Application of Iron-Bimetal Biochar for As and Cd Reduction and Soil Organic Carbon Preservation Under Varying Moisture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of RSB Composites
2.3. As and Cd Adsorption Kinetics by RSB-Fe/Mn and RSB-Fe/Mg
2.4. Soil Incubation Experiment
2.5. Fractionation of SOC
2.6. Determination of Iron Oxide Fractions
2.7. Characterization of the Materials
2.8. Statistical Analysis
3. Results
3.1. Cd/As Adsorption Kinetics and Isotherm on RSB-Fe/Mn and RSB-Fe/Mg
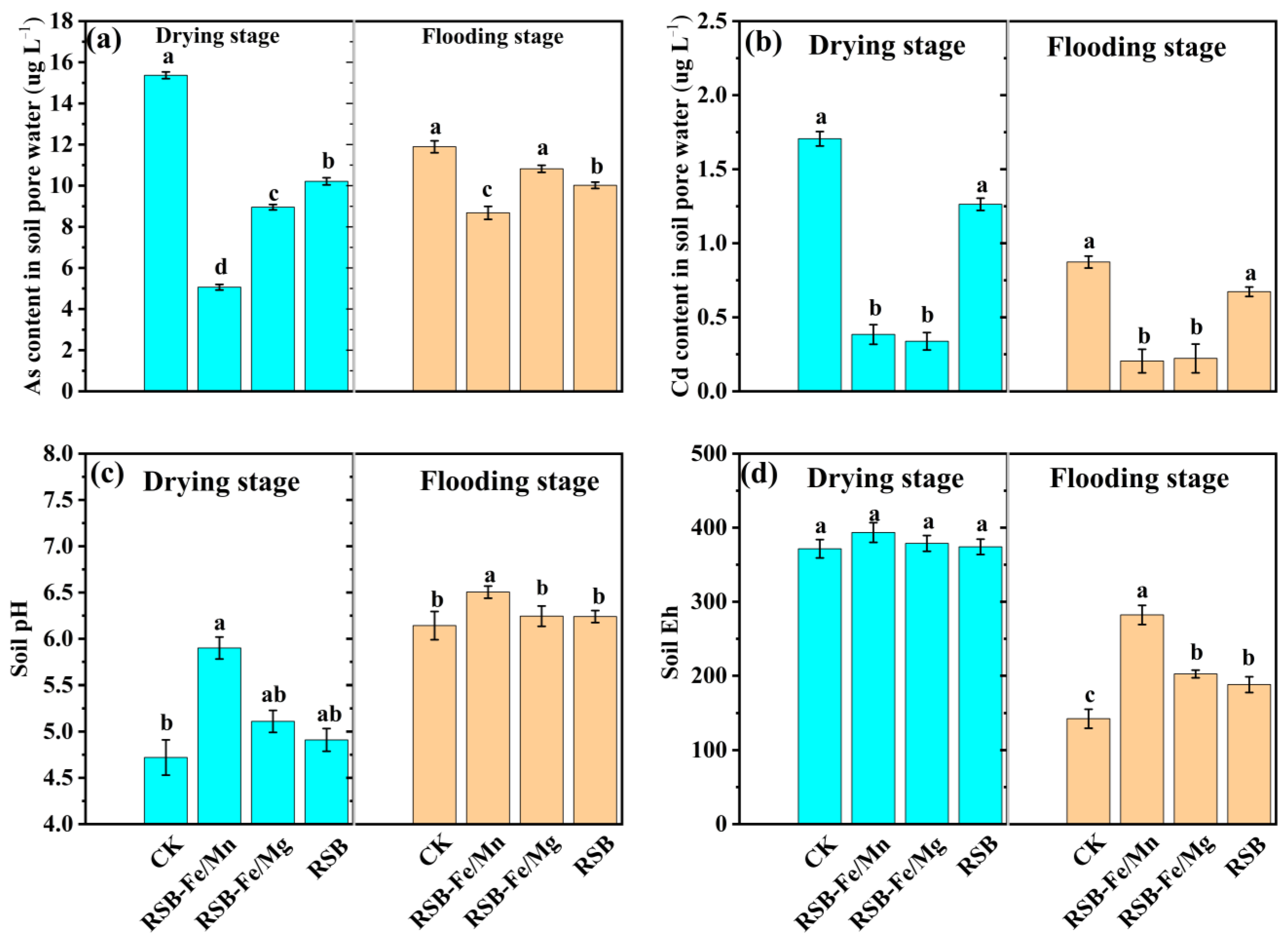
3.2. Influence of RSB-Fe/Mn and RSB-Fe/Mg Addition on Soil Cd/As Content, pH, and Eh
3.3. Evaluation of Kinetic Model Performance Using Error Metrics for Cd and As Adsorption
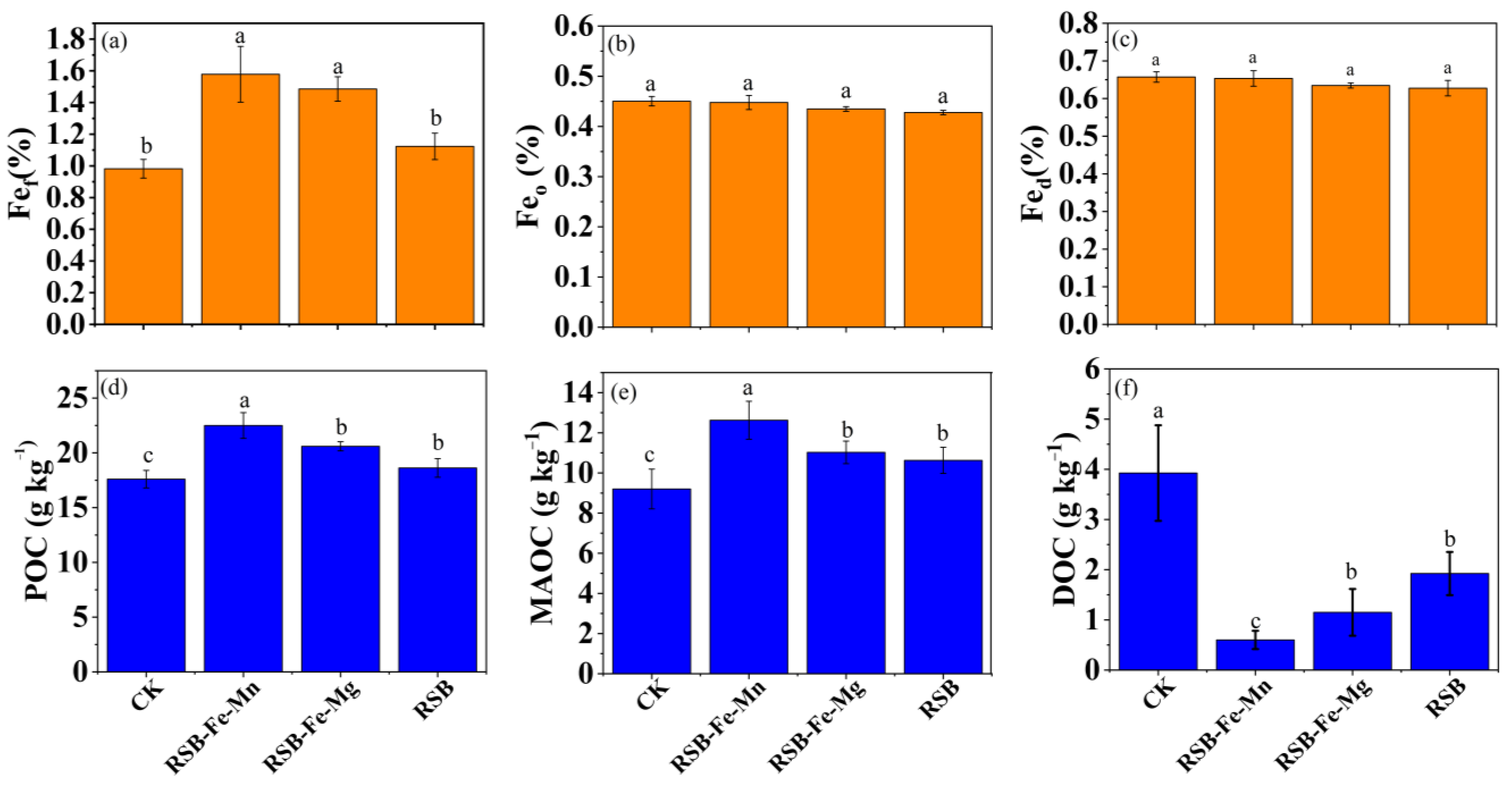
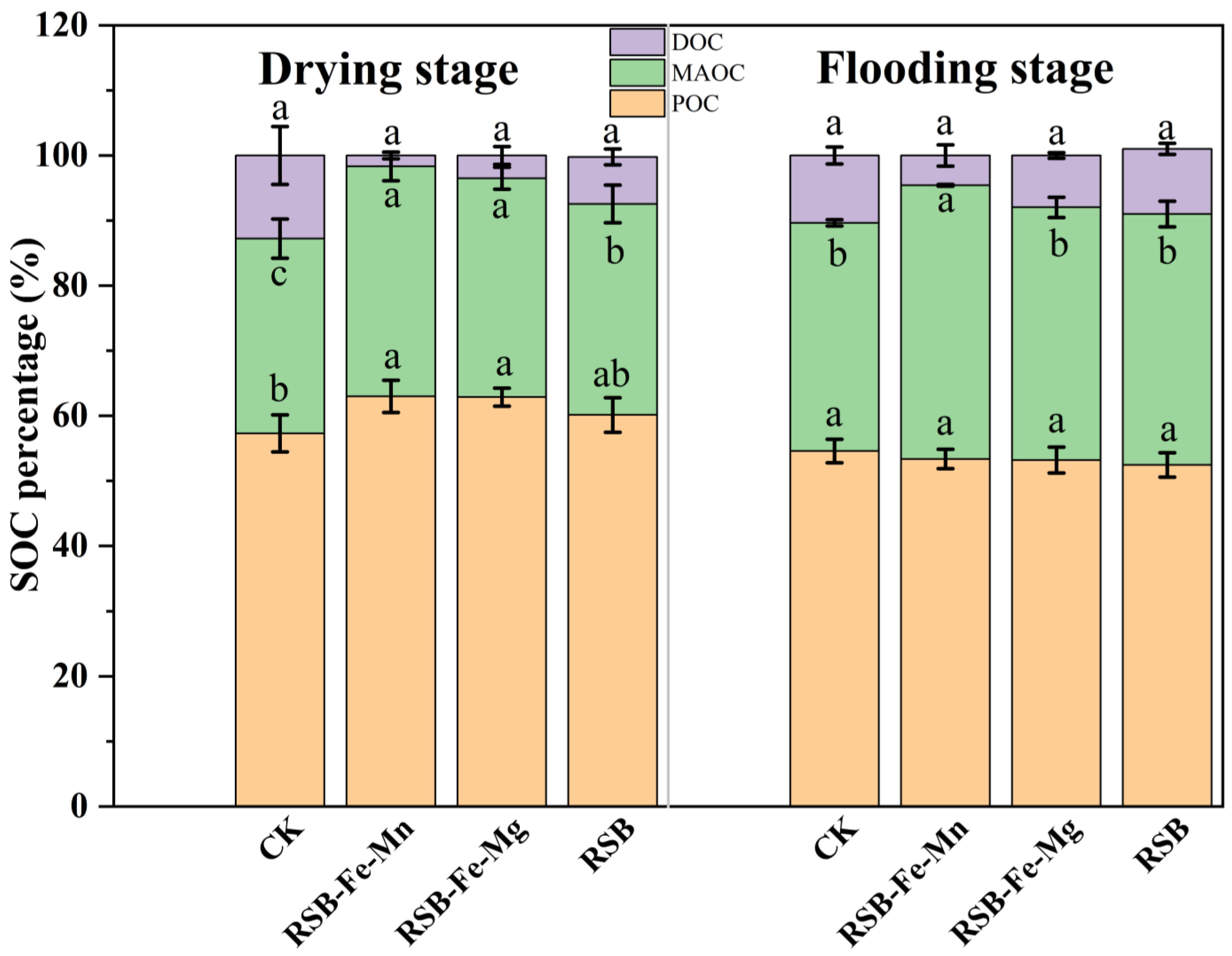
3.4. Influence of RSB-Fe/Mn and RSB-Fe/Mg on Fe-Oxides and SOC Fractions
3.5. Spectral Characterization Results
4. Discussion
4.1. Impact of RSB-Fe/Mn(Mg) on Soil As/Cd Content, pH, and Eh During Flooding and Drying Stages
4.2. Impact of RSB-Fe/Mn and RSB-Fe/Mg on the Protection of SOC
4.3. The Cd/As Interaction Mechanisms with RSB-Fe/Mn and RSB-Fe/Mg
4.4. Correlation Analysis of As/Cd Contents, SOC Fractions, and Soil Physicochemical Properties with Material Addition
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, P.N.; Lei, M.; Sun, G.; Huang, Q.; Lu, Y.; Deacon, C.; Meharg, A.A.; Zhu, Y.-G. Occurrence and partitioning of cadmium, arsenic, and lead in mine impacted paddy rice: Hunan, China. Environ. Sci. Technol. 2009, 43, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Guo, D.; Ali, A.; Mi, S.; Liu, T.; Ren, C.; Li, R.; Zhang, Z. Accumulation, ecological-health risks assessment, and source apportionment of heavy metals in paddy soils: A case study in Hanzhong, Shaanxi, China. Environ. Pollut. 2019, 248, 349–357. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Kim, J.-W.; Lee, S.-P.; Yang, J.-E.; Kim, S.-C. Effect of combined soil amendment on immobilizing bioavailable As and Pb in paddy soil. Toxics 2022, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Liu, C.; Zhu, J.; Li, F.; Deng, D.-M.; Wang, Q.; Liu, C. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef]
