Abstract
What is the potential impact on carbon storage of the native and introduced tree species commonly associated with coffee in the central Peruvian Amazon? Coffee is a pivotal crop within the Peruvian economy. Nevertheless, the establishment of new plantations—driven by the subsistence needs of smallholder farmers—has led to expansion into forested areas. Given the significance of this crop and the demonstrated ecosystem benefits of agroforestry systems (AFSs), the aim of this study was to evaluate the influence of native and introduced shade tree species on carbon storage in coffee plantations. This study was observational and exhibited characteristics of an unbalanced incomplete block design. Agroforestry systems (AFSs) with shade tree species such as Inga, Retrophyllum rospigliosii, Eucalyptus and Pinus, and three unshaded coffee plantations, were included in this study. The total carbon stored in each AFS was higher than in unshaded coffee plantations. Soil contributed between 47% and 91% to total carbon storage, shade trees (24–46%), coffee (2–7%), leaf litter (0.6–1.9%) and shrubs and herbaceous plants (0.02–0.3%). The AFS with R. rospigliosii achieved the highest carbon storage with 190.38 Mg ha−1, highlighting the compatibility of this species with coffee plantations, as well as its positive effect on climate change mitigation in deforested areas.
1. Introduction
World coffee demand has exhibited an upward trend in recent years, increasing from 9,534,000 Mg to 9,870,000 Mg of Arabica and Robusta coffee [1]. Peru is the fifth largest supplier of Arabica coffee worldwide (3.9% of global production) [1], with a production of 3.4 million 60 kg bags (204,000 Mg). Coffee is one of Peru’s main export products [2] and ranks third among the crops with the largest planted area with 79,000 km2 nationally, after potato and corn [3]. This particular crop involves more than 289,000 registered producers [3]. In the coffee-growing areas of the Peruvian central Amazon, the Junín and Pasco regions produce 27% of Peruvian coffee [4]. The Junín region is the second largest in terms of the area dedicated to coffee with, more than 157,000 ha; in contrast, the Pasco region with more than 12,000 hectares occupies the eighth largest. Both regions collectively encompass more than 59,000 coffee producers [3].
Despite the economic importance of coffee, the average yield remains low, prompting the expansion of new plantations into forested areas. The area under coffee cultivation in Peru increased from 399,000.6 ha to 422,000 ha, in the period of analysis from 2013 to 2022 [5]. Under these conditions, coffee cultivation has become a substantial driver for deforestation [6], by subsistence needs, and the lack of alternative sources of income. Although the global trend of deforestation has slowed compared to past decades [7], in Peru more than 3 million ha of Amazonian and dry forests have been deforested from 2021 to 2023 [8]. The deforestation of these regions has been demonstrated to inflict considerable harm and augment the vulnerability of ecosystems to changes in biotic and abiotic factors [9], in addition to increasing national emissions, which have risen from 20,000.9 Mg CO2 in 1990 to 53,000.3 Mg CO2 in 2019 [10]. The phenomenon of climate change is exerting its influence on agricultural systems with implications for the entire food system and for society around the world [11]. It poses a challenge to farmers maintaining future levels of agricultural production [12]. Climate-driven changes in coffee farming systems have notable implications for management decisions and farmer livelihoods [13].
Various studies have demonstrated that agroforestry systems (AFSs) are an effective strategy not only for mitigating environmental impacts but also for enhancing the profitability of priority agricultural crops within the Peruvian context [14,15]. The environmental benefits of carbon storage are substantiated by research in different regional and interregional contexts; Ref. [16] estimated 9 Mg C ha−1 for AFSs in semi-arid regions and 21, 50 and 63 Mg C ha−1 for sub-humid, humid and temperate areas, respectively. In the context of Mexican soil, the composition of soil C was influenced by tree age and the useful life of trees [17]. In the Amazon, AFSs also contributed to the mitigation of erosion, the conservation of biodiversity and the improvement of soil nutrient recycling [18]. In terms of economic benefits, agricultural diversification within AFSs enhances productivity and profitability, representing a viable and proven economic alternative for farmers [19].
A notable strength of coffee plantations is the potential for associativity with AFSs, forming an encouraging ecosystem for carbon storage. Consequently, coffee systems integrated with shade trees could have great potential for carbon sequestration as a means of mitigating and adapting to climate change. This crop is well-suited to cultivation alongside forest species, offering additional benefits through the trees [20], and the maintenance of biodiversity [4].
The trees most frequently used in agroforestry systems (AFSs) with coffee in Peru are native species of the genus Inga [21,22]. These trees have been shown to fix about 100 kg ha−1 of atmospheric nitrogen [23]; they are fast-growing, can withstand pruning more than once a year and multiply easily by seed [24]. Likewise, Retrophyllum rospigliosii, a native species that adapts to agroforestry systems, especially in association with coffee, is distinguished by its excellent quality of wood, small crown and straight trunk [25]. In addition, introduced species of the genus Eucalyptus and Pinus. R. rospigliosii is an endemic species to the tropical Andes of South America, found in countries such as Peru, Colombia, Bolivia, Venezuela, and Ecuador. This habitat is of significant ecological value, serving as a refuge for wildlife and facilitating the colonization of soils with low nutrient availability. This process facilitates the establishment of various Andean species beneath its canopy [26]. Additionally, it produces one of the most valuable and high-quality timbers; however, the species is currently threatened by indiscriminate logging practices [26,27,28]. In natural habitats, its trunk can exceed 15 m in height, highlighting its potential for carbon sequestration [27]. Despite these attributes, R. rospigliosii has attracted limited attention within coffee agroforestry systems due to the limited availability of material for its propagation, its slow vegetative growth and a general preference for simpler agricultural systems [25,29,30]. In contrast, there has been greater promotion and interest in Inga and Eucalyptus, whose species exhibit broader adaptability and prevalence. This trend has encouraged the use of fast-growing introduced species, such as Eucalyptus spp. and Pinus spp., valued for their rapid shading capacity, adaptability to variable climatic conditions, including drought and irregular rainfall, and their commercial potential for timber and other products [31,32]. These characteristics make them particularly attractive options for agroforestry systems.
Recent meta-analyses highlight the benefits of agroforestry systems in increasing soil organic carbon and dissolved organic carbon by more than 10%. Moreover, they reveal a notable improvement in the rates of change in stored carbon by diversified AFSs, compared to simple agrosystems [33,34]. At the global level, it is estimated that increasing tree coverage on agricultural land could capture more petagrams of carbon (PgC), with South America being the region with the greatest sequestration capacity, followed by Southeast Asia and western and central Africa [35]. However, there are still gaps and limited information regarding agroforestry systems in the central Peruvian Amazon. This study will contribute to the understanding of the benefits of native or introduced shade trees in coffee plantations and their ability to capture carbon as part of an ecosystem service. In order to guarantee the sustainability of coffee cultivation and the restoration of deforested areas intended for this crop. As noted by [36], the long-term viability of unshaded coffee cultivation is limited due to changing weather patterns and rising temperatures associated with climate change. Thus, quantifying carbon storage (Mg ha−1) in coffee plantations with shade trees is essential for recognizing and preserving these species as key contributors to climate change mitigation. Additionally, shade trees benefit coffee cultivation by creating a favorable microclimate and providing other advantages [12,37]. Our results indicate that the native species Retrophyllum rospigliosii (ulcumano) exhibited a superior carbon storage capacity, highlighting the value of incorporating this native tree into agroforestry systems in the central Peruvian Amazon.
2. Materials and Methods
2.1. Study Setup
This present study was conducted in the districts of the Villa Rica-Pasco region, and in the San Luis de Shuaro and Perené-Junín regions, Peru (Figure 1). A total of ten AFSs and three control coffee plantations (unshaded coffee) were evaluated. Each AFS and control was subdivided into three sections, resulting in a total of 39 observational units. The characteristics of the plots included coffee in the production stage, a plot with a minimum of one hectare in size and shade trees with a uniform distribution. The selection of plots was executed in according to the Guide for the Determination of Carbon in Small Rural Properties [38]. Figure 1 can be downloaded as Supplementary Materials.
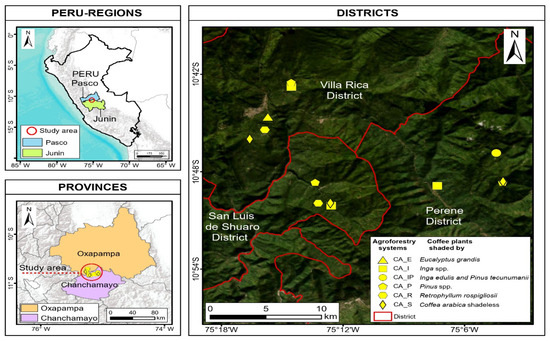
Figure 1.
Distribution of agroforestry systems and controls in Villa Rica, San Luis de Shuaro, and Perené.
The area was stratified based on topographic characteristics, with three subplots within each observational plot. All three subplots were nested in a concentric circle [39]. Each subplot had a defined area and specific purpose: Subplot 1 (1.13 m2) was used to evaluate shrubs, herbaceous plants, leaf litter and soil bulk density. Subplot 2 (28.27 m2) was designated for assessing coffee plants (Coffea arabica L.). Finally, Subplot 3 (314.16 m2) was to evaluate shade trees and collect soil sample for determining organic carbon content (Figure 2).
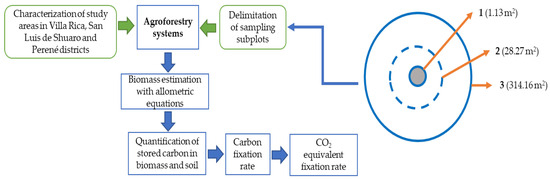
Figure 2.
Flowchart of carbon storage estimation in agroforestry systems.
2.2. Description of Study Area
The Villa Rica district is classified as part of the tropical premontane humid forest life zone (bh-PMT), based on the Holdridge World Life Zone Classification System [40]. This zone exhibits maximum and minimum temperatures ranging from 23.2 to 13.4 °C, relative humidity of 74.7% and average annual precipitation of 2591.5 mm year−1 [41]. One of the primary economic drivers is the cultivation of shade-grown coffee for export, in addition to livestock farming [21]. In the context of agroforestry systems, CA_I and CA_P presented a sandy loam texture with a very acidic pH (4.80). CA_E was found to have a loam soil with a notable acidic pH (5.00). CA_R presented a sandy loam soil with a pH of 6.20 (slightly acid). Finally, CA_S exhibited a sandy loam texture with a very strongly acidic pH (5.00). Coffee varieties: Catimor, Caturra, Obatá and Costa Rica 95.
The San Luis de Shuaro district, located in the very humid premontane tropical forest life zone (bmh-PMT) [40], with maximum and minimum temperatures from 32.9 to 20 °C. The district also experiences an average annual relative humidity of 74.7% and average annual precipitation of 1861.5 mm year−1 [41]. For this district, coffee cultivation represents a significant economic resource. In the San Luis de Shuaro district, the soil of the SLS-CG showed a pH of 4.20 (extremely acidic) and a sandy loam texture. CA_P was found to have a loam soil with a pH of 5.00. The CA_R area was characterized by sandy loam soil with a pH of 4.90. CA_S: The pH was 4.20 (extremely acidic) and the texture was classified as sandy clay loam. Coffee varieties: Caturra, Bourbon and Cluscateco.
