Abstract
As an innovative plant protection method in precision agriculture, electrostatic spray technology can increase the droplet coverage area by over 30% coMpared to conventional spraying. This technology not only achieves higher droplet deposition density and coverage but also enables water and pesticide savings while reducing environmental pollution. This study, combining theoretical analysis with experimental validation, reveals the critical role of electrode material selection in induction-based electrostatic spray systems. Theoretical analysis indicates that the Fermi level and work function of electrode materials fundamentally determine charge transfer efficiency, while corrosion resistance emerges as a key parameter affecting system durability. To elucidate the effects of different electrode materials on droplet charging, a coMparative study was conducted on nickel, copper, and brass electrodes in both pristine and moderately corroded states based on the corrosion classification standard, using a targeted mesh-based charge-to-mass measurement device. The results demonstrated that the nickel electrode achieved a peak charge-to-mass ratio of 1.92 mC/kg at 10 kV, which was 8.5% and 11.6% higher than copper (1.77 mC/kg) and brass (1.72 mC/kg), respectively. After corrosion, nickel exhibited the smallest reduction in the charge-to-mass ratio (19.2%), significantly outperforming copper (40.2%) and brass (21.6%). Droplet size analysis using a Malvern Panalytical Spraytec spray particle analyzer (measurement range: 0.1–2000 µm) further confirmed the atomization advantages of nickel electrodes. The volume median diameter (Dv50) of droplets produced by nickel was 4.2–8 μm and 6.8–12.3 um smaller than those from copper and brass electrodes, respectively. After corrosion, nickel showed a smaller increase in droplet size spectrum inhomogeneity (24.5%), which was lower than copper (30.4%) and brass (25.8%), indicating superior droplet uniformity. By establishing a multi-factor predictive model for spray droplet size after electrode corrosion, this study quantifies the correlation between electrode characteristics and spray performance metrics. It provides a theoretical basis for designing weather-resistant electrostatic spray systems suitable for agricultural pesticide application scenarios involving prolonged exposure to corrosive chemicals. This work offers significant technical support for sustainable crop protection strategies.
1. Introduction
The control of crop pests, diseases, and weeds is a critical component for safeguarding food security and maintaining the balance of agricultural ecosystems. According to statistics from the 2024 Statistical Yearbook of World Food and Agriculture published by the Food and Agriculture Organization of the United Nations (FAO), annual crop losses caused by plant diseases, insect pests, and weeds account for approximately 30 percent of global production. From 2000 to 2022, global pesticide usage increased by 70%. Chemical control remains the most effective emergency measure for managing biological disasters in field crops [1,2]. However, conventional hydraulic spraying technologies exhibit significant limitations: pesticide droplet utilization rates are generally below 50% due to environmental and intrinsic factors [3], with insufficient deposition density on leaf adaxial surfaces and deposition on abaxial surfaces amounting to only 15–20% of that on adaxial surfaces [4,5,6]. More critically, approximately 40–60% of pesticide formulations are lost to drift, bounce, and runoff, leading to environmental pollution and secondary disasters such as water eutrophication and soil heavy metal contamination [7,8]. Consequently, the development of precision and high-efficiency pesticide application technologies has become a pivotal challenge for sustainable agricultural development.
Electrostatic spraying technology offers a novel solution to the challenges of hydraulic pesticide application through the field-induced deposition effect of charged droplets. Currently, numerous scholars and enterprises are conducting theoretical and experimental research on this technology. For example, Wang Shilin and his team at China Agricultural University carried out experimental studies on a bipolar contact-type aerial electrostatic spray system. The results showed that the charge-to-mass ratio of the spray liquid is positively correlated with electrostatic voltage. The coefficient of variation for droplet deposition with electrostatic spraying was 30.43%, significantly lower than the 45.4% observed with conventional spraying. Electrostatic spray equipment co-produced by China’s Jereh Huachuang and US ESS establishes an electric field near the nozzle, transforming ordinary droplets into collectively charged droplets. This reduces droplet drift and enhances penetration, etc. Calculations show that electrostatic spraying can reduce pesticide usage by over 60%. Studies have demonstrated that charged droplets can enhance deposition efficiency on target surfaces by 40–60%, reduce spatial droplet size variability, and significantly improve spray uniformity [9]. The underlying mechanisms include Space charge effects increasing droplet dispersion by over 30% [10]; Image force effects amplifying abaxial leaf surface deposition by 2–3 times [11]; Electric field-driven velocity field reconstruction reducing drift losses by 45–55% [12,13,14]. However, existing electrostatic spray systems face a critical bottleneck in practical applications: charge efficiency attenuation rates of 25–40% during prolonged operation, severely limiting their industrial-scale adoption [15]. This performance degradation primarily stems from electrochemical degradation and corrosion-induced failure of electrode materials [16,17].
In recent years, optimization studies of electrostatic spraying systems have primarily focused on the parametric design of charging structures. Patel et al. [18] achieved a charge-to-mass ratio of 1.2 mC/kg through geometric optimization of nickel-plated copper electrodes. Zhang Haiyan’s team [19,20,21] developed a low-pressure cyclone nozzle that realized 85% droplet coverage in aerial pesticide applications. Chen Jie [22,23,24] demonstrated that inductive charging efficiency improved by 18% coMpared to contact charging at an electrode gap of 3 mm. However, these studies exhibit limitations: Predominant use of single-metal electrodes (copper or steel) without systematic analysis of the relationship between intrinsic material properties and charging efficiency [25]; Neglect of surface state density changes induced by electrochemical corrosion and their iMpact on charge transfer [26]; Short experimental durations (<100 h), insufficient to reflect long-term corrosion effects in real agricultural environments [27]. Recent research reveals that surface oxide layers formed during electrode corrosion can cause local electric field distortion and significant alterations in material work function, triggering charge leakage [28]. However, existing studies lack robust quantitative models to address these phenomena.
Building on this research foundation, this study proposes a multi-scale coupled material-corrosion-performance investigation, specifically examining the influence of electrode materials and their corrosion products on charging performance and atomization efficacy. We further develop predictive equations for charge-to-mass ratio and atomization performance under multi-parameter coupling, incorporating factors such as material work function (Φ). These advancements provide critical insights for rational electrode material selection and performance evaluation in electrostatic spraying systems, facilitating their application in complex agricultural scenarios.
2. Materials and Methods
2.1. Experimental Materials
- (1)
- Electrostatic Nozzle: The electrostatic spraying device used in this study was a self-designed inductive electrostatic spraying system, with its structural schematic and physical setup illustrated in Figure 1. The atomization component employed an ST110-03 fan nozzle (Lechler GmbH, Germany) with the following specifications: an orifice diameter of 0.3 mm, a spray angle of 110°, and an operating pressure range of 200–500 kPa. The electrode assembly was fabricated using three metallic materials: nickel (purity ≥ 99.5%), brass (CuZn37), and copper (T2 grade). Laboratory-simulated moderate corrosion treatment was applied to their surfaces (5% NaCl solution, 25 ± 2 °C, 48 h spray exposure). The spraying solution was 5% NaCl. The schematic diagram and physical setup of the device are shown in Figure 1.
