Hop Waste Seed Coating (Pilling) as Circular Bioeconomic Alternative to Improve Seed Germination and Trichoderma Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Trichoderma Isolates
2.2. In Vitro Evaluation of Trichoderma Strains in Hop Waste
2.3. Effect of Hop Waste Seed Coating on Seed Germination
2.3.1. Selected Seeds to Carry out the Pilling with Hop Waste
- Small seeds, <3 mm: broccoli, rapeseed, and alfalfa;
- Medium seeds, 3–10 mm: lentil and wheat;
- Large seeds, >10 mm: corn, bean, chickpea, sunflower, and melon.
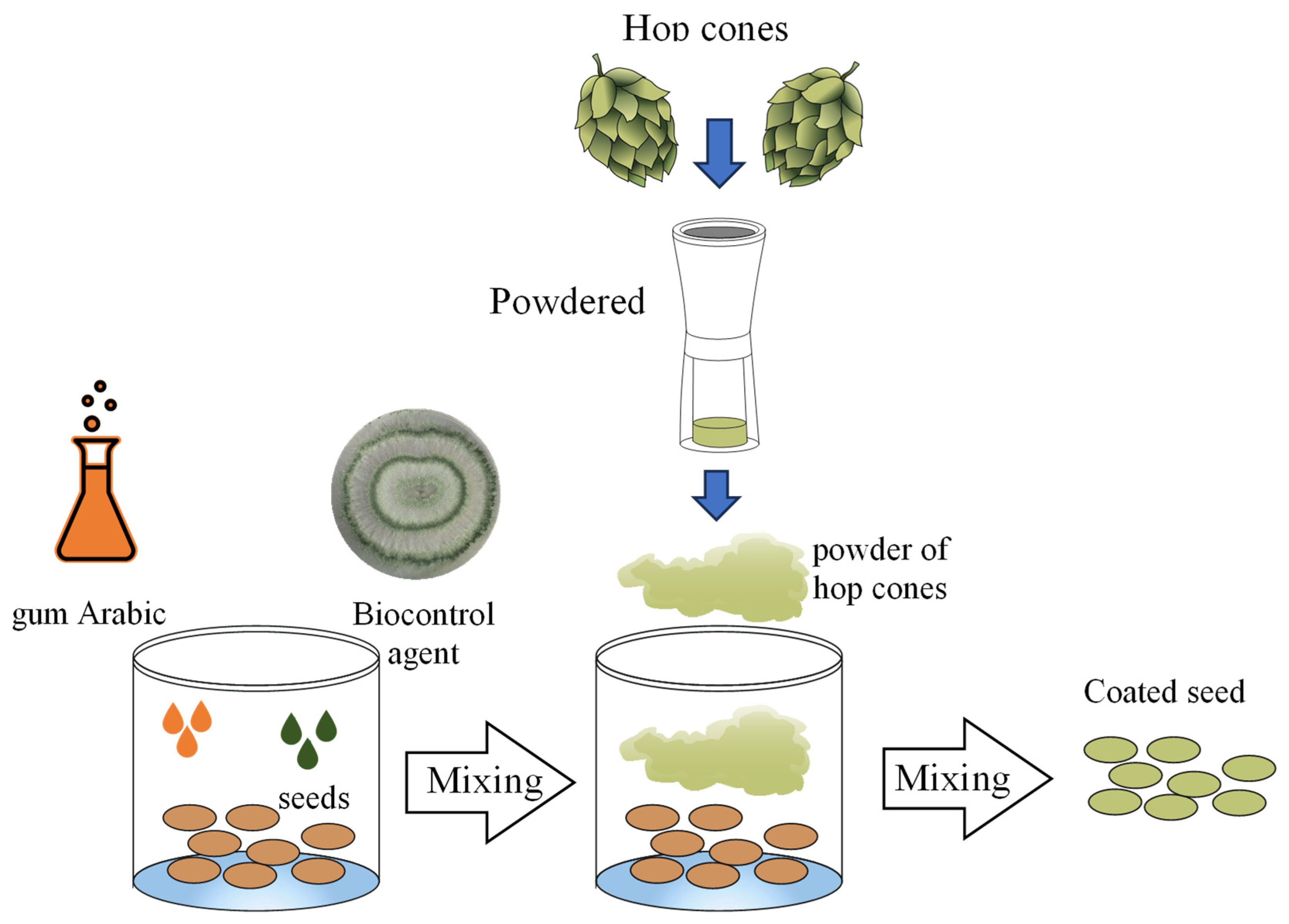
2.3.2. Seed Pelleting Process
2.3.3. Evaluation of Seeds Pelleted with Hop Waste
2.4. Effect of Hop Waste and Trichoderma Coating on Seed Germination in Pot
2.5. Evaluation of Hop Waste and Trichoderma Coating Seed in Bean Fields
3. Results
3.1. In Vitro Evaluation of Trichoderma Strains in Hop Waste
3.2. Effect of Hop Waste Seed Coating on Seed Germination
3.3. Effect of Hop Waste and Trichoderma Coating on Seed Germination in Pot
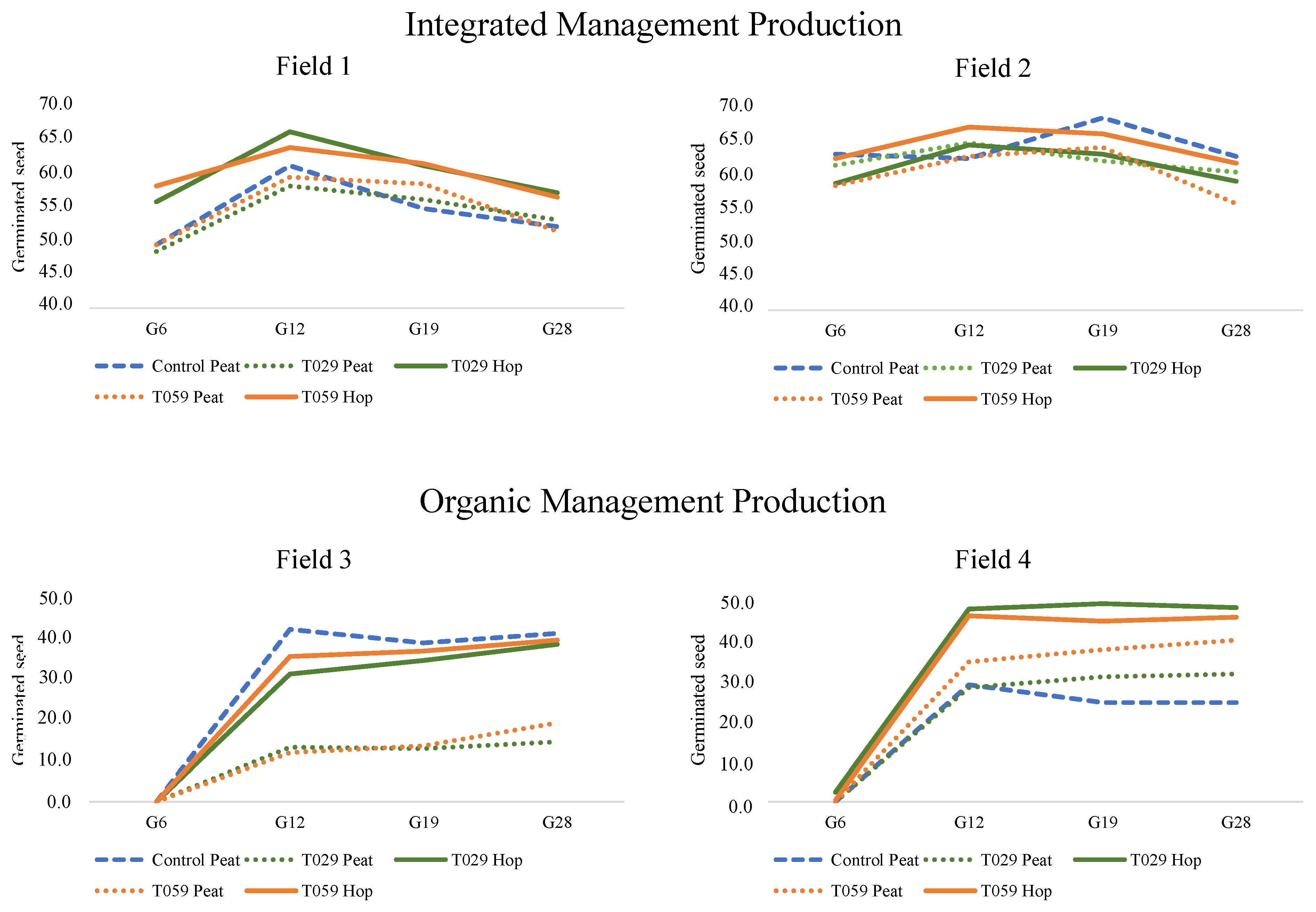
3.4. Evaluation of Hop Waste and Trichoderma Coating Seed in Bean Fields
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernández, J.P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil. 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and Assessment of Cell Viability in Formulation of Non-Sporulating Bacterial Inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Schneider, A.; Renault, P. Effects of Coating on Seed Imbibition: II. Effect of Coating Rates. Crop Sci. 1997, 37, 1850–1857. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Chamekh, Z.; Jallouli, S.; Ayadi, S.; Serret, M.D.; Araus, J.L.; Trifa, Y.; Hamada, W. Comparative Effect of Seed Treatment with Thyme Essential Oil and Paraburkholderia phytofirmans on Growth, Photosynthetic Capacity, Grain Yield, Δ15N and Δ13C of Durum Wheat under Drought and Heat Stress. Ann. Appl. Biol. 2022, 181, 58–69. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Pereira, A.d.E.S.; Aleksieienko, I.; do Carmo, G.C.; Gohari, G.; Santaella, C.; Fraceto, L.F.; Oliveira, H.C. Encapsulated Plant Growth Regulators and Associative Microorganisms: Nature-Based Solutions to Mitigate the Effects of Climate Change on Plants. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef]
- Sharratt, B.S.; Gesch, R.W. Emergence of Polymer-Coated Corn and Soybean Influenced by Tillage and Sowing Date. Agron. J. 2008, 100, 585–590. [Google Scholar] [CrossRef]
- Pedrini, S.; Bhalsing, K.; Cross, A.T.; Dixon, K.W. Protocol Development Tool (PDT) for Seed Encrusting and Pelleting. Seed Sci. Technol. 2018, 46, 393–405. [Google Scholar] [CrossRef]
- Asano, H. Semillas Recubiertas. ES2087104, 16 July 1996. [Google Scholar]
- Asrar, J.; Kohn, F.C. Seed Treatment with Combinations of Pyrethrins/Pyrethroids and Thiamethoxam. ES2287165, 16 December 2007. [Google Scholar]
- Batista, V.A.P.; Vieira, H.D.; Pires, J.I.C.; Acha, A.J. Sorghum Seed Coating with Zinc: Physiological Quality and Initial Performance of Plants. Acta Sci. Agron. 2022, 44, e53803. [Google Scholar] [CrossRef]
- Chen, Z.; Castaing, J.-C.; Ji, P.; Cristobal, H. Seed Coatings, Coating Compositions and Methods for Use. EP2680685A2, 28 August 2019. [Google Scholar]
- Peltonen, J.; Saarikko, E. Coated Seed and Process for Coating a Seed. ES2272734, 1 May 2007. [Google Scholar]
- Weimin, C. Preparation Method of Seed Coating Agent for Preventing Sunflower Black-Stem Disease. CN102783490A, 21 November 2012. Available online: https://patents.google.com/patent/CN102783490A/en (accessed on 17 June 2025).