In the Perené district, three plots were classified as bh-PMT and one plot was designated as tropical humid forest (bh-T) [40]. Perené experiences maximum and minimum temperatures between 32.9 and 20.0 °C, with a lative humidity of 74.7% and precipitation of 1861.5 mm year−1 [41]. The predominant economic source of this district is agriculture, with a focus on shade-grown coffee and citrus crops. In Perené, CA_I had loam soil with a pH of 6.00 was observed, indicating a moderately acidic condition. CA_P showed a sandy clay loam texture with a pH of 3.80 (extremely acidic). CA_IP exhibited clay loam with a pH of 4.80, indicating a markedly acidic nature. Finally, CA_S presented sandy loam soil with a pH of 4.10 (extremely acidic). Catuaí, Costa Rica 95 and Geisha were identified in the study.
In general, farmers provided information regarding the age of the tree species and the coffee crop, and a field study was conducted to collect data on the system, planting distance and density. With respect to the age of the pruned coffee plants, it was considered based on the time elapsed since the previous pruning (Table 1).

Table 1.
Characteristics of agroforestry systems.
2.3. Taxonomic Identification of Shade Trees
The taxonomic identification of the shade trees was carried out collecting botanical samples (leafy branches and reproductive structures), complemented with photographic images obtained in situ. The samples were analyzed in the Herbarium of the Department of Biology (MOL) of the La Molina National Agrarian University, according to APG IV [42] and Gymnosperms [43,44]. The identified species were named in Table 2. The results of the taxonomic identification are available in the Supplementary Materials.

Table 2.
Taxonomic identification of shade trees and location of agroforestry systems.
2.4. Assessment of Stored Carbon
The quantification of stored carbon in the AFS was achieved by estimating the shrub and herbaceous biomass, leaf litter and aboveground and root biomass using allometric equations for shade tree species and coffee. In addition, an estimation was made of the soil carbon storage. The diameter at breast height (DBH) at 130 cm from ground level was measured for trees and pruned coffee, and the diameter at 15 cm from the ground (D15) for unpruned coffee; by using a digital vernier caliper or measuring tape. The tree height was also calculated using a SUUNTO clinometer [38,39].
In Subplot 1, the shrub and herbaceous biomass (SHB) and leaf litter biomass (LLB) were measured in Mg ha−1 (1). All vegetation (shrubs smaller than 2.5 cm in diameter, grasses and other weeds) present within the circular frame was cut at ground level [45]. The sample underwent a drying process at a temperature of 75 °C for 72 h until it reached a constant dry weight [45,46] in the Soil, Water and Foliar Laboratory (LABSAF), of the Pichanaki Agrarian Experimental Station, at the National Institute for Agrarian Innovation (INIA), in Junín, Peru. Regarding LLB, all material with a size greater than 2 mm and smaller than the minimum diameter of 10 cm for dead wood (arranged on the soil surface) [47] was collected. The mulch layer, leaves, fruits, seeds, twigs, branches and stubble were included in different states of decomposition [39,45]. The samples were exposed to a drying process for 48 h until a constant dry weight [39,45,46] was reached. After calculating the biomass as modified from [48], stored carbon was quantified by multiplying the biomass values by the carbon fraction (CF), being on average 0.44 for shrubs and herbaceous plants [49] and 0.37 for leaf litter [47].
where DWS = dry weight of the collected sample (g); FWS = fresh weight of the collected sample (g); TFW = total fresh weight (g); A = sampling area (1.13 m2), 0.01 = conversion factor.
In Subplot 2, the aboveground biomass (AGB) (kg plant−1) of unpruned coffee plants was measured (2) [50], mentioned by [39], as well as that of pruned coffee plants (AGBp) (3) [37,51,52]. Considering the coffee plant as a perennial shrub [53], the calculation of its root biomass considered the root-to-shoot ratio, defined as the belowground biomass/aboveground biomass, with a value of 0.40 for tropical shrubs [47]. The biomass values were multiplied by CF, being 0.4718 for estimating the stored carbon in coffee plants [51].
where R2 = 0.93 coefficient of determination for AGB; R2 = 0.9455 for AGBp; R2 = 0.84 for Rb; D15 (cm) = diameter at 15 cm from the ground; DBH (cm) = diameter at breast height at 130 cm from ground level.
In Subplot 3, we estimated the aboveground and root biomass of tree species by the following formulas: for Inga species (4) [50], cited by [39]; Pinus patula (5) [54,55]; Pinus sp. applied to P. tecunumanii (6) [40,41]; Eucalyptus grandis (7) [56]; general formula applied to Retrophyllum rospigliosii (8) [45,48,57]. The root biomass (Rb) in Mg ha−1 was calculated by the allometric Equation (9), a formula applicable to tropical areas [58] recommended by [59]. All biomass values were multiplied by CF, being 0.47 for shade trees [47].
where AGB = aboveground biomass (kg tree−1), R2 = 0.94 for Inga and Pinus sp. applied to P. tecunumanii, R2 = 0.98 for Pinus patula, R2 = 0.99 for Eucalyptus grandis, DBH (cm) = diameter at breast height at 130 cm from ground level, Ln = natural logarithm, H = total height (m), exp = “e raised to the power of” and AGB from (9) in Mg ha−1.
Soil carbon storage was calculated using the bulk density (10) [45], soil volume weight (11) [46] and percentage of organic carbon (12) [46,60,61]. Bulk density was determined calculated by collecting samples from three soil horizons (0–10, 10–20 and 20–30 cm) [38,45] using metal cylinders of known volume. The samples were then oven-dried at 105 °C for 24 h, until a constant weight was obtained [38,46]. Soil volume weight was estimated using the total depth of the 0–30 cm soil horizon [38,39,47]. The percentage of organic carbon (%OC) was calculated from random soil samples (1 kg) extracted at a depth of 0–30 cm [39], using a sampling auger [38]. This sampling depth is justified by the greater influence of land use on the surface parameters [62,63]. Measures aimed at minimizing carbon loss and/or maximizing carbon retention in agricultural soils are predominantly focused on the surface soil layers (0–30 cm horizon) [63,64]. Soil organic matter (%OM) was analyzed by the wet oxidation chemical method described by [65]. Based on all gathered data, the soil carbon (SOC) (13) was calculated [45,48].
where SBD = bulk density of soil (g cm−3) of (10), NDWS = net dry weight of soil (g), CV = cylinder volume (constant) (cm3). SBD of (11) in Mg m−3, DSH = depth of soil horizon (m), 10,000 = area of one hectare of land (constant), %OC = soil organic carbon; %OM = soil organic matter, 1.724 = Van Bemmelen coefficient, WSV = weight of soil volume (Mg ha−1), SOC = soil carbon (Mg ha−1).
2.5. Carbon Fixation Rate and Carbon Dioxide Equivalent (CO2e)
The carbon fixation rate (CFR) (14) was calculated from the relationship between the carbon stored in the biomass (aboveground and root) and the average ages of the forest species and coffee plants. The carbon dioxide equivalent fixation rate (15) was estimated by multiplying the CFR by the molecular weight ratio constant of CO2 and C, having a value of 44/12 or 3.67 as given by [38,46,47,66].
where CFR = carbon fixation rate (Mg C ha−1 year−1); = carbon stored in aboveground biomass (Mg ha−1); = carbon stored in root biomass (Mg ha−1); = average age of the evaluated component (years); tCO2e = carbon dioxide equivalent fixation rate (Mg CO2e ha−1 year−1); 3.67 = molecular weight ratio of CO2 and C.
tCO2e = CFR × 3.67
2.6. Statistical Analysis
This observational study followed an unbalanced incomplete block design (UIBD). Consequently, generalized linear mixed models (GLMMs) were applied—an appropriate statistical approach that enables the simultaneous modeling of fixed effects (e.g., agroforestry system and control variables) and random effects (e.g., districts). The inclusion of random effects is suitable to account for simple or complex nesting structures, spatial or temporal correlations or when only a random sample of a given factor is available. The adopted models were as follows:
In both expressions, denotes the overall mean, represents the vector of coefficients associated with the control variables, corresponds to the fixed effects (types of agroforestry systems with coffee) and to the random effects (districts) and represents the residual error. Accordingly, Equation (16) corresponds to an ANOVA model that does not include control variables, whereas Equation (17) represents an ANCOVA model that incorporates at least one control variable. The control variables considered in this study were the coffee planting density (DSC), shade tree planting density and shade tree age. These variables were retained in the final model if they were statistically significant and exhibited a multicollinearity level of less than 5.
Data analysis was conducted using RStudio (version 4.4.1), developed by the R Core Team and maintained by the R Foundation for Statistical Computing, Vienna, Austria. The user interface RStudio was provided by Posit, PBC, Boston, MA, USA. Model fitting was performed using the glmmTMB package (generalized linear mixed models using the Template Model Builder) [67]. Multiple comparisons were performed using emmeans, model selection was guided by MuMIn [68], and model assumptions were evaluated using DHARMa [69]. For each variable, three to four models with different distributional assumptions were fitted. The optimal model was selected based on the corrected Akaike information criterion (AICc). The selected models assumed lognormal, gamma or beta distributions and satisfied the assumptions of uniformity, the absence of dispersion and the absence of outliers, verified in the residuals. Multiple comparisons were made using the false discovery rate (FDR) correction method with a significance threshold of 0.05. The general data and statistical analysis can be downloaded as Supplementary Materials.
3. Results
3.1. Biomass
The performance of agroforestry systems (AFSs) can be assessed through their biomass, as it reflects not only the structural growth of vegetation but also the system’s capacity for carbon storage and the provision of ecosystem services. The accumulation biomass is contingent upon the composition and structure of the AFS, making its analysis imperative for comprehending system functionality. To identify how biomass varies according to the type of AFS, statistical models were fitted for each compartment. Table 3 presents the selection of the model based on the AICc, which indicates that the lognormal distribution provides the optimal fit to the empirical data for the components of the coffee aboveground biomass (ACB), coffee root biomass (RCB) and leaf litter biomass (LB). Conversely, the gamma distribution emerged as a more suitable model for the aboveground biomass of shade trees (ATB), root biomass of shade trees (RTB), shrub and herbaceous biomass (HSB) and total biomass. These models enabled the evaluation of the AFS’s effects on the different biomass components.

Table 3.
Biomass components according to agroforestry systems: fixed factors, distribution selection and random factors.
The deviance values generated by the selected models were statistically significant (p < 0.05), indicating that the AFS had a significant effect on the evaluated compartments. The estimate of the variance attributed to the districts ranged from 0.00 to 0.36, indicating differences in the contribution of the random effect to the different components of the biomass. The models adjusted for HSB and LB showed zero variance ( = 0) between districts, indicating that fixed effects entirely explained the observed variability in these components. In contrast, ACB demonstrated a higher spatial heterogeneity ( = 0.36), indicating that its variation was attributable not only to fixed factors but also to district-specific characteristics.