- Qiao, J.-t.; Liu, T.-x.; Wang, X.-q.; Li, F.-b.; Lv, Y.-h.; Cui, J.-h.; Zeng, X.-d.; Yuan, Y.-z.; Liu, C.-p. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Li, Y.; Gao, L.; Wang, Y.; Cheng, S.; Wu, G.; Yang, X.; Wan, S. Development of an acidized biochar-supported hydrated Fe (III) oxides for highly efficient cadmium and copper sequestration from water. Sci. Total Environ. 2021, 784, 147017. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Y.; Zhou, Y.; Adeel, M.; Chen, Q. Effects of magnesium ferrite biochar on the cadmium passivation in acidic soil and bioavailability for packoi (Brassica chinensis L.). J. Environ. Manag. 2019, 251, 109610. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Luo, X.; Shi, J. Efficient adsorption of dyes from aqueous solution using a novel functionalized magnetic biochar: Synthesis, kinetics, isotherms, adsorption mechanism, and reusability. Bioresour. Technol. 2022, 360, 127526. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Ouyang, Z. Organic carbon and its fractions in paddy soil are affected by different nutrient and water regimes. Geoderma 2005, 124, 133–142. [Google Scholar] [CrossRef]
- Rocci, K.S.; Lavallee, J.M.; Stewart, C.E.; Cotrufo, M.F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 793, 148569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Zong, Y.; Yu, J.; Ding, H.; Kong, Y.; Ma, J.; Ding, L. Efficient removal of cadmium by salts modified-biochar: Performance assessment, theoretical calculation, and quantitative mechanism analysis. Bioresour. Technol. 2022, 361, 127717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Poulson, S.R.; Obrist, D.; Sumaila, S.; Dynes, J.J.; McBeth, J.M.; Yang, Y. Iron-bound organic carbon in forest soils: Quantification and characterization. Biogeosciences 2016, 13, 4777–4788. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chu, W.; Li, H.; Boyd, S.A.; Teppen, B.J.; Mao, J.; Lehmann, J.; Zhang, W. Quantification and characterization of dissolved organic carbon from biochars. Geoderma 2019, 335, 161–169. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Wan, D.; Ye, T.; Lu, Y.; Chen, W.; Cai, P.; Huang, Q. Iron oxides selectively stabilize plant-derived polysaccharides and aliphatic compounds in agricultural soils. Eur. J. Soil Sci. 2019, 70, 1153–1163. [Google Scholar] [CrossRef]
- Sodano, M.; Lerda, C.; Nisticò, R.; Martin, M.; Magnacca, G.; Celi, L.; Said-Pullicino, D. Dissolved organic carbon retention by co-precipitation during the oxidation of ferrous iron. Geoderma 2017, 307, 19–29. [Google Scholar] [CrossRef]
- Karlsson, T.; Persson, P.; Skyllberg, U.; Morth, C.-M.; Giesler, R. Characterization of iron (III) in organic soils using extended X-ray absorption fine structure spectroscopy. Environ. Sci. Technol. 2008, 42, 5449–5454. [Google Scholar] [CrossRef]
- Li, H.; Santos, F.; Butler, K.; Herndon, E. A critical review of the multiple roles of manganese in stabilizing and destabilizing soil organic matter. Environ. Sci. Technol. 2021, 55, 12136–12152. [Google Scholar] [CrossRef]
- Herndon, E.; Bidas, K.; Li, H.; Santos, F.; Sulman, B.N. The roles of manganese in stabilizing and destabilizing soil organic matter. In Proceedings of the Goldschmidt 2023 Conference, Lyon, France, 9–14 July 2023. [Google Scholar]
- Fasihnikoutalab, M.H.; Asadi, A.; Huat, B.K.; Ball, R.J.; Pourakbar, S.; Singh, P. Utilisation of carbonating olivine for sustainable soil stabilization. Environ. Geotech. 2017, 4, 184–198. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, G.; Liu, L.; He, F.; Arinzechi, C.; Liao, Q.; Yang, W.; Si, M. Simultaneous immobilization of lead, cadmium and arsenic in soil by iron-manganese modified biochar. Front. Environ. Sci. 2023, 11, 1281341. [Google Scholar] [CrossRef]
- Tang, J.-Q.; Zhang, X.; Huang, G.-Y.; Hu, H.-Q. Effect of water regimes on Pb and Cd immobilization by biochar in contaminated paddy soil. Huan Jing Ke Xue = Huanjing Kexue 2021, 42, 1185–1190. [Google Scholar] [PubMed]
