Effect of Exogenous Plant Growth Regulators on Antioxidant Defense in Zucchini Cotyledons Under Different Light Regimes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
- (1)
- Control (on distilled water),
- (2)
- 10−4 M MeJA,
- (3)
- 10−5 M BA,
- (4)
- 10−5 M 4PU-30,
- (5)
- MeJA+BA, containing combination of 10−4 M MeJA and 10−5 M BA, and
- (6)
- MeJA+4PU-30, containing combination of 10−4 M MeJA and 10−5 M 4PU-30.
2.2. Biochemical Analyses
2.2.1. Activity of Antioxidant Enzymes
2.2.2. Content of Hydrogen Peroxide and Proline
2.2.3. Leaf Pigment Content
2.2.4. Chemicals and Equipment Used
2.3. Statistics
3. Results
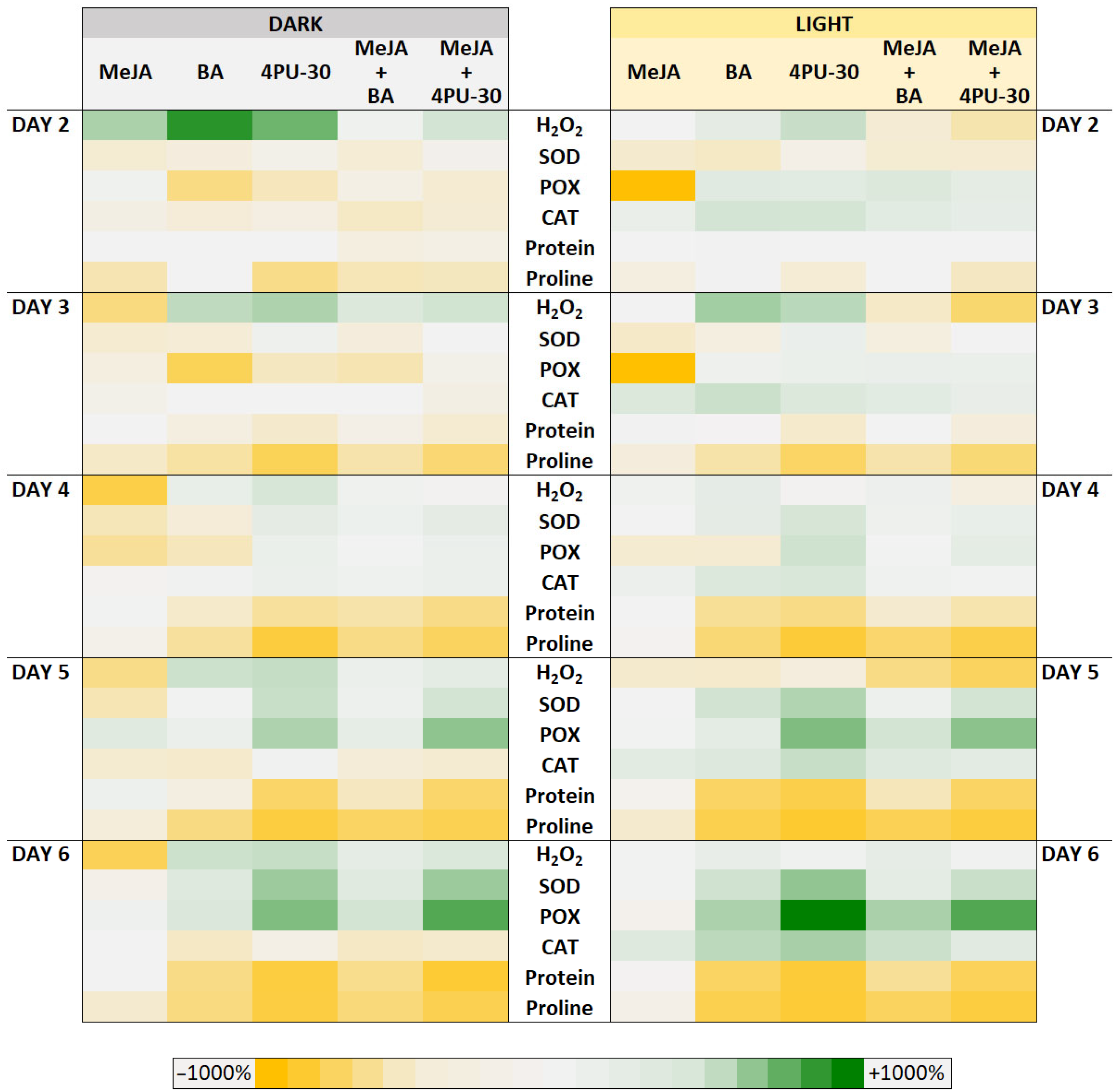
3.1. H2O2 Content
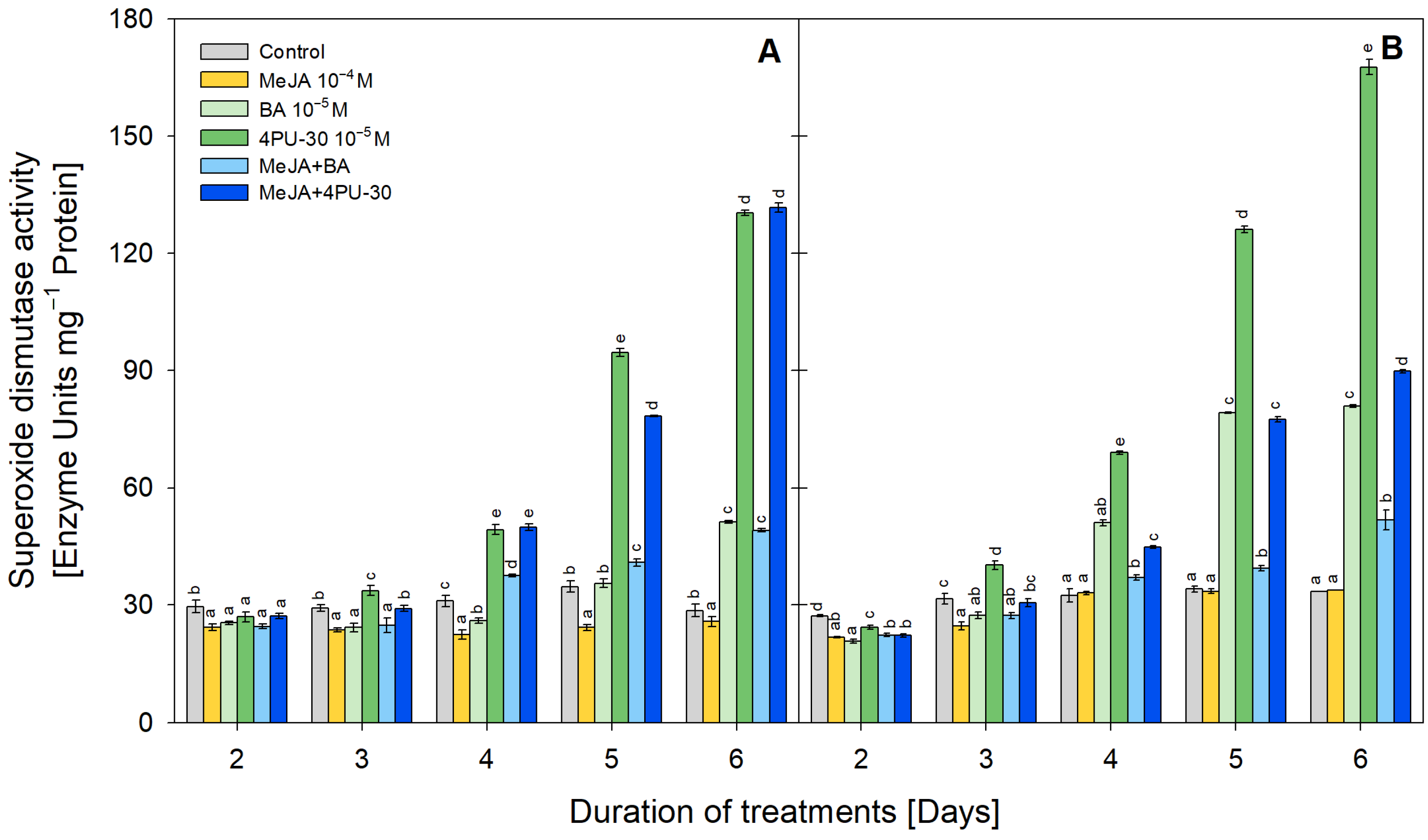
3.2. SOD Activity
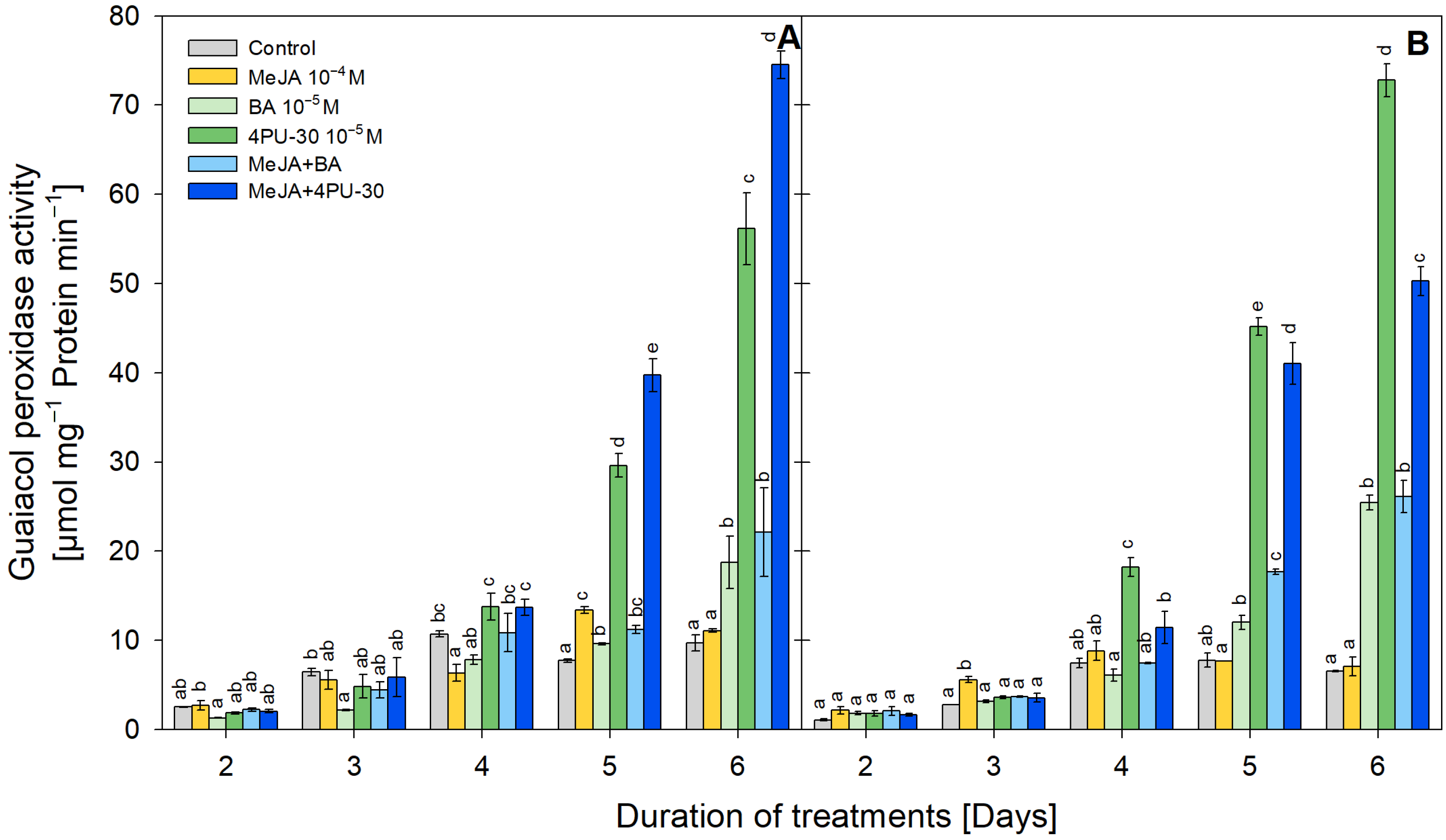
3.3. POX Activity
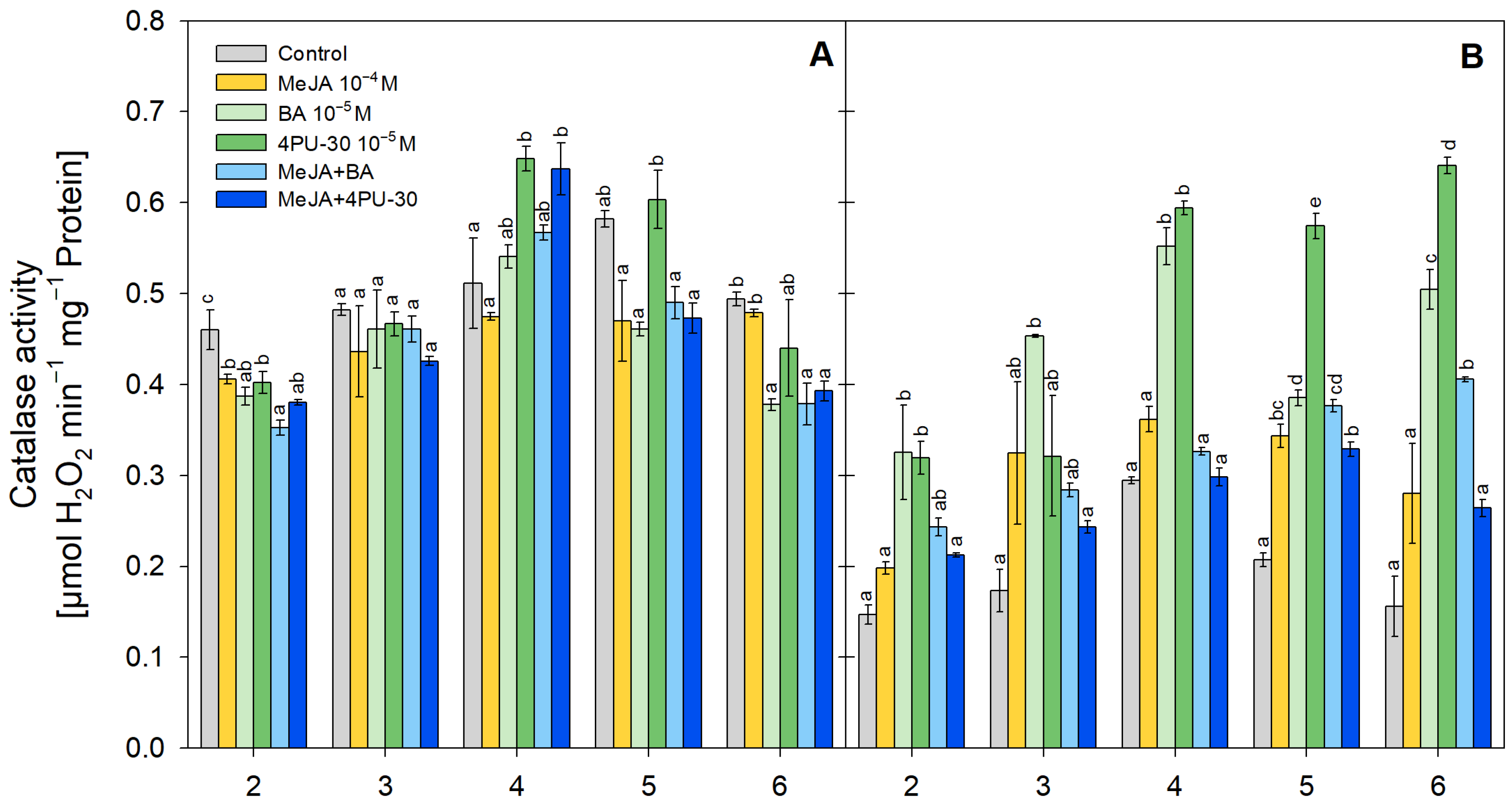
3.4. CAT Activity
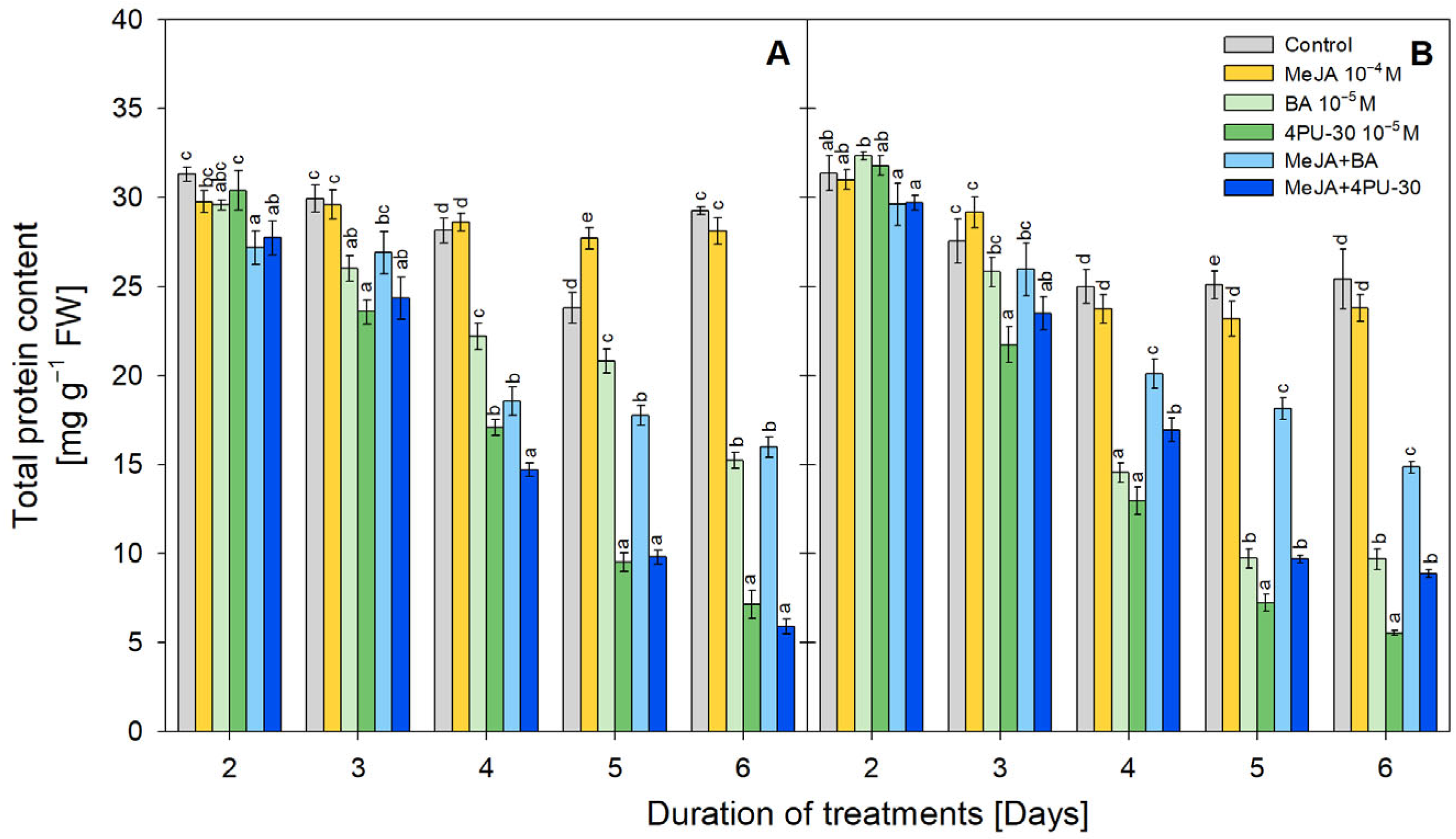
3.5. Protein Content
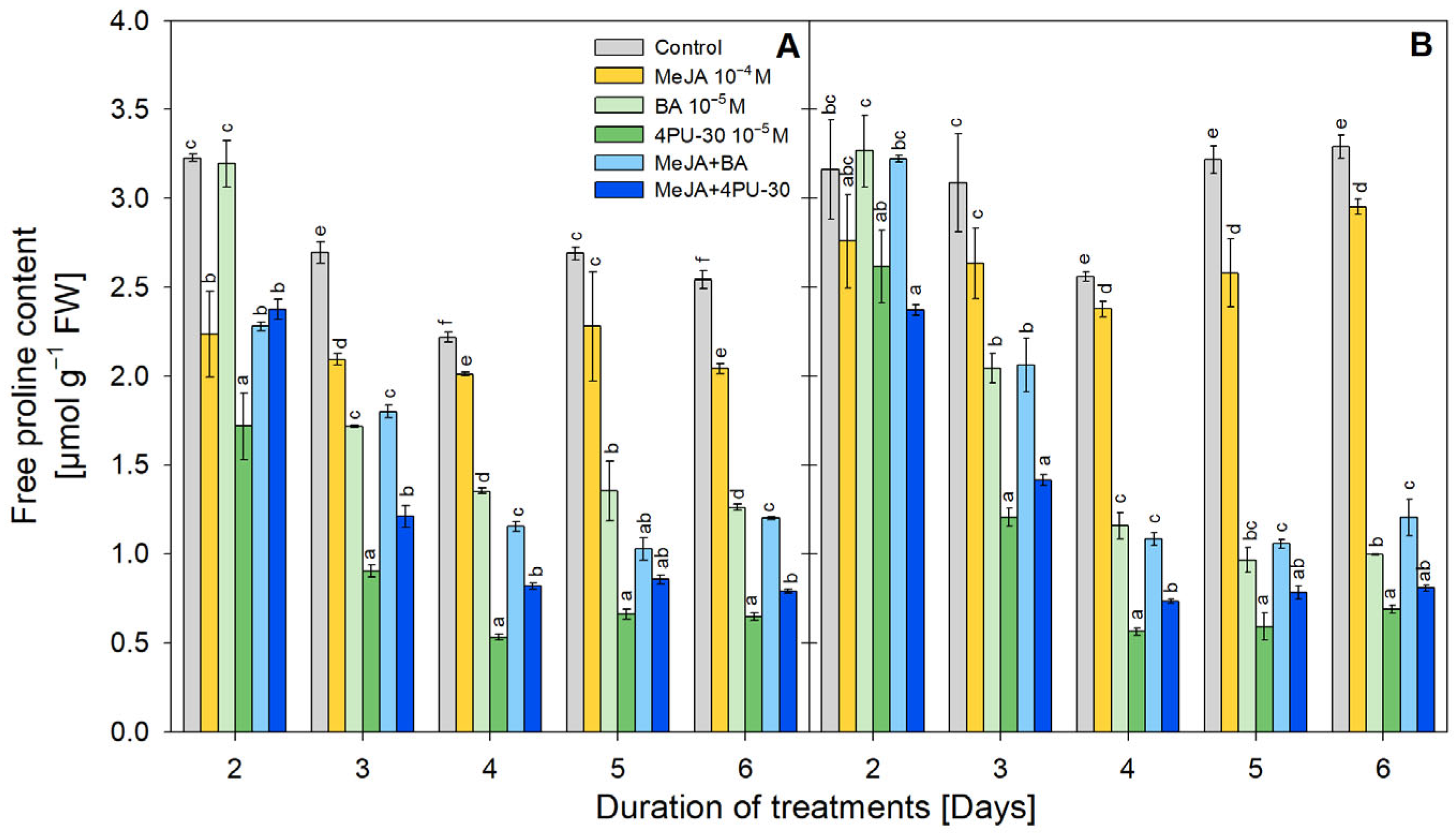
3.6. Free Proline Content
3.7. Chlorophyll and Carotenoids Content
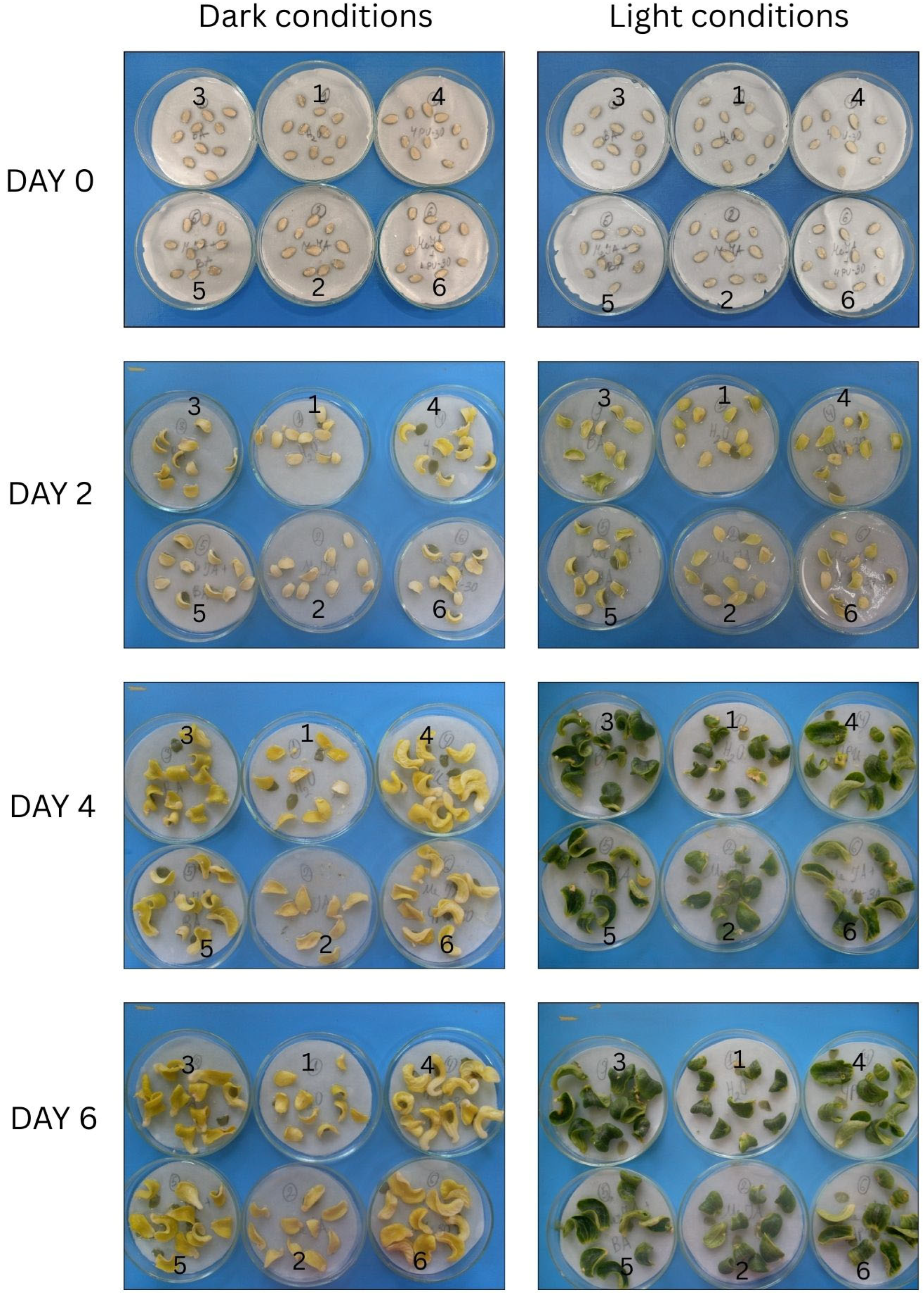
3.8. Dynamics of Phenotypic Alterations
4. Discussion
4.1. Alteration in Antioxidant Defense System in Dark-Grown Cotyledons
4.2. Alteration in Antioxidant Defense System and Leaf Pigments in Light-Grown Cotyledons
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BA | N6-benzyl adenine |
| CAT | catalase |
| CK | cytokinin |
| MeJA | methyl jasmonate |
| POX | guaiacol peroxidase |
| ROS | reactive oxygen species |
| 4PU-30 | N1-(2-chloropyridin-4-yl)-N2-phenylurea |
| SOD | superoxide dismutase |
| PAs | polyamines |
References
- Deepika; Ankit; Sagar, S.; Singh, A. Dark-induced hormonal regulation of plant growth and development. Front. Plant Sci. 2020, 11, 581666. [Google Scholar]
- Shimotohno, A.; Aki, S.S.; Takahashi, N.; Umeda, M. Regulation of the plant cell cycle in response to hormones and the environment. Ann. Rev. Plant Biol. 2021, 72, 273–296. [Google Scholar] [CrossRef]