The estimated means by the selected models revealed significant differences in biomass across its various components depending on the agroforestry system (Table 4). The AFS with Pinus exhibited the highest biomass accumulation in ACB and RTB (20.72 and 5.05 Mg ha−1, respectively), comparable to those observed in Inga + Pinus and significantly higher than in the other evaluated systems. In contrast, the AFS with Eucalyptus stored the lowest amounts of these components (6.93 and 1.88 Mg ha−1, respectively). However, the system with Eucalyptus accumulated the greatest biomass ATB (146.48 Mg ha−1) and RTB (28.41 Mg ha−1), with values analogous to those observed in Inga + Pinus and significantly higher than the rest of the assessed systems. Shadeless C. arabica showed no biomass storage in these compartments due to the absence of trees in its structural composition. Additionally, Eucalyptus displayed the highest biomass accumulation in LB, followed by Inga and Inga + Pinus with intermediate values. The lowest LB values were recorded in R. rospigliosii (3.61 Mg ha−1), Pinus (4.02 Mg ha−1) and C. arabica shadeless (3.26 Mg ha−1), with no significant differences detected among them. The unshaded coffee plants and Pinus system stored the highest biomass in the HSB component (0.91 and 0.63 Mg·ha−1, respectively), comparable to the values recorded in R. rospigliosii and Inga + Pinus and significantly higher than those in Inga and Eucalyptus systems, with the latter showing the lowest HSB biomass accumulation (0.08 Mg ha−1). In regard to the total biomass, the highest recorded value was attained by Inga + Pinus (209.69 Mg ha−1), statistically similar to Eucalyptus and R. rospigliosii. This value was significantly greater than those seen in Inga, Pinus and shadeless C. arabica, with the latter exhibiting the lowest total biomass (24.07 Mg ha−1).

Table 4.
Biomass in different components of the agroforestry system, adjusted by system type in coffee plantations.
The control variable DSC exerted a significant and negative impact on HSB (Figure 3A) ( = –0.32, p = 0.01), suggesting that a one-standard-deviation increase in this variable is associated with an average reduction of 27.39% in HSB. This effect may be due to a greater number of coffee plants, which has the potential to diminish the coverage or presence of secondary vegetation in the understory. In contrast, DSC had a significant positive effect on LB (Figure 3B) ( = 0.12, p = 0.02), indicating that a one-standard-deviation increase in DSC corresponds to an average increase of 12.75% in LB, although to a lesser extent. This may be attributed to the greater contribution of leaf material derived from the higher density of coffee plants.
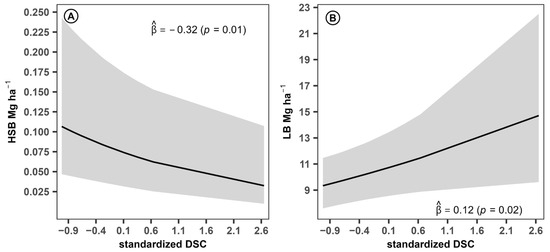
Figure 3.
Estimated effects of control variables on the biomass components of the coffee agroforestry system. HSB: shrub and herbaceous biomass, LB: leaf litter biomass, DSC: coffee planting density. Ⓐ Effect of standardized coffee planting density on shrub and herbaceous biomass. Ⓑ Effect of standardized coffee planting density on leaf litter biomass.
3.2. Carbon
The contribution of agroforestry system components to total carbon storage (Figure 4) indicates that soil organic carbon (SOC) represents the primary carbon reservoir across all evaluated systems. In the unshaded C. arabica system, SOC accounts for over 90% of the total carbon, while it remains the dominant component in shaded tree systems, albeit at relatively lower proportions. This pattern suggests that the inclusion of tree species reduces reliance on soil as the sole carbon reservoir by diversifying carbon storage across compartments.
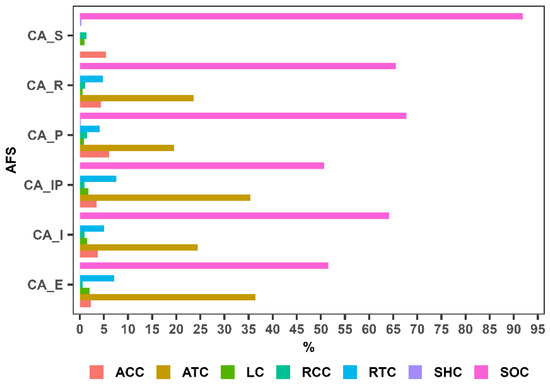
Figure 4.
Contribution of components to total carbon storage in each agroforestry system. AFS: agroforestry systems, ACC: carbon in coffee aboveground biomass, RCC: carbon in coffee root biomass, ATC: carbon in shade tree aboveground biomass, RTC: carbon in shade tree root biomass, SHC: carbon in shrub and herbaceous biomass, LC: carbon in leaf litter biomass, SOC: soil organic carbon.
In systems that incorporate shade trees, the tree aboveground biomass (ATC) contributes more substantially to the total carbon storage. The highest proportions of ATC were observed in the AFS with Eucalyptus (CA_E) (36.40%) and that with Inga + Pinus (CA_I + CA_P) with 35.40%. By contrast, compartments associated with coffee, such as the aboveground biomass (ACC), root biomass (RCC), shrub and herbaceous biomass (SHC), shade tree roots (RTC) and litter (LC), contribute less to the total carbon composition. This pattern suggests that both the presence and type of tree species influence the distribution of carbon among the different compartments of the system.
Given the observation that the system structure appears to influence the internal distribution of carbon, statistical models were fitted to assess the effect of the agroforestry system type on each compartment. Based on the AICc, the lognormal distribution was selected for the ACC, RCC, LC and SOC components; the gamma distribution for the ATC, RTC and SHC; and the normal distribution for total carbon. Model deviance indicated that the type of agroforestry system significantly affects (p < 0.05) carbon storage across these components. The variance between districts revealed a greater degree of heterogeneity in ACC ( = 0.36), indicating a more variable spatial distribution in this component and suggesting that the differences observed between districts are not solely attributed to fixed factors but also to district-specific characteristics. In contrast, LC exhibited no variance between districts ( = 0.00), implying that its variability was entirely explained by the model’s fixed effects (Table 5).

Table 5.
Carbon components according to agroforestry systems: fixed factors, distribution selection and random factors.
The statistical model results revealed significant differences in the estimated means of the AFS, depending on the type of system evaluated (Table 6). Pinus stored the highest carbon content in the ACC and RCC components (9.78 and 2.38 Mg ha−1, respectively), with values similar to the Inga + Pinus system and significantly higher than those in Inga, R. rospigliosii, Eucalyptus and unshaded C. arabica, the latter of which had the lowest carbon stocks (7.41 and 1.85 Mg ha−1, respectively). The Eucalyptus (CA_E) system exhibited the highest carbon accumulation in the ATC and RTC components (68.85 and 13.36 Mg ha−1, respectively), with no statistical differences from the Inga + Pinus system and significantly greater than the other systems. Unshaded C. arabica recorded zero values due to the absence of shade trees in its structure. Furthermore, E. grandis also stored the most carbon in LC (3.92 Mg ha−1), followed by Inga and Inga + Pinus with intermediate values, while the AFSs with R. rospigliosii, P. tecunumanii and unshaded C. arabica showed the lowest accumulations. Unshaded coffee stored the greatest amount of carbon in the SHC component (0.43 Mg·ha−1), with values similar to those observed in Pinus, R. rospigliosii and the Inga + Pinus association, and significantly higher than in Inga and Eucalyptus. Additionally, unshaded coffee sequestered more carbon in SOC (128.70 Mg ha−1), along with R. rospigliosii (129.58 Mg·ha−1). A comparative analysis revealed that these values did not differ significantly from those in Inga, Pinus or Eucalyptus. However, they were significantly higher than those observed in the Inga + Pinus system, which recorded the lowest carbon sequestration (76.95 Mg ha−1). In terms of total carbon, R. rospigliosii stood out in storage (190.38 Mg ha−1), with no significant differences from Inga, Pinus, Eucalyptus and Inga + Pinus, and significantly greater than that of the unshaded C. arabica, which registered the lowest total carbon sequestration amount (138.17 Mg ha−1).

Table 6.
Carbon content (Mg ha−1) in the different components of the AFS, adjusted by the type of system in coffee crops.
The control variable representing coffee planting density (DSC) exhibited a positive and statistically significant effect on the carbon stored in leaf litter biomass ( = 0.12, p = 0.02), (Figure 5), indicating that a one-standard-deviation increase in DSC was associated with an average 12.75% increase in litter carbon storage. Although limited in magnitude, this effect suggests that superior coffee planting density contributes modestly to carbon content, which could be attributed to the deposition of organic residues, particularly through the contribution of leaves and other plant debris.
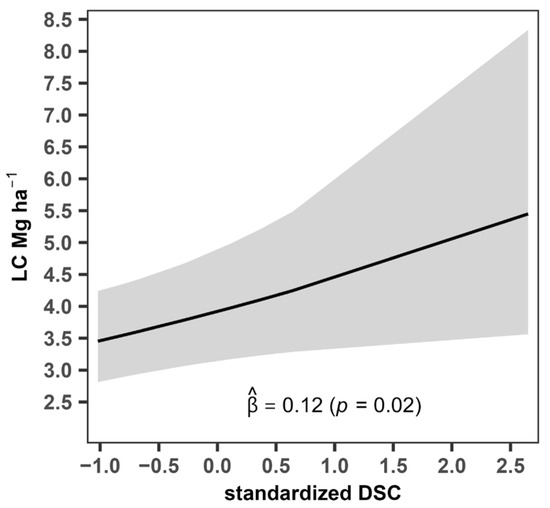
Figure 5.
Estimated effect of coffee planting density on carbon in leaf litter biomass within the coffee agroforestry system. LC: carbon in leaf litter biomass, DSC: coffee planting density.
3.3. Carbon Fixation Rate
The models that best fit the data for the different components of the carbon sequestration rate employed the gamma distribution for the carbon sequestration rate in coffee (CFC) and in shade trees (CFT) and the normal distribution for the total carbon sequestration rate. These models provided more reliable estimates, as evidenced by the AICc values presented in Table 7. The model deviances were statistically significant (p < 0.05), confirming that the agroforestry systems had a significant effect on all components of the carbon sequestration rate, including the total. The variance attributed to the district level was zero for both CFT ( = 0.00) and the total carbon sequestration rate ( = 0.00), suggesting that the agroforestry system entirely explained the variability observed in these components. Nevertheless, the total carbon sequestration rate exhibited a district-level variance of = 0.36, indicating a certain degree of spatial heterogeneity. This suggests that, in addition to the agroforestry system, district-specific characteristics also contributed to the observed variability in this component. The comparison between agroforestry systems is presented in Figure 6.

Table 7.
Components of the carbon fixation rate according to agroforestry systems: fixed factors, distribution selection and random factors.
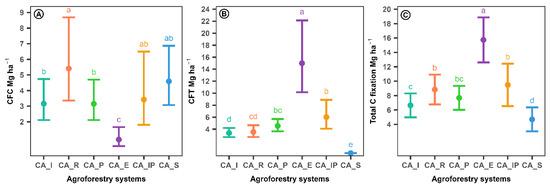
Figure 6.
The 95% confidence intervals for the components of the carbon fixation rate adjusted by agroforestry systems in coffee cultivation. CFC: carbon fixation rate in coffee; CFT: carbon fixation rate in shade trees. abcde: Different letters among agroforestry systems indicate statistically significant differences at the 0.05 significance level, based on multiple comparison testing with FDR correction. Ⓐ Carbon fixation rate in coffee per agroforestry system. Ⓑ Carbon fixation rate in shade trees per agroforestry system. Ⓒ Total carbon fixation rate per agroforestry system.
3.4. Carbon Dioxide Equivalent Fixation Rate
The most plausible models were selected based on the lowest AICc values, identifying the gamma distribution as optimal for the CO2eFC and CO2eFT components (Table 8), while the lognormal distribution provided the best fit for total CO2e fixation. In these models, the deviance of the data was statistically significant (p < 0.05), indicating that agroforestry systems have a significant influence on the aforementioned components. Furthermore, virtually no variance was observed between districts for the CO2eFT component and total CO2e fixation, suggesting high spatial homogeneity between sites and implying that the observed variability in these components is primarily attributable to the type of agroforestry system. In contrast, the CO2eFC component exhibited slight heterogeneity ( = 0.16), suggesting that its variability may be associated not only with the agroforestry system but also with district-specific characteristics.

