1. Introduction
Lettuce (
Lactuca sativa) is an economically important crop, with over 28.08 million tons of lettuce produced in open fields and greenhouses in 2023 [
1]. The global lettuce harvested area in 2023 was 1.26 million hectares [
1]. Global lettuce production had been increasing until 2018, but growth has slowed down in recent years. The United States was the second-largest lettuce-producing country in 2023, following mainland China, and the third-largest in harvested area, following mainland China and India. In 2023, lettuce production in the U.S. was 4.71 million tons, with a production area of 134 thousand hectares. In 2019, lettuce was the third most consumed food in the U.S., after potatoes and tomatoes, with 25.1 pounds consumed per capita per year [
2]. Charles et al. showed that the annual yield of lettuce was higher in a greenhouse than in a field [
3]. This is due to year-round production cycles in a greenhouse, while field production is limited by season.
There are a variety of production methods used in greenhouse lettuce cultivation, including nutrient film technique (NFT) and deep water culture (DWC) [
4]. The two methods differ mostly by the volume of water that the roots are grown in; NFT uses a shallow stream of water, while DWC fully submerges the root system. DWC is more stable with less fluctuation in nutrient concentration, pH, and water temperature due to its larger volume per plant [
4].
Tipburn is a significant physiological issue that reduces the quality and marketable value of lettuce. There are two types of tipburn: inner leaf tipburn and outer leaf marginal necrosis [
5]. Inner leaf tipburn is caused by an insufficient calcium supply to the growing young leaves. High humidity, lack of airflow, or poor root development will limit plant transpiration, resulting in insufficient calcium supply to the plants. There are three ways to prevent inner leaf tipburn. The first is to ensure that the nutrient solution contains sufficient calcium. The second is to promote transpiration of plants, using lower humidity and better ventilation to promote transpiration of the plants. Vertical air flow (VAF) fans can be used to provide sufficient air to the lettuce center. The third is to avoid excessive daily light integrals (DLI). Tipburn occurs more frequently when DLI exceeds 17 mol∙m
2∙d
−1 [
6].
Previous research has shown tipburn symptoms were greatest at electric conductivity (EC) 1.8 dS∙m
−1, while lower EC exhibited reduced symptoms [
7]. However, while lower EC reduced tipburn symptoms, it also resulted in reduced yield [
8]. The application of a foliar calcium chloride spray has been shown to reduce tipburn symptoms. However, this approach can lead to excess moisture on the surface of the leaves, potentially contributing to foliar diseases such as powdery mildew [
9].
Nutrient delivery can be adjusted both temporally (over time) and spatially (delivering different concentrations to different parts of the root system). Overall, studies have shown that, the higher the nutrient concentration, the more nutrients are absorbed [
7,
10]. Cresswell (1991) showed that, when nutrient concentrations were higher during the day than at night, shoot fresh weight did not differ, but tipburn was reduced compared to when concentrations were the same during the day and night for lettuce [
11]. Masuda et al. (1990) showed that tomatoes and cucumbers absorbed more water and nutrients during the day than at night [
12]. Also, the amount of nutrients per unit volume of water absorbed was higher at night than during the day. Both daytime and nighttime water absorption decrease with increased nutrient concentrations. Nutrient absorption, except for Ca, increased during the day and at night as nutrient concentrations were increased. Ca absorption during the day decreases with increasing nutrient solution concentration but increases at night. Albornoz (2016) et al. investigated whether the daily pattern of absorption of each nutrient was affected by the age of the plants [
13]. N, P, and K absorption decreased with age, but not for Ca, Mg, and S. In general, water and nutrients are absorbed by transpiration during the day and by osmotic pressure at night. Additionally, this study showed that all nutrients are released at some time during the day and night. Albornoz et al. (2015) showed that, at 25 °C and 600 PPF, the nighttime uptake rate of Ca, Mg, and S was negative, meaning that nutrients were released [
14]. In other words, the release of calcium at night may increase the risk of tipburn.