- Stoynova-Bakalova, E.; Petrov, P.I.; Gigova, L.; Baskin, T.I. Differential effects of methyl jasmonate on growth and division of etiolated zucchini cotyledons. Plant Biol. 2008, 10, 476–484. [Google Scholar] [CrossRef]
- Stoynova-Bakalova, E. Properties of plate meristem of growing epigeal cotyledons in an experimental system. Environ. Exp. Bot. 2007, 59, 76–83. [Google Scholar] [CrossRef]
- Stoynova-Bakalova, E.; Petrov, P.I. Control by cytokinins of the cellular behavior in the plate meristem of zucchini cotyledons. Planta 2006, 223, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Stoynova-Bakalova, E.; Petrov, P.; Gigova, L.; Ivanova, N. Differences in cytokinin control on cellular dynamics of zucchini cotyledons cultivated in two experimental systems. Plant Biol. 2011, 13, 22–27. [Google Scholar] [CrossRef]
- Stoynova-Bakalova, E.Z.; Petrov, P.I.; Karanov, E.N. Effects of benzylaminopurine and abscisic acid on distribution of rRNA in the palisade cells of excised Cucurbita pepo cotyledons. Biol. Plant. 2001, 44, 355–360. [Google Scholar] [CrossRef]
- Roitsch, T.; Ehneß, R. Regulation of source/sink relations by cytokinins. Plant Growth Regul. 2000, 32, 359–367. [Google Scholar] [CrossRef]
- Sergiev, I.; Todorova, D.; Somleva, M.; Alexieva, V.; Karanov, E.; Stanoeva, E.; Lachkova, V.; Smith, A.; Hall, M. Influence of cytokinins and novel cytokinin antagonists on the senescence of detached leaves of Arabidopsis thaliana. Biol. Plant. 2007, 51, 377–380. [Google Scholar] [CrossRef]
- Gao, S. Function and mechanism study of plant cytokinins. In Proceedings of the 2020 10th International Conference on Biomedical Engineering and Technology, Tokyo, Japan, 15–18 September 2020; pp. 80–84. [Google Scholar]
- Parthier, B. Hormone-induced alterations in plant gene expression. Biochem. Physiol. Pfl 1989, 185, 289–314. [Google Scholar] [CrossRef]
- Mok, M.C.; Mok, D.W.; Turner, J.E.; Mujer, C.V. Biological and biochemical effects of cytokinin-active phenylurea derivatives in tissue culture systems. HortScience 1987, 22, 1194–1197. [Google Scholar] [CrossRef]