Table 8.
Components of the carbon dioxide fixation rate according to agroforestry systems: fixed factors, distribution selection and random factors.
Based on the selected statistical models, marginal means were estimated for each agroforestry system evaluated, and multiple comparisons were conducted (Figure 7). The system incorporating R. rospigliosii exhibited the highest equivalent CO2 fixation in the coffee fraction (CO2eFC), with a value of 19.84 Mg ha−1 year−1. This was comparable to the values observed in Inga + Pinus and unshaded C. arabica, but significantly higher than those recorded for Inga + Pinus, which showed intermediate levels, and Eucalyptus, which had the lowest value at 3.15 Mg ha−1 year−1 (Figure 7A). Nevertheless, Eucalyptus demonstrated the highest CO2e fixation in the shade tree component (CO2eFT), reaching 55.03 Mg ha−1 year−1, substantially surpassing all other systems. Inga + Pinus ranked second, with values comparable to Pinus, whereas unshaded C. arabica exhibited no fixation in this compartment due to the absence of trees (Figure 7B). Overall, Eucalyptus recorded the highest total CO2e fixation (57.75 Mg ha−1 year−1), followed by R. rospigliosii and Inga + Pinus, between which no significant differences were observed. These values were notably higher than those recorded for Inga (24.37 Mg ha−1 year−1) and unshaded C. arabica (17.22 Mg ha−1 year−1), the latter showing the lowest total fixation (Figure 7C).
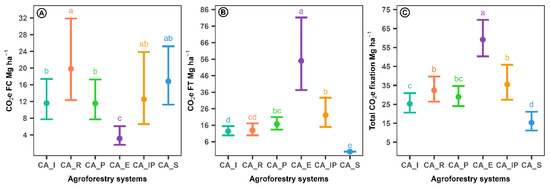
Figure 7.
The 95% confidence intervals for the components of the carbon dioxide fixation rate adjusted for agroforestry systems in coffee cultivation. CO2eFC: Equivalent carbon dioxide fixation rate in coffee; CO2eFT: Equivalent carbon dioxide fixation rate in trees. abcde: Different letters among agroforestry systems indicate statistically significant differences at the 0.05 significance level, based on multiple comparison testing with FDR correction. Ⓐ Equivalent carbon dioxide fixation rate in coffee per agroforestry system. Ⓑ Equivalent carbon dioxide fixation rate in trees per agroforestry system. Ⓒ Total equivalent carbon dioxide fixation rate per agroforestry system.
3.5. Other Variables
Other soil variables evaluated included the carbon percentage, organic matter percentage and porosity percentage, which were modeled using a beta distribution since they were proportional variables (Table 9). These models exhibited the highest likelihood fit to the empirical data based on the AICc and demonstrated statistically significant deviance (p < 0.05), indicating that agroforestry systems influence these soil properties. The estimated variance attributable to the district effect for carbon and organic matter percentages ( = 0.14) suggested that the differences were associated with characteristics specific to each geographical area, in addition to the fixed factors included in the model. In contrast, the porosity values remained consistent across districts, suggesting that they were solely influenced by the type of agroforestry system.

Table 9.
Percentage of carbon, organic matter and porosity according to agroforestry systems: fixed factors, distribution selection and random factors.
The selected models estimated the mean percentages of carbon, organic matter and porosity adjusted for agroforestry systems, revealing statistically significant differences (Table 10). The unshaded C. arabica system recorded the highest percentages of carbon and organic matter (4.79% and 8.27%, respectively), followed closely by the R. rospigliosii system (4.48% and 7.74%), with values statistically similar to each other and to those of the Pinus system. These three systems showed significantly higher values than those observed in Inga, Eucalyptus and the Inga + Pinus system, which did not differ significantly from each other and recorded the lowest concentrations. Regarding soil porosity, the highest values were recorded in the unshaded C. arabica system (66.75%), comparable to those observed in the R. rospigliosii and Pinus systems, but significantly greater than those in Inga, Inga + Pinus and Eucalyptus, with the latter exhibiting the lowest porosity (48.17%), even below that recorded in Pinus.

Table 10.
Carbon content in the different components of the agroforestry system, adjusted by system type in coffee crops.
The control variable DSC exhibited significant and distinct effects on the evaluated soil properties. Specifically, it had a negative impact on the carbon content (Figure 8A; = −0.13, p = 0.02), organic matter (Figure 8B; = −0.13, p = 0.02) and porosity (Figure 8C; = −0.14, p < 0.01). These results suggest that increasing DSC may detrimentally affect the soil quality of the system, potentially due to heightened resource competition, reduced organic matter inputs or increased soil compaction.
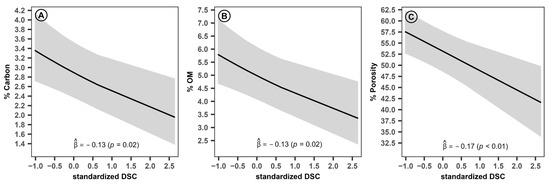
Figure 8.
Estimated effects of control variables on the percentage of carbon, organic matter and porosity in the coffee agroforestry system. DSC: coffee planting density. Ⓐ Effect of standardized coffee planting density on % carbon. Ⓑ Effect of standardized coffee planting density on % organic matter. Ⓒ Effect of standardized coffee planting density on % porosity.
4. Discussion
The findings in the central Peruvian Amazon demonstrate the substantial contribution of AFSs to the carbon stock. This assertion is corroborated by numerous other researchers [70,71,72].
In this sense, the total carbon stored in each AFS was higher than that found in coffee plots without shade trees (Table 6). The contrast found was 52.21 Mg ha−1 with the R. rospigliosii system, 40.3 Mg ha−1 with respect to the Eucalyptus system, and 24.68 Mg ha−1 in the case of Inga system. The authors of [21] found a difference of 62.6 Mg ha−1 in a coffee AFS with Eucalyptus spp. with respect to coffee without shade, in the district of Villa Rica. Additionally, Ref. [73] discovered a contrast of 114.97 Mg C ha−1 with Inga spp. in the Peruvian Amazon. The structural method used to evaluate C storage entailed the stratification and distribution of random points [74]. Each sampling point was formed into three concentric subplots [39] (Figure 2), which allowed the present biomass to be measured in detail, according to its distribution and soil carbon.
The shade trees accounted for 24% to 46% of the total carbon in their respective AFS, quantified with averages values ranging from 39.6 to 82.21 Mg C ha−1 (see Table 6). The values obtained in this study exceed those reported by [21] in species of the genera Inga, Eucalyptus and Pinus (from 27.5 to 57.5 Mg C ha−1). As indicated in the extant literature, variations in carbon storage may be due to planting density, the tree species and its development of biomass [75]. Inga + Pinus, R. rospigliosii and Eucalyptus are particularly noteworthy for their substantial aboveground and radicular biomass and their contribution to the total biomass of AFSs compared to unshaded coffee. Among these, Inga + Pinus exhibit the most prominent results (209.69 Mg ha−1). The results of this study are consistent with those reported by [73], who found a higher biomass in a coffee AFS planted with Inga sp. This is in contrast to polyculture coffee and coffee without shade trees, but only lower than in a secondary forest. The greater aboveground and radicular biomass of shade trees has been demonstrated to have beneficial effects on carbon storage [76,77], a finding that is corroborated by the carbon stock of the trees in Inga + Pinus, R. rospigliosii and Eucalyptus systems (Table 4). In addition to the benefit of incorporating long-lived species into carbon storage [78], shade trees also contribute to microclimate sustainability and production stabilization, which is crucial for the coffee sector [12]. These measures have been shown to offer protection against low water resources and synergistic biodiversity conservation [79]. This reflects the significance of the permanence and conservation of shade trees over time, creating a harmonious environment that increasingly resembles its original, native forest state [80].
Thus, it is crucial to emphasize the total carbon of the AFS with R. rospigliosii (190.38 Mg C ha−1), a species widely established in these regions due to the superior quality of the wood and its role in providing shade to coffee [25]. The use of native species is related to the years of knowledge, management practices, selection criteria and preferences of smallholder farmers. These play a key role in their economy, in terms of both the use of shade and wood. This knowledge can play an important role in AFSs by balancing local needs and conserving biodiversity [37]. It has been demonstrated that the presence of native species can have a substantial impact on mitigating the negative impacts of climate change on agricultural areas [12]. These species play a significance role, recovering the areas deforested due to agriculture.
As demonstrated in Table 6, the carbon storage values of coffee plants range between 4.16 and 12.16 Mg ha−1, corresponding to CA_E and CA_P, respectively. These values are lower than 48.5 Mg C ha−1 in a coffee AFS with Albizia sp. in Ethiopia [81]. However, our results exceed the 2.3 Mg C ha−1 found in an AFS with 6045 coffee plants ha−1 reported by [79], and the 9.1 Mg C ha−1 found in a coffee monoculture [82]. The contribution of coffee plants should not be underestimated, as they can contribute an average of 12.8% of the carbon in an AFS [81]. In this study, a maximum contribution of 7.4% was found in AFSs and 6.7% for coffee without shade. Litter carbon contributed a maximum of 2% to all the systems studied. Eucalyptus was the most significant contributor (3.92 Mg C ha−1), followed by Inga (2.67 and 2.41 Mg C ha−1 for CA_I and CA_IP, respectively). Inga species are traditionally utilized in AFSs with coffee. The authors of [76] found the same litter carbon stock values between a coffee AFS with Inga spuria and a deciduous forest.
Soil has been shown to be a significant contributor of C in all AFSs and coffee without shade trees, with averages ranging from 76.95 to 129.58 Mg ha−1 (CA_IP and CA_R, respectively) (Table 6), demonstrating that soil carbon stocks are a more persistent reservoir than biomass [83]. The soil C stored in the AFS with Inga was 105.44 Mg C ha−1, which is higher than the 87 Mg C ha−1 in a coffee AFS with Inga in the Peruvian Amazon [84], and the 91.5 Mg C ha−1 in a coffee AFS in Ethiopia [81]. It has been established that a minimum contribution of 47% of the total carbon is present. This finding is comparable to that of [75], where a contribution of 47% was also identified. The maximum soil contribution was recorded at 67% for AFS and 91% for unshaded coffee. In the case of systems with Inga spp., the soil contribution was 56% (CA_IP) and 64% (CA_I). Regarding the system with R. rospigliosii, the soil contribution was 67%, with Pinus 67% and with Eucalyptus 50%. These values are proximate to those ascertained by [21] for Inga (75%), Pinus (65%) and Eucalyptus (67%) and higher than those found (30.5%) by [81]. Soil carbon has been shown to benefit from the presence of organic matter such as leaves, branches, exudates, etc., favored by trees over an extended period [52,79]. Meanwhile, carbon variations observed among the distinct coffee systems are ascribed to the degree of disturbance and edaphic variables, which have been identified as the primary drivers of disparities in carbon stocks across these systems [85,86]. Ref. [87] observed that the least disturbed plant community, which exhibited a higher density of trees and shrubs, had the highest SOC stocks compared to the most disturbed communities.