- Ghandali, M.V.; Safarzadeh, S.; Ghasemi-Fasaei, R.; Zeinali, S. Heavy metals immobilization, and bioavailability in multimetal contaminated soil under ryegrass cultivation as affected by ZnO and MnO2 nanoparticle-modified biochar. Sci. Rep. 2024, 14, 10684. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wu, X.; Liang, A.; Li, S.; Gao, H.; Song, X.; Liu, X.; Jia, A.; Degré, A. Conservation tillage enhances the sequestration and iron-mediated stabilization of aggregate-associated organic carbon in Mollisols. Catena 2024, 243, 108197. [Google Scholar] [CrossRef]
- Patzner, M.S.; Mueller, C.W.; Malusova, M.; Baur, M.; Nikeleit, V.; Scholten, T.; Hoeschen, C.; Byrne, J.M.; Borch, T.; Kappler, A. Iron mineral dissolution releases iron and associated organic carbon during permafrost thaw. Nat. Commun. 2020, 11, 6329. [Google Scholar] [CrossRef]
- Coward, E.K.; Thompson, A.T.; Plante, A.F. Iron-mediated mineralogical control of organic matter accumulation in tropical soils. Geoderma 2017, 306, 206–216. [Google Scholar] [CrossRef]
- Pan, Y.; Koopmans, G.F.; Bonten, L.T.; Song, J.; Luo, Y.; Temminghoff, E.J.; Comans, R.N. Influence of pH on the redox chemistry of metal (hydr) oxides and organic matter in paddy soils. J. Soils Sediments 2014, 14, 1713–1726. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, L.; Rui, X.; Hu, J.; Zhu, N. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges. J. Hazard. Mater. 2022, 432, 128668. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Shi, H.; Liu, Y. Redox dependence of manganese controls cadmium isotope fractionation in a paddy soil-rice system under unsteady pe+ pH conditions. Sci. Total Environ. 2022, 806, 150675. [Google Scholar] [CrossRef]
- Yang, X.; Shaheen, S.M.; Wang, J.; Hou, D.; Ok, Y.S.; Wang, S.-L.; Wang, H.; Rinklebe, J. Elucidating the redox-driven dynamic interactions between arsenic and iron-impregnated biochar in a paddy soil using geochemical and spectroscopic techniques. J. Hazard. Mater. 2022, 422, 126808. [Google Scholar] [CrossRef]
