Abstract
This study focused on the seed germination of the local Cretan endemic Campanula cretica, an endangered and nationally protected species with ornamental value. To determine its seed germination requirements, high-resolution bioclimatic (temperature and precipitation) maps were integrated with geographic distribution data of C. cretica using Geographic Information Systems. The seed germination was tested at four constant temperatures (10, 15, 20, and 25 °C) with a photoperiod of 12 h light/12 h dark and under light/darkness and darkness at 15 °C. Pre-treatments with gibberellic acid solutions (500 and 1000 mg·L−1 GA3) and cold moist stratification at 5 °C were applied to investigate seed dormancy. Seed germination was significantly affected by the interaction of temperature and seed pre-treatments; without pre-treatment, the seeds germinated better (>85%) at 10 and 15 °C. The detected seed germination pattern matched the natural temperatures prevailing in situ during late autumn. Pre-treatments with GA3 solutions and cold stratification first reported herein widened the seed germination range at 20 and 25 °C. The seeds germinated better in light (94.38%) than in darkness (69.38%). The results of this investigation addressed existing research gaps (GIS-derived bioclimatic profiling, effects of incubation temperature, cold stratification, GA3, and light investigated for the first time), thus facilitating species-specific conservation efforts and enabling sustainable utilization strategies.
1. Introduction
Greece represents a significant center of evolution and diversity for members of the genus Campanula, hosting approximately 96 taxa (species and subspecies) out of the 448 Campanula taxa globally [1], of which ca. 60 are single-country endemics confined only to Greece [2,3]. This exceptional richness and uniqueness underscore the importance of Greek flora within this highly diverse and evolutionarily complex genus of Campanulaceae [2]. Many of the Greek endemic Campanula species are neglected and underutilized plants (NUPS) with potential applications across various sectors, such as the Cretan endemic C. pelviformis Lam. in the agro-alimentary and the medicinal-cosmetic sectors [4,5] or the latter as well as the Greek endemics C. incurva Aucher, C. cretica (A. DC.) D. Dietr., and C. laciniata L. in the ornamental-horticultural sector [6,7,8,9]. Such wild bellflowers provide a valuable genetic reservoir for developing new cropped cultivars and varieties [9]. Conserving and utilizing these species not only preserves Greece’s botanical heritage but also creates economic opportunities by enabling the development of value chains on local and global scales [10].
Crete, the largest island in Greece and the fifth largest in the Mediterranean Basin [11,12], is recognized as the most significant hot spot for plant endemism in the Mediterranean region [13], providing habitats for 11.5% of the Greek Campanula members among which eight taxa are local Cretan endemics and two are Greek endemics shared with other mainland or insular regions of Greece [3]. Designated by the International Union for Conservation of Nature (IUCN) as a Global Center of Plant Diversity [11], Crete’s unique biodiversity is largely attributed to its varied environmental conditions, complex topography, and long-term isolation due to troubled geological history (mountain building and tectonic movements) [11,12,14]. The dramatic terrain of this island with several mountains is peaked by three major mountain ranges—Lefka Ori, Psiloritis, and the Dikti Mountains—which serve as climatic refugia and are home to a significant number of threatened endemic species [12,13]. Among these, Lefka Ori hosts the highest concentration of endemic taxa assessed as threatened under IUCN criteria [13], as well as half of the Cretan endemic Campanula taxa [15].
Previous studies to date have investigated the effects of constant temperatures (10, 15, and 20 °C) on the germination of the local Cretan endemics Campanula hierapetrae Rech. fil., Campanula laciniata, and Campanula saxatilis L. subsp. saxatilis. The findings consistently indicate that the optimal temperature for seed germination in these single-island endemic species is 15 °C [16]. A more recent study on C. saxatilis subsp. saxatilis has assessed seed germination under four constant temperatures (10, 15, 20, and 25 °C) with a 12 h light/dark cycle. The results have revealed the highest germination percentage (85%) at 10 °C, with a slightly lower but faster germination rate observed at 15 °C [17]. Additionally, germination experiments involving five populations of the Cretan endemic bellflower C. pelviformis have demonstrated the highest germination percentages (>85%) at both 10 °C and 15 °C [8]. Collectively, these studies suggest that lower to moderate temperatures (10–15 °C) are generally favorable for the germination of Cretan endemic Campanula species. Temperature has significant effects on the induction and termination of dormancy and is considered the most influential environmental factor regulating the timing of seed germination [18,19]. By controlling germination under constant temperatures, both the range of germination temperatures and the germination season of a species can be determined [19]. In addition to the influence of temperature, the application of gibberellic acid (GA3) has been shown to enhance germination in various Campanula species. For example, treating C. incurva seeds with 400 mg·L⁻1 GA3 at 21 ± 1 °C has significantly improved the seed germination percentage [6]. Similarly, germination stimulation through GA3 pre-treatment has been observed in C. persicifolia L. (1000 mg·L⁻1 GA3) at alternating temperatures of 25/15 °C, C. rumeliana (Hampe) Vatke at 20/10 °C [20], and C. punctata Lam. var. punctata at both 15/6 °C and 25/15 °C [21]. Cold stratification is widely recognized as a critical pre-germination treatment for overcoming seed dormancy and enhancing germination percentages in many temperate species [22]. Previous studies have confirmed its effectiveness in various members of the genus Campanula. Notably, the sub-Balkan endemic Campanula trachelium L. subsp. athoa (Boiss. & Heldr.) Hayek has demonstrated a remarkable germination percentage of 99% at alternating temperatures of 25/15 °C after undergoing one month of cold stratification [20]. Similarly, in C. punctata var. punctata, seeds subjected to cold stratification for 4, 8, or 12 weeks, followed by four weeks of incubation at a constant 25 °C, have exhibited high germination percentages ranging from 77.0% to 87.0% [21]. These findings underscore the importance of temperature regulation, gibberellic acid application, and cold stratification as key strategies in promoting successful seed germination and seedling establishment in Campanula species, highlighting their potential utility in conservation and propagation efforts.
For the development of species-specific bioclimatic profiles, Geographic Information Systems (GIS) provide an essential tool offering valuable insights into the abiotic factors that prevail in the natural habitats of wild-growing populations of specific local endemic species. These profiles help assess whether such conditions can be replicated in human-made environments, thereby supporting ex situ conservation and cultivation efforts [17,23,24]. By defining the environmental conditions necessary for successful germination, growth, and acclimatization of specific species, bioclimatic (ecological) profiles are instrumental not only in species reintroduction and in situ conservation but also in the development of sustainable exploitation strategies [24]. In recent years, such bioclimatic (ecological) profiles have been established for several Greek endemic species of conservation concern (for example, [6,8,9,25]), underscoring their significance for ex situ conservation. Consequently, such GIS applications in plant conservation play a critical role in optimizing species management efforts [26].