Contrary to expectations, coffee without shade trees achieved an SOC (128.70 Mg ha−1) statistically equal to that of R. rospigliosii (Table 6). This result is higher than that found by [70], for two coffee plantations without shade trees, at a soil depth of 0 - 30 cm (56.16 and 61.96 Mg C ha−1). The SOC of shadeless coffee is consistent with the % porosity and % OM, in which CA_R and CA_S stand out. This phenomenon can be attributed to a substantial positive correlation between SOC and total soil porosity [87,88]. The high soil organic carbon (SOC) content and the greater density of coffee plants present in the system (7692 and 3787 plants ha−1) likely favored a symbiotic association between coffee plants and arbuscular mycorrhizal fungi (AMF). The increased number of AMF spores observed in shadeless coffee is analogous to the findings of [89], who reported that the abundance and richness of AMF, particularly Glomerospora, were greater in agroforestry systems than in forest ecosystems. Moreover, the presence of AMF plays a pivotal role in glomalin accumulation [90], thereby promoting the greater accumulation and preservation of organic carbon in soil aggregates and carbon reserves [91]. Likewise, our results could be due to plant residues or remnants left at the study locations [92]. This occurrence can be attributed to the high forest density that prevailed in the past. Considering the capacity of soils to sequester carbon for up to 500 years, the high levels of soil organic carbon observed could be explained by this process [93,94]. Similarly, the phenomenon may be attributable to management practices. For example, Ref. [75] found that ground cover contributed more to soil organic carbon content when compared to the impact of shade trees.
Regarding the CO2e fixation rate, Eucalyptus (CA_E) stands out. However, the values obtained by the coffee plants in this AFS are the lowest. This result could be due to the elevated density or spatial arrangement of the system (625 trees ha−1), which might impede the growth of coffee trees, generating competition for nutrients and other resources. This is noticeable in the lower average biomass of coffee trees (8.81 Mg ha−1), in contrast to their counterparts in other AFSs (Table 4). This situation highlights the importance of the optimal distribution and density of the crop and shade trees [95], as well as pruning practices [96]. These practices have been demonstrated to enhance coffee productivity and foster the development of a sustainable ecosystem [97,98]. It is not appropriate to endorse a single practice that would have adverse consequences for other components of the system, as this could compromise the integrity of the carbon storage [73]. In general, all agroforestry systems (AFSs) outperformed shade-free coffee, with values exceeding 6.64 Mg ha−1 year−1. This finding stands in contrast to the rates reported under neotropical conditions [16], where C fixation rates vary from 1,5 to 3.5 Mg ha−1 year−1 for the AFSs of small producers. The findings of this study suggest that the implementation of coffee and shade trees may offer certain benefits to ecosystems in terms of climate change mitigation. The results of this study indicate a positive association between these two factors. This phenomenon has been thoroughly validated in other tropical conditions analogous to our own, where erosion was found to be reduced, biodiversity conservation was enhanced and soil nutrient recycling was observed to occur [18].
The genus Eucalyptus has been observed to exhibit rapid growth, resulting in elevated levels of biomass generation [99] and enhanced carbon fixation rates, as evidenced by recent research findings. However, R. rospigliosii distinguishes itself through its association with coffee, a relationship that enables the attainment of the highest carbon and carbon dioxide equivalents fixation rates (Figure 6A and Figure 7A). Apart of that, the potential use of wood from this species should direct our attention to the conservation and use of this native species as part of the biodiversity in Peruvian areas [27].
The variability of climatic events should redirect us in our coffee-growing practices. The confluence of extreme weather changes [100], coupled with the trend of shade-free coffee production, high crop density, nutrient leaching and agrochemical overuse [101], poses significance threats to agroecosystems. In a projection to 2060, Ref. [102] found that climate change could lead to a 20–60% reduction in coffee yields under full sun and a 4–25% decrease in agroforestry coffee systems. AFSs have been demonstrated to exert an impact on the improvement and stabilization of crop yields and sustainability, generating greater capacity to suppress pests and pathogenic transmissions [103]. Furthermore, AFSs have been proven to play a role in the protection of production by providing a cushion against damage from climate variability [9] and soil conservation [104]. This underscores the critical importance of transitioning to sustainable agricultural practices. It is imperative to identify and integrate tree species that are compatible with coffee plantations and provide ecosystem benefits in their growing areas. A notable example is Retrophyllum rospigliosii which has been observed in the central Peruvian Amazon and the tropical Andes of South America.
5. Limitations
Carbon stocks vary depending on the type of vegetation where the native tree Retrophyllum rospigliosii (ulcumano) is present. This species is notable for its capacity to store carbon, assessed at a given time and under certain climatic conditions. However, it is important to note that the findings of this study may not be generalizable to cases in which measurements are conducted over an extended period or if changes to ecosystems (such as deforestation, crop changes, poor agricultural practices, etc.) dramatically increase their CO2 emissions. The limited number of levels in the random effect (districts) impedes the robust estimation of spatial variance in mixed models. This restriction reduces the ability to generalize and requires a cautious approach when interpreting variability among sites. It would be advisable for future studies to consider a random sample of districts with more units to strengthen the spatial inference. Likewise, it is imperative to identify and implement appropriate allometric equations for each species. In our research, we faced the limitation of not having a species-specific equation for R. rospigliosii. In addition, it is recommended to consider additional variables such as the attributes and characteristics of the entire plant community for each plot (size, density and frequency), the interaction of microorganisms, glomalin and its relationship with soil organic carbon [73]. These elements are critical to strengthening the understanding of the carbon cycle in agroforestry systems.
6. Conclusions
Although soil represents the largest contributor to total carbon storage, the presence of trees distinguishes agroforestry systems from unshaded coffee plantations, occupying second place with a share ranging from 24% to 46%. Our research indicates that the native tree Retrophyllum rospigliosii exhibits a superior carbon storage capacity, underscoring the significance of incorporating this native species into coffee-based agroforestry systems in the central Peruvian Amazon. Consequently, we recommend implementing coffee systems that integrate this native species. Agroforestry systems including Eucalyptus have demonstrated robust adaptability within the study area. The findings suggest that R. rospigliosii functions as a suitable model for shade-grown coffee plantations, and its implementation should be replicated in the area. However, long-term, large-scale measurements and carbon dioxide emissions in these study systems are needed to fully understand their climate impacts, which can lead to policy recommendations for the sustainable management of ecosystems in the central Peruvian Amazon. In addition, it is advised that analogous research be conducted incorporating study factors such as season, diverse soil utilization and measurement variables including soil proteins, respiration, microbial biomass and the economic potential of long-term carbon sequestration in coffee trees. This research should prioritize the resilience of sustainable livelihoods of farmers within the context of poverty alleviation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture15131415/s1. All supplementary material was submitted to the journal in the form of digital files.
Author Contributions
Conceptualization, N.S.V., L.E.R.-C. and R.S.A.; methodology, N.S.V., E.S.H.P., C.d.C.I.P., L.E.R.-C., G.V.-T. and U.A.P.; investigation, L.E.R.-C., N.S.V., E.S.H.P. and C.d.C.I.P.; data curation, U.A.P., C.d.C.I.P., E.S.H.P., J.M.C.-C. and L.E.R.-C.; writing—review and editing, L.E.R.-C., G.V.-T., N.S.V., E.S.H.P., J.M.C.-C., C.d.C.I.P. and U.A.P.; formal analysis, U.A.P., J.M.C.-C., R.S.A. and G.V.-T.; supervision and review, R.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The National Institute of Agrarian Innovation—INIA, Perú, funded this research within the framework of the investment project “Improvement of research and technology transfer services in the management and recovery of degraded agricultural soils and irrigation water in small and medium-sized agriculture in the departments of Lima, Ancash, San Martín, Cajamarca, Lambayeque, Junín, Ayacucho, Arequipa, Puno and Ucayali”, with CUI N° 2487112.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to the farmers who gave us the facilities to carry out this research in their plots, as well as to César Padilla, Kenyi Quispe, Edilson Requena and Raihil Rengifo for their valuable constructive suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AFS | Agroforestry system |
| CA_I | Coffee and Inga system |
| CA_R | Coffee and R. rospigliossi system |
| CA_E | Coffee and Eucalyptus system |
| CA_P | Coffee and Pinus system |
| CA_IP | Coffee and Inga + Pinus system |
| CA_S | Coffea arabica L. shadeless |
| ACB | Aboveground biomass of coffee |
| RCB | Root biomass of coffee |
| ATB | Aboveground biomass of shade trees |
| RTB | Root biomass of shade trees |
| HSB | Herbaceous and shrub biomass |
| LB | Litter biomass |
| ACC | Carbon in the aboveground biomass of coffee |
| RCC | Carbon in the root biomass of coffee |
| ATC | Carbon in the aboveground biomass of shade trees |
| RTC | Carbon in the root biomass of shade trees |
| SHC | Carbon in the shrub and herbaceous biomass |
| LC | Carbon in the litter biomass |
| SOC | Soil organic carbon |
| CFC | Carbon fixation rate of coffee |
| CFT | Carbon fixation rate of shade trees |
| CO2e FC | CO2e fixation rate of coffee |
| CO2e FT | CO2e fixation rate of shade trees |
| %C | %carbon |
| %OM | %organic matter |
References
- USDA. Coffee: World Markets and Trade. 2023. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/m900nt40f/73667k581/qz20v7955/coffee.pdf (accessed on 28 March 2025).
- MIDAGRI. Observatorion de COOMDITIES. 2024. Available online: https://cdn.www.gob.pe/uploads/document/file/6461268/5316298-commodities-cafe-n-01-2024.pdf?v=1718054404 (accessed on 28 March 2025).
- SEIA Padrón de Productores Agrarios, Actualizado al mes de Julio del 2024. Available online: https://observatorio-ppa.midagri.gob.pe/ (accessed on 13 January 2025).
- Jezeer, R.; Verweij, P. Café en sistemas Agroforestales- Doble Dividendo para la Biodiversidad y los Pequeños Agricultores en Perú. Available online: https://www.researchgate.net/publication/301694184_Cafe_en_sistemas_Agroforestales-_Doble_dividendo_para_la_biodiversidad_y_los_pequenos_agricultores_en_Peru (accessed on 28 March 2025).
- MIDAGRI. Observatorio de COMMODITIES. 2023. Available online: https://cdn.www.gob.pe/uploads/document/file/5524126/4327863-commodities-cafe-abr-jun-2023.pdf?v=1701812688 (accessed on 28 March 2025).
- Díaz, C.V.; Carmen, M.W. Línea de Base del Sector Café en el Perú. Available online: https://www.undp.org/es/latin-america/publicaciones/linea-de-base-del-sector-cafe-en-el-peru (accessed on 13 January 2025).
- FAO. FRA 2020 Remote Sensing Survey; FAO: Rome, Italy, 2022; ISBN 978-92-5-136147-4. Available online: http://www.fao.org/documents/card/en/c/cb9970en (accessed on 28 March 2025).
- Geoboques Bosque y Pérdida de Bosque. Ministerio del Ambiente—Perú. Available online: https://geobosques.minam.gob.pe/geobosque/view/perdida.php (accessed on 28 March 2025).
- Burgess, A.J.; Correa Cano, M.E.; Parkes, B. The deployment of intercropping and agroforestry as adaptation to climate change. Crop Environ. 2022, 1, 145–160. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2773126X22000223 (accessed on 7 January 2025). [CrossRef]
- Chirinos, R.A.G. En este Artículo se Presenta Información Sobre la Emisión de Dióxido de Carbono (CO2) del Perú y las Acciones que se Pueden Tomar Para Mitigar sus Efectos en el Medio Ambiente y el Planeta. 2021. Volume 5. Available online: https://www.bcrp.gob.pe/docs/Publicaciones/Revista-Moneda/moneda-188/moneda-188-12.pdf (accessed on 7 January 2025).