- Houssa, C.; Bernier, G.; Pieltain, A.; Kinet, J.M.; Jacqmard, A. Activation of latent DNA-replication origins: A universal effect of cytokinins. Planta 1994, 193, 247–250. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones: Physiology, Biochemistry and Molecular Biology; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 1–12. [Google Scholar]
- Hare, P.D.; Cress, W.A.; Van Staden, J. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regul. 1997, 23, 79–103. [Google Scholar] [CrossRef]
- Cortleven, A.; Schmülling, T. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Han, F.; Gao, F.; Gan, F.; Huang, K.; Li, Z. ROS, an important plant growth regulator in root growth and development: Functional genes and mechanism. Biology 2024, 13, 1033. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef] [PubMed]
- Shudo, K. Chemistry of phenylurea cytokinins. In Cytokynins: Chemistry, Activity and Function; Mok, D.W.S., Mok, M.C., Eds.; CRC Press: Boca Raton, FL, USA; Ann Arbor, MI, USA; London, UK; Tokyo, Japan, 1994; Chapter 3; pp. 35–42. [Google Scholar]
- Pan, J.; Wang, H.; You, Q.; Cao, R.; Sun, G.; Yu, D. Jasmonate-regulated seed germination and crosstalk with other phytohormones. J. Exp. Bot. 2023, 74, 1162–1175. [Google Scholar] [CrossRef]
- Wang, C.Y.; Buta, J.G. Methyl jasmonate reduces chilling injury in Cucurbita pepo through its regulation of abscisic acid and polyamine levels. Environ. Exp. Bot. 1994, 34, 427–432. [Google Scholar] [CrossRef]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef]
- Damyanova, M.; Todorova, D.; Sergiev, I. Polyamine alterations in isolated zucchini cotyledons grown in presence of cytokinins and Cu2+. Am. J. Plant Sci. 2014, 5, 2141–2147. [Google Scholar] [CrossRef]
- Damyanova, M.; Todorova, D.; Sergiev, I. Endogenous polyamine profiles of isolated zucchini cotyledons incubated on solutions of Cu2+ and methyl jasmonate. Oxid. Commun. 2015, 38, 104–113. [Google Scholar]