One notable example of Crete’s unique flora is Campanula cretica (Campanulaceae), an impressive wildflower species that is locally endemic to Crete and primarily found at the foothills of the Lefka Ori mountain range, being classified as endangered (EN) [13] and assigned a protection status under the Greek Presidential Decree 67/1981 [3]. Beyond the biological rarity of C. cretica and its ecological role, a recent study has demonstrated that the species possesses strong antioxidant and anti-inflammatory properties [4], thus suggesting a new potential source of bioactive compounds. Additionally, the plant has considerable ornamental and horticultural value and has sparked commercial interest [10] since it is already catalogued and traded through the Internet by foreign nurseries, being available online as both living plants and seeds [11]. This uncontrolled commercialization raises concerns about potential overcollection from its natural habitats and/or its unauthorized propagation and trade in terms of the EU Regulation 511/2014 enforcing the Nagoya Protocol [10,11]. Therefore, this study specifically focused on C. cretica, aiming to develop a comprehensive and effective seed germination protocol for authorized use and correctly assign the species’ seed dormancy class. To this end, the effects of four different temperatures (10, 15, 20, and 25 °C) under alternating light/dark conditions, as well as the effects of different lighting conditions (light/darkness and darkness) at 15 °C, on seed germination behavior were investigated. Additionally, to explore seed dormancy in C. cretica, pre-treatments with gibberellic acid solutions (500 and 1000 mg·L−1) and cold moist stratification at 5 °C were applied. Furthermore, a bioclimatic (ecological) profile was constructed to support both the conservation and sustainable utilization of this showy Cretan bellflower.
2. Materials and Methods
2.1. Plant Material and Value
Campanula cretica (Figure 1) is a hemicryptophyte with a restricted range, found exclusively on the island of Crete, Greece [15]. It primarily grows in the gorges located in the southern and western foothills of the Lefka Ori mountains, with a recent discovery in a gorge in eastern Crete [15]. This species thrives in the shady crevices and ledges of limestone cliffs within ravines and is occasionally found on rocky road embankments in macchie and in gaps of Castanea sativa Mill. forests [15]. Its altitude range spans from sea level to 700 m above sea level and less frequently up to 1400 m, blooming from May to July [15] and occasionally (but to a lesser extent) in autumn. This impressive local endemic plant has relatively large and narrowly bell-shaped, white to deep violet-blue flowers developed on simple and erect flowering stems up to 60 cm (often bent downward when growing on inclined rocky surfaces), which are surrounded by large and vivid green, heart-shaped basal leaves; for detailed morphology, see [15].

Figure 1.
Wild-growing individuals of Campanula cretica in their shady rocky (A) and/or forest (B) natural habitats in the island of Crete, Greece, with vivid green heart-shaped leaves (C) and ascending (A) or pending inflorescences (D) with large showy sympetalous white to deep violet-blue corollas (E).
2.2. GIS Ecological Profiling
To establish a bioclimatic (ecological) profile for the local Cretan endemic C. cretica, it was essential to accurately depict its natural range in Crete (in total, 15 dot occurrences; see [27]). Utilizing digitized geographic data from a previous study built on a grid cell size of 8.25 × 8.25 km [28], the main distribution areas for C. cretica wild-growing populations (n = 8) were georeferenced through GIS (Geographical Information Systems). High-resolution bioclimatic maps were then employed and linked accordingly, with a pixel size of 30 arcsec and a spatial resolution of 1 km2. These maps provided detailed data on the minimum, maximum, and average monthly temperatures and precipitation levels, along with 19 additional bioclimatic variables for the natural distribution areas depicting the overall range of C. cretica. The climate data, covering the period from 1970 to 2000, were obtained from the open-access WorldClim database [29].
2.3. Seed Collection and Storage
Using an authorized collection permit (YPEN/DPD/15539/845 of 24 February 2022) issued by the competent Greek authorities (domestic Ministry of Environment and Energy) and following taxonomic verification, mature fruits (capsules) of C. cretica (Figure 2) were collected prior to their natural dispersal from a wild-growing population in Crete on 27 August 2022 found in Kakopetros, Chania, Crete Island (N35.419.784, E23.740.193, 518 m above sea level).
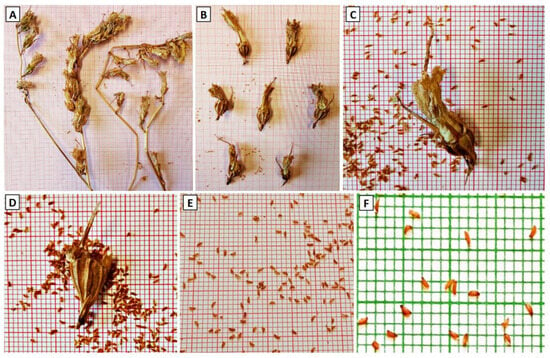
Figure 2.
Dried inflorescences of Campanula cretica as collected from the wild habitats (A), separated mature capsules (B), crashed capsule with desiccated corolla before sieving (C), corolla-free capsule ready for final sieving (D), isolated small seeds after sieving (E), and morphology of individual seeds (F).
Upon collection, the capsules were placed on filter paper and were maintained for a week under laboratory conditions. Subsequently, manual cleaning of the seeds was performed using a series of sieves with varying mesh sizes, according to the dimensions of the seeds. The taxonomically identified and manually cleaned seeds were assigned the IPEN (International Plant Exchange Network) accession number GR-BBGK-1-22,128. After thorough cleaning, the seeds were stored in glass containers at a temperature of 3–5 °C in a low-humidity environment (<15%) until the initiation of the experiments. To further ensure the conservation of the species’ genetic variability, new authorized seed collections were made in different areas in Crete during 2023–2025 (YPEN/DPD/38262/2306 of 2 August 2023, 80381/5557 of 9 August 2024, and 22236/1535 of 10 April 2025), resulting in representative seed lots stored for long-term conservation and further future experimentation.