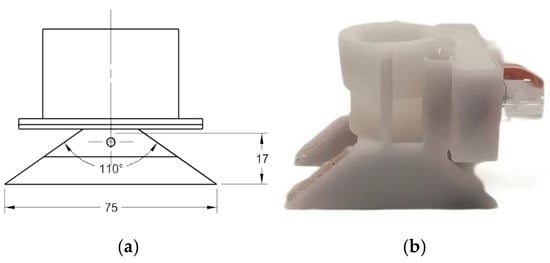 Figure 1. Device schematic and physical setup image. (a) Device schematic. (b) Physical setup image.
Figure 1. Device schematic and physical setup image. (a) Device schematic. (b) Physical setup image.
- (2)
- Charging Mechanism of Electrode Materials: CoMpared to conventional hydraulic spray, electrostatic spray fundamentally alters the generation, movement, and deposition patterns of droplets by introducing a high-voltage electrostatic field and an electrode charging mechanism, thereby achieving improved spray performance. The electrostatic spraying process involves two critical stages:
- (a)
- Pressure Atomization Stage: Primary atomization of the liquid is achieved through hydraulic shear forces;
- (b)
- Electrostatic Atomization Stage: Under a high-voltage electric field, charge accumulation on droplet surfaces induces Coulomb repulsion. Coupled with synergistic effects of gravity, shear forces, and surface tension, this drives secondary droplet breakup to form Taylor cone structures. According to classical atomization theory, the charge-to-mass ratio (q/m)—a key electrospray parameter—is primarily influenced by the combined effects of solution physicochemical properties (surface tension γ and electrical conductivity σ) and the charge transfer efficiency of electrode materials. Based on the Maxwell–Wagner interfacial polarization theory, the dielectric relaxation time (τ) of the solution can be characterized by the following equation:where ε0 is the vacuum permittivity, εr is the relative permittivity (dielectric constant) of the liquid solution, and σ is the electrical conductivity of the metal. The equation indicates that the charge-carrying capacity of the solution is related to its dielectric constant and conductivity. When droplet charging occurs within the polarization regime, the liquid’s relaxation time must be shorter than the atomization timescale. Since the total induced charge correlates with the electron density at the electrode material surface, the electron emission capability of the electrode material directly governs charge transfer efficiency. Thus, electrode materials critically influence the magnitude of the charge-to-mass ratio.
This study employs solid-state band theory to elucidate the intrinsic charging characteristics of electrode materials: the interfacial charge transfer barrier is jointly determined by the metal work function and Fermi energy level. Previous studies suggest the following work function order for the three metallic materials: copper > brass > nickel. However, under agricultural spraying conditions, electrochemical oxidation occurs at electrode surfaces, leading to the formation of metal oxide semiconductor layers that significantly alter the effective work function. To address this, Kelvin probe force microscopy was utilized to quantify surface potential variations before and after oxidation, providing critical operational parameters for subsequent experiments. The electrode materials are shown in Figure 2.
Figure 2.
Electrode materials. (a) C11000 Copper (T2). (b) Nickel 200 (N6). (c) CuZn37 Brass (H62).
2.2. Experimental Equipment and Methods
The droplet charge-to-mass ratio measurement experiments for electrostatic spraying were conducted at the College of Plant Protection Machinery, HARDI Factory, Heilongjiang Bayi Agricultural University. The charge-to-mass ratio of the electrostatic spray droplets was measured using a dedicated charge-to-mass ratio testing system. As illustrated in Figure 3, the system comprises an electrostatic spray test bench, a target grid droplet collection device, a microcurrent meter, a timer, and a computer.
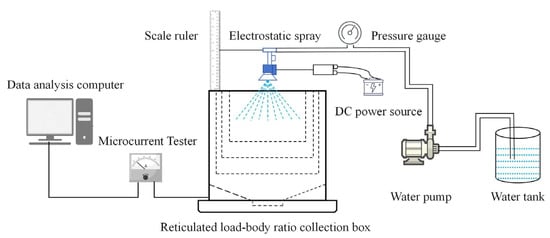
Figure 3.
Structure of the droplet charge-to-mass ratio measurement system.
The liquid charging current was monitored using a EST121 Digital Microcurrent Tester (Yiaidi Technology Co., Ltd., Beijing, China) (±0.5% accuracy). The collector, positioned beneath the nozzle, is a target grid box designed to capture droplets in real-time while acquiring their charge. The three-dimensional structure of the target grid collection device is shown in Figure 3. Constructed from stainless steel, the device integrates a three-layer brass mesh with decreasing aperture sizes (top to bottom) to ensure precise current measurement and prevent liquid accumulation on the metal grids. Current signals from the bottom electrode are transmitted to a precision ammeter. The microcurrent meter processes these signals and uploads the data to a current recording application on the computer for storage and analysis.
It should be noted that several potential sources of uncertainty exist in the measurement process: environmental humidity may interfere with charge conduction; collisions, rebound, or coalescence of droplets among the multilayer metal meshes may lead to partial charge loss; residual droplets on the mesh or device walls may introduce minor current errors; and the precision of the microammeter in extremely low-current ranges may affect the reliability of final readings. These factors collectively influence the calculation results of the charge-to-mass ratio, and their iMpact should be considered during analysis.
2.3. Experimental Design
2.3.1. Single-Factor Experiment on Charging Performance of Electrostatic Nozzle
A ST110-03 fan nozzle (Lechler GmbH, Metzingen, Germany) was selected for the experiments. Single-factor experiments were conducted to analyze the effects of spray pressure, charging voltage, and spray height on the droplet charge-to-mass ratio (C/m), investigating their influence patterns under different electrode materials (pristine and moderately corroded).
Fixed-point measurements were performed to assess variations in q/m across three electrode materials—nickel, brass, and copper—and their moderately corroded counterparts. The test plane was positioned vertically below the nozzle within a height range of 100–1100 mm. Spray height experiment: Charging voltage fixed at 10 kV and spray pressure at 0.30 Mpa. Spray pressure experiment: Charging voltage fixed at 10 kV, spray height at 500 mm, and pressure varied from 0.2 to 0.4 Mpa. Charging voltage experiment: Spray pressure fixed at 0.30 Mpa (typical operational value), spray height at 500 mm, and voltage adjusted from 7 to 13 kV. Testing conditions: Laboratory temperature, 18–21 °C; humidity, 64–69%.