A technique known as split root system (SRS) has been used in plant biology studies for decades, allowing for adjusted spatial delivery of nutrients to plant root systems. The oldest study of the split root system is by Wiersum in 1958 [
15]. This was a study of the effect of peas on roots in response to single ions in pot culture. Tomatoes grown in different concentrations of nutrient solution under SRS had higher yields and lower blossom end rot (BER) than tomatoes grown in uniform concentrations of nutrient solution [
16]. Furthermore, the calcium content in leaves and stems was higher at uneven concentrations. Italian ryegrass was supplied with nitrate to only half of the root system via SRS, showing that nitrate-containing roots were able to absorb more nitrate to make up for the deficiency [
17]. Root biomass was higher for roots that had uneven nitrate supply than for roots that had even nitrate supply. Corina et al. explored the effects of locally high concentrations of phosphorus on plant growth and nitrate uptake [
18]. The results showed that nitrate absorption was inhibited when the entire root was highly phosphate. However, localized high concentrations of phosphate resulted in the same amount of nitrate absorption as plants whose roots were exposed to medium concentrations. Cucumbers grown in SRS with calcium and phosphorus were supplied separately to prevent sedimentation, resulting in increased chlorophyll concentration and stomatal conductance, improved photosynthesis, and increased biomass and yield [