2.4. Seed Germination Experiment
The two germination experiments for C. cretica seeds commenced in December 2022 and were conducted at the Laboratory of Floriculture, Department of Agriculture, Aristotle University of Thessaloniki (Thermi, Greece). In the first experiment, the seeds were subjected to the following different pre-treatments: (i) no treatment (control), (ii) seeds immersed in a solution of gibberellic acid (GA3) at a concentration of 500 mg·L−1 for 24 h, (iii) seeds immersed in a solution of GA3 at a concentration of 1000 mg·L−1 for 24 h, and (iv) seeds subjected to cold and moist stratification at 5 °C for 25 days. In the present study, the seeds of C. cretica were not stratified for more than 25 days, due to a pre-experiment conducted showing that germination of seeds stored for about four months was observed by the end of the fourth week of cold stratification (Subsequently, the germination response of the pre-treated seeds was evaluated across four constant temperatures, 10 °C, 15 °C, 20 °C, and 25 °C, using temperature-controlled growth chambers (CRW-500SD, Chrisagis, Athens, Greece). At each incubation temperature and for each pre-treatment, four replications were employed each with 40 individual seeds. The seeds were placed on filter paper moistened with distilled water in 9 cm sterile plastic Petri dishes. A layer of sterile, wet river sand about 1 cm thick was placed under the filter paper. The Petri dishes were randomly arranged on the shelves of the growth chambers. To maintain moisture, distilled water was added to the filter paper as needed throughout the experiment. Within the chambers, light was provided by fluorescent lamps at an intensity of 82 μmol m⁻2 s⁻1, with a photoperiod of 12 h light and 12 h dark, and relative humidity (RH) was maintained at 75 ± 1%. Germination was recorded every four days over a 36-day period, with germinated seeds being removed at each observation. Seed germination was defined by the visible emergence of the radicle from the seed coat (Figure 3).
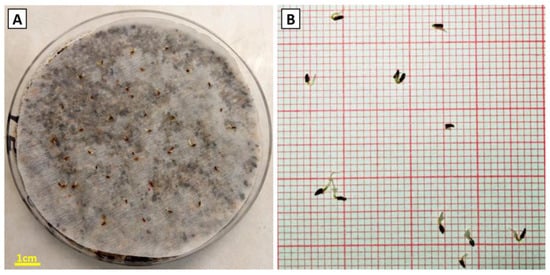
Figure 3.
Detected seed germination in Petri dishes (A) and germinated individual seeds of Campanula cretica (B).
Immediately after the completion of the germination test of control seeds of the first experiment (which lasted for 36 days), a second experiment was conducted to assess the light requirements for the germination of C. cretica seeds. Seeds of the seedlot used in the first experiment were subjected to germination at 15 °C. For each light treatment, a sample of four replications of 40 seeds each was used. The Petri dishes were placed in a chamber under either alternating light/dark conditions (12 h light/12 h dark) or continuous darkness. For the dark treatment, Petri dishes were covered with double layers of aluminum foil, which was removed only briefly for the counting of germinated seeds and then replaced. The mean germination time (MGT) for each treatment (light/dark and dark) was calculated based on the average of four replicates. The MGT for each replicate was determined using the following equation [30]:
where n is the number of seeds that germinated on day D, and D is the number of days counted from the onset of the experiment.
2.5. Statistical Analysis
In the first germination experiment, the experimental design employed a completely randomized factorial design in a split-plot arrangement with the factors being the incubation temperature (4 levels, 10, 15, 20, and 25 °C) and the seed pre-treatments (4 conditions, control, 500 mg·L−1 GA3, 1000 mg·L−1 GA3, and cold stratification). The incubation temperature was the main plot factor, and the treatments were the subplots. Two-way ANOVA [31] was conducted to evaluate the effects of the applied factors (temperature and pre-treatment) and their interaction on germination of C. cretica seeds, and the comparisons of the means were conducted using the least significance difference (LSD) test at significance level a = 0.05 [32]. The data were first checked for normality (Kolmogorov–Smirnov and Shapiro–Wilk test) and homogeneity of variances (Levene’s test) prior to analysis. In the second experiment, a t-test was conducted to evaluate the effects of light conditions on the germination percentage and mean germination time (days) of C. cretica seeds incubated at 15 °C. All statistical analyses were carried out using SPSS 28.0 (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. GIS-Derived Bioclimatic (Ecological) Profile
A GIS-based bioclimatic (ecological) profile was developed for C. cretica, utilizing natural occurrence records to capture the abiotic conditions prevailing in its natural habitats, particularly temperature and precipitation (Figure 4). According to historical climate data, the lowest average temperature for C. cretica was recorded in January (7.18 ± 1.74 °C) and February (7.38 ± 1.74 °C). From March to May, the average monthly temperatures gradually increased (8.85 ± 1.69 °C, 12.16 ± 1.62 °C, and 16.19 ± 1.49 °C, respectively), reaching 20.54 ± 1.44 °C at the beginning of summer (June). Notably, the highest average temperatures were observed in July (22.46 ± 1.45 °C) and August (22.21 ± 1.45 °C). Following the peak in temperatures, a gradual decrease was noted from September (19.72 ± 1.50 °C) to December (8.84 ± 1.71 °C). The bioclimatic profile of C. cretica (Figure 4) indicated that the minimum mean temperatures could reach 4.37 ± 2.20 °C in February, while the maximum mean temperatures could reach 18.14 ± 1.58 °C in July. The mean recorded diurnal range was 7.25 ± 0.15 °C, and the annual mean temperature was 14.44 ± 1.60 °C. The relatively low values of these two bioclimatic variables, combined with the absence of extreme temperatures throughout the seasons—historically not dropping below 0 °C or rising above 30 °C (minimum of Tmin = 1.09 °C in February and maximum of Tmax = 29.40 °C in July)—suggested that C. cretica wild-growing populations may thrive in relatively favorable environmental conditions in terms of plant growth, with a mild winter season in the study area.
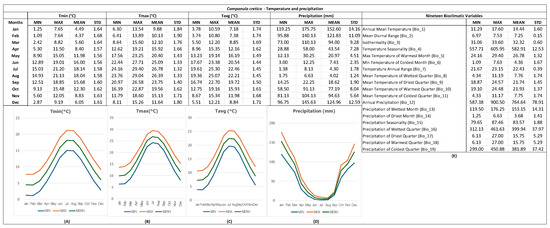
Figure 4.
Bioclimatic (ecological) profile across the natural distribution range of Campanula cretica wild-growing populations (n = 8) in Crete, Greece, linked in GIS with geodatabases (WorldClim version 2.1) presenting data for (A) minimum temperatures per month (°C), (B) maximum temperatures per month (°C), (C) average temperatures per month (°C), (D) precipitation per month (mm), and (E) calculated values for 19 bioclimatic variables. For (A–E), the minimum, maximum, average, and standard deviation are displayed based on data from 1970 to 2000. The colors of the plotted lines indicate the minimum (blue), maximum (orange), and mean (green) monthly values for temperature (°C) and precipitation (mm).
In terms of precipitation, the bioclimatic (ecological) profiling indicated that the rainy season may extend from October to March (Figure 4), with most precipitation occurring during this period. Historically, the rainiest months in the species’ natural range were herein shown to be December, January, and February, with January standing out as the month with the highest rainfall (152.60 ± 14.16 mm). From April onward, precipitation was shown to decrease, leading to the onset of the dry season by June (7.43 ± 2.35 mm). July and August were found to be the two driest months (4.30 ± 1.78 and 4.02 ± 1.24 mm, respectively). From September onward, the precipitation patterns were reported as slightly increasing (18.62 ± 1.90 mm), and they were gradually more pronounced during October and November (77.19 ± 8.04 and 94.63 ± 5.64 mm, respectively).