- Stoynova-Bakalova, E.; Ivanova, N.; Bakalov, D.; Gigova, L. Modifying effects of some plant hormones on zucchini cotyledons subjected to high temperature and excess copper. Bot. Lith. 2020, 26, 28–39. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges—A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.A.D.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Phua, S.Y.; De Smet, B.; Remacle, C.; Chan, K.X.; Van Breusegem, F. Reactive oxygen species and organellar signaling. J. Exp. Bot. 2021, 72, 5807–5824. [Google Scholar] [CrossRef]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Madadkar Haghjou, M.; Najafi-Kakavand, S.; Zhuang, W. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 961872. [Google Scholar] [CrossRef]
- Oracz, K.; Karpinski, S. Phytohormones signaling pathways and ROS involvement in seed germination. Front. Plant Sci. 2016, 7, 864. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Bielach, A.; Hrtyan, M. Redox regulation at the site of primary growth: Auxin, cytokinin and ROS crosstalk. Plant Cell Environ. 2017, 40, 2586–2605. [Google Scholar] [CrossRef]
- Ochatt, S.J. Less frequently used growth regulators in plant tissue culture. Methods Mol. Biol. 2024, 2827, 109–143. [Google Scholar]
- Nowakowska, K.; Pińkowska, A.; Siedlecka, E.; Pacholczak, A. The effect of cytokinins on shoot proliferation, biochemical changes and genetic stability of Rhododendron ‘Kazimierz Odnowiciel’ in the in vitro cultures. Plant Cell Tissue Organ Cult. 2022, 149, 675–684. [Google Scholar] [CrossRef]
- Dias, I.; Costa, M. Effect of low salt concentration on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Aebi, M. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase. Improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Frew, J.E.; Jones, P.; Scholes, F. Spectrophotometric determination of hydrogen peroxide and organic hydroperoxide at low concentrations in aqueous solution. Anal. Chim. Acta 1983, 155, 139–150. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, M. Role and activity of jasmonates in plants under in vitro conditions. Plant Cell Tissue Organ Cult. 2021, 146, 425–447. [Google Scholar] [CrossRef]
- Ślesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Kärkönen, A.; Dewhirst, R.A.; Mackay, C.L.; Fry, S.C. Metabolites of 2,3-diketogulonate delay peroxidase action and induce non-enzymic H2O2 generation: Potential roles in the plant cell wall. Arch. Biochem. Biophys. 2017, 620, 12–22. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Parra-Lobato, M.C.; Fernandez-Garcia, N.; Olmos, E.; Alvarez-Tinaut, M.C.; Gómez-Jiménez, M.C. Methyl jasmonate-induced antioxidant defence in root apoplast from sunflower seedlings. Environ. Exp. Bot. 2009, 66, 9–17. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sreenivasulu, N.E.S.E. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Hima Kumari, P.; Sunita, M.S.L.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef]
- Thomas, J.C.; McElwain, E.F.; Bohnert, H.J. Convergent induction of osmotic stress-responses: Abscisic acid, cytokinin, and the effects of NaCl. Plant Physiol. 1992, 100, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; Smigocki, A.C.; Bohnert, H.J. Light-induced expression of ipt from Agrobacterium tumefaciens results in cytokinin accumulation and osmotic stress symptoms in transgenic tobacco. Plant Mol. Biol. 1995, 27, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Zou, Z.R.; Yang, S.L. Effect of exogenous methyl jasmonate on osmotic adjustment capacity and proline metabolism of Jatropha curcas seedlings under salt stress. Acta Bot. Boreal-Occid Sin. 2023, 43, 794–804. [Google Scholar]
- Javadipour, Z.; Balouchi, H.; Movahhedi Dehnavi, M.; Yadavi, A. Physiological responses of bread wheat (Triticum aestivum) cultivars to drought stress and exogenous methyl jasmonate. J. Plant Growth Regul. 2022, 41, 3433–3448. [Google Scholar] [CrossRef]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Hassan, M.N.; Roberts, T.H. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate droughtinduced stress in maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef]
