; Martínez de Alba, A.E.; Nicolás, C.; Monte, E.; Hermosa, R. Effect of Inorganic N Top Dressing and Trichoderma harzianum Seed-Inoculation on Crop Yield and the Shaping of Root Microbial Communities of Wheat Plants Cultivated Under High Basal N Fertilization. Front. Plant Sci. 2020, 11, 575861. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Cobb, A.B.; Luo, G.; Zhou, J.; Yang, G.; Wilson, G.W.T.; Zhang, Y. Trichoderma biofertilizer links to altered soil chemistry, altered microbial communities, and improved grassland biomass. Front. Microbiol. 2018, 9, 848. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Nafady, N.A.; Sultan, R.; El-Zawahry, A.M.; Mostafa, Y.S.; Alamri, S.; Mostafa, R.G.; Hashem, M.; Hassan, E.A. Effective and Promising Strategy in Management of Tomato Root-Knot Nematodes by Trichoderma harzianum and Arbuscular Mycorrhizae. Agronomy 2022, 12, 315. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Gérard, L.; Milhau, C.; Vinale, F.; Dufossé, L.; Fouillaud, M. Salinity and Temperature Influence Growth and Pigment Production in the Marine-Derived Fungal Strain Talaromyces albobiverticillius 30548. Microorganisms 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Alborino, V.; Lanzuise, S.; Lombardi, N.; Marra, R.; Balestrieri, A.; Ritieni, A.; Woo, S.L.; Vinale, F. Trichoderma enzymes for degradation of aflatoxin B1 and ochratoxin A. Molecules 2022, 27, 3959. [Google Scholar] [CrossRef]
- Nicoletti, R.; Vinale, F. Bioactive Compounds from Marine-Derived Aspergillus, Penicillium, Talaromyces and Trichoderma Species. Mar. Drugs 2018, 16, 408. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef]
- Lombardi, N.; Salzano, A.M.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Caira, S.; Lorito, M.; d’Errico, G.; et al. Effect of Trichoderma bioactive metabolite treatments on the production, quality, and protein profile of strawberry fruits. J. Agric. Food Chem. 2020, 68, 7246–7258. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2021, 9, 749–759. [Google Scholar] [CrossRef]
- Comite, E.; El-Nakhel, C.; Rouphael, Y.; Ventorino, V.; Pepe, O.; Borzacchiello, A.; Vinale, F.; Rigano, D.; Staropoli, A.; Lorito, M.; et al. Bioformulations with Beneficial Microbial Consortia, a Bioactive Compound and Plant Biopolymers Modulate Sweet Basil Productivity, Photosynthetic Activity and Metabolites. Pathogens 2021, 10, 870. [Google Scholar] [CrossRef]
- Lazazzara, V.; Vicelli, B.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Trichoderma spp. volatile organic compounds protect grapevine plants by activating defense-related processes against downy mildew. Physiol. Plant. 2021, 172, 1950–1965. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.M.; Martins Filho, A.P.; da Costa, D.P.; de Barros, J.A.; da França, R.F.; de Sousa Lima, J.R.; Duda, G.P.; da Silva, M.M.; Araujo, A.S.F.; Medeiros, E.V.d. Winery Residues Transformed into Biochar and Co-Applied with Trichoderma Increase Grape Productivity and Soil Quality. Sustainability 2025, 17, 4150. [Google Scholar] [CrossRef]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Lotito, D.; Orazzo, G.; Matera, R.; Musco, N.; Staropoli, A.; Vinale, F. Beneficial fungal microbes as novel ecosustainable tools for forage crops. Acta IMEKO 2024, 13, 1–5. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Francioli, D.; Strack, T.; Dries, L.; Voss-Fels, K.P.; Geilfus, C.M. Roots of resilience: Optimizing microbe-rootstock interactions to enhance vineyard productivity. Plants People Planet 2024, 7, 524–535. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial-fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Young, E.H.; Unc, A. A review of nematodes as biological indicators of sustainable functioning for northern soils undergoing land-use conversion. Appl. Soil. Ecol. 2023, 183, 104762. [Google Scholar] [CrossRef]