- Ahmed, S.; Brinkley, S.; Smith, E.; Sela, A.; Theisen, M.; Thibodeau, C.; Warne, T.; Anderson, E.; Van Dusen, N.; Giuliano, P.; et al. Climate Change and Coffee Quality: Systematic Review on the Effects of Environmental and Management Variation on Secondary Metabolites and Sensory Attributes of Coffea arabica and Coffea canephora. Front. Plant Sci. 2021, 12, 708013. Available online: https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2021.708013/full (accessed on 28 April 2025). [CrossRef]
- Gomes, L.C.; Bianchi, F.J.J.A.; Cardoso, I.M.; Fernandes, R.B.A.; Filho, F.E.I.; Schulte, R.P.O. Agroforestry systems can mitigate the impacts of climate change on coffee production: A spatially explicit assessment in Brazil. Agric. Ecosyst. Environ. 2020, 294, 106858. Available online: https://research.wur.nl/en/publications/agroforestry-systems-can-mitigate-the-impacts-of-climate-change-o (accessed on 15 January 2025). [CrossRef]
- Boehm, R.; Kitchel, H.; Ahmed, S.; Hall, A.; Orians, C.M.; Stepp, J.R.; Robbat, J.; Griffin, T.S.; Cash, S.B. Is Agricultural Emissions Mitigation on the Menu for Tea Drinkers? Sustainability 2019, 11, 4883. [Google Scholar] [CrossRef]
- Tinoco-Jaramillo, L.; Vargas-Tierras, Y.; Habibi, N.; Caicedo, C.; Chanaluisa, A.; Paredes-Arcos, F.; Viera, W.; Almeida, M.; Vásquez-Castillo, W. Agroforestry Systems of Cocoa (Theobroma cacao L.) in the Ecuadorian Amazon. Forests 2024, 15, 195. [Google Scholar] [CrossRef]
- Murga-Orrillo, H.; Lobo, F.D.A.; Santos Silva Amorim, R.; Fernandes Silva Dionisio, L.; Nuñez Bustamante, E.; Chu-Koo, F.W.; López, L.A.A.; Arévalo-Hernández, C.O.; Abanto-Rodriguez, C. Increased Production of Tara (Caesalpinia spinosa) by Edaphoclimatic Variation in the Altitudinal Gradient of the Peruvian Andes. Agronomy 2023, 13, 646. [Google Scholar] [CrossRef]
- Montagnini, F.; Nair, P.K.R. Carbon sequestration: An underexploited environmental benefit of agroforestry systems. Agrofor. Syst. 2004, 61, 281–295. [Google Scholar] [CrossRef]
- López-Hernández, J.C.; Aryal, D.R.; Villanueva-López, G.; Pinto-Ruiz, R.; Reyes-Sosa, M.B.; Hernández-López, A.; Casanova-Lugo, F.; Venegas-Venegas, J.A.; Medina-Jonapa, F.J.; Guevara-Hernández, F.; et al. Carbon storage and sequestration rates in Leucaena leucocephala-based silvopasture in Southern Mexico. Agrofor. Syst. 2024, 98, 1105–1121. [Google Scholar] [CrossRef]
- Villa, P.M.; Martins, S.V.; de Oliveira Neto, S.N.; Rodrigues, A.C.; Hernández, E.P.; Kim, D.-G. Policy forum: Shifting cultivation and agroforestry in the Amazon: Premises for REDD+. For. Policy Econ. 2020, 118, 102217. Available online: https://www.sciencedirect.com/science/article/pii/S1389934119305441 (accessed on 13 January 2025). [CrossRef]
- Shibu, J. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. Available online: http://link.springer.com/10.1007/s10457-009-9229-7 (accessed on 13 January 2025). [CrossRef]
- Villarreyna, R.; Avelino, J.; Cerda, R. Adaptación basada en ecosistemas: Efecto de los árboles de sombra sobre servicios ecosistémicos en cafetales1. Agron. Mesoam. 2020, 31, 499–516. Available online: http://www.scielo.sa.cr/scielo.php?script=sci_abstract&pid=S1659-13212020000200499&lng=en&nrm=iso&tlng=es (accessed on 9 January 2025). [CrossRef]
- Ehrenbergerová, L.; Cienciala, E.; Kučera, A.; Guy, L.; Habrová, H. Carbon stock in agroforestry coffee plantations with different shade trees in Villa Rica, Peru. Agrofor. Syst. 2016, 90, 433–445. [Google Scholar] [CrossRef]
- Rezende, M.Q.; Venzon, M.; dos Santos, P.S.; Cardoso, I.M.; Janssen, A. Extrafloral nectary-bearing leguminous trees enhance pest control and increase fruit weight in associated coffee plants. Agric. Ecosyst. Environ. 2021, 319, 107538. Available online: https://www.sciencedirect.com/science/article/pii/S0167880921002425 (accessed on 28 March 2025). [CrossRef]
- Leblanc, H. Dinitrogen-fixation by three neotropical agroforestry tree species under semi-controlled field conditions. Plant Soil 2007, 291, 199–209. Available online: https://www.academia.edu/29714381/Dinitrogen_fixation_by_three_neotropical_agroforestry_tree_species_under_semi_controlled_field_conditions (accessed on 28 March 2025). [CrossRef]
- Rapidel, B.; Allinne, C.; Cerdan, C.; Meylan, L.; de Melo, V.F.E.; Avelino, J.; Sistemas Agroforestales. Funciones Productivas, Socioeconómicas y Ambientales. CATIE. Efectos Ecológicos y Productivos del Asocio de Árboles de Sombra con Café en Sistemas Agroforestales. 2015. Available online: https://agritrop.cirad.fr/575470/ (accessed on 28 March 2025).
- More, P.; Cuellar, J.; Salazar, E. Propagación vegetativa de Retrophyllum rospigliosii (Pilg.) C.N. Page “ulcumano” en cámara de subirrigación en Chanchamayo/Perú. Ecol. Apl. 2021, 20, 15–23. Available online: https://revistas.lamolina.edu.pe/index.php/eau/article/view/1687 (accessed on 28 March 2025). [CrossRef]
- Marín Vélez, A.; Vásquez Velásquez, G.; Monsalve, M.; Orozco, F.; Carbonell, J.A.; Villegas, C.; Ramírez Ospina, J.; Romero, J.L. Ecología y Silvicultura de las Podocarpáceas Andinas de Colombia. Cali: Smurfit Cartón de Colombia. 1998. Available online: https://catalogo.jbb.gov.co/cgi-bin/koha/opac-detail.pl?biblionumber=2808 (accessed on 5 June 2025).
- Ruiz-Erazo, C.E.; Riascos-Acosta, R.I.; Guerrero-Martínez, E.S.; Marín-Vélez, A.M.; Sierra, C.A.; Ramírez-Correa, J.A. Potencial de captura de carbono en plantaciones de Retrophyllum rospigliosii (Pilg.) C. N. Page con fines de restauración en la región Andina colombiana. Rev. Chapingo Ser. Cienc. For. Ambiente 2025, 31, e24009. Available online: https://revistas.chapingo.mx/forestales/article/view/1327 (accessed on 5 June 2025). [CrossRef]
- Gardner, M.; Thomas, P. IUCN Red List of Threatened Species: Retrophyllum rospigliosii. IUCN Red List Threat Species. 2013. Available online: https://www.iucnredlist.org/en (accessed on 5 June 2025).
- Baselly-Villanueva, J.R.; Goycochea-Casas, G.; Carvalho, A.M.M.L.; Roncal-Briones, W.R.; Chumbimune-Vivanco, S.Y.; Chavesta-Custodio, M. CARACTERIZACIÓN Y DIFERENCIAS ANATÓMICAS DE MADERAS DE Retrophyllum rospigliosii (Pilg.) C.N. Page Y Prumnopitys harmsiana (Pilg.) de Laub. (PODOCARPACEAE) PROCEDENTES DE LA PROVINCIA DE SAN IGNACIO, PERÚ. Folia Amaz. 2021, 30, 137–148. Available online: https://revistas.iiap.gob.pe/index.php/foliaamazonica/article/view/587 (accessed on 5 June 2025). [CrossRef]
- Camarena-Yupanqui, R.C.; Orellana-Mendoza, E.; Bernaola-Paucar, R.M.; Ames-Martínez, F.N.; Loardo-Tovar, H.; Quispe-Melgar, H.R. Seedling Production of Retrophyllum rospigliosii in Nurseries and Potential Reforestation Areas Using Modeling Techniques. Forests 2024, 15, 2179. [Google Scholar] [CrossRef]
- Loewe-Muñoz, V.; del Río, R.; Delard, C.; Balzarini, M. Short-term stem diameter variations in irrigated and non-irrigated stone pine (Pinus pinea L.) trees in a xeric non-native environment. Ann. For. Sci. 2021, 78, 99. Available online: https://annforsci.biomedcentral.com/articles/10.1007/s13595-021-01114-8 (accessed on 28 March 2025). [CrossRef]
- Sülüsoglu, M. The Management of Villagers Owned Stone Pine (Pinus pinea L.) Plantations In Kozak Region, Turkey: A Case Study. Available online: https://www.fao.org/4/j4821e/j4821e00.htm (accessed on 28 March 2025).
- Gomes, V.M.; Miranda Júnior, M.S.; Silva, L.J.; Teixeira, M.V.; Teixeira, G.; Schossler, K.; Freitas, D.A.; Oliveira, D.M. A Global Meta-Analysis of Soil Carbon Stock in Agroforestry Coffee Cultivation. Agronomy 2025, 15, 480. [Google Scholar] [CrossRef]
- Pan, J.; Chen, S.; He, D.; Zhou, H.; Ning, K.; Ma, N.; Li, K.; Liao, D.; Mi, W.; Wu, Q.; et al. Agroforestry increases soil carbon sequestration, especially in arid areas: A global meta-analysis. Catena 2025, 249, 108667. Available online: https://www.sciencedirect.com/science/article/pii/S0341816224008646 (accessed on 5 June 2025). [CrossRef]
- Zomer, R.J.; Bossio, D.A.; Trabucco, A.; van Noordwijk, M.; Xu, J.; Zomer, R.J.; Bossio, D.A.; Trabucco, A.; van Noordwijk, M.; Xu, J. Global carbon sequestration potential of agroforestry and increased tree cover on agricultural land. Circ. Agric. Syst. 2022, 2, 1–10. Available online: https://www.maxapress.com/article/doi/10.48130/CAS-2022-0003 (accessed on 5 June 2025). [CrossRef]
- Nguyen, M.P.; Vaast, P.; Pagella, T.; Sinclair, F. Local Knowledge about Ecosystem Services Provided by Trees in Coffee Agroforestry Practices in Northwest Vietnam. Land 2020, 9, 486. [Google Scholar] [CrossRef]
- Flores-Ortiz, C.M.; Davila, P.; Rodríguez-Arevalo, I.; Manson, R.H.; Toledo-Garibaldi, M.; Cabrera-Santos, D.; Salguero, M.A.; Vázquez, F.G.; Cobos-Silva, J.; Gianella, M.; et al. Prioritisation of native trees for enhancing carbon sequestration in shade-grown coffee plantations in the State of Veracruz (México): Linking conservation and ecological traits to community needs. Agrofor. Syst. 2025, 99, 55. [Google Scholar] [CrossRef]
- Rügnitz, M.T.; Chacón León, M.; Porro, R. Guía Para la Determinación de Carbono en Pequeñas Propiedades Rurales; Manual Técnico; ICRAF: Lima, Peru, 2009; ISBN 978-92-9059-254-9. [Google Scholar]
- Jurado, M.A.J.R.; Jurado, H.R.O.; Burbano, T.C.L. Evaluación de la captura de carbono en sistemas productivos de café (Coffea arabica L.), Consacá, Nariño, Colombia. Luna Azul 2020, 51, 166–181. Available online: https://revistasojs.ucaldas.edu.co/index.php/lunazul/article/view/5342 (accessed on 14 January 2025). [CrossRef]
- Instituto Nacional de Recursos Naturales Inrena. Mapa Ecológico del Perú: Guía Explicativa; INRENA: Lima, Peru, 1995. Available online: https://app.ingemmet.gob.pe/biblioteca/pdf/Lib-215.pdf (accessed on 14 January 2025).