- Repkina, N.; Ignatenko, A.; Holoptseva, E.; MiszalskI, Z.; Kaszycki, P.; Talanova, V. Exogenous methyl jasmonate improves cold tolerance with parallel induction of two cold-regulated (COR) genes expression in Triticum aestivum L. Plants 2021, 10, 1421. Plants 2021, 10, 1421. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A unified mechanism in plant development and stress response? Plants 2025, 14, 2. [Google Scholar] [CrossRef]
- Galili, G.; Tang, G.; Zhu, X.; Gakiere, B. Lysine catabolism: A stress and development super-regulated metabolic pathway. Curr. Opin. Plant Biol. 2001, 4, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Agarwal, R.; Gupta, R. Unraveling the crosstalk among ethylene, nitric oxide, and polyamines in tailoring the abiotic stress resilience in plants. Stress Biol. 2025, 5, 20. [Google Scholar] [CrossRef]
- Sergiev, I.; Todorova, D.; Katerova, Z.; Brambilla, I.; Mapelli, S.; Simova, S. Polyamines and amino acids in triticale plants grown on humic acids enriched nutrient solution and treated with UV-B irradiation. Theor. Exp. Plant Physiol. 2018, 30, 153–163. [Google Scholar] [CrossRef]
- Legocka, J.; Żarnowska, A. Role of polyamines in the cytokinindependent physiological processes II. Modulation of polyamine levels during cytokinin-stimulated expansion of cucumber cotyledons. Acta Physiol. Plant 2000, 22, 395–401. [Google Scholar] [CrossRef]
- Biondi, S.; Scoccianti, V.; Scaramagli, S.; Ziosi, V.; Torrigani, P. Auxin and cytokinin modify methyl jasmonate effects on polyamine metabolism and ethylene biosynthesis in tobacco leaf discs. Plant Sci. 2003, 165, 95–101. [Google Scholar] [CrossRef]
- Haggag, W.M.; Abd-El-Kareem, F. Methyl jasmonate stimulates polyamines biosynthesis and resistance against leaf rust in wheat plants. Arch. Phytopathol. Plant Prot. 2009, 42, 16–31. [Google Scholar] [CrossRef]