19]. In a tomato experiment with SRS supplying nutrient solution and clear water for 30 days, shoot and root dry weight were not found to be significantly different from nutrient solution only [
20]. Another tomato experiment found that the combination of high salinity nutrient solution and clear water increased yield for both high salinity concentrations, but the combination of medium salinity concentration and clear water did not increase it [
21]. No research on hydroponic SRS lettuce production has been reported to date. Further, most previous research on SRS has focused on scientific aspects of root nutrient uptake rather than crop yield and quality. For this study, we developed a Split Root Nutrient film technique (SR-NFT) where an NFT channel is divided longitudinally into two separate channels with their own input and drain line. In the system, plant roots can be intentionally divided to supply different nutrient solutions to each half without mixing them.
This study aims to inform about the potential benefits and limitations of SR-NFT, especially in relation to nutrient management strategies. In that aim, our experiment had two specific objectives. First, we investigated the impact of SR-NFT on yield and tipburn compared to conventional NFT with the same nutrient concentrations. We hypothesized that uniform nutrient concentrations would not affect plants in NFT and SR-NFT. In other words, there would be no difference in plant response between NFT and SR-NFT. Second, we investigated the effects of SR-NFT on yield and tipburn at non-uniform nutrient concentrations. We hypothesized that, when SRS had uniform nutrient concentrations, their growth would not be affected vs. traditional NFT with a single nutrient concentration. However, when SRS had uneven nutrient concentrations, plant growth and quality would be impacted.
4. Discussion
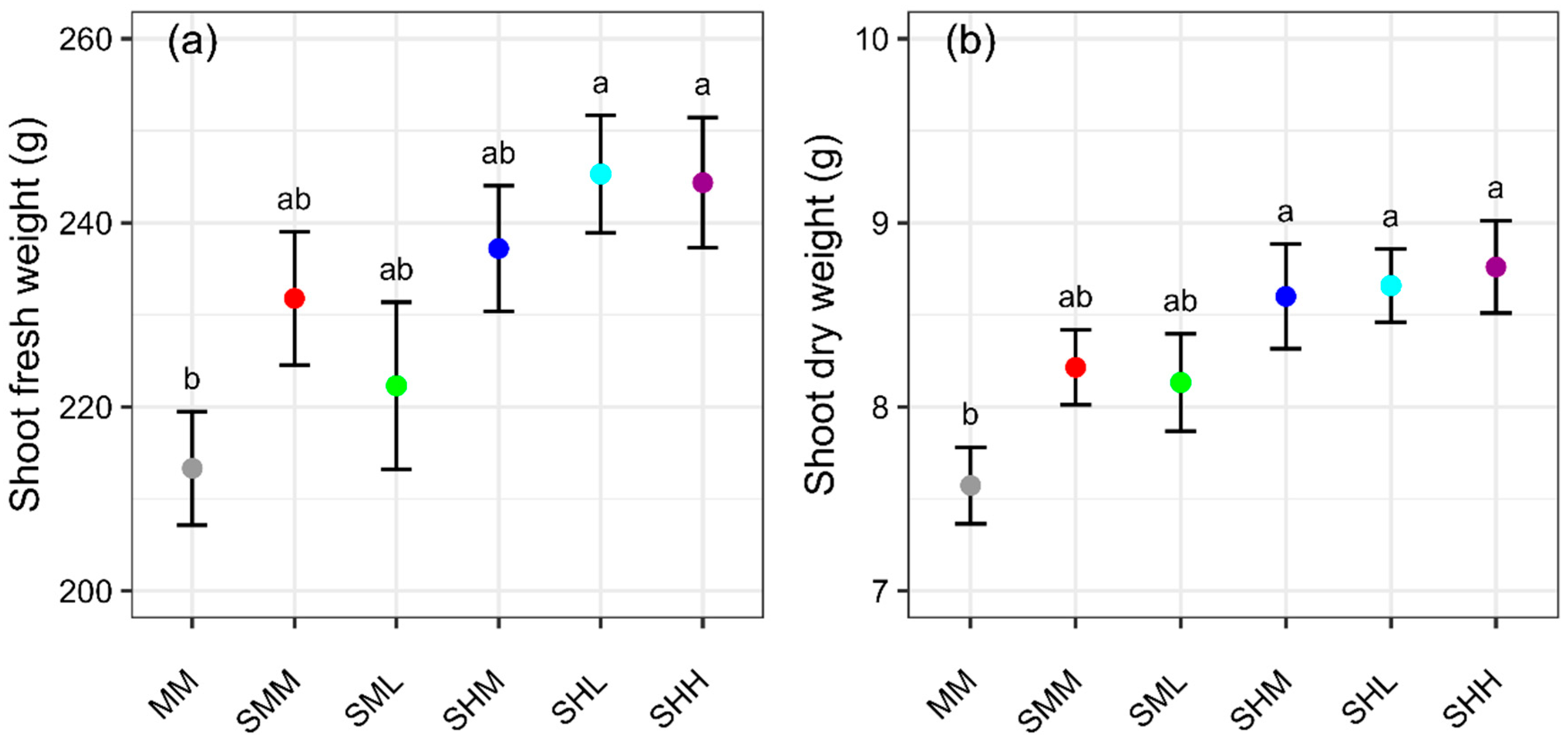
Comparing MM, SMM, and SHL (all had the same average EC of 1.8 dS∙m
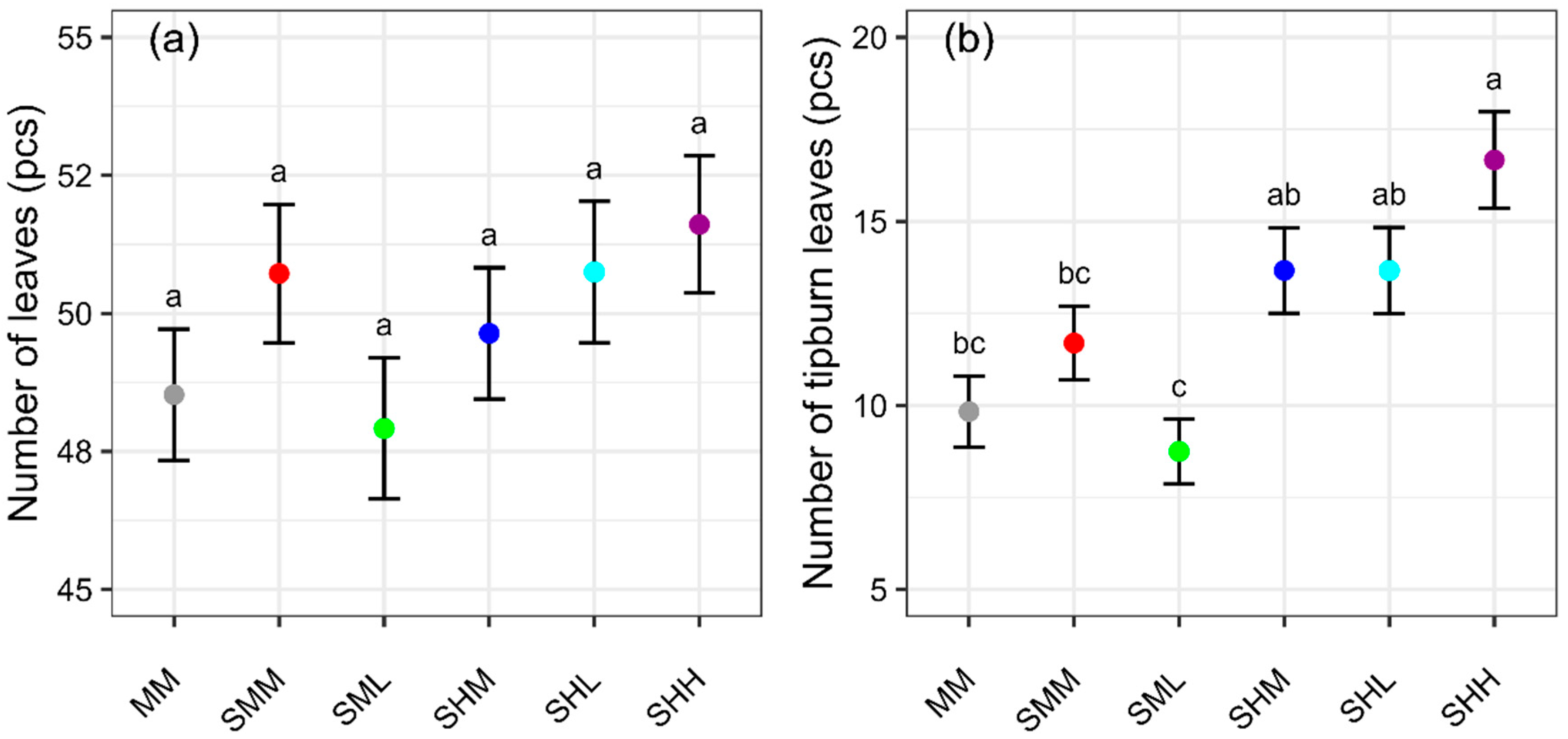
−1), SFW was significantly higher (by 15%) for SHL compared to MM. This result indicated that SFW increased with SRS at uneven concentrations. The number of tipburn leaves and calcium concentration in leaf tissue were not significantly different in SHL and MM. This is an interesting finding as typical lettuce plants with a larger biomass have a greater incidence of tipburn [
23]. Growing tomatoes under uneven nutrient concentrations resulted in increased SFW and lower incidence of blossom end rot (BER) caused by calcium deficiency, more than under uniform nutrient concentrations [
17]. There is a strong negative correlation between the incidence of BER and the amount of exudation [