- Campos-Herrera, R.; Půža, V.; Jaffuel, G.; Blanco-Pérez, R.; Čepulyte Rakauskiene, R.; Turlings, T. Unraveling the intraguild competition between Oscheius spp. nematodes and entomopathogenic nematodes: Implications for their natural distribution in Swiss agricultural soils. J. Invertebr. Pathol. 2015, 132, 216–227. [Google Scholar] [CrossRef]
- Castro-Ortega, I.R.; Caspeta-Mandujano, J.M.; Suárez-Rodríguez, R.; Peña-Chora, G.; Ramírez Trujillo, J.A.; Cruz-Pérez, K.; Sosa, I.A.; Hernández-Velázquez, V.M. Oscheius myriophilus (Nematoda: Rhabditida) isolated in sugar cane soils in Mexico with potential to be used as entomopathogenic nematode. J. Nematol. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Jamal, W.; Somvanshi, V.S.; Chauhan, K.; Mumtaz, S. Description of Oscheius indicus n. sp. (Rhabditidae: Nematoda) from India. J. Nematol. 2019, 51, e2019-04. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S.; Tóth, T.; Ádám, S.; Lakatos, T.; Majić, I. Discovery of Oscheius myriophilus (Nematoda: Rhabditidae) in Gastropods and Its Similar Virulence to Phasmarhabditis papillosa against Arion vulgaris, Deroceras reticulatum, and Cernuella virgata. Agronomy 2023, 13, 1386. [Google Scholar] [CrossRef]
- Wei, K.; Wang, J.; Yuan, C.; Tang, J.; Zhu, B. Relationships of bacterial-feeding nematodes, phosphatase-producing bacteria, phosphatase activity and their effects on soil organic phosphorus mineralization under straw return. Appl. Soil. Ecol. 2024, 196, 105280. [Google Scholar] [CrossRef]
- Li, Y.; Tang, K.; Zhang, L.; Zhao, Z.; Xie, X.; Chen, C.T.A.; Wang, D.; Jiao, N.; Zhang, Y. Coupled carbon, sulphur, and nitrogen cycles mediated by microorganisms in the water column of a shallow-water hydrothermal ecosystem. Front. Microbiol. 2018, 9, 406029. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Ei Mon Myo, E.M.; Boutaj, H.; Guerra Sierra, B.E.; et al. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: Advances in soil, plant, and microbial multifactorial interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Silva, V.; Brito, I.; Alexandre, A. The Vineyard Microbiome: How Climate and the Main Edaphic Factors Shape Microbial Communities. Microorganisms 2025, 13, 1092. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, X.; Li, X.; Zhang, Y.; Jiang, L.; Li, J.; Guan, Z.; Cai, Y.; Liao, X. The rhizospheric microbial community structure and diversity of deciduous and evergreen forests in Taihu Lake area, China. PLoS ONE 2017, 12, e0174411. [Google Scholar] [CrossRef]
- Lee, J.H.; Anderson, A.J.; Kim, Y.C. Root-Associated Bacteria Are Biocontrol Agents for Multiple Plant Pests. Microorganisms 2022, 10, 1053. [Google Scholar] [CrossRef]
- Kumar, K.K.; Dara, S.K. Fungal and bacterial endophytes as microbial control agents for plant-parasitic nematodes. Int. J. Environ. Res. Public Health 2021, 18, 4269. [Google Scholar] [CrossRef]
- Gade, R.M.; Koche, M.D. Enhancing the growth and disease suppression ability of Pseudomonas fluorescens. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier Science Publishers: Amsterdam, The Netherlands, 2022; pp. 351–368. [Google Scholar]
- Hao, Y.; You, Y.; Chen, Z.; Li, J.; Liu, G.; Wen, Y. Avermectin B1a production in Streptomyces avermitilis is enhanced by engineering aveC and precursor supply genes. Appl. Microbiol. Biotech. 2022, 106, 2191–2205. [Google Scholar] [CrossRef]
- Wang, K.; Ke, S.; Fang, W.; Liu, F.; Zhang, Z. Agroactive volatile organic compounds from microbes: Chemical diversities and potentials of application in crop protection. Adv. Agrochem. 2023, 2, 39–57. [Google Scholar] [CrossRef]