2.3.2. Orthogonal Experiment on Influencing Factors of Electrostatic Nozzle Charging Performance
Building upon the single-factor experiments, a response surface methodology (RSM) was employed with optimized factor levels to investigate the influence of electrode material, spray pressure, and charging voltage on the droplet charge-to-mass ratio, aiming to identify the optimal operating parameters for different electrode materials (nickel, copper, and brass) to achieve peak spraying performance. Based on prior analyses, the experimental factors and ranges were defined as follows: electrode material: nickel, copper, brass; charging voltage: 8–12 kV; spray pressure: 0.20–0.30 Mpa; and spray height: 100–1000 mm. A three-factor, three-level orthogonal experiment was designed using Design-Expert (v10) software. The experimental factors and their levels are detailed in Table 1.

Table 1.
Factors and levels of the orthogonal experiment on nozzle charging performance.
2.3.3. Experiment on Droplet Size Variation Trends Before and After Electrode Material Corrosion
Electrode materials influence droplet size by modulating charge transfer efficiency. Droplet size critically governs spray performance, including droplet trajectory, distribution uniformity, deposition efficacy, and pest control effectiveness on target crops. While electrostatic charging reduces droplet surface tension, promoting secondary atomization into finer droplets (beneficial for water and pesticide conservation), excessively small droplets (<50 μm) are prone to drift under field conditions—particularly in aerial applications where rotor downwash and wind exacerbate off-target losses, resulting in chemical waste and phytotoxicity risks. Thus, optimizing droplet size within operational standards is essential for balancing efficacy and environmental safety.
A Spraytec laser diffraction analyzer (Malvern Instruments Ltd., Malvern, UK) (measurement range: 0.1–2000 μm) was employed to quantify droplet size distributions under varying electrode materials (pristine and moderately corroded), spray pressures, and charging voltages. This analysis aimed to elucidate droplet size variation patterns across operational parameters.
- (1)
- Single-Factor Experiment on Droplet Size Before and After Electrode Material Corrosion Experimental Protocol: The experiment was conducted in the Droplet Size Laboratory at the Center for Pesticide Application Equipment and Technology, China Agricultural University. A Malvern laser diffraction analyzer (Spraytec model) was used for indoor measurements, with the test plane positioned 500 mm vertically below the nozzle. Three electrode materials—nickel, brass, and copper—along with their moderately corroded counterparts, were tested. Spray pressure experiment: Charging voltage fixed at 10 kV, spray pressure varied from 0.2 to 0.35 Mpa. Charging voltage experiment: Spray pressure fixed at 0.25 Mpa (typical operational value), voltage adjusted from 7 to 13 kV. To evaluate the sensitivity of droplet size results to minor fluctuations in key parameters such as charging voltage and spray pressure, a ±5% parameter deviation test was conducted under baseline conditions (voltage: 10 kV, pressure: 0.25 Mpa). Testing conditions: Laboratory temperature 21–25 °C, humidity 60–66%. Each experimental group was triplicated, with results averaged.Analytical Methods: The Relative Span Factor (RSF)—an industry-standard metric for evaluating droplet uniformity—is defined as:where RSF denotes the Relative Span Factor, is the droplet diameter at the 90th percentile of the cumulative volume distribution (μm), is the diameter at the 10th percentile (μm), and (Volume Median Diameter) is the diameter at the 50th percentile (μm). The RSF is calculated as:
- (2)
- Multi-Factor Experiment on Droplet Size with Corroded Electrode Materials: To further investigate the effects and relative contributions of various factors on spray atomization after electrode material corrosion, a multi-factor experiment was designed based on single-factor findings. The independent variables included electrode material, spray pressure, and charging voltage, while the dependent variable was droplet volume median diameter. To facilitate model quantification, the work function (Φ) of each electrode material was measured and incorporated as an independent variable. This study adopted a 3-factor, 3-level full factorial experimental design to systematically evaluate interactions among variables.
3. Results
3.1. Single-Factor Analysis of Droplet Charge-to-Mass Ratio
3.1.1. Effect of Spray Pressure on Droplet Charge-to-Mass Ratio
The variation curve of droplet charge-to-mass ratio under different spray pressures is shown in Figure 4. Analysis of charge-to-mass ratio (q/m) curves for three metallic electrodes (nickel, brass, copper) before and after corrosion reveals that electrode corrosion severely iMpairs charge transfer capability. Among the three metal electrode materials, the charge capacity can be ranked as follows: nickel > copper > brass. Experimental results indicate an overall increasing trend in q/m with rising spray pressure. However, beyond approximately 0.35 Mpa, the rate of q/m increase diminishes. It should be noted that the measurement results are subject to a ±0.02 Mpa systematic error in the pressure sensing system, ±5% fluctuations in microammeter readings, and ±8% charge-to-mass ratio deviation caused by charge accumulation on the metal mesh. This combined uncertainty interval may affect the precise determination of the 0.35 Mpa inflection point and the statistical significance of material performance rankings. This phenomenon is attributed to interactions among charging relaxation time, pressure-induced atomization time, and nozzle flow rate. In electrostatic spraying, the charging relaxation time remains relatively constant. At low-to-medium spray pressures (0.2–0.35 Mpa), the pressure-induced atomization time is shorter than the charging relaxation time, and higher pressures reduce droplet size, enhancing charge acquisition. However, as pressure exceeds 0.35 Mpa, the nozzle flow rate escalates, causing the atomization time to approach the charging relaxation time. This reduces the effective charging zone and leads to stagnation or attenuation of liquid charging and atomization.
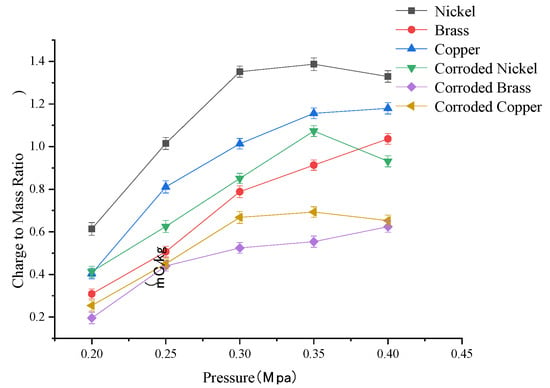
Figure 4.
Curve of the change in droplet charge-to-mass ratio under different spray pressures.
CoMparative analysis of charge variations across three metallic electrodes reveals that when spray pressure reaches 0.35 Mpa, nickel electrodes exhibit the smallest decline in the charge-to-mass ratio before and after corrosion. This indicates shorter liquid relaxation times when using nickel electrodes, whether pristine or corroded, enabling faster attainment of maximum charge-to-mass ratio and superior charging efficiency. In high-pressure, high-flow-rate spraying operations, nickel outperforms copper and brass as an electrode material. Post-corrosion, all electrodes showed a reduced charge-to-mass ratio: Nickel: 30% decrease in charge capacity after moderate corrosion. Copper and Brass: 40% reduction under similar corrosion conditions.