3.2. Seed Germination Experiment and Seed Germination Success
3.2.1. Effects of Incubation Temperature and Different Pre-Treatments
According to the results of two-way analysis of variance, the incubation temperature and pre-treatment factors, as well as their interaction, had a significant effect (p < 0.05) on seed germination of C. cretica (Table 1).

Table 1.
Results of two-way ANOVA regarding the effects of temperature, pre-treatment, and their interaction on final germination percentage of Campanula cretica seeds.
In the light of the significant effect of the interaction of temperature × pre-treatment on the percentage of germinated seeds (p < 0.05), only the latter was analyzed (single-factor effects were not analyzed). Explicitly, at 10 °C, high germination percentages (>86%) were observed across all pre-treatments (Figure 5). However, seeds pre-treated with 500 mg·L−1 GA3 exhibited a higher percentage of germinated seeds compared with those pre-treated with 1000 mg·L−1 GA3. It was noteworthy that in the cold stratification treatment, the first germinated seeds were observed on the fourth day, whereas in the other treatments, they were observed on the eighth day from the starting day of the germination experiment. Seed germination was completed by the 20th day in the 500 mg·L−1 GA3 pre-treatment and by the 24th day for the other pre-treatments.
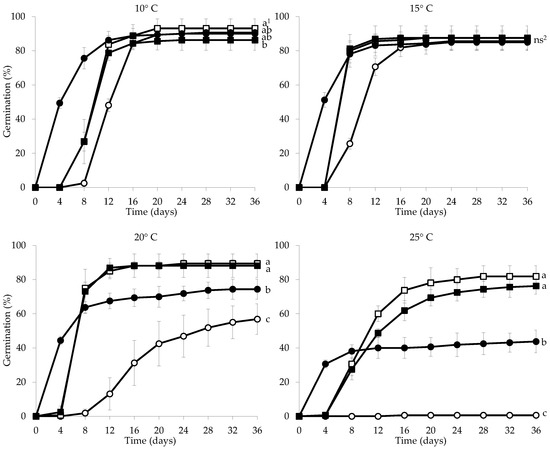
Figure 5.
Cumulative germination percentage diagrams of Campanula cretica seeds incubated at 10, 15, 20, and 25 °C without pre-treatment (○) or pretreated with 500 mg·L−1 GA3 (□), 1000 mg·L−1 GA3 (■), and cold stratification (●). 1 In each incubation temperature, the mean final germination percentages were statistically different at p < 0.05 when they did not share a common letter; ns2, mean final germination percentages that were not statistically significantly different (p > 0.05). The comparisons were performed using the LSD test.
At 15 °C, the germination percentages were very high across all treatments with no detected statistically significant differences (Figure 5). In the cold-stratified seeds, germination began before the 4th day and was completed by the 24th day from the starting day of the germination experiment. In the control seeds and in the seeds treated with 500 mg·L−1 and 1000 mg·L−1 GA3, the first germinated seeds were observed on the 8th day, and their germination was completed by the 24th, 20th, and 16th days, respectively.
At 20 °C and 25 °C, the pre-treatment of seeds with the GA3 regardless of solution concentration resulted in the highest germination percentages, with no statistically significant differences observed between them, while the lowest germination percentage was recorded in the control seeds (Figure 5). Specifically, at 25 °C, only one seed germinated out of 120 control seeds. At 20 °C, germination in the control seeds began on the eighth day from the starting day of the germination experiment. Whereas, in seeds treated with 500 and 1000 mg·L−1 GA3 or cold-stratified ones, the germination began before the 4th day and was completed by the 16th or 28th, respectively. At 25 °C, germination began on the 4th day for seeds treated with 500 and 1000 mg·L−1 GA3 or cold-stratified ones and was completed after the 24th day.
In the control, the highest germination was observed at 10 °C (90.00%) and 15 °C (85.00%), with no statistically significant differences between them. In contrast, a significant reduction in the number of germinated seeds was observed at 20 °C (56.88%) and especially at 25 °C (0.63%) (Table 2). In the seeds treated with 500 mg·L⁻1 GA3, statistically significant differences in germination percentages were observed only between 10 °C and 25 °C incubation temperature (93.13 and 81.88%, respectively). Furthermore, in the 1000 mg·L−1 GA3 pre-treatment, the germination percentages at 10 °C (86.25%), 15 °C (87.50%), and 20 °C (88.13%) did not differ significantly in statistical terms (Table 2), while a significant reduction in germination was observed at 25 °C (76.25%). Finally, in the seeds subjected to the cold stratification treatment, the highest germination percentages were observed at incubation temperatures of 10 °C and 15 °C (90.63 and 85.63%, respectively), with no statistically significant differences between them, while the increase in incubation temperature to 20 °C and 25 °C resulted in a significant reduction in the germinated seed percentages (74.38 and 43.75%, respectively) (Table 2).

Table 2.
Effect of incubation temperature on the germination percentage of Campanula cretica seeds without pre-treatment (control) and pretreated with 500 mg·L−1 GA3, 1000 mg·L−1 GA3, and cold stratification (CS). Means ± standard deviation values are given.
3.2.2. Effects of Light Treatment
In the experiment assessing the effect of light on the germination of C. cretica seeds, statistically significant differences were observed in both the germination percentage and the mean germination time (Table 3). Seeds that germinated under alternating light/dark conditions exhibited a higher germination rate (94.38%) compared with those germinated in continuous darkness (69.38%). Additionally, the mean germination time for seeds in alternating light/dark conditions was 9.7 days, whereas in darkness, the mean germination time was 15.34 days.

Table 3.
Effect of light conditions on germination of Campanula cretica seeds incubated at 15 °C. Means ± standard deviation values are given.
4. Discussion
The conservation of threatened and protected local Cretan endemic plants such as the herein investigated C. cretica requires a deep understanding of species-specific propagation and acclimatization techniques. Successful conservation strategies must incorporate both in situ assessments focused on wild-growing populations and ex situ approaches with a particular focus on developing effective seed germination protocols [7,33]. Sexual propagation is critical for maintaining genetic diversity, yet seed dormancy poses a significant challenge to large-scale plant production [34,35,36], especially in Campanulaceae [25]. For this reason, in the frame of the project CON-CRETE supported by the Hellenic Foundation for Research and Innovation and the Natural Environment & Climate Change Agency), additional seed lots of Campanula cretica were collected from geographically distinct populations (e.g., Samaria gorge, Lefka Ori, and Kakopetros) and different habitats (rocky outcrops and woodland) to be stored ex situ for future targeted seed germination experiments.
