Converting Agroforestry Biowaste into Stable Near-Natural Chars via Hydrothermal Humification and Pyrolysis for Immobilizing Plasticizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of CHAs
2.3. Characterization of CHAs
2.4. Sorption of DEP onto CHAs
2.5. High-Performance Liquid Chromatography Analysis
3. Results and Discussion
3.1. Specific Properties of CHAs
3.2. Sorption Kinetics of DEP onto CHAs
3.3. Sorption Isotherm of DEP onto CHAs
3.4. Sorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.; Jiang, Z.Y.; Li, P.F.; Wang, J.; Zhou, X. Contamination of phthalic acid esters in China’s agricultural soils: Sources, risk, and control strategies. Agronomy 2025, 15, 433. [Google Scholar] [CrossRef]
- Liang, J.F.; Ji, X.M.; Feng, X.X.; Su, P.J.; Xu, W.Z.; Zhang, Q.Z.; Ren, Z.H.; Li, Y.L.; Zhu, Q.Q.; Qu, G.B.; et al. Phthalate acid esters: A review of aquatic environmental occurrence and their interactions with plants. J. Hazard. Mater. 2024, 470, 134187. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanesh, M.; Farjadfard, S.; Ahmadi, M.; Ramavandi, B.; Fatahi, M.; Sanati, A.M. Review of toxicity and global distribution of phthalate acid esters in fish. Sci. Total Environ. 2024, 953, 175966. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Tao, Y.; Guo, X.Y.; Cui, Y.H.; Li, Z.X. Phthalates (PAEs) and reproductive toxicity: Hypothalamic-pituitary-gonadal (HPG) axis aspects. J. Hazard. Mater. 2023, 459, 132182. [Google Scholar] [CrossRef]
- He, W.; Yang, H.; Pu, Q.K.; Li, Y. Novel control strategies for the endocrine-disrupting effect of PAEs to pregnant women in traffic system. Sci. Total Environ. 2022, 851, 158269. [Google Scholar] [CrossRef]
- Cui, Z.G.; Shi, C.; Zha, L.T.; Liu, J.M.; Guo, Y.C.; Li, X.H.; Zhang, E.; Yin, Z.H. Phthalates in the environment of China: A scoping review of distribution, anthropogenic impact, and degradation based on meta-analysis. Ecotox. Environ. Safe. 2025, 289, 117659. [Google Scholar] [CrossRef]
- Bai, H.C.; Lu, P.L.; Zhang, L.L.; Li, Y.T.; Li, Y.; Zhao, H.Q.; Wang, J. New insight into the contribution of humic substance to degradation of phthalate acid esters in soil: Link batch experiment to model study. J. Environ. Chem. Eng. 2022, 10, 108731. [Google Scholar] [CrossRef]