17]. When nutrients are unevenly distributed, exudation increases due to low osmotic pressure in low-concentration areas, resulting in a lower incidence of BER. Supplying low concentrations of nutrients or only calcium nitrate at night reduced tipburn without reducing lettuce yields [
11]. The calcium content of young leaves was also found to be higher when supplied at lower concentrations or only with calcium nitrate during the night. The research team hypothesized that supplying low concentrations or calcium nitrate at night increased root pressure, causing the plants to absorb more calcium during the night. Increasing root pressure flow successfully prevented leaf tipburn in cabbage [
29]. In an experiment with strawberries, low concentrations of nutrient solution at night cause positive water pressure in the xylem, which results in greater calcium translocation to the leaves [
30]. Therefore, we conclude that our experimental SHL treatment decreased tipburn, in part due to the low EC nutrient solution facilitating greater calcium uptake at night. Francisco et al. showed that when nutrient concentrations were higher at night than during the day, shoot fresh weight did not differ, but nutrient content decreased compared to when concentrations were the same during the day and at night for lettuce [
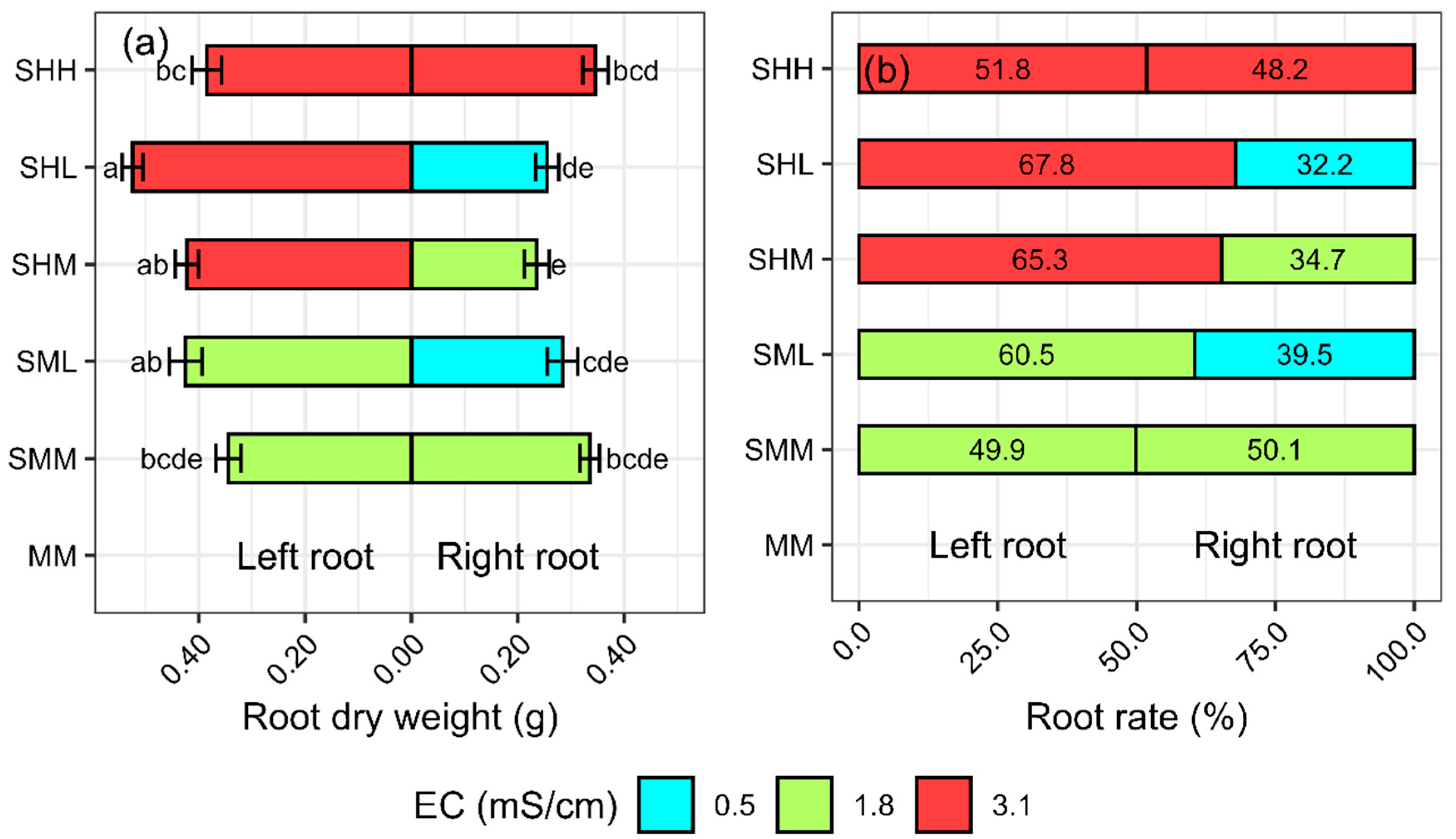
31]. This allows the same yield with less fertilizer. The total root dry weight was 25% higher for SHL than for MM. The dry weight of the SHL root at the higher concentration was 5% greater than at the lower concentration.