The high seed germination results of non-treated seeds at 10 °C and 15 °C (90.00% and 85.00%, respectively) and the significantly decreased germination at 20 °C and 25 °C (58.75% and <1%, respectively) detected in this investigation were consistent with a previous study on C. cretica [20]. However, in the present study, fresh seeds of C. cretica were not used in the germination test. The used seeds were stored for about 4 months, and this may have affected to some extent the dormancy of the seeds. Therefore, a further germination experiment with freshly collected seeds will need to be carried out to draw more secure conclusions regarding the germination requirements of the species. Similarly, high germination percentages at 10 and 15 °C have also been observed in other Cretan endemic species of Campanula, such as C. saxatilis subsp. saxatilis [17] and C. pelviformis [8]. Comparable results were observed in Petromarula pinnata (L.) A. DC. (also Campanulaceae), with relatively high percentages (>50%) of germinated seeds at 20 °C, a dramatic decrease (<3%) at 25 °C, and optimal seed germination at 10 and 15 °C [25]. These findings also agree with an earlier study indicating that many species within the Greek Campanulaceae germinating without pre-treatments prefer low temperatures, specifically (5)10–15 °C [20]. In the natural habitats of C. cretica, such germination trends probably occur from late autumn to early winter (November and December) when monthly temperatures decreased enough to fall within the range of those required for germination, as herein indicated by GIS-based bioclimatic (ecological) profiling. During this period, the increased soil moisture resulting from higher rainfall likely created favorable conditions for seed germination, while the relatively mild winter temperatures facilitated the growth and survival of seedlings. The uneven distribution of rainfall (mainly limited during the winter months) and the high summer temperatures in the Mediterranean (Greek) ecosystems were probably strongly associated with the adaptation of the plant life cycle of Greek native Campanula members to these climatic conditions. This comprehensive profiling offered valuable insights into the in situ climatic requirements of C. cretica in the wild, thus providing a deeper understanding of the species’ biological cycle and the specific conditions met in natural habitats (sexual reproduction of wild-growing populations) or man-made settings (ex situ seed germination).
Apart from informing the selection of appropriate temperature intervals for seed gemination experiments, the development of seed germination protocols informed by GIS-bioclimatic profiles can also be perceived as a solution for creating efficient propagation protocols for threatened endemic plants and, subsequently, as an opportunity to produce large numbers of individuals in man-made settings. This leads to the establishment of ex situ collections of plants of wild origin, which offer the opportunity to be used for translocation and reinforcement of wild-growing populations in situ with the aim to preserve or restore the species genetic diversity [36,37,38], facilitation of possible commercial interest for horticulture and floriculture [10,39,40,41,42,43,44], or exploration of agro-alimentary [45,46] and pharmacognostic interest [47,48,49,50,51] in a sustainable fashion, which is not based on plant material directly sourced from the wild. The insights presented herein regarding this rare, endangered, and valuable endemic plant of Crete can be effectively harnessed for conservation strategies and may be used to support its sustainable utilization, given its recognized ornamental value [10,11] and the newly discovered medicinal properties [4]. Accordingly, these GIS-facilitated germination protocols can be employed to generate adequate plant material of C. cretica for conservation actions, as well as for pilot-scale pot or field cultivation in nurseries and/or botanical gardens. The latter end was met in the frame of the agricultural research project titled “Establishment of an operational group to promote new flower crops from rare and seldom-seen autochthonous plants of Greece for cut flower production by precision fertilization” (acronym: Greek cut-flowers). In this project, upon successful application of the seed germination protocols herein developed and the acclimatization of the produced young plants, the first pilot cultivations were established recently (2024) at the premises of the Institute of Plant Breeding and Genetic Resources of the Hellenic Agricultural Organization Demeter in Thermi, metropolitan Thessaloniki, Greece (pot cultivation), and, with the co-operation of local cut-flower producers, in another two locations in Attica, Greece (greenhouse and outdoor field cultivations).
In general, seed dormancy is a mechanism that many plants have evolved to regulate the time of seed germination, thus ensuring successful seedling establishment and growth under favorable conditions [22,52,53,54,55]. Given that genes influencing seed germination and dormancy are among those under the strongest selection pressure in plants [56], germination and dormancy mechanisms hold significant ecological importance [52]. The failure to consider these traits in population restoration planning often leads to unsuccessful plant establishment [35,57]. Understanding the species-specific seed dormancy and the respective germination requirements of C. cretica is therefore essential for both conservation and ex situ cultivation efforts. In the present study, pre-treating C. cretica seeds with GA3 (500 mg·L−1 and 1000 mg·L−1) and applying cold stratification (5 °C) were implemented for the first time. These treatments significantly enhanced the germination percentages of the seeds at temperatures of 20 and 25 °C compared with the control. The increase in germination percentage following GA3 pre-treatment has also been reported in other species of the genus Campanula. For example, the seeds of C. incurva exhibit increased germination percentages following pre-treatment with 400 mg·L⁻1 GA3 at 21 ± 1 °C [6]. Similarly, enhanced germination percentages after 1000 mg·L⁻1 GA3 pre-treatment have been reported for C. persicifolia L. at alternating temperatures 25/15 °C, for C. rumeliana (Hampe) Vatke at 20/10 °C [20], and for C. punctata var. punctata at 15/6 °C and 25/15 °C [21]. Furthermore, cold moist stratification for 25 days resulted in higher germination percentages at 20 °C and 25 °C compared with seeds of C. cretica that did not undergo cold stratification. This increase in germination percentages following cold stratification (5 °C) is consistent with findings for the eastern Asiatic C. punctata var. punctata [21] and the sub-Βalkan endemic C. trachelium L. subsp. athoa (Boiss. Heldr.) Hayek presenting 99% germination at 25/15 °C after one month of cold stratification [20].
In this study, the germination behavior observed in C. cretica seeds indicated that both cold stratification and GA3 treatment resulted in the loss of the effect of temperature on germination, and the seeds germinated over a wider range of temperatures. At the incubation temperatures tested herein (10, 15, 20, and 25 °C), the germination of cold stratified seeds was observed in the first four days, whereas the seeds treated with GA3, as well as the control seeds, started to germinate before the eighth day (see Figure 5). At the incubation temperature of 15 °C, the pre-treatments applied to the seeds in the present study (GA3, cold stratification) resulted in an increase in germination speed; specifically, about 80% of seeds germinated on the eighth day from the beginning of germination experiment. According to previous studies [22], seeds are considered to exhibit physiological dormancy when cold stratification reduces the specific temperature requirement for germination or enhances the germination rate. Additionally, the application of gibberellins has been shown to be effective in breaking this type of dormancy [22]. Nevertheless, accurately classifying the type of dormancy in a species requires investigation of the pre-germination embryo development [22,58,59]. In the present study, this aspect was not examined, as measuring embryo size was not feasible due to the extremely small size of C. cretica seeds (see Figure 2). It has been reported that seeds of Campanulaceae exhibit either morphological dormancy (MD) or morphophysiological dormancy (MPD), with embryo growth occurring at temperatures of 15 °C or higher [58]. For example, seeds of C. americana L. exhibit morphological dormancy (MD) [22,58,60] as the embryo increases in size by 103% prior to germination when seeds are placed at 25/15 °C under light conditions without any dormancy-breaking treatment [60]. Similarly, in seeds of various Campanulaceae species from mountainous regions of Hawaii (USA), embryo growth ranging from 87% to 179% has been reported prior to radicle emergence, depending on the species [61]. On the other hand, the east Asiatic C. punctata Kam. var. punctata (syn. C. takesimana Nikai) demonstrates morphophysiological dormancy (MPD) [21] as its seeds possess underdeveloped embryos at dispersal and require GA3 treatment or short-term cold stratification to break physiological dormancy. Undoubtedly, further research on C. cretica is recommended to accurately determine the specific type of seed dormancy.