- Tang, R.D.; Gong, D.X.; Deng, Y.C.; Xiong, S.; Zheng, J.F.; Li, L.; Zhou, Z.P.; Su, L.; Zhao, J. π-π stacking derived from graphene-like biochar/g-C3N4 with tunable band structure for photocatalytic antibiotics degradation via peroxymonosulfate activation. J. Hazard. Mater. 2022, 423, 126944. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, Y.; Cheng, K.; Wang, L.L.; Dong, W.C.; Liu, Z.Q.; Yang, F. Artificial humic acid mediated migration of phosphorus in soil: Experiment and modelling. CATENA 2024, 238, 107896. [Google Scholar] [CrossRef]
- Li, X.N.; Zhi, Y.C.; Jia, M.H.; Wang, X.W.; Tao, M.N.; Wang, Z.Y.; Xing, B.S. Properties and photosynthetic promotion mechanisms of artificial humic acid are feedstock-dependent. Carbon Res. 2024, 3, 4. [Google Scholar] [CrossRef]
- Shao, Y.C.; Bao, M.G.; Huo, W.Z.; Ye, R.; Liu, Y.Q.; Lu, W.J. Production of artificial humic acid from biomass residues by a non-catalytic hydrothermal process. J. Clean. Prod. 2022, 335, 130302. [Google Scholar] [CrossRef]
- Li, Y.Z.; Gupta, R.; Zhang, Q.Z.; You, S.M. Review of biochar production via crop residue pyrolysis: Development and perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Cheng, H.; Pan, D.; Wu, Y.R.; Ji, R.T.; Li, W.; Jiang, X.; Han, J.G. One-pot pyrolysis of a typical invasive plant into nitrogen-doped biochars for efficient sorption of phthalate esters from aqueous solution. Chemosphere 2021, 280, 130712. [Google Scholar] [CrossRef]
- Lin, S.P.; Shen, Z.Q.; Pan, D.; Ji, R.T.; Bian, Y.R.; Han, J.G.; Jiang, X.; Song, Y.; Cheng, H.; Xue, J.M. Marine macroalgae-derived multielement-doped porous biochars for efficient removal of sulfamethoxazole from aqueous solution: Sorption performance and governing mechanisms. J. Anal. Appl. Pyrol. 2023, 171, 105963. [Google Scholar] [CrossRef]
- Kayranli, B.; Bilen, M.; Seckin, I.Y.; Yilmaz, T.; Dinc, A.; Akkurt, F.; Simsek, H. Peanut shell biochar for Rhodamine B removal: Efficiency, desorption, and reusability. Chemosphere 2024, 364, 143056. [Google Scholar] [CrossRef]