- Deaker, R.; Roughley, R.J.; Kennedy, I.R. Legume Seed Inoculation Technology—A Review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The Use of Microbial Inoculants for Biological Control, Plant Growth Promotion, and Sustainable Agriculture: A Review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Ma, Y. Seed Coating with Beneficial Microorganisms for Precision Agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Growth and Nutrition of Cowpea (Vigna unguiculata) under Water Deficit as Influenced by Microbial Inoculation via Seed Coating. J. Agron. Crop Sci. 2019, 205, 447–459. [Google Scholar] [CrossRef]
- FAO Phytosanitary Glossary. FAO Terminology Portal. Available online: https://www.fao.org/faoterm/collection/phytosanitary-glossary/en/ (accessed on 22 April 2024).
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–Plant–Pathogen Interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Campelo, M.P.; Lorenzana, A.; Rodríguez-González, A.; Reinoso, B.; Gutiérrez, S.; Casquero, P.A. Antifungal Activity and Bean Growth Promotion of Trichoderma Strains Isolated from Seed vs Soil. Eur. J. Plant Pathol. 2020, 158, 817–828. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Porteous-Álvarez, A.J.; Rodríguez-González, Á.; Gutiérrez, S.; Casquero, P.A. Evaluation of Substrates and Additives to Trichoderma harzianum Development by QPCR. Agron. J. 2020, 112, 3188–3194. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Rodríguez-González, Á.; Lorenzana, A.; Gutiérrez, S.; Casquero, P.A. Influence of Substrates in the Development of Bean and in Pathogenicity of Rhizoctonia solani JG Kühn. Agronomy 2020, 10, 707. [Google Scholar] [CrossRef]
- Mayo, S.; Gutierrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in Growth of Bean (Phaseolus vulgaris L.) and in the Induction of Plant Defense-Related Genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Porteous-Álvarez, A.J.; Mezquita-García, S.; Rodríguez-González, Á.; Carro-Huerga, G.; del Ser-Herrero, S.; Gutiérrez, S.; Casquero, P.A. Influence of Physicochemical Characteristics of Bean Crop Soil in Trichoderma spp. Development. Agronomy 2021, 11, 274. [Google Scholar] [CrossRef]
- Mayer, A.; Haas, W.; Wiedenhofer, D.; Krausmann, F.; Nuss, P.; Blengini, G.A. Measuring Progress towards a Circular Economy: A Monitoring Framework for Economy-Wide Material Loop Closing in the EU28. J. Ind. Ecol. 2019, 23, 62–76. [Google Scholar] [CrossRef]
- Amoroso, G.; Idbella, M.; Motti, R.; Gemini, A.; Cozzolino, A.; Bonanomi, G. Biochar, Beneficial Microbes, and Agro-Industrial Byproducts in Seed Coatings: Improving Germination and Biomass in Multiple Crops. Horticulturae 2025, 11, 554. [Google Scholar] [CrossRef]
- Engelson, M.; Solberg, M.; Karmas, E. Antimycotic Properties of Hop Extract in Reduced Water Activity Media. J. Food Sci. 1980, 45, 1175–1178. [Google Scholar] [CrossRef]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A Comparison of Antioxidant and Antimicrobial Activity between Hop Leaves and Hop Cones. Ind. Crops Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Rivière, C. An Overview of the Antimicrobial Properties of Hop. In Natual Antimicrobial Agents; Mérillon, J.-M., Riviere, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 31–54. ISBN 978-3-319-67045-4. [Google Scholar]
- Jacquin, J.; Moureu, S.; Deweer, C.; Hakem, A.; Paguet, A.S.; Bonneau, N.; Bordage, S.; Dermont, C.; Sahpaz, S.; Muchembled, J.; et al. Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora infestans. Agronomy 2022, 12, 2826. [Google Scholar] [CrossRef]
- Inside Beer Germany Reclaims Top Spot in Global Hop Production After 9 Years. Available online: https://www.inside.beer/news/detail/germany-reclaims-top-spot-in-global-hop-production-after-9-years (accessed on 9 June 2025).
- IHPS (Inštitut za Hmeljarstvo in Pivovarstvo Slovenije) Project LIFE BioTHOP. Available online: https://www.life-biothop.eu/ (accessed on 9 June 2025).