Species of Campanulaceae typically produce small to very small seeds that require light for germination [20]. The findings of the present study are consistent with previous research reporting that the final germination rate of C. cretica seeds in light conditions may reach 99% at a constant temperature of 15 °C, while in darkness, it can only reach 24% [20]. Other species within the genus Campanula such as the Balkan endemics C. spatulata Sm. subsp. spatulata and subsp. spruneriana (Hampe) Hayek and the sub-Balkan species C. versicolor Andrews and C. sparsa Friv. also show higher germination rates in light compared with darkness [20]. It is noteworthy that although an earlier study has reported higher germination rates for C. pelviformis seeds under light conditions (98%) compared with continuous darkness (11%) [20], a more recent study has found no statistically significant differences in germination percentage and mean germination time between the two conditions [8]. In the latter study [8], both light treatments exhibited high germination percentages and similar MGT values (98.75% and 10.95 days under light/dark conditions and 95% and 11.32 days in dark conditions) at the incubation temperature of 15 °C. A comparable germination pattern has also been observed in the seeds of Petromarula pinata [25].
5. Conclusions
This study provides a comprehensive analysis of the abiotic environmental conditions under which the wild-growing populations of C. cretica (Cretan bellflower) have evolved, focusing on their natural distribution across diverse habitats in Crete, Greece. Utilizing GIS-derived bioclimatic (ecological) profiling, this research established crucial environmental parameters that can support both the in situ conservation efforts and ex situ cultivation of this endangered Cretan endemic species. The insights gained herein are vital for developing strategies to integrate C. cretica into either natural habitats or artificial environments, thereby facilitating both conservation purposes and its sustainable utilization in man-made settings for different purposes. Additionally, the study addressed significant gaps in the understanding of the germination requirements of C. cretica, specifically investigating the effects of incubation temperature, cold stratification, gibberellic acid (GA3), and light exposure (pre-treatments investigated for the first time). The findings revealed optimal conditions for seed germination, which are instrumental in formulating effective propagation protocols. These protocols are essential for initiatives aimed at reinforcing natural populations or restoring degraded habitats. Moreover, the study identified the ideal incubation parameters necessary for maximizing germination success, which is a critical step toward the ex situ conservation of this species in seed banks and botanical gardens. In conclusion, the results of this research not only contribute to the preservation of C. cretica’s genetic diversity but also provide insights into its potential utilization in the ornamental-horticultural and medicinal-cosmetic sectors. In addition, this research serves ongoing efforts to support integrated conservation actions for this endangered local endemic plant species and facilitates its sustainable management as a unique but neglected and underutilized Cretan phytogenetic resource.
Author Contributions
Conceptualization, N.K., G.T., E.P., S.K. and S.H.; methodology, T.-N.P., I.A., E.P., S.K., S.H., N.K. and G.T.; software, I.A.; validation, I.A., S.H., N.K. and E.P.; formal analysis, T.-N.P., E.P. and I.A.; investigation, N.K., T.-N.P. and I.A.; resources, S.H., S.K., N.K. and G.T.; data curation, T.-N.P., E.P. and I.A.; writing—original draft preparation, T.-N.P., E.P. and I.A.; writing—review and editing, S.K., S.H., G.T. and N.K.; visualization, T.-N.P. and I.A.; supervision, S.H. and S.K.; project administration, N.K. and G.T.; funding acquisition, N.K. and G.T. All authors have read and agreed to the published version of the manuscript.
Funding
The investigation of G.T. and N.K. was conducted in co-operation with the project titled “Establishment of an operational group to promote new flower crops from rare and seldom-seen autochthonous plants of Greece for cut flower production by precision fertilization” (acronym: Greek cut-flowers; Μ16ΣΥΝ2-00419), which has been co-funded under Measure 16—Cooperation (16.1–16.5) by Greece and the European Union (European Agricultural Fund for Rural Development 2014–2020). The scientific work of N.K., G.T., E.P., S.H., S.K., and I.A. is partially supported by the Hellenic Foundation for Research and Innovation and the Natural Environment & Climate Change Agency under the project titled “DNA Barcoding, ecological profiles, and ex-situ conservation actions regarding threatened and/or non-evaluated local endemic plants of Crete” (acronym CON-CRETE; Project 28047).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data supporting the results of this study are included in the manuscript, and the datasets are available upon request.
Acknowledgments
The authors would like to thank the anonymous reviewers for their constructive comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: https://powo.science.kew.org/ (accessed on 13 April 2025).
- Liveri, E.; Kalachanis, D.; Bareka, P.; Grammatikopoulos, G.; Kamari, G. Contribution to the seed morphology of some Greek Campanula species of Sect. Quinqueloculares (Campanulaceae). Flora Medit. 2020, 30, 347–363. [Google Scholar] [CrossRef]
- Flora of Greece Web. Available online: https://portal.cybertaxonomy.org/flora-greece/intro (accessed on 13 April 2025).