- Paul, S.; Selvasembian, R. Potential of pyrolyzed and co-pyrolyzed biomass-derived biochar for the removal of ciprofloxacin. J. Anal. Appl. Pyrol. 2025, 186, 106884. [Google Scholar] [CrossRef]
- Leng, L.J.; Huang, H.J. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.L.; Zhu, L.Z. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar] [CrossRef]
- Xu, Y.L.; Chen, B.L. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sust. Energ. Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Potnuri, R.; Surya, D.V.; Rao, C.S.; Yadav, A.; Sridevi, V.; Remya, N. A review on analysis of biochar produced from microwave-assisted pyrolysis of agricultural waste biomass. J. Anal. Appl. Pyrol. 2023, 173, 106094. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R.L. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.Y.; Chan, Y.H.; Chin, B.L.F.; Lock, S.S.M.; Yee, C.Y.; Yiin, C.L.; Peng, W.X.; Lam, S.S. Production of biochar from rice straw and its application for wastewater remediation−An overview. Bioresour. Technol. 2022, 360, 127588. [Google Scholar] [CrossRef]
- Li, X.R.; Cen, K.H.; Wang, L.C.; Jia, D.X.; Zhu, X.F.; Chen, D.Y. Co-pyrolysis of cellulose and lignin: Effects of pyrolysis temperature, residence time, and lignin percentage on the properties of biochar using response surface methodology. Ind. Crop. Prod. 2024, 219, 119071. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.Q.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Raju, T.D.; Badhulika, S. Green synthesis of nitrogen, sulfur-co-doped worm-like hierarchical porous carbon derived from ginger for outstanding supercapacitor performance. Carbon 2020, 168, 209–219. [Google Scholar] [CrossRef]
- Zou, X.; Zhai, M.; Liu, G.N.; Wang, T.Y.; Guo, L.; Zhang, Y.; Liaquat, R. In-depth understanding of the microscopic mechanism of biochar carbonaceous structures during thermochemical conversion: Pyrolysis, combustion and gasification. Fuel 2024, 361, 130732. [Google Scholar] [CrossRef]
- Yang, H.P.; Yu, Y.M.; Zhang, H.; Wang, W.W.; Zhu, J.J.; Chen, Y.Q.; Zhang, S.H.; Chen, H.P. Effect mechanism of phosphorous-containing additives on carbon structure evolution and biochar stability enhancement. Biochar 2024, 6, 39. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhang, X.; Chen, W.; Yang, H.P.; Chen, H.P. The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresour. Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef]