- Ministerio de Agricultura Pesca Y Alimentacion. Información General Del Sector Del Lúpulo. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/cultivos-herbaceos/lupulo/Informacion%20general%20del%20sector%20del%20lupulo.aspx (accessed on 9 June 2025).
- Rodríguez-González, Á.; Carro-Huerga, G.; Mayo-Prieto, S.; Lorenzana, A.; Gutiérrez, S.; Peláez, H.J.; Casquero, P.A. Investigations of Trichoderma spp. and Beauveria bassiana as Biological Control Agent for Xylotrechus arvicola, a Major Insect Pest in Spanish Vineyards. J. Econ. Entomol. 2018, 111, 2585–2591. [Google Scholar] [CrossRef]
- Gálvez, A.; López-Galindo, A.; Peña, A. Effect of Different Surfactants on Germination and Root Elongation of Two Horticultural Crops: Implications for Seed Coating. N. Z. J. Crop Hortic. Sci. 2019, 47, 83–98. [Google Scholar] [CrossRef]
- Oleszczuk, P. Phytotoxicity of Municipal Sewage Sludge Composts Related to Physico-Chemical Properties, PAHs and Heavy Metals. Ecotoxicol. Environ. Saf. 2008, 69, 496–505. [Google Scholar] [CrossRef]
- Valenciano, J.B.; Casquero, P.A.; Boto, J.A.; Marcelo, V. Evaluation of the Occurrence of Root Rots on Bean Plants (Phaseolus vulgaris) Using Different Sowing Methods and with Different Techniques of Pesticide Application. N. Z. J. Crop Hortic. Sci. 2006, 34, 291–298. [Google Scholar] [CrossRef]
- Agake, S.-i.; Artigas Ramirez, M.D.; Kojima, K.; Ookawa, T.; Ohkama-Ohtsu, N.; Yokoyama, T. Seed Coating by Biofertilizer Containing Spores of Bacillus pumilus TUAT1 Strain Enhanced Initial Growth of Oryza sativa L. Agron. J. 2021, 113, 3708–3717. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Temprano, F.J. Use of Sinorhizobium (Ensifer) fredii for Soybean Inoculants in South Spain. Eur. J. Agron. 2009, 30, 205–211. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.; Beltrán-Acosta, C.; Zapata-Narvaez, Y.; Chaparro, M.; Gómez, M.; Cruz-Barrera, M. Seed Coating as a Delivery System for the Endophyte Trichoderma koningiopsis Th003 in Rice (Oryza sativa). Appl. Microbiol. Biotechnol. 2021, 105, 1889–1904. [Google Scholar] [CrossRef]
- O’Callaghan, M. Microbial Inoculation of Seed for Improved Crop Performance: Issues and Opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Quilichini, T.D.; Gao, P.; Yu, B.; Bing, D.; Datla, R.; Fobert, P.; Xiang, D. The Seed Coat’s Impact on Crop Performance in Pea (Pisum sativum L.). Plants 2022, 11, 2056. [Google Scholar] [CrossRef]
- Jarecki, W.; Migut, D. Comparison of Yield and Important Seed Quality Traits of Selected Legume Species. Agronomy 2022, 12, 2667. [Google Scholar] [CrossRef]
- Cojocaru, A.; Carbune, R.V.; Teliban, G.C.; Stan, T.; Mihalache, G.; Rosca, M.; Rusu, O.R.; Butnariu, M.; Stoleru, V. Physiological, Morphological and Chemical Changes in Pea Seeds under Different Storage Conditions. Sci. Rep. 2024, 14, 28191. [Google Scholar] [CrossRef]
- Ehteshamul-Haque, S.; Sultana, V.; Ara, J.; Athar, M. Cultivar Response against Root-Infecting Fungi and Efficacy of Pseudomonas aeruginosa in Controlling Soybean Root Rot. Plant Biosyst. 2007, 141, 51–55. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Sharma, P.; Yadav, R. Delivery Systems for Introduction of Microbial Inoculants in the Field. In Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 2: Functional Applications; Springer: Chennai, India, 2016; Volume 2, pp. 199–218. ISBN 9788132226444. [Google Scholar]
- Ghiasi, F.; Gahruie, H.H.; Eskandari, M.H.; Golmakani, M.T.; Khaneghah, A.M. Natural Gums. In Physicochemical and Enzymatic Modification of Gums: Synthesis, Characterization and Application; Gahruie, H.H., Eskandari, M.H., Khaneghah, A.M., Ghiasi, F., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 3–29. ISBN 9783030879969. [Google Scholar]
- Kyei-Boahen, S.; Slinkard, A.E.; Walley, F.L. Rhizobial Survival and Nodulation of Chickpea as Influenced by Fungicide Seed Treatment. Can. J. Microbiol. 2011, 47, 585–589. [Google Scholar] [CrossRef]
- Singh, A.; Jain, A.; Sarma, B.K.; Upadhyay, R.S.; Singh, H.B. Rhizosphere Competent Microbial Consortium Mediates Rapid Changes in Phenolic Profiles in Chickpea during Sclerotium rolfsii Infection. Microbiol. Res. 2014, 169, 353–360. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Prévost, D. Development of Emulsion from Rhizobial Fermented Starch Industry Wastewater for Application as Medicago sativa Seed Coat. Eng. Life Sci. 2010, 10, 248–256. [Google Scholar] [CrossRef]