- SENAMHI. Datos Hidrometeorológicos a Nivel Nacional. Available online: https://www.senamhi.gob.pe/?p=estaciones (accessed on 14 January 2025).
- Angiosperm Phylogeny Group; Chase, M.W.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Reveal, J.L.; Farjon, A.; Gardner, M.F.; Mill, R.R.; Chase, M.W. A new classification and linear sequence of extant gymnosperms. Phytotaxa 2011, 19, 55–70. Available online: https://www.biotaxa.org/Phytotaxa/article/view/phytotaxa.19.1.3 (accessed on 14 January 2025). [CrossRef]
- Yang, Y.; Ferguson, D.K.; Liu, B.; Mao, K.-S.; Gao, L.-M.; Zhang, S.-Z.; Wan, T.; Rushforth, K.; Zhang, Z.-X. Recent advances on phylogenomics of gymnosperms and a new classification. Plant Divers. 2022, 44, 340–350. Available online: https://www.sciencedirect.com/science/article/pii/S2468265922000440 (accessed on 14 January 2025). [CrossRef]
- Zavala, W.; Merino, E.; Peláez, P. Influencia de tres sistemas agroforestales del cultivo de cacao en la captura y almacenamiento de carbono. Sci. Agropecu. 2018, 9, 493–501. Available online: http://www.scielo.org.pe/scielo.php?script=sci_abstract&pid=S2077-99172018000400004&lng=es&nrm=iso&tlng=es (accessed on 14 January 2025). [CrossRef]
- Cuellar, J.E.B.; Salazar, E.J.H. Dinámica del Carbono Almacenado en los Diferentes Sistemas de uso de la Tierra del Perú, Base para una Estrategia de Mitigación ante el Cambio Climático. 2016. Available online: https://repositorio.inia.gob.pe/handle/20.500.12955/363 (accessed on 14 January 2025).
- IPCC Directrices del IPCC de 2006 para los Inventarios Nacionales de Gases de Efecto Invernadero Volumen 4 Agricultura, Silvicultura y otros Usos de la Tierra. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/spanish/vol4.html (accessed on 14 January 2025).
- Arevalo, L.A.; Alegre, J.C.; Montoya Vilcahuaman, L.J. Metodologia Para Estimar o Estoque de Carbono em Diferentes Sistemas de uso da Terra. 2002. Available online: http://www.infoteca.cnptia.embrapa.br/handle/doc/308054 (accessed on 15 January 2025).
- Razo Zárate, R.; Gordillo Martínez, A.J.; Rodríguez Laguna, R.; Maycotte Morales, C.C.; Acevedo Sandoval, O.A. Coeficientes de carbono para arbustos y herbáceas del bosque de oyamel del Parque Nacional El Chico. Rev. Mex. Cienc. For. 2018, 6, 58–67. Available online: https://cienciasforestales.inifap.gob.mx/index.php/forestales/article/view/195 (accessed on 28 March 2025). [CrossRef]
- Quilio, A.; Castellanos, E.; Pons, D. Estudio de Línea Base de Carbono en Cafetales; Universidad del Valle de Guatemala: Guatemala, 2010; Volume 46. [Google Scholar]
- Marroquín, P.M.; Pérez, J.J.; Yamallel, J.I.Y.; García, R.S. Almacenamiento de carbono en Coffea arabica L. en la Sierra Madre de Chiapas. Rev. Mex. Cienc. Agríc. 2024, 15, e3315. Available online: https://cienciasagricolas.inifap.gob.mx/index.php/agricolas/article/view/3315 (accessed on 14 January 2025). [CrossRef]
- van Noordwijk, M.; Rahayu, S.; Hairiah, K.; Wulan, Y.C.; Farida, A.; Verbist, B. Carbon stock assessment for a forest-to-coffee conversion landscape in Sumber-Jaya (Lampung, Indonesia): From allometric equations to land use change analysis. Sci. China Ser. C-Life Sci. 2002, 45, 75–86. Available online: https://www.semanticscholar.org/paper/Carbon-stock-assessment-for-a-forest-to-coffee-in-Noordwijk-Rahayu/d167089d33268097a56adad7775ab28350c5012e (accessed on 16 January 2025).
- MIDAGRI Requerimientos Agroclimáticos del Cultivo de Café. 2019. Available online: https://repositorio.midagri.gob.pe/bitstream/20.500.13036/237/1/ficha-tecnica-11-cultivo-cafe%20%281%29.pdf (accessed on 5 June 2025).
- Rodríguez, R.L.; Jiménez-Pérez, J.; Aguirre-Calderón, Ó.A.; Treviño-Garza, E.J.; Razo-Zárate, R. Estimación De Carbono Almacenado En El Bosque De Pino-Encino En La Reserva De La Biosfera El Cielo, Tamaulipas, México. 2009. Available online: https://www.redalyc.org/articulo.oa?id=46111817006 (accessed on 15 January 2025).
- Rojas-García, F.; De Jong, B.H.J.; Martínez-Zurimendí, P.; Paz-Pellat, F. Database of 478 allometric equations to estimate biomass for Mexican trees and forests. Ann. For. Sci. 2015, 72, 835–864. Available online: https://annforsci.biomedcentral.com/articles/10.1007/s13595-015-0456-y (accessed on 15 January 2025). [CrossRef]
- Winck, R.A.; Fassola, H.E.; Barth, S.R.; Crechi, E.H.; Keller, A.E.; Videla, D.; Zaderenko, C. Modelos predictivos de biomasa aérea de eucalyptus grandis para el noreste de Argentina. Ciênc. Florest. 2015, 25, 595–606. Available online: https://periodicos.ufsm.br/cienciaflorestal/article/view/19611 (accessed on 15 January 2025). [CrossRef]
- Callo, D.C.; Krishnamurthy, L.; Alegre, J. Secuestro de carbono por sistemas agroforestales amazónicos. Rev. Chapingo Ser. Cienc. For. Ambiente 2002, 8, 101–106. Available online: https://www.redalyc.org/articulo.oa?id=62980202 (accessed on 15 January 2025).
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Guía de Buenas Prácticas Para el uso de la Tierra, el Cambio de uso de la Tierra y la Silvicultura. Inventarios Nacionales de Gases de Efecto Invernadero. Métodos Complementarios y Orientación sobre las Buenas Prácticas Derivados del Protocolo de Kyoto. Available online: https://www.ipcc-nggip.iges.or.jp/public/gpglulucf/gpglulucf_languages.html (accessed on 15 January 2025).
- Van Bemmelen, J.M. Ueber die Bestimmung des Wassers, des Humus, des Schwefels, der in den Colloidalen Sillikaten Gebunden Kieselsäure, des Mangans u.s.w. im Ackerboden. 1879. Available online: https://edepot.wur.nl/211282 (accessed on 15 January 2025).
- Eyherabide, M.; Saínz Rozas, H.; Barbieri, P.; Echeverría, H.E. Comparación de métodos para determinar carbono orgánico en suelo. Cienc. Suelo 2014, 32, 13–19. Available online: https://www.scielo.org.ar/scielo.php?script=sci_abstract&pid=S1850-20672014000100002&lng=es&nrm=iso&tlng=es (accessed on 15 January 2025).
- Rolando, J.L.; Dubeux, J.C.B.; de Souza, T.C.; Mackowiak, C.; Wright, D.; George, S.; Pires, T.; Santos, E. Organic carbon is mostly stored in deep soil and only affected by land use in its superficial layers: A case study. Agrosystems Geosci. Environ. 2021, 4, e20135. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/agg2.20135 (accessed on 5 June 2025). [CrossRef]
- Button, E.S.; Pett-Ridge, J.; Murphy, D.V.; Kuzyakov, Y.; Chadwick, D.R.; Jones, D.L. Deep-C storage: Biological, chemical and physical strategies to enhance carbon stocks in agricultural subsoils. Soil Biol. Biochem. 2022, 170, 108697. Available online: https://www.sciencedirect.com/science/article/pii/S0038071722001547 (accessed on 5 June 2025). [CrossRef]
- Yost, J.L.; Hartemink, A.E. How deep is the soil studied—An analysis of four soil science journals. Plant Soil 2020, 452, 5–18. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. Available online: https://journals.lww.com/soilsci/citation/1934/01000/an_examination_of_the_degtjareff_method_for.3.aspx (accessed on 16 January 2025). [CrossRef]
- Marín, M.D.; Andrade, H.J.; Sandoval, A.P. Fijación de carbono atmosférico en la biomasa total de sistemas de producción de cacao en el departamento del Tolima, Colombia. Rev. UDCA Actual. Divulg. Científica 2016, 19, 351–360. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0123-42262016000200012 (accessed on 14 January 2025).
- Brooks, M.E.; Bolker, B.M.; Kristensen, K.; Machler, M.; Magnusson, A.; Skaug, H.J.; Nielsen, A.; Berg, C.; van Bentham, H.; Sadat, N.; et al. glmmTMB: Generalized linear mixed models using Template Model Builder. 2025. Available online: https://cran.r-project.org/web/packages/glmmTMB/index.html (accessed on 31 May 2025).
- Bartoń, K. MuMIn: Multi-Model Inference. 2025. p. 1.48.11. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 31 May 2025).
- Hartig, F.; Lohse, L.; de Souza, M. DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) Regression models. 2024. Available online: https://cran.r-project.org/web/packages/DHARMa/index.html (accessed on 31 May 2025).
- Soto-Pinto, L.; Aguirre-Dávila, C.M.; Anzueto-Martínez, M.J. Almacenes de carbono en cafetales con distintos manejos en el Norte de Chiapas, México. Estado ctual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2015. Texcoco, Estado de México, México. 2015, pp. 256–263. Available online: https://pmcarbono.org/pmc/simposio/files/Memorias_Res_Cortos-2015.pdf (accessed on 15 January 2025).
- Salas, V.M.; Paz Pellat, F.; Rojas-García, F.; Bolaños-González, M.A. Almacenes de Carbono en Sistemas Agroforestales Cafetaleros de la Sierra Madre de Chiapas. En: Estado Actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis 2018. 2018. Available online: https://www.researchgate.net/publication/330384193_Almacenes_de_carbono_en_sistemas_agroforestales_cafetaleros_de_la_Sierra_Madre_de_Chiapas (accessed on 15 January 2025).