- Chen, D.Z.; Fang, Z.; Wei, Y.F.; Xu, J.; Xu, K.; Jiang, L.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Micro-Raman spectroscopy and Petrography for unraveling the complex heterogeneous physicochemical structures of biochar from the scale of bulk to micro: A comparison and discussion. J. Anal. Appl. Pyrol. 2025, 188, 107057. [Google Scholar] [CrossRef]
- Khiari, B.; Ghouma, I.; Ferjani, A.I.; Azzaz, A.A.; Jellali, S.; Limousy, L.; Jeguirim, M. Kenaf stems: Thermal characterization and conversion for biofuel and biochar production. Fuel 2020, 262, 116654. [Google Scholar] [CrossRef]
- Wei, F.; Jin, S.L.; Yao, C.Y.; Wang, T.H.; Zhu, S.P.; Ma, Y.B.; Qiao, H.; Shan, L.X.; Wang, R.C.; Lian, X.X.; et al. Revealing the combined effect of active sites and intra-particle diffusion on adsorption mechanism of methylene blue on activated red-pulp pomelo peel biochar. Molecules 2023, 28, 4426. [Google Scholar] [CrossRef] [PubMed]
- Na, P.T.L.; Tuyen, N.D.K.; Dang, B.T. Sorption of four antibiotics onto pristine biochar derived from macadamia nutshell. Bioresour. Technol. 2024, 394, 130281. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhong, W.Z.; Qiu, R.B.; Han, L.J. Kinetics and modeling of Pb (II) adsorption in pellet biochar based on micro-computed tomography characterization. Bioresour. Technol. 2023, 387, 129645. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.W.; Yang, Q.; Dang, Z.; Zhang, L.J. Effect of carbon chain structure on the phthalic acid esters (PAEs) adsorption mechanism by mesoporous cellulose biochar. Chem. Eng. J. 2019, 362, 383–391. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, J.; Chen, X.W.; Mosa, A.; Ling, W.T.; Gao, Y.Z. Interaction and sorption mechanisms of phthalate plasticizers and Cd2+ on biochar. Environ. Pollut. 2025, 373, 126176. [Google Scholar] [CrossRef]
- Song, X.L.; Zhang, Y.; Yan, C.Y.; Jiang, W.J.; Chang, C.M. The Langmuir monolayer adsorption model of organic matter into effective pores in activated carbon. J. Colloid Interf. Sci. 2013, 389, 213–219. [Google Scholar] [CrossRef]