- Accinelli, C.; Abbas, H.K.; Morena, C.; Bruno, V.; Khambhati, V.H.; Paulk, R.T.; Little, N.S.; Bellaloui, N.; Forbes, W.; Shier, W.T. Use of a Biochar-Based Formulation for Coating Corn Seeds. Cogent Food Agric. 2023, 9, 2283956. [Google Scholar] [CrossRef]
- Amirkhani, M.; Netravali, A.N.; Huang, W.; Taylor, A.G. Investigation of Soy Protein–Based Biostimulant Seed Coating for Broccoli Seedling and Plant Growth Enhancement. HortScience 2016, 51, 1121–1126. [Google Scholar] [CrossRef]
- Zvinavashe, A.T.; Laurent, J.; Mhada, M.; Sun, H.; Fouda, H.M.E.; Kim, D.; Mouhib, S.; Kouisni, L.; Marelli, B. Programmable Design of Seed Coating Function Induces Water-Stress Tolerance in Semi-Arid Regions. Nat. Food 2021, 2, 485–493. [Google Scholar] [CrossRef]
- Zvinavashe, A.T.; Mardad, I.; Mhada, M.; Kouisni, L.; Marelli, B. Engineering the Plant Microenvironment to Facilitate Plant-Growth-Promoting Microbe Association. J. Agric. Food Chem. 2021, 69, 13270–13285. [Google Scholar] [CrossRef]
- Mulas, D.; García-Fraile, P.; Carro, L.; Ramírez-Bahena, M.-H.; Casquero, P.; Velázquez, E.; González-Andrés, F. Distribution and Efficiency of Rhizobium leguminosarum Strains Nodulating Phaseolus vulgaris in Northern Spanish Soils: Selection of Native Strains That Replace Conventional N Fertilization. Soil Biol. Biochem. 2011, 43, 2283–2293. [Google Scholar] [CrossRef]
- Parish, F.; Sirin, A.; Charman, D.; Joosten, H.; Minaeva, T. Assessment on Peatlands, Biodiversity and Climate Change; Wetlands International: Wageningen, The Netherlands, 2008; ISBN 983-43751-0-2. [Google Scholar]
- Elsharkawy, M.M. The Potential of Chitosan Nanoparticles to Control Plant Pathogens. In Nanotechnology in Plant Health; Rai, M., Avila-Quezada, G., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 180–195. ISBN 9781040027950. [Google Scholar]
- Somsri, A.; Saelao, P.; Thongsen, N.; Kenkhunthot, T.; Pilasombut, K.; Urairong, H.; Rumjuankiat, K. In Vitro Biocontrol Potential of Natural Substance Combination against Plant Pathogens. Int. J. Agric. Technol. 2025, 21, 685–696. [Google Scholar] [CrossRef]
- Jíménez-Arias, D.; Morales-Sierra, S.; Silva, P.; Carrêlo, H.; Gonçalves, A.; Ganança, J.F.T.; Nunes, N.; Gouveia, C.S.S.; Alves, S.; Borges, J.P.; et al. Encapsulation with Natural Polymers to Improve the Properties of Biostimulants in Agriculture. Plants 2023, 12, 55. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, X.; Gao, Q.; Zou, Y.; Xing, D.; Chen, R.; He, X.; Li, Q. Preparation and Chemical Properties of Microencapsulation Developed with Mulberry Anthocyanins and Silk Fibroin. Ind. Crops Prod. 2024, 212, 118383. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Izydorczyk, G.; Taf, R.; Moustakas, K.; Chojnacka, K. Cellulose-Based Fertilizers for Sustainable Agriculture: Effective Methods for Increasing Crop Yield and Soil Health. Ind. Crops Prod. 2023, 205, 117500. [Google Scholar] [CrossRef]
- Firmanda, A.; Fahma, F.; Syamsu, K.; Suryanegara, L.; Wood, K. Controlled/Slow-Release Fertilizer Based on Cellulose Composite and Its Impact on Sustainable Agriculture: Review. Biofuels Bioprod. Biorefining 2022, 16, 1909–1930. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; He, H.; Zhu, W.; Yuan, X.; Wang, H. Engineering High-Performance and Multifunctional Seed Coating Agents from Lignocellulosic Components. Ind. Crops Prod. 2024, 222, 119768. [Google Scholar] [CrossRef]
- Schnabl, H.; Polus, G.L. Biological Plant Protection Agent with Resistance-Promoting Action and Method for Producing Same. ES2221357, 16 December 2004. [Google Scholar]
- Yvin, J.-C.; Chabot, R. New Compositions of Marine Algae Derivatives, Process to Obtain Them and Applications in Agriculture. ES2041605, 1 December 1993. [Google Scholar]
- Green, K. Seed Product with Plant Nutrient Fine Powder Coating and Method of Preparing Thereof. ES2680899, 11 September 2018. [Google Scholar]
- Wang, C.; Zhang, Y.; Kuang, W.; Zhang, X.; Chen, J.; Mao, W. Seed Coating Agent of Trichoderma Fungi and Preparation Method Thereof—Google Patents. CN103039439A, 15 April 2015. Available online: https://patents.google.com/patent/CN103039439A/en?q=(%22Seed+Coating+Agent+of+Trichoderma+Fungi+and+Preparation+Method+Thereof%22)&oq=%22Seed+Coating+Agent+of+Trichoderma+Fungi+and+Preparation+Method+Thereof%22 (accessed on 17 June 2025).