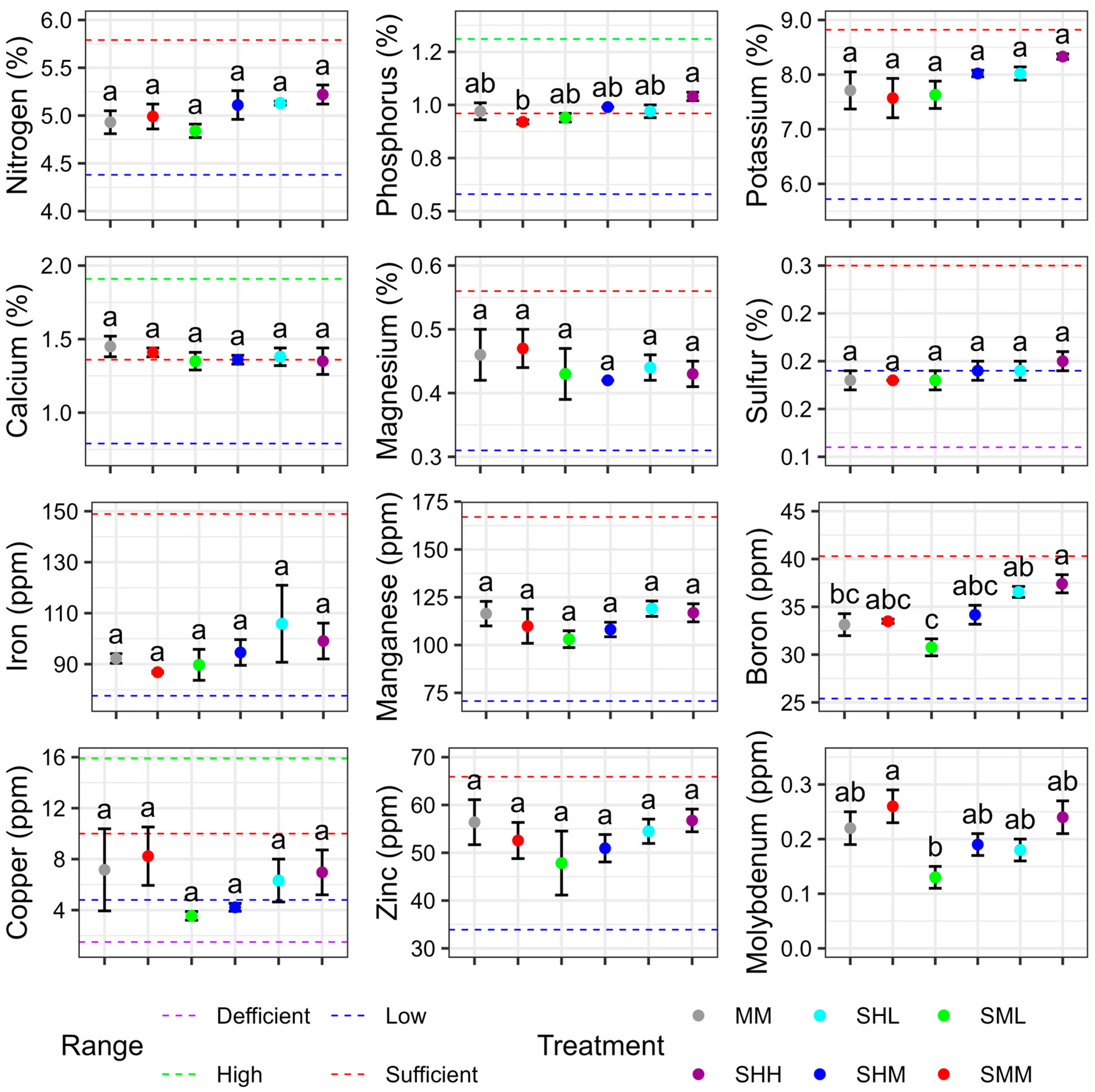
All nutrients in leaf tissue were not significantly different between SHL and MM. When P. Laine et al. used SR-NFT to locally supply nitrate to plant roots, root dry weight was greater in the nutrient solution containing nitrate than in the nutrient solution without nitrate [
18]. Furthermore, the rate of nitrate uptake was the same as plants that were uniformly supplied with nitrate. These assisted in supplementing the nitrate deficiency. Anna et al. showed that a localized supply of nitrate increased the rate of nitrate uptake [
32]. Therefore, localized supply of nitrate, or non-uniform concentration, increases the dry weight of roots and the rate of nitrate absorption. The increased dry weight of the roots is also thought to increase the root surface area, and, therefore, other nutrients may have been absorbed to a greater extent.
Comparing SMM (EC 1.8 dS∙m
−1) and SHH (EC 3.1 dS∙m
−1), which were supplied in an even concentration to both sides of the roots, SFW and SDW were higher for SHH than SMM, but not significantly different. The number of tipburn leaves was significantly greater for SHH than SMM. However, Samarakoon et al. (2020) showed that SFW and tipburn increased from EC 1.4 to 1.8 dS∙m
−1 yet decreased from EC 1.8 to 2.0 dS∙m
−1 [
8]. Serio et al. (2001) showed that SFW reaches a maximum at EC 1.5 dS∙m
−1 and decreases as EC increases (EC 2.5, 3.5 dS∙m
−1) [
33]. Scuderi et al. (2009) conducted experiments under high EC (2.8, 3.8, 4.8 dS∙m
−1) and showed that the yield was maximum at EC 2.8 dS∙m
−1 and decreased with increasing EC [
34]. Previous research has shown that too high EC decreases SFW, but the suitable EC depends on the cultivar. If the Mattson et al. (2014) nutrient solution recipe we referred to used water with EC 0 dS∙m
−1, the solution would be EC 1.8 dS∙m
−1 [
22]. This would suggest that EC 3.1 dS∙m
−1 is too high and, therefore, SFW would decrease. However, our experiments showed the opposite result. This result may have been affected by the cultivar or SR-NFT, but further research is needed.