3.1.2. Effect of Charging Voltage on Droplet Charge-to-Mass Ratio
The variation curve of droplet charge-to-mass ratio under different charging voltages is shown in Figure 5. Single-factor experimental results demonstrate a positive correlation between the charge-to-mass ratio and charging voltage within the 7–12 kV range. However, beyond 12 kV, the charge-to-mass ratio exhibits a declining trend with further voltage increases. This phenomenon can be attributed to the Rayleigh limit of droplets and the reduced breakdown threshold of humid air at elevated voltages, both of which iMpair droplet charging efficacy. Post-corrosion, the charge-to-mass ratio decline becomes pronounced: Nickel: 20% reduction in charge-to-mass ratio after moderate corrosion coMpared to pristine electrodes. Copper and Brass: 40% decrease under similar conditions. These findings confirm that electrode corrosion severely degrades charge transfer capability.
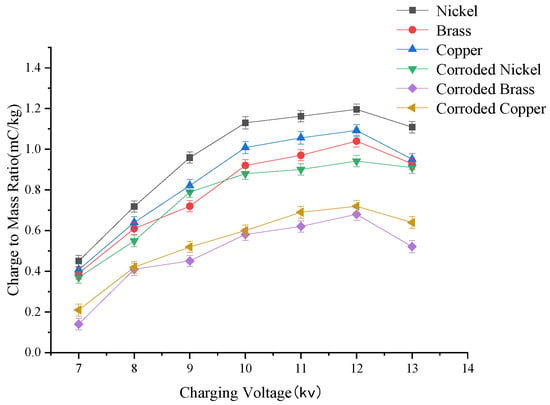
Figure 5.
Curve of change in droplet charge-to-mass ratio at different charging voltages.
Analysis of charge-to-mass ratio curves for three metallic electrodes (pristine and moderately corroded) reveals the following ranking of charge-to-mass ratio values: nickel> copper > brass > moderately corroded nickel > moderately corroded copper > moderately corroded brass. Notably, moderately corroded nickel exhibits charge-to-mass ratio values coMparable to those of pristine brass. Under identical operating conditions, nickel electrodes demonstrate superior charge retention before and after corrosion coMpared to copper and brass. Post-corrosion, nickel electrodes achieved charge-to-mass ratio values of 0.04–0.14 mC/kg and 0.11–0.24 mC/kg, higher than corroded copper and brass electrodes, respectively. Furthermore, nickel electrodes exhibited more stable charging performance under high-voltage conditions.
3.1.3. Effect of Spray Height on Droplet Charge-to-Mass Ratio
The variation curve of droplet charge-to-mass ratio at different spray heights is shown in Figure 6. The results from single-factor experiments on spray height effects indicate a negative correlation between the charge-to-mass ratio and spray height. In the 100–700 mm range, the charge-to-mass ratio exhibits a pronounced decline, with an average reduction of 40%. In the 700–1100 mm range, the decline rate diminishes significantly, and the charge-to-mass ratio stabilizes within 0.43–0.75 mC/kg at the 1100 mm spray height.
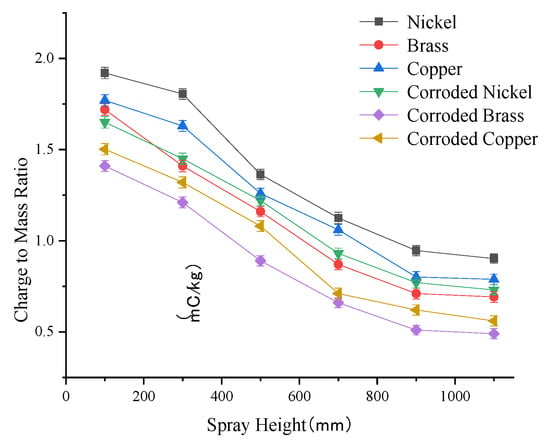
Figure 6.
Curve of change in droplet charge-to-mass ratio at different spray heights.
Under identical operating conditions, the peak charge-to-mass ratios for nickel, copper, and brass electrodes were 1.92 mC/kg, 1.77 mC/kg, and 1.72 mC/kg, respectively. Post-corrosion, all electrodes exhibited reduced charge-to-mass ratios, with nickel showing the smallest decline (19.2%), followed by brass (21.6%) and copper (40.2%). Across all tested spray heights, nickel electrodes—both pristine and corroded—achieved higher charge-to-mass ratios values coMpared to copper and brass. The higher initial charge levels of nickel electrodes help mitigate rapid charge attenuation caused by increased spray heights, maintaining sufficient droplet charge for effective long-distance transport.
3.1.4. Orthogonal Test Results of Spray Charge-to-Mass Ratio for Nickel Metal Electrodes
With spray height (H), spray pressure (P), and charging voltage (V) as independent variables, and the charge-to-mass ratio of the electrostatic nozzle as the dependent variable, the experimental design and results are summarized in Table 2. A multiple linear regression analysis of the data in Table 2 yielded the regression model (Equation (4)) for factors influencing the charge-to-mass ratio of the nickel metal electrode electrostatic nozzle. The analysis of variance (ANOVA) results for the model are provided in Table 3.

Table 2.
Experimental design and response variables for nickel as the electrode material.

Table 3.
ANOVA results for nickel.
The experimental data from Table 2 were subjected to multiple regression analysis using Design-Expert software to develop a response surface model for the droplet charge-to-mass ratio. The significance of the established regression model was evaluated through analysis of variance (ANOVA), with results presented in Table 3.
As shown in Table 3, the p-value of the droplet charge-to-mass ratio response model is <0.05, confirming the statistical significance of the established model. Based on the significance levels (p < 0.05) of individual factors in Table 3, the model was optimized by retaining only statistically significant factors. The optimized results are as follows:
Herein, X1, X2, and X3 correspond to spray height, pressure, and voltage, respectively. Analysis of Table 4 and the formula derivation process reveals the following characteristics of the fitted model:

Table 4.
Multi-factor experimental data on droplet size distribution.
- ①
- The quadratic coefficient for height is the largest, indicating its dominant role;
- ②
- The pressure–voltage interaction term was eliminated;
- ③
- The height–pressure interaction term reveals their synergistic enhancement mechanism.
These results demonstrate that spray height, spray pressure, and electrostatic voltage significantly influence the charge-to-mass ratio model.