- Donald, L.; Pipite, A.; Subramani, R.; Owen, J.; Keyzers, R.A.; Taufa, T. Streptomyces: Still the biggest producer of new natural secondary metabolites, a current perspective. Microbiol. Res. 2022, 13, 418–465. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. The use of plant growth-promoting bacteria to prevent nematode damage to plants. Biology 2020, 9, 381. [Google Scholar] [CrossRef]
- Topalović, O.; Hussain, M.; Heuer, H. Plants and Associated Soil Microbiota Cooperatively Suppress Plant-Parasitic Nematodes. Front. Microbiol. 2020, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Savazzini, F.; Longa, C.M.O.; Pertot, I. Impact of the biocontrol agent Trichoderma atroviride SC1 on soil microbial communities of a vineyard in northern Italy. Soil. Biol. Biochem. 2009, 41, 1457–1465. [Google Scholar] [CrossRef]
- Carrascosa, M.; Sanchez-Moreno, S.; Alonso-Prados, J.L. Effects of organic and conventional pesticides on plant biomass, nematode diversity and the structure of the soil food web. Nematology 2015, 17, 11–26. [Google Scholar] [CrossRef]
- Moosavi, M.R.; Zare, R. Fungi as biological control agents of plant-parasitic nematodes. In Plant Defence: Biological Control, Progress in Biological Control; Mérillon, J., Ramawat, K., Eds.; Springer: Dordrecht, The Netherlands, 2020; pp. 333–384. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.; Van Wees, S.C. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef]
- Medeiros, H.A.; Araújo Filho, J.V.; Freitas, L.G.; Castillo, P.; Rubio, M.B.; Hermosa, R.; Monte, E. Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride. Sci. Rep. 2017, 7, 40216. [Google Scholar] [CrossRef]
- Csótó, A.; Kovács, C.; Pál, K.; Nagy, A.; Peles, F.; Fekete, E.; Karaffa, L.; Kubicek, C.P.; Sándor, E. The Biocontrol Potential of Endophytic Trichoderma Fungi Isolated from Hungarian Grapevines, Part II, Grapevine Stimulation. Pathogens 2022, 12, 2. [Google Scholar] [CrossRef]
- Yilmaz, T.; Ates, F.; Turan, M.; Hatterman-Valenti, H.; Kaya, O. Dynamics of Sugars, Organic Acids, Hormones, and Antioxidants in Grape Varieties ‘Italia’ and ‘Bronx Seedless’ during Berry Development and Ripening. Horticulturae 2024, 10, 229. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Fortes, A.M.; Pais, M.S.; Choi, Y.H.; Verpoorte, R. Monitoring biochemical changes during grape berry development in Portuguese cultivars by NMR spectroscopy. Food Chem. 2011, 124, 1760–1769. [Google Scholar] [CrossRef]
- Li, R.; Liu, K.; Liang, Z.; Lu, H.; Wang, T.; An, J.; Wang, Q.; Li, X.; Guan, Y.; Xiao, Y.; et al. Unpruning improvement the quality of tea through increasing the levels of amino acids and reducing contents of flavonoids and caffeine. Front. Nutr. 2022, 9, 1017693. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Arneborg, N.; Toldam-Andersen, T.B.; Petersen, M.A.; Bredie, W.L. Effect of sequential fermentations and grape cultivars on volatile compounds and sensory profiles of Danish wines. J. Sci. Food Agric. 2017, 97, 3594–3602. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]

| 30 cm | 60 cm | |
| Fungi | (total fungal CFU: 1.83 105 cfu/g ± 2.46 × 104) | (total fungal CFU: 1.25 × 105 cfu/g ± 1.23 × 104) |
| Albifimbria verrucaria | Aspergillus aeropaus | |
| Aspergillus awamori | Clonostachys rosea | |
| A. kevei | Fusarium circinatum | |
| A. luchuensis | Neonectria radicicola | |
| Clonostachys rosea | Ochroconis tshawytschae | |
| C. rosea f. catenulata | P. ardesiacum | |
| Didymella rosea | P. senticosum | |
| F. equiseti strain D3 | Penicillium sp. | |
| F. oxysporum | ||
| F. oxysporum isolate F34 | ||
| F. oxysporum f. sp. vasinfectum | ||
| F. solani FS-4P | ||
| Penicillium sp. | ||
| Pleosporales sp. | ||
| Rhizopus oryzae | ||
| Sirastachyi castanedae | ||
| Talaromyces pinophilus | ||
| Bacteria | (total bacterial CFU: 1.34 × 106 cfu/g ± 7.66 × 105) | (total bacterial CFU: 1.56 × 106 cfu/g ± 1.01 × 105) |