- Xue, C.; Peng, L.; Tang, J.P.; Lei, M.; Chen, A.W.; Shao, J.H.; Luo, S.; Mu, Y.S. Screening the main factors affecting phthalate esters adsorption on soils, humic acid, and clay organo-mineral complexes. Ecotoxicol. Environ. Saf. 2020, 190, 109143. [Google Scholar] [CrossRef]
- Jing, F.Q.; Guan, J.J.; Tang, W.; Chen, J.W. Mechanistic insight into adsorptive removal of ionic NOR and nonionic DEP organic contaminates by clay-biochar composites. Environ. Pollut. 2022, 310, 119881. [Google Scholar] [CrossRef]
- Fan, X.H.; Wang, X.Q.; Zhao, B.; Wan, J.F.; Tang, J.W.; Guo, X.Y. Sorption mechanisms of diethyl phthalate by nutshell biochar derived at different pyrolysis temperature. J. Environ. Chem. Eng. 2022, 10, 107328. [Google Scholar] [CrossRef]
- Bordoloi, N.; Goswami, R.; Kumar, M.; Kataki, R. Biosorption of Co (II) from aqueous solution using algal biochar: Kinetics and isotherm studies. Bioresour. Technol. 2017, 244, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Kończak, M.; Rakowska, M.; Oleszczuk, P. Engineered biochars from organic wastes for the adsorption of diclofenac, naproxen and triclosan from water systems. J. Clean. Prod. 2021, 288, 125686. [Google Scholar] [CrossRef]
- Luo, M.K.; Jiang, X.; Liu, Y.L.; Liu, Y.Q.; Yu, H.Q.; Niu, Y.; Meng, X.F.; Wang, L.; Niu, Y. Enhanced adsorption complexation of biochar by nitrogen-containing functional groups. J. Environ. Chem. Eng. 2023, 11, 111194. [Google Scholar] [CrossRef]
- Liu, Y.X.; Lonappan, L.S.; Brar, S.K.; Yang, S.M. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70. [Google Scholar] [CrossRef]
- Thomas, E.; Borchard, N.; Sarmiento, C.; Atkinson, R.; Ladd, B. Key factors determining biochar sorption capacity for metal contaminants: A literature synthesis. Biochar 2020, 2, 151–163. [Google Scholar] [CrossRef]
- Guo, S.J.; Zou, Z.Y.; Chen, Y.; Long, X.X.; Liu, M.; Li, X.P.; Tan, J.H.; Chen, R.Z. Synergistic effect of hydrogen bonding and π-π interaction for enhanced adsorption of rhodamine B from water using corn straw biochar. Environ. Pollut. 2023, 320, 121060. [Google Scholar] [CrossRef]