- Valdés-Velarde, E.; Vázquez-Domínguez, L.P.; Tinoco-Rueda, J.Á.; Sánchez-Hernández, H.; Salcedo-Pérez, E.; Lagunes-Fortiz, E. Servicio ecosistémico de carbono almacenado en cafetales bajo sombra en sistema agroforestal. Rev. Mex. De Cienc. Agrícolas 2022, 13, 287–297. [Google Scholar] [CrossRef]
- Vallejos-Torres, G.; Gaona-Jimenez, N.; Pichis-García, R.; Ordoñez, L.; García-Gonzales, P.; Quinteros, A.; Lozano, A.; Saavedra-Ramírez, J.; Tuesta-Hidalgo, J.C.; Reategui, K.; et al. Carbon reserves in coffee agroforestry in the Peruvian Amazon. Front. Plant Sci. 2024, 15, 1410418. Available online: https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2024.1410418/full (accessed on 16 January 2025). [CrossRef]
- Castellanos, E.; Quilo, A.; Mato, R. Metodología Estimación Carbono Bosques Guatemala. Available online: https://studylib.es/doc/7798569/metodología-para-la-estimación-del-contenido-de-carbono-en (accessed on 5 June 2025).
- Lugo-Pérez, J.; Hajian-Forooshani, Z.; Perfecto, I.; Vandermeer, J. The importance of shade trees in promoting carbon storage in the coffee agroforest systems. Agric. Ecosyst. Environ. 2023, 355, 108594. Available online: https://www.sciencedirect.com/science/article/pii/S0167880923002530 (accessed on 16 January 2025). [CrossRef]
- Espinoza-Domínguez, W.; Krishnamurthy, L.; Vázquez-Alarcón, A.; Torres-Rivera, A. Almacén de carbono en sistemas agroforestales con café. Rev. Chapingo Ser. Cienc. For. Ambiente 2012, 18, 57–70. Available online: https://www.redalyc.org/pdf/629/62924537005.pdf (accessed on 15 January 2025). [CrossRef]
- Nelson, K.C.; De Jong, B.H. Making Global Initiatives Local Realities: Carbon Mitigation Projects in Chiapas, Mexico. Available online: https://colab.ws/articles/10.1016%2Fs0959-3780%2802%2900088-2 (accessed on 16 January 2025).
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. Available online: https://www.sciencedirect.com/science/article/pii/S0167880903001385 (accessed on 28 March 2025). [CrossRef]
- Häger, A. The effects of management and plant diversity on carbon storage in coffee agroforestry systems in Costa Rica. Agrofor. Syst. 2012, 86, 159–174. [Google Scholar] [CrossRef]
- de Carvalho, F.F.; Barreto-Garcia, P.A.B.; Pérez-Maluf, R.; Monroe, P.H.M.; Pereira, F.R.; Almeida, T.C.; Nunes, M.R. Effects of Coffee arabica cultivation systems on tropical soil microbial biomass and activity in the northeast region of Brazil. Agrofor. Syst. 2024, 98, 2397–2410. [Google Scholar] [CrossRef]
- Niguse, G.; Iticha, B.; Kebede, G.; Chimdi, A. Contribution of coffee plants to carbon sequestration in agroforestry systems of Southwestern Ethiopia. J. Agric. Sci. 2022, 160, 440–447. Available online: https://www.cambridge.org/core/journals/journal-of-agricultural-science/article/contribution-of-coffee-plants-to-carbon-sequestration-in-agroforestry-systems-of-southwestern-ethiopia/320308C9F0281FB4911D64988E75394E (accessed on 16 January 2025). [CrossRef]
- Hergoualc’h, K.; Blanchart, E.; Skiba, U.; Henault, C.; Harmand, J.-M. Changes in Carbon Stocks and Greenhouse Gas Balance in a Coffee (Coffea arabica) Monoculture Versus an Agroforestry System with Inga Densiflora, in Costa Rica. CIFOR-ICRAF. 2012. Available online: https://www.cifor-icraf.org/knowledge/publication/5154/ (accessed on 16 January 2025). [CrossRef]
- Negash, M.; Starr, M. Biomass and soil carbon stocks of indigenous agroforestry systems on the south-eastern Rift Valley escarpment, Ethiopia. Plant Soil 2015, 393, 95–107. [Google Scholar] [CrossRef]
- Solis, R.; Vallejos-Torres, G.; Arévalo, L.; Marín-Díaz, J.; Ñique-Alvarez, M.; Engedal, T.; Bruun, T.B. Carbon stocks and the use of shade trees in different coffee growing systems in the Peruvian Amazon. J. Agric. Sci. 2020, 158, 450–460. Available online: https://www.cambridge.org/core/journals/journal-of-agricultural-science/article/carbon-stocks-and-the-use-of-shade-trees-in-different-coffee-growing-systems-in-the-peruvian-amazon/EFBC4767DB3C355403F162F6286E2373 (accessed on 28 March 2025). [CrossRef]
- Gebeyehu, G.; Soromessa, T.; Bekele, T.; Teketay, D. Carbon stocks and factors affecting their storage in dry Afromontane forests of Awi Zone, northwestern Ethiopia. J. Ecol. Environ. 2019, 43, 7. [Google Scholar] [CrossRef]
- Asrat, F.; Soromessa, T.; Bekele, T.; Kurakalva, R.M.; Guddeti, S.S.; Smart, D.R.; Steger, K. Effects of Environmental Factors on Carbon Stocks of Dry Evergreen Afromontane Forests of the Choke Mountain Ecosystem, Northwestern Ethiopia. Int. J. For. Res. 2022, 2022, 9447946. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1155/2022/9447946 (accessed on 28 April 2025). [CrossRef]
- Birhane, E.; Ahmed, S.; Hailemariam, M.; Negash, M.; Rannestad, M.M.; Norgrove, L. Carbon stock and woody species diversity in homegarden agroforestry along an elevation gradient in southern Ethiopia. Agrofor. Syst. 2020, 94, 1099–1110. [Google Scholar] [CrossRef]
- Fukumasu, J.; Jarvis, N.; Koestel, J.; Kätterer, T.; Larsbo, M. Relations between soil organic carbon content and the pore size distribution for an arable topsoil with large variations in soil properties. Eur. J. Soil Sci. 2022, 73, e13212. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/ejss.13212 (accessed on 28 April 2025). [CrossRef]
- Polo-Marcial, M.H.; Solís-Ramos, L.Y.; Murillo-Cruz, R.; Ávila-Arias, C.; Andrade-Torres, A. Mycorrhizal and endophytic richness and colonization in Cedrela odorata L., in agroforestry systems and secondary forest from southeastern Costa Rica. Agrofor. Syst. 2023, 97, 647–658. [Google Scholar] [CrossRef]
- Wang, Y.-J.; He, X.-H.; Meng, L.-L.; Zou, Y.-N.; Wu, Q.-S. Extraradical Mycorrhizal Hyphae Promote Soil Carbon Sequestration through Difficultly Extractable Glomalin-Related Soil Protein in Response to Soil Water Stress. Microb. Ecol. 2023, 86, 1023–1034. [Google Scholar] [CrossRef]
- Son, Y.; Martínez, C.E.; Kao-Kniffin, J. Three important roles and chemical properties of glomalin-related soil protein. Front. Soil Sci. 2024, 4, 1418072. Available online: https://www.frontiersin.org/journals/soil-science/articles/10.3389/fsoil.2024.1418072/full (accessed on 5 June 2025). [CrossRef]
- Paz-Pellat, F.; Salas-Aguilar, V.M.; Sánchez-Sánchez, C.D.; Libert-Amico, A.; Bolaños-González, M.A. Caracterización de los almacenes de carbono, estructura y diversidad de los cafetales bajo sombra y vegetación natural en la Sierra Madre de Chiapas, México. Elem. Para Políticas Públicas 2022, 6, 101–122. Available online: https://www.elementospolipub.org/ojs/index.php/epp/article/view/46 (accessed on 16 January 2025).
- Brevik, E.C.; Burgess, L.C. Soils and Human Health; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4398-4454-0. [Google Scholar]
- Belay, B.; Pötzelsberger, E.; Sisay, K.; Assefa, D.; Hasenauer, H. The Carbon Dynamics of Dry Tropical Afromontane Forest Ecosystems in the Amhara Region of Ethiopia. Forests 2018, 9, 18. [Google Scholar] [CrossRef]
- Somarriba, E.; Cerda, R.; Orozco, L.; Cifuentes, M.; Dávila, H.; Espin, T.; Mavisoy, H.; Ávila, G.; Alvarado, E.; Poveda, V.; et al. Carbon stocks and cocoa yields in agroforestry systems of Central America. Agric. Ecosyst. Environ. 2013, 173, 46–57. Available online: https://www.sciencedirect.com/science/article/pii/S0167880913001230 (accessed on 28 March 2025). [CrossRef]
- Canal-Daza, D.; Andrade-Castañeda, H. Adaptation to Climate Change in Coffee Production Systems in Tolima. Floresta E Ambiente 2019, 26, e20171165. Available online: https://www.scielo.br/j/floram/a/cmnsMbWD9wksFyKJ4zRjksM/?lang=en (accessed on 28 March 2025). [CrossRef]
- Merle, I.; Villarreyna-Acuña, R.; Ribeyre, F.; Roupsard, O.; Cilas, C.; Avelino, J. Microclimate estimation under different coffee-based agroforestry systems using full-sun weather data and shade tree characteristics. Eur. J. Agron. 2022, 132, 126396. Available online: https://www.sciencedirect.com/science/article/pii/S1161030121001672 (accessed on 28 March 2025). [CrossRef]
- Kimaro, O.D.; Desie, E.; Verbist, B.; Kimaro, D.N.; Vancampenhout, K.; Feger, K.-H. Soil organic carbon stocks and fertility in smallholder indigenous agroforestry systems of the North-Eastern mountains, Tanzania. Geoderma Reg. 2024, 36, e00759. Available online: https://ui.adsabs.harvard.edu/abs/2024GeodR..36E00759/abstract (accessed on 16 January 2025). [CrossRef]
- Mada, G.; Anjulo, A.; Gelaw, A. Estimation of biomass and carbon sequestration capacity of the Surra mountain plantation forest in Gamo Highlands, Southern Ethiopia. Food Energy Secur. 2022, 11, e399. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/fes3.399 (accessed on 5 June 2025). [CrossRef]
- Reay, D. Climate-Smart Coffee. In Climate-Smart Food; Reay, D., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 93–104. ISBN 978-3-030-18206-9. [Google Scholar] [CrossRef]
- Perfecto, I.; Jiménez-Soto, M.E.; Vandermeer, J. Coffee Landscapes Shaping the Anthropocene: Forced Simplification on a Complex Agroecological Landscape. Curr. Anthropol. 2019, 60, S236–S250. Available online: https://www.journals.uchicago.edu/doi/full/10.1086/703413 (accessed on 28 March 2025). [CrossRef]
- Gidey, T.; Crous-Duran, J.; Oliveira, T.S. Using the Yield-SAFE Model to Assess the Impacts of Climate Change on Yield of Coffee (Coffea arabica L.) Under Agroforestry and Monoculture Systems; ResearchGate: Berlin, Germany, 2020; Available online: https://www.researchgate.net/publication/344649153_Using_the_yield-SAFE_model_to_assess_the_impacts_of_climate_change_on_yield_of_coffee_Coffea_arabica_L_under_agroforestry_and_monoculture_systems (accessed on 28 March 2025).
- Lin, B.B. Resilience in Agriculture through Crop Diversification: Adaptive Management for Environmental Change. BioScience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- Pandey, D.N. Carbon sequestration in agroforestry systems. Clim. Policy 2002, 2, 367–377. Available online: https://www.tandfonline.com/doi/abs/10.3763/cpol.2002.0240 (accessed on 13 January 2025). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).