- Todorova, D.; Kamenova, S.; Ananieva, K. Changes of endogenous polyamines in excised Cucurbita pepo L. (zucchini) cotyledons cultivated in the presence of benzyladenine and methyl jasmonate. Genet. Plant Physiol. 2015, 5, 39–47. [Google Scholar]
- Kusnetsov, V.V.; Doroshenko, A.S.; Kudryakova, N.V.; Danilova, M.N. Role of phytohormones and light in de-etiolation. Russ. J. Plant Physiol. 2020, 67, 971–984. [Google Scholar] [CrossRef]
- Armarego-Marriott, T.; Sandoval-Ibañez, O.; Kowalewska, Ł. Beyond the darkness: Recent lessons from etiolation and de-etiolation studies. J. Exp. Bot. 2020, 71, 1215–1225. [Google Scholar] [CrossRef]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez-Juez, E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2022, 233, 2000–2016. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D. Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol. 2008, 49, 1092–1111. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, A.; Nowak, A.; Ozimek, E.; Dresler, S.; Plak, A.; Sujak, A.; Reszczyńska, E.; Strzemski, M. Effect of copper stress on Phaseolus coccineus in the presence of exogenous methyl jasmonate and/or Serratia plymuthica from the Spitsbergen soil. J. Hazard. Mater. 2022, 436, 129232. [Google Scholar] [CrossRef] [PubMed]
- Ananieva, K.; Ananiev, E.D.; Mishev, K.; Georgieva, K.; Malbeck, J.; Kamínek, M.; Van Staden, J. Methyl jasmonate is a more effective senescence-promoting factor in Cucurbita pepo (zucchini) cotyledons when compared with darkness at the early stage of senescence. J. Plant Physiol. 2007, 164, 1179–1187. [Google Scholar] [CrossRef]
- Kim, S.J.; Tran, B.Q.; Jung, S. Methyl jasmonate-induced senescence results in alterations in the status of chlorophyll precursors and enzymatic antioxidants in rice plants. Biochem. Biophys. Res. Commun. 2023, 671, 38–45. [Google Scholar] [CrossRef]
- Hudeček, M.; Nožková, V.; Plíhalová, L.; Plíhal, O. Plant hormone cytokinin at the crossroads of stress priming and control of photosynthesis. Front. Plant Sci. 2023, 13, 1103088. [Google Scholar] [CrossRef]