- Dimitriadis, K.M.; Karavergou, S.; Hadjipavlou-Litina, D.; Krigas, N.; Lazari, D. Phytochemical and antioxidant evaluation of the ex-situ cultivated species Petromarula pinnata (L.) A. DC. and Campanula cretica (A.DC.) Dietr. (Campanulaceae) from Crete (Greece). Planta Med. 2022, 88, 1523. [Google Scholar] [CrossRef]
- Tsiftsoglou, O.S.; Lagogiannis, G.; Psaroudaki, A.; Vantsioti, A.; Mitić, M.N.; Mrmošanin, J.M.; Lazari, D. Phytochemical analysis of the aerial parts of Campanula pelviformis Lam. (Campanulaceae): Documenting the dietary value of a local endemic plant of Crete (Greece) traditionally used as wild edible green. Sustainability 2023, 15, 7404. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Maloupa, E. GIS-Facilitated ex situ conservation of the rare Greek endemic Campanula incurva Aucher: Seed germination requirements and effect of growth regulators on in vitro proliferation and rooting. Plant Biosyst. 2014, 148, 1169–1177. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Krigas, N.; Tsoktouridis, G.; Maloupa, E.; Grigoriadou, K. Seed germination trials and ex situ conservation of local prioritized endemic plants of Crete (Greece) with commercial interest. Seeds 2022, 1, 279–302. [Google Scholar] [CrossRef]
- Anestis, I.; Pipinis, E.; Kostas, S.; Papaioannou, E.; Karapatzak, E.; Dariotis, E.; Tsoulpha, P.; Koundourakis, E.; Chatzileontari, E.; Tsoktouridis, G.; et al. GIS-Facilitated germination of stored seeds from five wild-growing populations of Campanula pelviformis Lam. and fertilization effects on growth, nutrients, phenol content and antioxidant potential. Horticulturae 2023, 9, 877. [Google Scholar] [CrossRef]
- Panagiotidou, T.-N.; Pipinis, E.; Anestis, I.; Kostas, S.; Tsoulpha, P.; Karapatzak, E.; Tsoktouridis, G.; Hatzilazarou, S.; Krigas, N. Integrated ex-situ conservation and ornamental evaluation of the Vulnerable and protected Greek endemic Campanula laciniata L.: A multifaceted approach. Agronomy 2024, 14, 1665. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Bourgou, S. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Menteli, V.; Krigas, N.; Avramakis, M.; Turland, N.; Vokou, D. Endemic plants of Crete in electronic trade and wildlife tourism: Current patterns and implications for conservation. J. Biol. Res. 2019, 26, 10. [Google Scholar] [CrossRef]
- Médail, F. Plant biogeography and vegetation patterns of the Mediterranean islands. Bot. Rev. 2022, 88, 63–129. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant endemism centres and biodiversity hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant diversity patterns and conservation implications under climate-change scenarios in the Mediterranean: The case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Strid, A. Atlas of the Aegean Flora Part 1 (Text & Plates) & Part 2 (Maps), 1st ed.; Englera 33 (1 & 2); Botanic Garden and Botanical Museum; Freie Universität: Berlin, Germany, 2016. [Google Scholar]
- Fournaraki, C. Conservation of Threatened Plants of Crete—Seed Ecology, Operation and Management of a Gene Bank. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2010. (In Greek). [Google Scholar]
- Hatzilazarou, S.; Anestis, I.; Pipinis, E.; Kostas, S.; Avramakis, M.; Greveniotis, V.; Dariotis, E.; Tsoktrouridis, G.; Krigas, N. GIS-Facilitated seed germination of six local endemic plants of Crete (Greece) and multifaceted evaluation in three economic sectors. J. Biol. Res. 2023, 30, 5. [Google Scholar] [CrossRef]
- Geneve, R. Impact of temperature on seed dormancy. HortScience 2003, 38, 336–341. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Koutsovoulou, A. Adaptation Strategies and Ecophysiology of Seed Germination in the Campanulaceae Family. Ph.D. Thesis, National and Kapodistrian University of Athens (EKPA), Athens, Greece, 2014. [Google Scholar]
- Kim, H.M.; Kim, J.H.; Lee, J.H.; Kim, G.M.; Lee, M.H.; Park, C.Y.; Kim, D.H.; Lee, D.H.; Kim, K.M.; Na, C.S. Dormancy-release and germination improvement of Korean bellflower (Campanula takesimana Nakai), a rare and endemic plant native to the Korean peninsula. PLoS ONE 2023, 18, e0292280. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination, 2nd ed.; Elsevier: San Diego, CA, USA, 2014. [Google Scholar]
- Parra Quijano, M.; Iriondo, J.; Torres, E. Review. Applications of ecogeography and geographic information systems in conservation and utilization of plant genetic resources. Span. J. Agric. Res. 2012, 10, 419–429. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Huang, L.; Meng, X.; Hu, H.; Luo, L.; Chen, S. A new GIS model for ecologically suitable distributions of medicinal plants. Chin. Med. 2019, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Anestis, I.; Pipinis, E.; Kostas, S.; Karapatzak, E.; Dariotis, E.; Paradeisopoulou, V.; Greveniotis, V.; Tsoktouridis, G.; Hatzilazarou, S.; Krigas, N. GIS-Facilitated germination of stored seeds from four wild-growing populations of Petromarula pinnata (L.) A. DC.—A valuable, yet Vulnerable local endemic Plant of Crete (Greece). Agronomy 2024, 14, 274. [Google Scholar] [CrossRef]
- Draper, D.; Rosselló-Graell, A.; Garcia, C.; Tauleigne Gomes, C.; Sérgio, C. Application of GIS in plant conservation programmes in Portugal. Biol. Conserv. 2003, 113, 337–349. [Google Scholar] [CrossRef]
- Cretan Flora. Available online: https://www.cretanflora.com/campanula_cretica.html (accessed on 13 April 2025).
- Lazarina, M.; Charalampopoulos, A.; Psaralexi, M.; Krigas, N.; Michailidou, D.-E.; Kallimanis, A.S.; Sgardelis, S.P. Diversity patterns of different life forms of plants along an elevational gradient in Crete, Greece. Diversity 2019, 11, 200. [Google Scholar] [CrossRef]
- WorldClim. Available online: https://www.worldclim.org/data/worldclim21.html (accessed on 13 January 2020).