- Mansfeldt, T. Redox potential of bulk soil and soil solution concentration of nitrate, manganese, iron, and sulfate in two Gleysols. J. Plant Nutr. Soil Sci. 2004, 167, 7–16. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, Y.; Dionysiou, D.D.; Sharma, V.K.; Ma, X. Biochar as a novel carbon-negative electron source and mediator: Electron exchange capacity (EEC) and environmentally persistent free radicals (EPFRs): A review. Chem. Eng. J. 2022, 429, 132313. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Chacón, F.J.; Sánchez-Monedero, M.A.; Lezama, L.; Cayuela, M.L. Enhancing biochar redox properties through feedstock selection, metal preloading and post-pyrolysis treatments. Chem. Eng. J. 2020, 395, 125100. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Tran, H.N.; Nguyen, T.V.; Vigneswaran, S.; Trinh, V.T.; Nguyen, T.D.; Nguyen, T.H.H.; Mai, T.N.; Chao, H.-P. Single-step removal of arsenite ions from water through oxidation-coupled adsorption using Mn/Mg/Fe layered double hydroxide as catalyst and adsorbent. Chemosphere 2022, 295, 133370. [Google Scholar] [CrossRef]
- Irshad, M.K.; Chen, C.; Noman, A.; Ibrahim, M.; Adeel, M.; Shang, J. Goethite-modified biochar restricts the mobility and transfer of cadmium in the soil-rice system. Chemosphere 2020, 242, 125152. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.; Li, X.; Yang, P. Meta-analysis compares the effectiveness of modified biochar on cadmium availability. Front. Environ. Sci. 2024, 12, 1413047. [Google Scholar] [CrossRef]
- Beiyuan, J.; Qin, Y.; Huang, Q.; Wang, J.; Sarkar, B.; Bolan, N.; Wu, X.; Xu, W.; Liu, J.; Chen, X. Modified biochar for arsenic immobilization in soil: A critical review. Rev. Environ. Contam. Toxicol. 2023, 261, 20. [Google Scholar] [CrossRef]
- Huang, Y.; Mubeen, S.; Yang, Z.; Wang, J. Cadmium Contamination in Agricultural Soils and Crops. In Theories and Methods for Minimizing Cadmium Pollution in Crops: Case Studies on Water Spinach; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–30. [Google Scholar]