- Ashraf, M.H.P.J.C.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Stoynova-Bakalova, E.; Petrov, P.; Tsukaya, H. Cell division and cell enlargement in isolated Cucurbita cotyledons grown in darkness and in light. J. Plant Res. 2002, 115, 375–380. [Google Scholar] [CrossRef]
- Werner, T.; Holst, K.; Pörs, Y.; Guivarc’h, A.; Mustroph, A.; Chriqui, D.; Grimm, B.; Schmülling, T. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot. 2008, 59, 2659–2672. [Google Scholar] [CrossRef]
- Loudya, N.; Mishra, P.; Takahagi, K.; Uehara-Yamaguchi, Y.; Inoue, K.; Bogre, L.; Mochida, K.; Lopez-Juez, E. Cellular and transcriptomic analyses reveal two staged chloroplast biogenesis underpinning photosynthesis build-up in the wheat leaf. Genome Biol. 2021, 22, 151. [Google Scholar] [CrossRef]
- Lopez-Juez, E.; Pyke, K.A. Plastids unleashed: Their development and their integration in plant development. Int. J. Dev. Biol. 2005, 49, 557–577. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, A.; Katerova, Z.; Sergiev, I.; Todorova, D. Effect of Exogenous Plant Growth Regulators on Antioxidant Defense in Zucchini Cotyledons Under Different Light Regimes. Agriculture 2025, 15, 1258. https://doi.org/10.3390/agriculture15121258
Petrova A, Katerova Z, Sergiev I, Todorova D. Effect of Exogenous Plant Growth Regulators on Antioxidant Defense in Zucchini Cotyledons Under Different Light Regimes. Agriculture. 2025; 15(12):1258. https://doi.org/10.3390/agriculture15121258
Chicago/Turabian StylePetrova, Asya, Zornitsa Katerova, Iskren Sergiev, and Dessislava Todorova. 2025. "Effect of Exogenous Plant Growth Regulators on Antioxidant Defense in Zucchini Cotyledons Under Different Light Regimes" Agriculture 15, no. 12: 1258. https://doi.org/10.3390/agriculture15121258
APA StylePetrova, A., Katerova, Z., Sergiev, I., & Todorova, D. (2025). Effect of Exogenous Plant Growth Regulators on Antioxidant Defense in Zucchini Cotyledons Under Different Light Regimes. Agriculture, 15(12), 1258. https://doi.org/10.3390/agriculture15121258