| Acineobacter sp. | Bordetella sp. | |
| Ensifer adhaerens | Pseudomonas mandelii | |
| Phyllobacterium ifriglyense | ||
| P. kilonensis | ||
| P. mandelii | ||
| P. salicylatoxidans | ||
| Pseudomonas sp. | ||
| Variovorax boronicumulans |
| Compound | p | Regulation lnFC vs. Ctrl Group | ||
|---|---|---|---|---|
| 6PP | M10 | T22 | ||
| D-(-)-Fructofuranose, 5TMS | 0.00825 | −0.4727 | −1.4630 | 0.7274 |
| D-Fructose, 5TMS | 0.00168 | −0.5724 | −8.4206 | −1.5036 |
| α-Tocopherol, TMS | 0.00268 | −9.0224 | −9.0224 | −9.0224 |
| Sucrose, 8TMS | 0.00890 | −9.3990 | −5.5547 | 4.8418 |
| 1-Octacosanol, TMS | 0.01940 | −6.3610 | −6.3610 | −6.3610 |
| Ascorbic acid, 4TMS | 8.45 × 10−4 | 4.2507 | 2.9247 | 15.7171 |
| Palmitic Acid, TMS | 0.02052 | −7.5909 | −3.2879 | −9.0512 |
| Fumaric acid, 2TMS | 0.00489 | −0.2869 | 10.7907 | 9.0419 |
| D-Psicopyranose, 5TMS | 0.03078 | −3.6017 | −3.6017 | 4.1862 |
| Hexacosanoic acid, TMS | 0.01730 | −5.7551 | −7.5577 | −7.5577 |
| L-Threonic acid, 4TMS | 0.03779 | −3.5858 | −7.3303 | −7.3303 |
| E-15-Heptadecenal | 0.01922 | −5.0534 | −5.0534 | −5.0534 |
| m-Hydroxybenzoic acid, TMS | 0.02732 | 1.5677 | 0.07934 | 8.3108 |
| Pregnane, TMS | 0.01922 | 4.7985 | −5.9307 | −3.5480 |
| Phenoxyethanol, TMS | 0.01802 | 6.2527 | 1.5192 | −1.6813 |
| Nonanoic acid, TMS | 2.63 × 10−4 | 7.6230 | −1.4803 | −1.8459 |
| Compound | p | Regulation lnFC vs. Ctrl Group | ||
|---|---|---|---|---|
| 6PP | M10 | T22 | ||
| N,N-Bis (2-hydroxyethyl) dodecanamide, 2TMS | 0.001622 | 2.9861 | 3.4179 | 3.0195 |
| D-Tagatose, 5TMS | 0.001303 | −2.4122 | −1.7898 | −1.5254 |
| Fructofuranose, 5TMS | 0.01134 | −2.6365 | 3.8001 | 3.0195 |
| Glucose, 5TMS | 0.001364 | 3.7694 | ns | ns |
| Allofuranose, 5TMS | 0.003398 | 1.3820 | ns | 3.6132 |
| Tartaric acid, 4TMS | 0.003418 | 3.9502 | 3.8215 | 2.8845 |
| Malic acid, 3TMS | 0.002851 | 6.2322 | 3.6132 | 3.1609 |
| Methylsuccinic acid, 2TMS | 0.003211 | 7.1832 | 6.4005 | 6.2037 |
| Glycerol, 3TMS | 0.001463 | −1.2569 | −3.4210 | −3.0801 |
| 3-Hydroxybenzoic acid, 2TMS | 0.004762 | −8.6090 | −6.2364 | −6.2815 |
| Flavan, 5TMS | 0.01142 | 7.9556 | 7.3708 | 7.6264 |
| Epigallocatechin, 6TMS | 0.006272 | ns | 3.3885 | 3.2239 |
| Catechine, 5TMS | 0.002674 | ns | 7.3846 | 8.4570 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, I.; d’Errico, G.; Troiano, E.; Gigliotti, C.; Vassetti, A.; Lotito, D.; Staropoli, A.; Parrella, G.; d’Errico, F.P.; Lorito, M.; et al. Combined Metagenomic and Metabolomic Analysis to Evaluate the Comprehensive Effects of Trichoderma and 6PP on Vineyard Ecosystems. Agriculture 2025, 15, 1441. https://doi.org/10.3390/agriculture15131441
Dini I, d’Errico G, Troiano E, Gigliotti C, Vassetti A, Lotito D, Staropoli A, Parrella G, d’Errico FP, Lorito M, et al. Combined Metagenomic and Metabolomic Analysis to Evaluate the Comprehensive Effects of Trichoderma and 6PP on Vineyard Ecosystems. Agriculture. 2025; 15(13):1441. https://doi.org/10.3390/agriculture15131441
Chicago/Turabian StyleDini, Irene, Giada d’Errico, Elisa Troiano, Claudio Gigliotti, Anastasia Vassetti, Daria Lotito, Alessia Staropoli, Giuseppe Parrella, Francesco P. d’Errico, Matteo Lorito, and et al. 2025. "Combined Metagenomic and Metabolomic Analysis to Evaluate the Comprehensive Effects of Trichoderma and 6PP on Vineyard Ecosystems" Agriculture 15, no. 13: 1441. https://doi.org/10.3390/agriculture15131441
APA StyleDini, I., d’Errico, G., Troiano, E., Gigliotti, C., Vassetti, A., Lotito, D., Staropoli, A., Parrella, G., d’Errico, F. P., Lorito, M., & Vinale, F. (2025). Combined Metagenomic and Metabolomic Analysis to Evaluate the Comprehensive Effects of Trichoderma and 6PP on Vineyard Ecosystems. Agriculture, 15(13), 1441. https://doi.org/10.3390/agriculture15131441