- Shaheen, S.M.; Mosa, A.; Natasha; Abdelrahman, H.; Niazi, N.K.; Antoniadis, V.; Shahid, M.; Song, H.; Kwon, E.E.; Rinklebe, J. Removal of toxic elements from aqueous environments using nano zero-valent iron-and iron oxide-modified biochar: A review. Biochar 2022, 4, 24. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L. Removal mechanisms of Cd from water and soil using Fe–Mn oxides modified biochar. Environ. Res. 2022, 212, 113406. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, Y.; Sun, G.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L. Application of ferromanganese functionalized biochar simultaneously reduces Cd and Pb uptake of wheat in contaminated alkaline soils. Ecotoxicol. Environ. Saf. 2023, 257, 114930. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Li, Y.; Dai, X.; Zhang, Q.; Zhang, M.; Zhang, Z.; Tao, Y.; Chen, W.; Zhang, M. Improved immobilization of soil cadmium by regulating soil characteristics and microbial community through reductive soil disinfestation. Sci. Total Environ. 2021, 778, 146222. [Google Scholar] [CrossRef]
- Lee, H.-S.; Shin, H.-S. Competitive adsorption of heavy metals onto modified biochars: Comparison of biochar properties and modification methods. J. Environ. Manag. 2021, 299, 113651. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Lu, Y.; Zhou, J.; Zhang, J.; Duan, Y.; Dong, C.; Wu, W. Simultaneous removal of Cd (II) and As (V) by ferrihydrite-biochar composite: Enhanced effects of As (V) on Cd (II) adsorption. J. Environ. Sci. 2024, 139, 267–280. [Google Scholar] [CrossRef]
- Su, J.; Guo, Z.; Zhang, M.; Xie, Y.; Shi, R.; Huang, X.; Tuo, Y.; He, X.; Xiang, P. Mn-modified bamboo biochar improves soil quality and immobilizes heavy metals in contaminated soils. Environ. Technol. Innov. 2024, 34, 103630. [Google Scholar] [CrossRef]