- Ghanbarpour Mamaghani, Z.; Hawboldt, K.A.; MacQuarrie, S.; Katz, M.J. Wood biochar as a point source CO2 adsorbent-impact of humidity on performance. Fuel 2024, 361, 130737. [Google Scholar] [CrossRef]
- Li, N.; Rao, F.; He, L.L.; Yang, S.M.; Bao, Y.J.; Huang, C.J.; Bao, M.Z.; Chen, Y.H. Evaluation of biochar properties exposing to solar radiation: A promotion on surface activities. Chem. Eng. J. 2020, 384, 123353. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Zeng, G.M.; Cheng, M.; Gong, X.M.; Wan, J.; Luo, H. Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour. Technol. 2018, 261, 265–271. [Google Scholar] [CrossRef]
- Xu, X.Y.; Schierz, A.; Xu, N.; Cao, X.D. Comparison of the characteristics and mechanisms of Hg(II) sorption by biochars and activated carbon. J. Colloid Interf. Sci. 2016, 463, 55–60. [Google Scholar] [CrossRef]
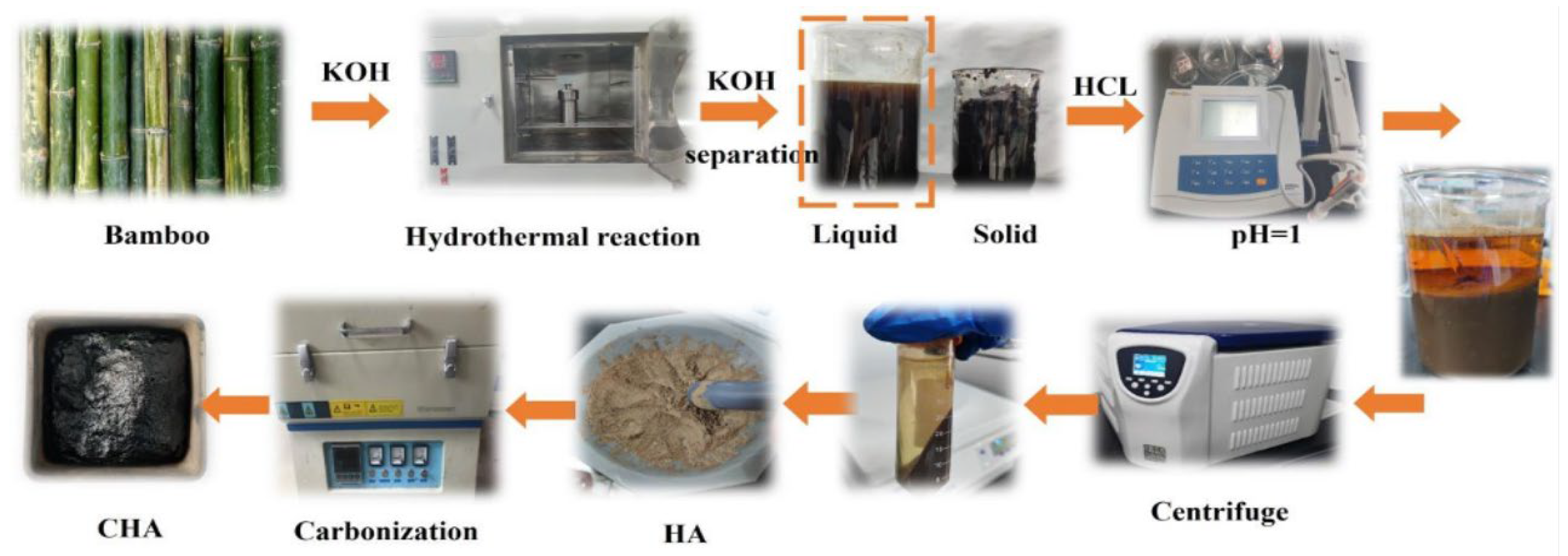


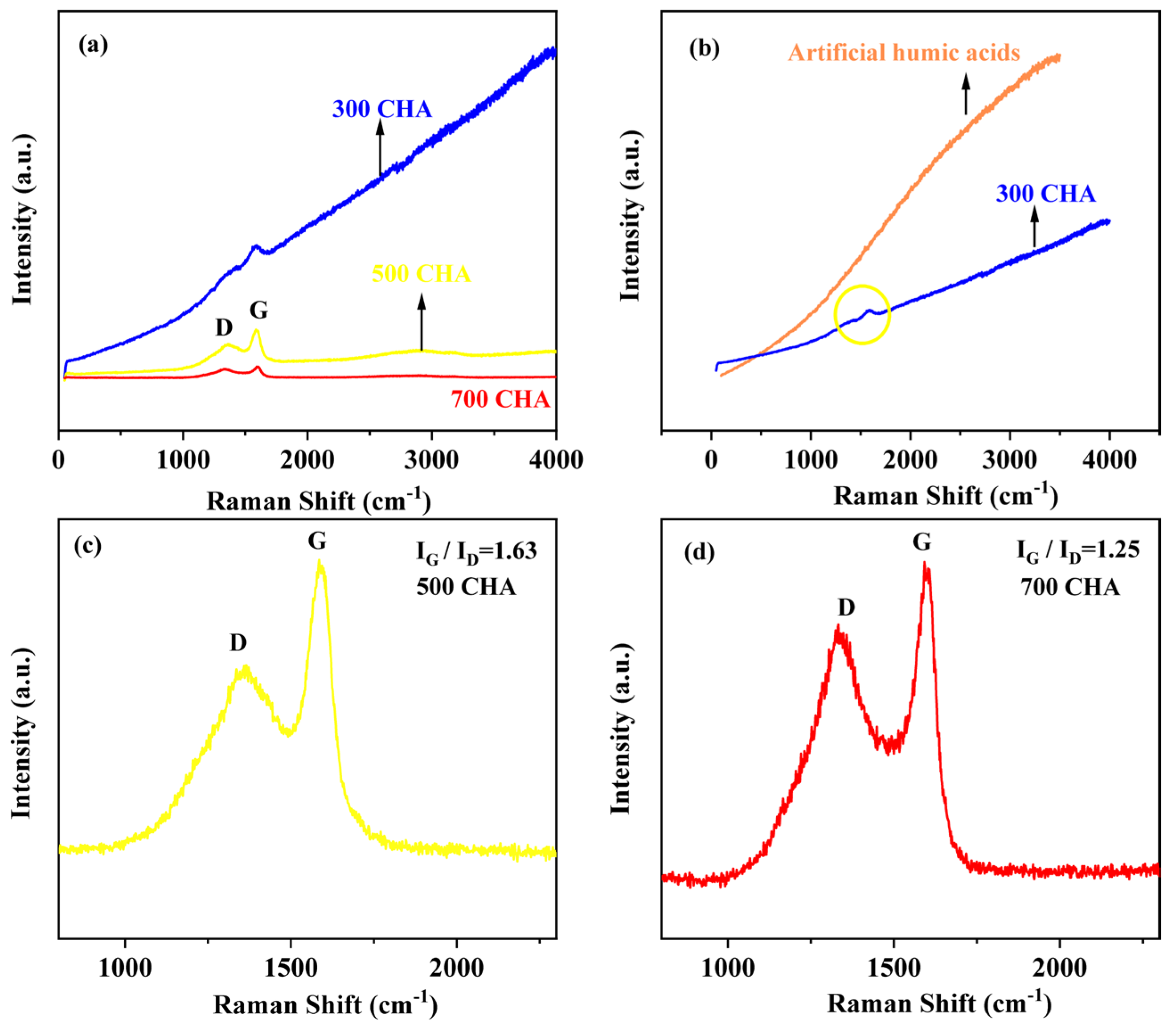


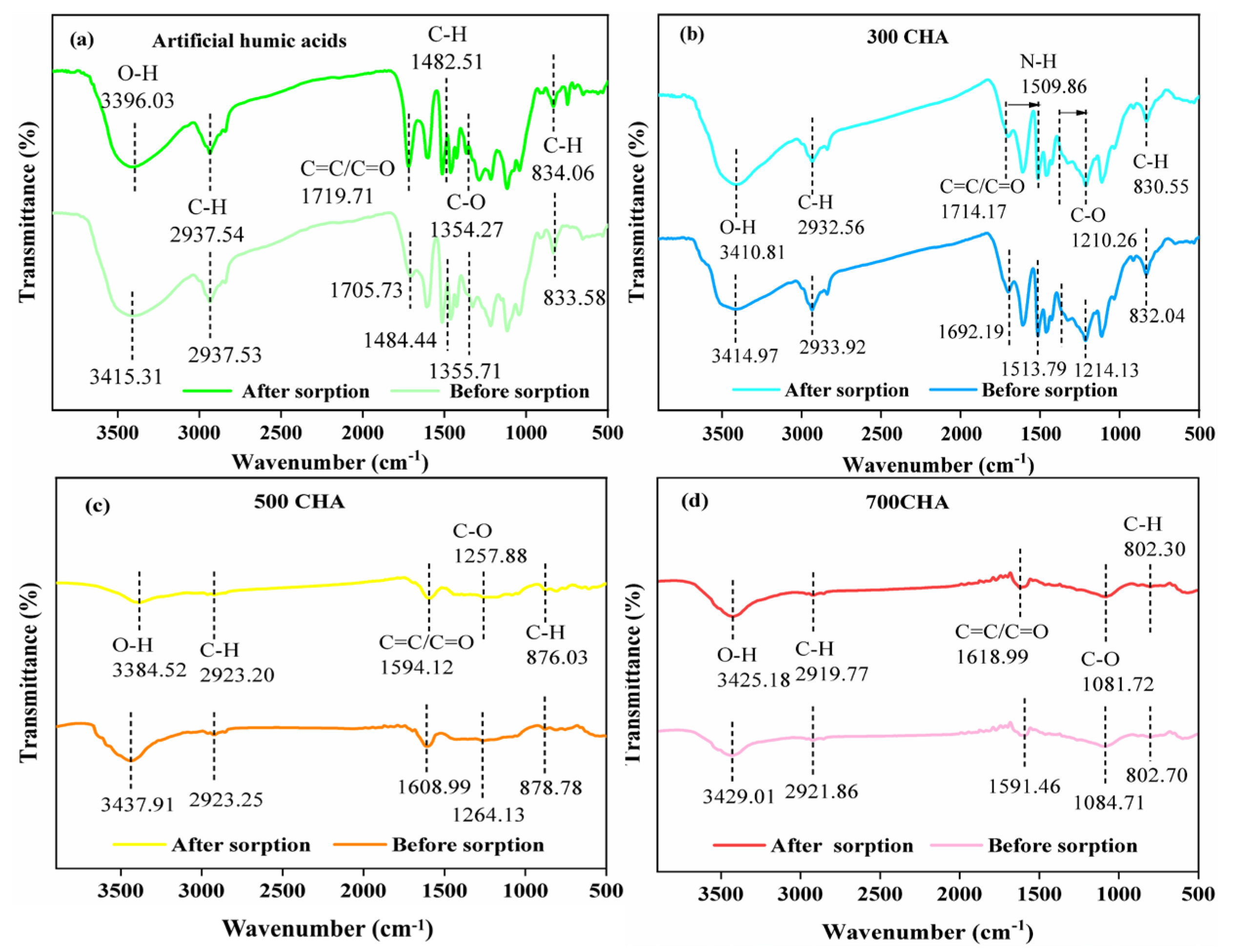

| Materials | Yield (%) | Elementary Composition (%) | Ash (%) | Atomic Ratio (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | O/C | H/C | (O + N)/C | |||
| Artificial humic acids | -- | 57.42 | 5.77 | 0.31 | 34.09 | 2.07 | 0.45 | 1.21 | 0.45 |
| 300CHA | 67.50 | 67.28 | 5.28 | 0.36 | 21.64 | 5.43 | 0.24 | 0.94 | 0.25 |
| 500CHA | 42.50 | 76.72 | 2.96 | 0.47 | 10.02 | 9.83 | 0.10 | 0.46 | 0.10 |
| 700CHA | 37.50 | 81.35 | 1.40 | 0.58 | 6.65 | 10.02 | 0.06 | 0.21 | 0.07 |
| Materials | ki1 | ci1 | R2 | ki2 | ci2 | R2 | ki3 | ci3 | R2 |
|---|---|---|---|---|---|---|---|---|---|
| Artificial humic acids | 778.84 | −34.35 | 0.958 | 140.24 | 1089.98 | 0.924 | −0.14 | 1898.78 | 0.835 |
| 300CHA | 150.17 | 12.76 | 0.944 | 31.69 | 0.69 | 0.884 | 11.31 | 5.65 | 0.847 |
| 500CHA | 75.37 | 11.41 | 0.944 | 11.85 | 1.47 | 0.931 | 5.60 | 5.60 | 0.851 |
| 700CHA | 52.13 | 7.06 | 0.955 | 8.83 | 8.83 | 0.907 | 0.23 | 5.10 | 0.888 |
| Materials | Pseudo-First-Order | Pseudo-Second-Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| kf | qfe | R2 | ks | qs | R2 | a | b | R2 | |
| Artificial humic acids | 0.45 | 1774.43 | 0.980 | 1.63 | 1904.66 | 0.994 | 5658.36 | 0.0037 | 0.981 |
| 300CHA | 0.18 | 268.30 | 0.903 | 0.01 | 291.38 | 0.946 | 5.19 × 102 | 1.93 × 10−3 | 0.984 |