More specifically, building on the regression analysis, response surfaces were generated using Design-Expert software to evaluate the effects of multiple influencing factors. The interaction effects of these factors on the droplet charge-to-mass ratio are illustrated in Figure 7. Figure 7 reveals significant interactions among the variables. For nickel electrodes, the charge-to-mass ratio increases with higher spray pressure and charging voltage. The optimal parameters were identified as spray pressure: 0.30 Mpa, charging voltage: 10.00 kV, and spray height: 100.00 mm. Under these conditions, the maximum charge-to-mass ratio of 1.92 mC/kg was achieved. These findings offer practical guidance for field-based electrostatic spraying operations: When applying nickel-electrode nozzles for crop protection, maintaining a spray height of 0.8–1.2 m while increasing pressure to 0.3 Mpa combined with 10 kV charging voltage achieves an optimized balance between canopy penetration and charged deposition efficiency.
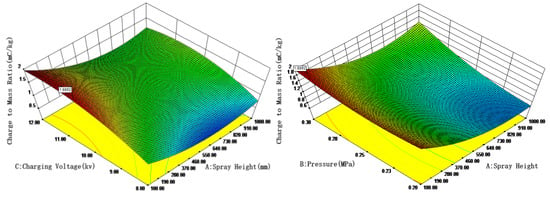
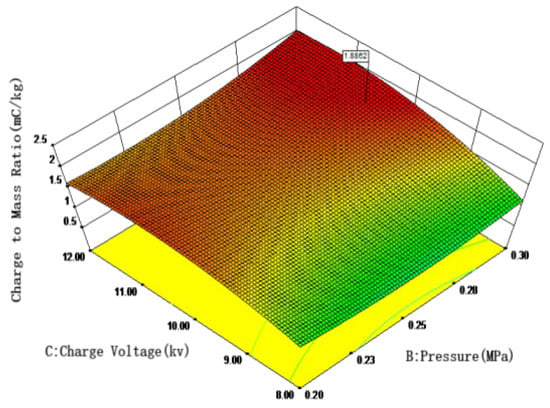
Figure 7.
Effects of spray pressure, charging voltage, and spray height on the charge-to-mass ratio with nickel electrodes.
3.2. Single-Factor Experimental Results of Droplet Size Before and After Electrode Material Corrosion
3.2.1. Effect of Spray Pressure on Droplet Size
As shown in Figure 8, droplet size exhibits a negative correlation with spray pressure for both pristine and moderately corroded electrode materials. When spray pressure exceeds 0.35 Mpa–0.40 Mpa, the rate of droplet size reduction diminishes significantly coMpared to lower pressures (<0.35 Mpa), indicating that the influence of spray pressure on droplet size weakens beyond this threshold. Under identical operating conditions, droplet sizes can be ranked from smallest to largest across electrode materials as follows: nickel < copper < brass < moderately corroded nickel < moderately corroded copper < moderately corroded brass. Key findings: Nickel electrodes produced droplets 4.2–8 μm and 6.8–12.3 μm smaller than copper and brass electrodes, respectively. Nickel maintained the smallest droplet size even after moderate corrosion, outperforming corroded copper and brass electrodes.
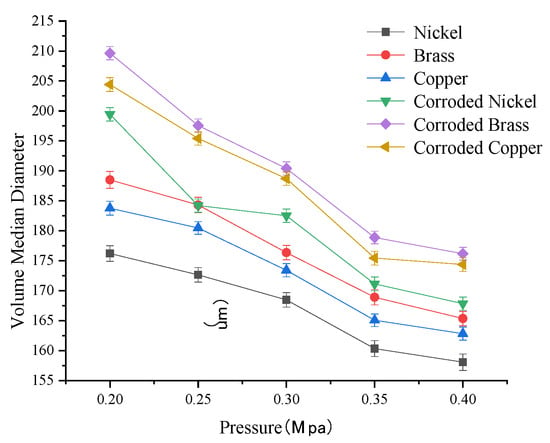
Figure 8.
Fog droplet diameter change curve under different spray pressures.
For the same metal electrodes, the larger droplet size observed after corrosion coMpared to before corrosion indicates that electrode material corrosion significantly iMpairs charging performance, thereby indirectly affecting droplet atomization. Furthermore, analysis of droplet size variation patterns across three electrode materials (pristine and corroded) reveals that corrosion critically iMpacts the atomization performance of metal electrodes. Among the tested materials, nickel electrodes demonstrated superior atomization performance for charged droplets, even after corrosion.
3.2.2. Effect of Charging Voltage on Droplet Size
As shown in Figure 9, droplet size exhibits a negative correlation with charging voltage for both pristine and moderately corroded electrode materials. The correlation coefficients between voltage and droplet size for nickel electrodes before and after corrosion were −0.92 and −0.65, respectively, indicating the iMpact of corrosion on electrode performance degradation. Under identical operating conditions, droplet sizes can be ranked from smallest to largest as follows: nickel < copper < brass < moderately corroded nickel < moderately corroded copper < moderately corroded brass.
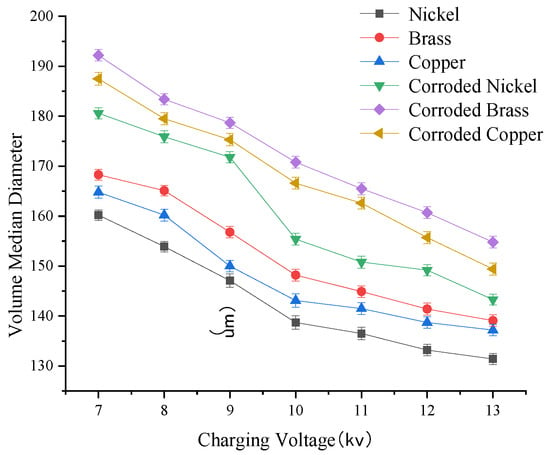
Figure 9.
Fog droplet diameter change curve under different charging voltages.
Key observations (Box 1):
Box 1. Test results.
- ①
- Voltage increase from 7 kV to 10 kV:Nickel: Droplet size decreased by 21.5 μm.Copper: Droplet size decreased by 21.7 μm.Brass: Droplet size decreased by 20.1 μm.
- ②
- Voltage increase from 10 kV to 13 kV:Nickel: Droplet size decreased by 7.3 μm.Copper: Droplet size decreased by 5.9 μm.Brass: Droplet size decreased by 9.1 μm.
These results demonstrate that the initial voltage increase (7–10 kV) induces a more pronounced reduction in droplet size, indicating enhanced atomization efficiency.
Post-corrosion effects: Moderately corroded electrodes exhibit larger droplet sizes coMpared to their pristine counterparts. Post-corrosion droplet size trends mirror those of pristine electrodes, with the following ranking: moderately corroded nickel < moderately corroded copper < moderately corroded brass. Analysis confirms that nickel electrodes deliver the best charge retention among the three metals, making them the optimal choice for electrode materials.