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures in Agricultural Research, 2nd ed.; Wiley: New York, NY, USA, 1984; ISBN 0-471-87092-7. [Google Scholar]
- Klockars, A.; Sax, G. Multiple Comparisons; Sage Publications: Newbury Park, CA, USA, 1986; p. 87. ISBN 0-8039-2051-2. [Google Scholar]
- Sharrock, S.; Jones, M. Saving Europe’s threatened flora: Progress towards GSPC Target 8 in Europe. Biodivers. Conserv. 2009, 20, 325–333. [Google Scholar] [CrossRef]
- Turner, S.R.; Steadman, K.J.; Vlahos, S.; Koch, J.M.; Dixon, K.W. Seed treatment optimizes benefits of seed bank storage for restoration-ready seeds: The feasibility of prestorage dormancy alleviation for mine-site revegetation. Restor. Ecol. 2013, 21, 186–192. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.O.; Chapman, T.; Sacco, A.D.; Mondoni, A.; Turner, S.R.; Cross, A.T. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28, S256–S265. [Google Scholar] [CrossRef]
- Rajpurohit, D.; Jhang, T. In situ and ex situ conservation of plant genetic resources and traditional knowledge. In Plant Genetic Resources and Traditional Knowledge for Food Security; Salgotra, R.K., Gupta, B.B., Eds.; Springer: Singapore, 2016; pp. 137–162. ISBN 978-981-10-0060-7. [Google Scholar]
- Di Martino, L.; Di Cecco, V.; Di Cecco, M.; Di Santo, M.; Ciaschetti, G.; Marcantonio, G. Use of native plants for ornamental purposes to conserve plant biodiversity: Case of study of Majella National Park. J. Nat. Conserv. 2020, 56, 125839. [Google Scholar] [CrossRef]
- Fernandes, M.P.; Pinto-Cruz, C.; Almeida, E.; Emídio, M.; Simões, M.P.; Gazarini, L.; Belo, A.D. Seed germination of six Iberian endemic species—A contribution to enhance plant conservation. Plant Biosyst. 2021, 155, 1146–1152. [Google Scholar] [CrossRef]
- Seglie, L.; Scariot, V.; Larcher, F.; Devecchi, M.; Chiavazza, P.M. In vitro seed germination and seedling propagation in Campanula spp. Plant Biosyst. 2012, 146, 15–23. [Google Scholar] [CrossRef]
- Scariot, V.; Gaino, W.; Devecchi, M. Propagation and cultivation protocols for wild creeping bellflowers (Campanula rapunculoides L.). In Proceedings of the International Symposium on Advanced Technologies and Management Towards Sustainable Greenhouse Ecosystems: Greensys 2011, Athens, Greece, 5–10 June 2011; Volume 952, pp. 265–272. [Google Scholar] [CrossRef]
- Scariot, V.; Seglie, L.; Gaino, W.; Devecchi, M. Evaluation of European native bluebells for sustainable floriculture. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Advances in Ornamentals, Landscape and Urban Horticulture, Lisbon, Portugal, 22–27 August 2010; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2012; Volume 937, pp. 273–279. [Google Scholar] [CrossRef]
- De Pascale, S.; Romano, D. Potential Use of Wild Plants in Floriculture. Acta Hortic. 2019, 1240, 87–98. [Google Scholar] [CrossRef]
- Facciuto, G.; Pannuzio, M.; Puerta, A.; Sanchez, M. Cut foliage: Potentiality of native Argentine ferns as new ornamental crops. Ornam. Hortic. 2021, 27, 566–574. [Google Scholar] [CrossRef]
- Luera, P.; Gabler, C.A. Combined Effects of scarification, phytohormones, stratification, and soil type on the germination and/or seedling performance of three tamaulipan thornscrub forest species. Plants 2022, 11, 2687. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWR) Priority in Italy: Distribution, ecology, in situ and ex situ conservation and expected actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
- Medeiros, M.B.; Valls, J.F.M.; Abreu, A.G.; Heiden, G.; Ribeiro-Silva, S.; José, S.C.B.R.; Santos, I.R.I.; Passos, A.M.A.; Burle, M.L. Status of the ex situ and in situ conservation of brazilian crop wild relatives of rice, potato, sweet potato, and finger millet: Filling the gaps of germplasm collections. Agronomy 2021, 11, 638. [Google Scholar] [CrossRef]
- Gomes, A.; Pimpão, R.C.; Fortalezas, S.; Figueira, I.; Miguel, C.; Aguiar, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Clemente, A.; et al. Chemical characterization and bioactivity of phytochemicals from Iberian endemic Santolina semidentata and strategies for ex situ propagation. Ind. Crop. Prod. 2015, 74, 505–513. [Google Scholar] [CrossRef]
- Ayuso, M.; García-Pérez, P.; Ramil-Rego, P.; Gallego, P.P.; Barreal, M.E. In vitro culture of the endangered plant Eryngium viviparum as dual strategy for its ex situ conservation and source of bioactive compounds. Plant Cell Tiss. Organ Cult. 2019, 138, 427–435. [Google Scholar] [CrossRef]
- Asgher, M.; Verma, S.; Khan, N.A.; Vyas, D.; Kumari, P.; Rashid, S.; Khan, S.; Qadir, S.; Ajmal Ali, M.; Ahmad, P. Physiological, biochemical and reproductive studies on Valeriana wallichii, a critically endangered medicinal plant of the Himalayan Region grown under in-situ and ex-situ conditions. Plants 2020, 9, 131. [Google Scholar] [CrossRef]
- Graikou, K.; Mpishinioti, A.; Tsafantakis, N.; Maloupa, E.; Grigoriadou, K.; Chinou, I. Comparative phytochemical analyses of flowers from Primula veris subsp. veris growing wild and from ex situ cultivation in Greece. Foods 2023, 12, 2623. [Google Scholar] [CrossRef]
- Sana; Aftab, T.; Khan, M.M.A.; Naeem, M. Chapter 7—Overexploitation and Conservation Strategies for Medicinal and Aromatic Plants. In Essential Oil-Bearing Plants; Naeem, M., Khan, M.M.A., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 95–105. ISBN 978-0-443-24860-3. [Google Scholar]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Dormancy and the Control of Germination. In Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Bewley, J.D., Bradford, K.J., Hilhorst, H.W.M., Nonogaki, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 247–297. [Google Scholar] [CrossRef]
- Willis, C.G.; Baskin, C.C.; Baskin, J.M.; Auld, J.R.; Venable, D.L.; Cavender-Bares, J.; Donohue, K.; de Casas, R.R.; The NESCent Germination Working Group. The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol. 2014, 203, 300–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, L.; Baskin, C.C.; Baskin, J.M.; Cao, M.; Yang, J. Seed dormancy in space and time: Global distribution, paleoclimatic and present climatic drivers, and evolutionary adaptations. New Phytol. 2022, 234, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Xiang, Y.; Tong, X.; Wojtyla, L.; Wang, Y. Editorial: Molecular basis of seed germination and dormancy. Front. Plant Sci. 2023, 14, 1242428. [Google Scholar] [CrossRef]
- Penfield, S. Seed Dormancy and Germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef]
- Wagner, M.; Pywell, R.F.; Knopp, T.; Bullock, J.M.; Heard, M.S. The germination niches of grassland species targeted for restoration: Effects of seed pre-treatments. Seed Sci. Res. 2011, 21, 117–131. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M.; Yoshinaga, A.; Wolkis, D. Seed dormancy in Campanulaceae: Morphological and morphophysiological dormancy in six species of Hawaiian Lobelioids. Botany 2020, 98, 327–332. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A Classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Underdeveloped embryos in dwarf seeds and implications for assignment to dormancy class. Seed Sci. Res. 2005, 15, 357–360. [Google Scholar] [CrossRef]
- Baskin, C.; Baskin, J.; Yoshinaga, A. Morphophysiological dormancy in seeds of six endemic Lobelioid shrubs (Campanulaceae) from the Montane Zone in Hawaii. Can. J. Bot. 2011, 83, 1630–1637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).