| 500CHA | 0.99 | 152.74 | 0.944 | 0.00 | 160.50 | 0.980 | 1.13 × 104 | 8.86 × 10−5 | 0.989 |
| 700CHA | 0.62 | 117.82 | 0.957 | 0.01 | 123.08 | 0.986 | 3.00 × 103 | 3.34 × 10−4 | 0.987 |
| Materials | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| kl | Qm | Rl | R2 | kf | 1/n | R2 | |
| Artificial humic acids | 1.77 × 10−2 | 26,529.73 | 0.37 | 0.993 | 606.29 | 1.235 | 0.989 |
| 300CHA | 2.62 × 10−2 | 3344.67 | 0.24 | 0.992 | 215.58 | 1.828 | 0.977 |
| 500CHA | 2.83 × 10−2 | 2855.11 | 0.23 | 0.990 | 190.40 | 1.826 | 0.976 |
| 700CHA | 2.97 × 10−2 | 2478.55 | 0.23 | 0.990 | 176.34 | 1.873 | 0.974 |
| Materials | E | β | LnqD-R | R2 |
|---|---|---|---|---|
| Artificial humic acids | 3.19 × 10−3 | 5.08 × 10−6 | 8.87 | 0.940 |
| 300CHA | 4.71 × 10−3 | 1.11 × 10−5 | 7.53 | 0.976 |
| 500CHA | 4.99 × 10−3 | 1.24 × 10−5 | 7.42 | 0.985 |
| 700CHA | 2.56 × 10−3 | 3.28 × 10−6 | 7.30 | 0.981 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, T.; Li, Y.; Chen, Z.; Kong, C.; Feng, B.; Zhu, C.; Zhang, Y.; Xue, J.; Cheng, H. Converting Agroforestry Biowaste into Stable Near-Natural Chars via Hydrothermal Humification and Pyrolysis for Immobilizing Plasticizer. Agriculture 2025, 15, 1177. https://doi.org/10.3390/agriculture15111177
Xue T, Li Y, Chen Z, Kong C, Feng B, Zhu C, Zhang Y, Xue J, Cheng H. Converting Agroforestry Biowaste into Stable Near-Natural Chars via Hydrothermal Humification and Pyrolysis for Immobilizing Plasticizer. Agriculture. 2025; 15(11):1177. https://doi.org/10.3390/agriculture15111177
Chicago/Turabian StyleXue, Tao, Yi Li, Zimo Chen, Chao Kong, Biyun Feng, Changyin Zhu, Yinlong Zhang, Jianming Xue, and Hu Cheng. 2025. "Converting Agroforestry Biowaste into Stable Near-Natural Chars via Hydrothermal Humification and Pyrolysis for Immobilizing Plasticizer" Agriculture 15, no. 11: 1177. https://doi.org/10.3390/agriculture15111177
APA StyleXue, T., Li, Y., Chen, Z., Kong, C., Feng, B., Zhu, C., Zhang, Y., Xue, J., & Cheng, H. (2025). Converting Agroforestry Biowaste into Stable Near-Natural Chars via Hydrothermal Humification and Pyrolysis for Immobilizing Plasticizer. Agriculture, 15(11), 1177. https://doi.org/10.3390/agriculture15111177