- Lewis, J.A.; Lumsden, R.D. Biocontrol of Damping-off of Greenhouse-Grown Crops Caused by Rhizoctonia solani with a Formulation of Trichoderma spp. Crop Prot. 2001, 20, 49–56. [Google Scholar] [CrossRef]
- Wijesinghe, C.J.; Wijeratnam, R.S.W.; Samarasekara, J.K.R.R.; Wijesundera, R.L.C. Development of a Formulation of Trichoderma asperellum to Control Black Rot Disease on Pineapple Caused by Thielaviopsis paradoxa. Crop Prot. 2011, 30, 300–306. [Google Scholar] [CrossRef]
- Sriram, S.; Roopa, K.P.; Savitha, M.J. Extended Shelf-Life of Liquid Fermentation Derived Talc Formulations of Trichoderma harzianum with the Addition of Glycerol in the Production Medium. Crop Prot. 2011, 30, 1334–1339. [Google Scholar] [CrossRef]
- El-Katatny, M.; Hetta, A.; Shaban, G.; El-Komy, H. Improvement of Cell Wall Degrading Enzymes Production by Alginate Encapsulated Trichoderma spp. Food Technol. Biotechnol. 2003, 41, 219–225. [Google Scholar]
- Bae, Y.S.; Knudsen, G.R. Soil Microbial Biomass Influence on Growth and Biocontrol Efficacy of Trichoderma harzianum. Biol. Control 2005, 32, 236–242. [Google Scholar] [CrossRef]
- Jin, X.; Custis, D. Microencapsulating Aerial Conidia of Trichoderma harzianum through Spray Drying at Elevated Temperatures. Biol. Control 2011, 56, 202–208. [Google Scholar] [CrossRef]
- Batta, Y.A. Postharvest Biological Control of Apple Gray Mold by Trichoderma harzianum Rifai Formulated in an Invert Emulsion. Crop Prot. 2004, 23, 19–26. [Google Scholar] [CrossRef]
- Kolombet, L.V.; Zhigletsova, S.K.; Kosareva, N.I.; Bystrova, E.V.; Derbyshev, V.V.; Krasnova, S.P.; Schisler, D. Development of an Extended Shelf-Life, Liquid Formulation of the Biofungicide Trichoderma asperellum. World J. Microbiol. Biotechnol. 2008, 24, 123–131. [Google Scholar] [CrossRef]
- Santos, A.; García, M.; Cotes, A.M.; Villamizar, L. Efecto de La Formulación Sobre La Vida Útil de Bioplaguicidas a Base de Dos Aislamientos Colombianos de Trichoderma koningiopsis Th003 y Trichoderma asperellum Th034. Rev. Iberoam. Micol. 2012, 29, 150–156. [Google Scholar] [CrossRef]
- Swaminathan, J.; van Koten, C.; Henderson, H.V.; Jackson, T.A.; Wilson, M.J. Formulations for Delivering Trichoderma atroviridae Spores as Seed Coatings, Effects of Temperature and Relative Humidity on Storage Stability. J. Appl. Microbiol. 2016, 120, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Jin, B.; Li, Y.; Chen, J.; Li, Z. Utilization of Winery Wastes for Trichoderma viride Biocontrol Agent Production by Solid State Fermentation. J. Environ. Sci. 2008, 20, 353–358. [Google Scholar] [CrossRef]
- Schoss, K.; Glavač, N.K.; Koce, J.D.; Anžlovar, S. Supercritical CO2 Plant Extracts Show Antifungal Activities against Crop-Borne Fungi. Molecules 2022, 27, 1132. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Tyśkiewicz, R.; Konkol, M.; Gruba, M.; Kowalski, R. Optimization of Antifungal Properties of Hop Cone Carbon Dioxide Extracts Based on Response Surface Methodology. Molecules 2024, 29, 2554. [Google Scholar] [CrossRef]
- Carbone, K.; Gervasi, F. An Updated Review of the Genus Humulus: A Valuable Source of Bioactive Compounds for Health and Disease Prevention. Plants 2022, 11, 3434. [Google Scholar] [CrossRef]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the Potential of Microwaves and Ultrasounds in the Green Extraction of Bioactive Compounds from Humulus lupulus for the Food and Pharmaceutical Industry. Ind. Crops Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- MacWilliam, I.C. Chemistry of Hop Constituents. IV. The Free Sugars. J. Inst. Brew. 1953, 59, 142–147. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Marra, R.; Vinale, F.; Rodríguez-González, Á.; Woo, S.L.; Lorito, M.; Gutiérrez, S.; Casquero, P.A. Effect of Trichoderma velutinum and Rhizoctonia solani on the Metabolome of Bean Plants (Phaseolus vulgaris L.). Int. J. Mol. Sci. 2019, 20, 549. [Google Scholar] [CrossRef]