3.3. Influence on Droplet Distribution Uniformity
3.3.1. Effect of Spray Pressure on Droplet Distribution Uniformity
Spray pressure significantly affects droplet size, and alterations in droplet size inevitably influence spray uniformity. Through experiments, droplet size distributions under different spray pressures were analyzed to evaluate the effects of electrode materials (pristine and corroded) on droplet distribution uniformity. Experimental conditions: Charging voltage: fixed at 10 kV; spray pressure: varied from 0.2 to 0.35 Mpa; environment: controlled indoor setting without natural wind interference. Figure 10 illustrates the variation curves of droplet uniformity under different spray pressures.
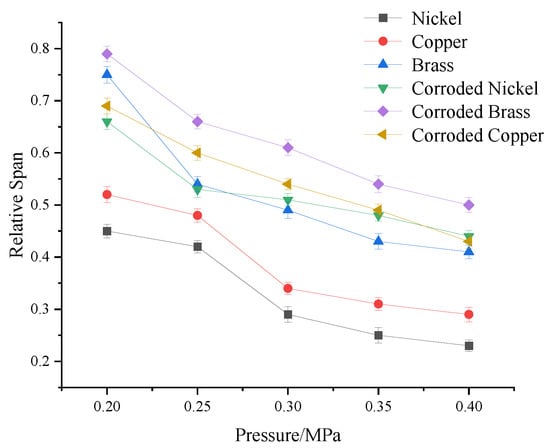
Figure 10.
Variation curve of droplet uniformity under different spray pressures.
Analysis of droplet uniformity variation curves under different spray pressures reveals that pristine nickel, copper, and brass electrodes exhibit similar trends in influencing droplet distribution uniformity. As spray pressure increases, the Uniformity Deviation Index (S) decreases. The Relative Span Factor narrows, indicating enhanced droplet uniformity. When spray pressure increases from 0.2 Mpa to 0.40 Mpa, spray uniformity improves by approximately 40%. Beyond 0.35 Mpa, the uniformity trend stabilizes. Under identical spray pressures, nickel electrodes achieve the highest uniformity. However, increasing spray pressure induces no statistically significant differences in uniformity improvement across electrode materials. Post-corrosion observations: Moderately corroded electrodes follow uniformity trends similar to their pristine counterparts. Moderately corroded nickel electrodes outperform pristine brass electrodes within the 0.30–0.35 Mpa spray pressure range. CoMparative analysis confirms that nickel electrodes—both pristine and moderately corroded—deliver superior uniformity coMpared to copper and brass electrodes. Based on the corrosion resistance advantages of nickel electrodes, it is recommended to prioritize nickel-electrode nozzles in electrostatic spraying operations, setting the working pressure within the optimal range of 0.30–0.35 Mpa. This approach can further improve pesticide utilization efficiency in electrostatic spraying while maintaining deposition uniformity.
3.3.2. Effect of Charging Voltage on Droplet Distribution Uniformity
Single-factor experimental analysis of droplet size reveals that droplet size decreases gradually with increasing charging voltage, thereby influencing spray uniformity. By measuring the cumulative volume median diameters (Dv10, Dv50, and Dv90) for nickel, copper, and brass electrodes, as well as their moderately corroded counterparts under varying charging voltages, the Relative Span Factor was calculated to quantify uniformity. The variation curves of droplet uniformity under different charging voltages are shown in Figure 11. The experimental parameters in this study were consistent with those in the single-factor experiment investigating the effect of charging voltage on droplet size. The spray pressure was set to 0.25 Mpa, and the charging voltage ranged from 7 to 13 kV. The entire experiment was conducted indoors under controlled conditions without natural wind interference.
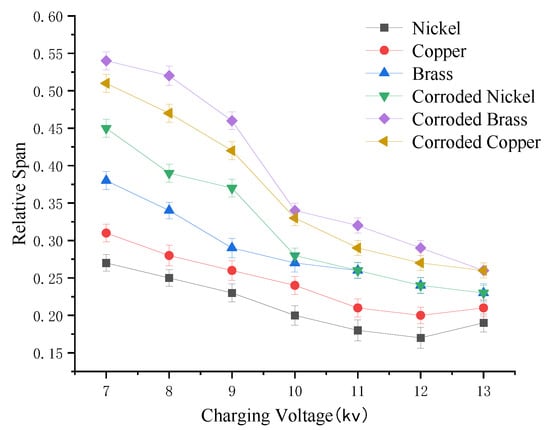
Figure 11.
Variation curve of droplet uniformity under different electrostatic voltages.
Analysis of droplet uniformity variation curves under different charging voltages reveals that pristine nickel, copper, and brass electrodes exhibit consistent trends. As charging voltage increases, the Uniformity Deviation Index decreases. Droplet uniformity improves. When charging voltage rises from 7 kV to 12 kV, the S value for all three electrodes (nickel, copper, brass) decreases, indicating enhanced uniformity. Among the materials, nickel electrodes achieve the highest droplet uniformity. Post-corrosion effects: All three electrodes show reduced uniformity, with the following declines in uniformity span: Nickel: 24.5% reduction, Copper: 30.4% reduction, Brass: 25.8% reduction. Moderately corroded nickel electrodes maintain superior uniformity coMpared to corroded copper and brass electrodes. CoMparative analysis of uniformity before and after corrosion confirms that nickel electrodes outperform copper and brass in both pristine and moderately corroded states.
3.3.3. Multi-Factor Experimental Results of Droplet Size Distribution for Pre-Corroded Electrode Materials
In practical plant protection spraying operations, the corrosion of metal electrodes is a continuous process, occurring both during and after spraying. Based on single-factor experimental results for spray uniformity, the moderate corrosion of electrodes significantly iMpacts droplet size and uniformity, with both parameters deteriorating (increased droplet size and reduced uniformity), thereby compromising spraying efficacy. To further investigate the magnitude and extent of factors influencing droplet size under different electrode materials, a multi-factor experiment was conducted with the following variables: independent variables: spray pressure, electrostatic voltage, and work function. Dependent variable: volume median diameter (Dv50). The experimental design and results are detailed in Table 4. Using multiple linear regression on the data from Table 4, the droplet size regression model and its predictions are illustrated in Figure 12. Coefficients of the fitted equation are listed in Table 5, and the analysis of variance (ANOVA) for model significance is provided in Table 6.
Figure 12.
Regression model for droplet size distribution.

Table 5.
Regression equation coefficients table.

Table 6.
Analysis of variance (ANOVA) for model significance.