There were statistically significant differences observed in the number of tipburn leaves in SR-NFT (SML vs. SHM, SHL, SHH). As mentioned above, this result may be due to the lower EC nutrient solution side, which promotes calcium uptake at night, thus leading to reduced tipburn. There were no statistically significant differences found in SFW and SDW with the SR-NFT treatments (SMM, SML, SHM, SHL, SHH). However, numerically, SFW and SDW were greater with higher average EC, except for SHL. This result matched that of the tomato experiment, where the combination of high concentration and clear water increased yield more than both medium concentrations, but the combination of medium concentration and clear water did not increase yield [
21]. These results indicate that the use of clear water on one side is expected to suppress tipburn or increase yield.
We evaluated the comparative performance of traditional NFT (i.e., MM, no split root with EC 1.8 dS∙m
−1) and SR-NFT (i.e., SMM, EC of 1.8 dS∙m
−1 on both sides of the channel). There were no statistically significant differences in all parameters (volume, chlorophyll content index, tipburn index,
SFW,
SDW, number of leaves, number of tipburn leaves, water content, RDW, and percent dry matter root). However, numerically,
SFW,
SDW, and RDW were 8–9% greater for SMM vs. MM. The Pearson correlation, which measures the degree of correlation between two variables, showed a value of 0.62 (
p < 0.001) for
SFW and RDW. The increase in RDW is thought to have increased the root surface area. Root length and root surface area are related to the plant’s ability to uptake nutrients [
35]. These indicate that the roots absorb more water and nutrients by growing more, resulting in increased
SFW and
SDW.
Future research should focus on clarifying the absorption mechanism of SR-NFT at uneven concentrations to increase the shoot fresh and dry weight and suppress tipburn. In addition, multiple cultivars should be compared to see if there is a consistent SRS response from one cultivar to another. Our experiments were conducted with hydroponics in greenhouses, but, in the future, this strategy could be adopted for outdoor field production. As Jing (2020) indicated, irrigating every other row would meet the transpiration demands of the crop, and providing a small amount of nutrients in the remaining rows would allow for increased yields and tipburn suppression in fields [
36]. In addition, if recirculating aquaponics and conventional hydroponics are used with SR-NFT, it could allow aquaponic nutrients to be supplemented with conventional nutrients, which are often lacking in aquaponics solutions. The commercial use of SR-NFT requires more pumps and solution tanks than usual, and there are also problems with the need to separate the roots when transplanting, which requires skilled techniques and funds. Therefore, we believe that a practical SR-NFT should be developed.
5. Conclusions
This is the first paper, to our knowledge, to examine the effect of split root nutrient applications on hydroponic lettuce. For the same average concentration of solution (EC 1.8 dS∙m−1), the SR-NFT that supplied different concentrations of solution on the left and right side (i.e., SHL, Left; EC 0.5 dS∙m−1, Right; EC 3.1 dS∙m−1) can increase the shoot fresh weight by 15%, shoot dry weight by 14%, and root dry weight by 25% without increasing tipburn compared to conventional NFT (i.e., MM, EC 1.8 dS∙m−1).
Furthermore, there were no statistically significant differences in all parameters between SR-NFT with the same concentration on the left and right sides (i.e., SMM) and conventional NFT (i.e., MM). This suggests that SRSs are more effective when different nutrient concentrations are used.
There were no significant differences in SFW and SDW for SHL vs. SMM, but biomass tended to increase as the average nutrient solution concentration increased in all conditions, with the exception of SHL, where the concentration difference was greatest. In addition, the lowest concentration (SML) reduced tipburn without reducing the shoot fresh weight in all conditions except SMM. In other words, the use of low EC water on one side is expected to suppress tipburn or increase yield.