| Isolate | Culture Collection | Trichoderma spp. | References |
|---|---|---|---|
| T019 | PAULET38 | T. harzianum | [21,24,35] |
| T029 | PAULET44 | T. velutinum | |
| T059 | PAULET74 | T. harzianum |
| Substrate | DNA Concentration (µg/mL) | ||
|---|---|---|---|
| T. harzianum T019 | T. harzianum T059 | T. velutinum T029 | |
| Leftover leaves | 5.274 | 10.947 | 0.003 |
| Remains of cones or flowers | 3358.898 | 73.386 | 2.492 |
| Seed | Germination (%) | RG 1 (%) | Biomass (mg) | RB 2 (%) | Root Length (mm) | RRL 3 (%) | GR 4 (%) | |
|---|---|---|---|---|---|---|---|---|
| Small size | ||||||||
| Alfalfa | C 5 | 58.33 ± 3.33 a | 105.71 | 2.49 ± 0.14 a | 114.62 | 24.72 ± 1.81 b | 134.05 | 141.71 |
| T 6 | 61.67 ± 10.93 a | 2.86 ± 0.17 a | 33.14 ± 2.82 a | |||||
| Broccoli | C | 65.00 ± 5.00 a | 43.59 | 3.67 ± 0.23 b | 269.48 | 36.73 ± 3.03 a | 47.70 | 43.59 |
| T | 28.33 ± 3.33 b | 9.89 ± 3.53 a | 17.52 ± 2.93 b | |||||
| Rapeseed | C | 90.00 ± 10.00 a | 55.56 | 4.13 ± 0.24 a | 97.66 | 54.79 ± 3.93 a | 77.65 | 55.56 |
| T | 50.00 ± 2.89 b | 4.03 ± 0.26 a | 42.55 ± 4.07 b | |||||
| Medium size | ||||||||
| Lentil | C | 88.33 ± 4.41 a | 94.34 | 7.47 ± 0.13 a | 99.17 | 13.60 ± 0.61 a | 84.94 | 94.34 |
| T | 83.33 ± 7.26 a | 7.41 ± 0.17 a | 11.55 ± 0.54 b | |||||
| Wheat | C | 100.00 ± 0.00 a | 96.67 | 7.29 ± 0.16 a | 91.64 | 17.43 ± 0.63 a | 85.54 | 96.67 |
| T | 96.67 ± 1.67 a | 6.68 ± 0.16 b | 14.91 ± 0.86 b | |||||
| Big size | ||||||||
| Chickpea | C | 98.33 ± 1.67 a | 77.97 | 74.84 ± 0.78 a | 97.64 | 13.96 ± 0.67 a | 37.49 | 77.97 |
| T | 76.67 ± 3.33 b | 73.07 ± 0.77 a | 5.23 ± 0.28 b | |||||
| Sunflower | C | 95.00 ± 0.00 a | 85.96 | 17.91 ± 0.32 a | 98.92 | 21.16 ± 1.02 a | 66.82 | 85.96 |
| T | 81.67 ± 6.01 b | 17.71 ± 0.40 a | 14.14 ± 1.27 b | |||||
| Bean | C | 98.33 ± 1.67 a | 94.92 | 114.72 ± 2.63 a | 99.13 | 43.07 ± 3.44 a | 73.53 | 94.92 |
| T | 93.33 ± 3.33 a | 113.72 ± 2.80 a | 31.67 ± 2.59 b | |||||
| Corn | C | 95.00 ± 0.00 a | 94.74 | 48.80 ± 0.96 a | 100.07 | 21.12 ± 1.40 a | 81.19 | 94.74 |
| T | 90.00 ± 2.89 b | 48.83 ± 1.13 a | 17.15 ± 1.38 b | |||||
| Melon | C | 73.33 ± 14.53 a | 54.55 | 12.68 ± 0.53 b | 118.19 | 41.77 ± 4.50 b | 139.81 | 54.55 |
| T | 40.00 ± 5.77 b | 14.99 ± 0.66 a | 58.39 ± 5.75 a | |||||
| Seed | Germination (%) | RG 1 (%) | Biomass (mg) | RB 2 (%) | Root Length (mm) | RRL 3 (%) | GR 4 (%) | |
|---|---|---|---|---|---|---|---|---|
| Small size | ||||||||
| Alfalfa | C 5 | 36.67 ± 8.82 a | 145.45 | 4.71 ± 0.69 a | 67.25 | 45.94 ± 6.97 a | 98.19 | 142.82 |
| T 6 | 53.33 ± 16.67 a | 3.17 ± 0.36 b | 45.11 ± 5.97 a | |||||
| Broccoli | C | 73.33 ± 6.67 a | 90.91 | 3.05 ± 0.37 a | 82.32 | 28.44 ± 5.52 a | 95.57 | 86.89 |
| T | 66.67 ± 6.67 a | 2.51 ± 0.35 a | 27.18 ± 4.76 a | |||||
| Rapeseed | C | 63.33 ± 17.64 a | 68.42 | 3.21 ± 0.37 a | 87.05 | 56.98 ± 13.76 a | 112.63 | 77.06 |
| T | 43.33 ± 8.82 a | 2.80 ± 0.46 a | 64.17 ± 13.93 a | |||||
| Medium size | ||||||||
| Lentil | C | 79.26 ± 0.74 a | 113.55 | 10.73 ± 0.39 a | 88.28 | 18.69 ± 1.45 a | 71.10 | 80.73 |
| T | 90.00 ± 10.00 a | 9.47 ± 0.32 b | 13.29 ± 1.00 b | |||||