The regression model formula for droplet size in electrostatic nozzle spray is shown in Equation (6) as follows:
In the equation, DV50 represents the volume median diameter (VMD) in micrometers (μm), P is the pressure in megapascals (Mpa), V denotes the voltage in kilovolts (kV), and Φ stands for the work function in electron volts (eV). Analysis of the formula derivation reveals the following model characteristics:
- ①
- The pressure coefficient exhibits the largest absolute value, confirming its dominant role;
- ②
- The positive coefficient of work function indicates that particle size increases with higher material work function (Ni > Cu > Brass);
- ③
- Although the voltage term has a smaller magnitude, it validates the auxiliary atomization effect of the electrostatic field.
As shown in Table 5, spray pressure, electrostatic voltage, and electrode work function significantly influenced the droplet size regression model. Spray pressure was the primary governing factor determining the droplet size in electrostatic nozzle spray, with droplet diameter progressively decreasing as pressure increased. The electrode work function emerged as a secondary influencing factor, where higher work function values correlated with increased droplet sizes. Although electrostatic voltage exhibited a relatively minor effect coMpared to pressure and work function, a clear decreasing trend in droplet size was observed with elevated voltage under identical operating conditions. These experimental findings align with single-factor test results, demonstrating the auxiliary droplet refinement effect of electrostatic fields. The regression model for nozzle droplet size yielded an adjusted coefficient of determination (R2adj) of 0.895, indicating high predictive reliability of the proposed model.
4. Discussion
As a novel plant protection method in precision agriculture, electrostatic spray technology has been widely recognized for its ability to significantly enhance spray atomization and deposition performance. Current research primarily focuses on the relationships between charging methods, nozzle configurations, operational parameters, and spray quality. While researchers acknowledge the critical role of atomization efficacy in pest/weed control, often employing atomization control effects to validate the superiority of electrostatic spray parameters, insufficient attention has been given to chargeability and atomization characteristics under diverse electrode materials. Additionally, challenges persist in selecting optimal electrode materials for agricultural operations under extreme conditions and maintaining droplet charge retention.
This study adopts a combined theoretical–experimental approach to investigate multi-parameter coupling effects involving electrode materials and corrosion fusion in inductive electrostatic spray systems. A systematic analysis was conducted on the interactive mechanisms governing charging voltage, spray pressure, spray height, and electrode materials regarding charge-to-mass ratio and droplet size distribution. Theoretical and experimental results demonstrate that the work function of electrode materials governs charge transfer efficiency, while corrosion resistance emerges as the key determinant of system durability. Among the three tested electrode materials, the nickel-equipped electrostatic nozzle exhibited superior long-term charge retention and atomization performance, confirming the critical role of electrode materials in ensuring inductive electrostatic spray efficacy.
Theoretically, this work elucidates the dynamic equilibrium mechanism between charging efficiency and atomization characteristics under multi-parameter coupling with corrosion effects, providing design guidelines for critical components of electrostatic spray systems. Practically, durable inductive electrode assembly reduces droplet drift by 22–35% (laboratory measurements), thereby enhancing pesticide utilization efficiency and ecological safety. Furthermore, the findings of this study can be extended to industrial applications such as electrostatic coating. For instance, adopting nickel-based electrodes in automotive painting can significantly improve coating material utilization rates. Future research should explore charge retention mechanisms of electrode materials in extreme industrial environments, including high-temperature, high-humidity, and acid-base conditions. Although nickel electrodes incur approximately 40% higher costs coMpared to copper electrodes, prolonged salt spray testing confirms their substantially lower corrosion rate. This extended service life reduces overall operational costs. In large-scale applications, the prolonged replacement cycle for nickel-electrode nozzles combined with optimized pressure–voltage parameters further decreases chemical application costs per unit area. The response surface model constructed via Box–Behnken design enables the real-time optimization of operational parameters. This study has the following limitations: (1) laboratory conditions inadequately simulated field complexities such as turbulent airflow and fluctuating humidity; (2) the tests utilized NaCl solutions without evaluating interference from fluid properties (viscosity and conductivity) on charge–atomization synergy. Future work will aim to conduct the following: (1) establish quantitative electrode geometry-charging efficiency mappings through CFD and multiphysics simulations; (2) validate drift suppression and the deposition uniformity of electrode materials in field trials; and (3) extend research to the charge–atomization behaviors of suspension concentrates and emulsifiable oils.
5. Conclusions
To quantitatively analyze the effects of electrode materials, spray pressure, and electrostatic voltage on the charge-to-mass ratio and atomization performance of electrostatic sprays, inductive electrodes of varying materials were integrated with ST-type hydraulic nozzles. This study investigated the charging efficacy and droplet characteristics of inductive electrostatic spraying systems combined with conventional hydraulic nozzles. The key findings are summarized as follows:
- (1)
- The combined use of an electrostatic charge-to-mass ratio detection system and a Malvern laser particle size analyzer enabled the rapid and effective measurement of droplet charging and atomization characteristics across different inductive electrode materials. Single-factor and multi-factor orthogonal experiments systematically revealed the iMpacts of operational parameters on charging efficiency and atomization performance, elucidating dominant controlling factors and their interactive mechanisms.
- (2)
- Based on multi-factor orthogonal experiments and the polynomial regression modeling of charge-to-mass ratios, nickel electrodes achieved peak charge capacity (1.92 mc/kg) under the following optimized parameters: a spray pressure of 0.30 Mpa, a charging voltage of 10.00 kV, and a spray height of 100.00 mm. Charge-to-mass ratios increased with elevated pressure and voltage but decreased with greater spray heights. Main effect contributions are ranked as follows: spray height > spray pressure > electrostatic voltage.
- (3)
- A multivariate linear regression model incorporating spray pressure, electrostatic voltage, and electrode work function demonstrated high significance (p < 0.0001, R2adj = 0.895) through ANOVA, proving effective for predicting atomized droplet sizes in inductive electrostatic nozzles. This work provides theoretical and experimental foundations for designing high-performance electrostatic spray systems with enhanced charging and atomization capabilities.
Author Contributions
J.H., A.Z. and Y.L. conceived the idea of the experiment; Y.L., A.Z. and C.L. performed the field test; A.Z., Y.L. and C.L. analyzed the data; J.H., A.Z., Y.L., C.L., Q.L., S.Z., Y.W. and W.Z. wrote and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Heilongjiang Bayi Agricultural University “San Zong” Research Support Program Project (No. ZRCPY202205). Open Fund Project of Heilongjiang Provincial Key Research and Development Program-Major Project (2023ZX01A06), Heilongjiang Provincial Natural Science Foundation Joint Guidance Project (LH2023E106), Heilongjiang Provincial “Double First-Class” Discipline Collaborative Innovation Achievement Project (LJGXCG2023-045), China University Industry-University-Research Collaboration Innovation Fund-Funding Project (2023RY059), Heilongjiang Bayi Agricultural University Talent Introduction Scientific Research Startup Fund Project (XYB202504) and Key Laboratory of Green Pesticides (Guizhou University, GPLKF202511).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly accessible due to their integral role in an ongoing research program. Restrictions also apply to third-party data availability, as all experimental results were obtained through proprietary testing with a self-developed device.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, J.C. Growth Inhibition of Citrus Sour Rot Pathogen by Monochromatic Visible Light. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar]
- Wang, G.; Ou, M.X.; Jia, W.D.; Zhou, H.; Dai, S.; Dong, X.; Wang, X.; Jiang, L.; Lu, W.; Wang, M. The dynamic evaporation characteristics of thiophanate-methyl droplets and their enhancement under different wind conditions. Horticulturae 2022, 8, 721. [Google Scholar] [CrossRef]
- Qi, Y.S. Research on Air-Assisted Electrostatic Pesticide Spraying Technology. Ph.D. Thesis, Hebei Agricultural University, Baoding, China, 2008. [Google Scholar]
- Liu, J.P.; Liu, X.Y.; Zhu, X.C.; Yuan, S. Droplet characterisation of a complete fluidic sprinkler with different nozzle dimensions. Biosyst. Eng. 2016, 148, 90–100. [Google Scholar] [CrossRef]
- Appah, S.; Jia, W.D.; Ou, M.X.; Wang, P.; Gong, C. Investigation of optimum applied voltage, liquid flow pressure, and spraying height for pesticide application by induction charging. Appl. Eng. Agric. 2019, 35, 795–804. [Google Scholar] [CrossRef]
- Guo, J.; Dong, X.; Qiu, B. Analysis of the factors affecting the deposition coverage of air-assisted electrostatic spray on tomato leaves. Agronomy 2024, 14, 1108. [Google Scholar] [CrossRef]
- Ru, Y.; Zheng, J.Q.; Zhou, H.P. Research and prospects of air-assisted electrostatic spray technology in forest pest control. World For. Res. 2005, 18, 38–42. [Google Scholar]
- Lin, J.; Cai, J.; Ouyang, J.; Xiao, L.; Qiu, B. The influence of electrostatic spraying with waist-shaped charging devices on the distribution of long-range air-assisted spray in greenhouses. Agronomy 2024, 14, 2278. [Google Scholar] [CrossRef]
- Zhou, L.F.; Zhang, L.; Xue, X.Y. Advances and application status analysis of pesticide electrostatic spraying technology. Trans. Chin. Soc. Agric. Eng. 2018, 34, 1–11. [Google Scholar]
- Xi, T.Y.; Li, C.L.; Qiu, W.; Wang, H.; Lv, X.; Han, C.; Ahmad, F. Droplet deposition behavior on a pear leaf surface under wind-induced vibration. Appl. Eng. Agric. 2020, 36, 913–926. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, B.; Jiang, L. Effect of UAV spray adjuvants on rice disease control. Agrochemicals 2018, 57, 299–301. [Google Scholar]
- Zeng, G.Z.; Ma, X.; Guo, L.J. Novel contact-type electrostatic spray device based on conical electrode. J. Agric. Mech. Res. 2020, 42, 58–63. [Google Scholar]
- Gao, J.; Xu, K.; He, R.; Chen, X.; Tunio, M.H. Development and experiments of low frequency ultrasonic electrostatic atomizing nozzle with double resonators. Int. J. Agric. Biol. Eng. 2022, 15, 39–48. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Li, Y.; Li, M. Effect of high-voltage electrostatic field on inorganic nitrogen uptake by cucumber plants. Trans. ASABE 2016, 59, 25–29. [Google Scholar]
- Jiang, Z.A.; Wang, Y.P.; Xu, F. Experimental study on atomization characteristics and dust suppression capability of gas-water nozzles in metal mines. J. Min. Eng. 2020, 36, 123–130. [Google Scholar]
- Zeng, Y.; Wu, Z.J.; Li, Y.F. Research progress in plant protection electrostatic spraying technology. J. Agric. Sci. Technol. 2020, 22, 52–58. [Google Scholar]
- Gong, C.; Kang, C.; Jia, W.; Yang, W.; Wang, Y. The effect of spray structure of oil-based emulsion spray on the droplet characteristics. Biosyst. Eng. 2020, 198, 78–90. [Google Scholar] [CrossRef]
- Patel, M.K.; Kundu, M.; Sahoo, H.K.; Nayak, M.K. Enhanced performance of an air-assisted electrostatic nozzle: Role of electrode material and its dimensional considerations in spray charging. Eng. Agric. Environ. Food 2016, 9, 332–338. [Google Scholar] [CrossRef]
- Zhang, H.Y. Development and Experimental Study of Electrostatic Spray System for Plant Protection UAV. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2018. [Google Scholar]
- Zhu, C.Y.; Wang, B.X. Development and discussion on aerial spray plant protection technology. Plant Prot. 2014, 5, 1–7. [Google Scholar]
- Patel, M.K.; Kundu, M.; Sahoo, H.K. Electrostatic spraying of pesticides: A review. J. Agric. Eng. 2017, 54, 1–15. [Google Scholar]
- Jiang, Y.; Chen, C.; Li, H.; Xiang, Q. Influences of nozzle parameters and low-pressure on jet breakup and droplet characteristics. Int. J. Agric. Biol. Eng. 2016, 9, 22–32. [Google Scholar]
- Qin, W.C.; Xue, X.Y.; Zhang, S.C.; Gu, W.; Chen, C. Optimization and experiment of pesticide application parameters for P20 multi-rotor UAV based on response surface methodology. J. Jiangsu Univ. (Nat. Sci. Ed.) 2016, 37, 548–555. [Google Scholar]
- Chen, J. Parameter Optimization of Electrostatic Spray Electrode for Fan-Shaped Nozzle and Experimental Study on Poultry House Electrostatic Spraying. Master’s Thesis, Zhejiang University, Hangzhou, China, 2019. [Google Scholar]
- Jia, Z.C. Research on Aerial Spray Test System and Spray Flow Field of Small Unmanned Helicopter. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2018. [Google Scholar]
- Song, J.L.; Liu, Y.J.; He, X.K.; Meng, Y.H.; Zhou, G.Q.; Wu, C.B. Pesticide Spraying System for Single-Rotor Plant Protection. China Patent CN203946276U, 19 November 2014. [Google Scholar]
- Li, Z.F. Study on Simulation of Fixed-Wing Aircraft Spray Flow Field and Droplet Distribution Patterns. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2019. [Google Scholar]
- Zhang, H.; Li, Y.; Wang, X. Low-pressure swirl nozzle design for aerial spraying. Biosyst. Eng. 2022, 215, 45–57. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).