| Wheat | C | 93.33 ± 3.33 a | 100.00 | 9.88 ± 0.41 a | 93.73 | 22.97 ± 1.63 a | 101.08 | 101.08 |
| T | 93.33 ± 3.33 a | 9.26 ± 0.31 a | 23.22 ± 1.29 a | |||||
| Big size | ||||||||
| Chickpea | C | 83.33 ± 3.33 b | 120.00 | 86.34 ± 1.90 a | 94.42 | 14.44 ± 1.77 a | 88.66 | 106.39 |
| T | 100.00 ± 0.00 a | 81.52 ± 1.24 b | 12.80 ± 1.27 a | |||||
| Sunflower | C | 80.00 ± 10.00 a | 66.67 | 34.14 ± 1.21 a | 108.34 | 28.03 ± 2.57 a | 135.79 | 90.53 |
| T | 53.33 ± 12.02 b | 36.99 ± 3.93 a | 38.06 ± 6.13 a | |||||
| Bean | C | 93.33 ± 3.33 a | 85.71 | 113.64 ± 7.00 a | 104.12 | 25.13 ± 3.41 a | 82.13 | 70.39 |
| T | 80.00 ± 0.00 b | 118.32 ± 5.97 a | 20.64 ± 2.40 a | |||||
| Corn | C | 100.00 ± 0.00 a | 90.00 | 52.46 ± 1.27 a | 104.12 | 29.91 ± 2.47 a | 68.11 | 61.29 |
| T | 90.00 ± 5.77 b | 54.62 ± 1.55 a | 20.37 ± 1.89 b | |||||
| Melon | C | 20.00 ± 10.00 b | 250.00 | 24.12 ± 0.91 a | 74.77 | 28.74 ± 7.90 b | 172.56 | 431.40 |
| T | 50.00 ± 0.00 a | 18.04 ± 1.05 b | 49.59 ± 4.47 a | |||||
| Seed | Germination (%) | RG 1 (%) | Biomass (mg) | RB 2 (%) | Root Length (mm) | RRL 3 (%) | GR 4 (%) | |
|---|---|---|---|---|---|---|---|---|
| Small size | ||||||||
| Rapeseed | C 5 | 23.33 ± 3.33 b | 271.43 | 34.76 ± 7.53 a | 99.47 | 46.11 ± 9.00 a | 64.86 | 176.04 |
| T 6 | 63.33 ± 17.64 a | 34.57 ± 10.27 a | 29.91 ± 9.38 a | |||||
| Medium size | ||||||||
| Wheat | C | 23.33 ± 12.02 b | 414.29 | 130.84 ± 12.55 b | 159.47 | 100.43 ± 8.07 b | 157.21 | 651.31 |
| T | 96.67 ± 3.33 a | 208.65 ± 9.99 a | 157.89 ± 3.14 a | |||||
| Big size | ||||||||
| Bean | C | 90.00 ± 10.00 a | 103.70 | 1990.51 ± 136.04 b | 152.61 | 117.93 ± 8.19 b | 137.04 | 142.12 |
| T | 93.33 ± 3.33 a | 3037.73 ± 158.27 a | 161.62 ± 4.20 a | |||||
| Source of Variation | df 1 | Day 28 |
|---|---|---|
| Trichoderma strains (Ts) | 2 | 84.028 |
| Coating (C) | 1 | 1220.083 * |
| Ts x C | 1 | 5.333 |
| Error | 55 | 225.989 |
| Total | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayo-Prieto, S.; Porteous-Álvarez, A.J.; Carro-Huerga, G.; Zanfaño, L.; Ramírez-Lozano, D.; Rodríguez-González, Á.; Lorenzana de la Varga, A.; Casquero, P.A. Hop Waste Seed Coating (Pilling) as Circular Bioeconomic Alternative to Improve Seed Germination and Trichoderma Development. Agriculture 2025, 15, 1328. https://doi.org/10.3390/agriculture15131328
Mayo-Prieto S, Porteous-Álvarez AJ, Carro-Huerga G, Zanfaño L, Ramírez-Lozano D, Rodríguez-González Á, Lorenzana de la Varga A, Casquero PA. Hop Waste Seed Coating (Pilling) as Circular Bioeconomic Alternative to Improve Seed Germination and Trichoderma Development. Agriculture. 2025; 15(13):1328. https://doi.org/10.3390/agriculture15131328
Chicago/Turabian StyleMayo-Prieto, Sara, Alejandra J. Porteous-Álvarez, Guzmán Carro-Huerga, Laura Zanfaño, Daniela Ramírez-Lozano, Álvaro Rodríguez-González, Alicia Lorenzana de la Varga, and Pedro A. Casquero. 2025. "Hop Waste Seed Coating (Pilling) as Circular Bioeconomic Alternative to Improve Seed Germination and Trichoderma Development" Agriculture 15, no. 13: 1328. https://doi.org/10.3390/agriculture15131328
APA StyleMayo-Prieto, S., Porteous-Álvarez, A. J., Carro-Huerga, G., Zanfaño, L., Ramírez-Lozano, D., Rodríguez-González, Á., Lorenzana de la Varga, A., & Casquero, P. A. (2025). Hop Waste Seed Coating (Pilling) as Circular Bioeconomic Alternative to Improve Seed Germination and Trichoderma Development. Agriculture, 15(13), 1328. https://doi.org/10.3390/agriculture15131328