- Feng, J.; Jiang, L.; Yuan, B.; Zhang, L.; Zhang, A. Enhanced removal of aqueous phosphorus by Zr–Fe-, Mn–Fe-, and Mn–Zr–Fe-modified natural zeolites: Comparison studies and adsorption mechanism. Environ. Eng. Sci. 2020, 37, 572–584. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Anca-Couce, A.; Frišták, V.; Moreno-Jiménez, E.; Bacher, M.; Bucheli, T.D.; Cimò, G.; Conte, P.; Hagemann, N.; Haller, A. Designing biochar properties through the blending of biomass feedstock with metals: Impact on oxyanions adsorption behaviour. Chemosphere 2019, 214, 743–753. [Google Scholar] [CrossRef]
- Behnam, H.; Firouzi, A.F. Effects of synthesis method, feedstock type, and pyrolysis temperature on physicochemical properties of biochar nanoparticles. Biomass Convers. Biorefinery 2023, 13, 13859–13869. [Google Scholar] [CrossRef]
- Lützow, M.v.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Estes, E.; Andeer, P.; Nordlund, D.; Wankel, S.; Hansel, C. Biogenic manganese oxides as reservoirs of organic carbon and proteins in terrestrial and marine environments. Geobiology 2017, 15, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Neupane, A.; Herndon, E.M.; Whitman, T.; Faiia, A.M.; Jagadamma, S. Manganese effects on plant residue decomposition and carbon distribution in soil fractions depend on soil nitrogen availability. Soil Biol. Biochem. 2023, 178, 108964. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-mediated soil organic carbon stabilization. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhu, J.; Shi, L.; Fu, Q.; Hu, H.; Huang, Q. Influence mechanisms of iron, aluminium and manganese oxides on the mineralization of organic matter in paddy soil. J. Environ. Manag. 2022, 301, 113916. [Google Scholar] [CrossRef]
- Johnson, K.; Purvis, G.; Lopez-Capel, E.; Peacock, C.; Gray, N.; Wagner, T.; März, C.; Bowen, L.; Ojeda, J.; Finlay, N. Towards a mechanistic understanding of carbon stabilization in manganese oxides. Nat. Commun. 2015, 6, 7628. [Google Scholar] [CrossRef]
- Novair, S.B.; Cheraghi, M.; Faramarzi, F.; Lajayer, B.A.; Senapathi, V.; Astatkie, T.; Price, G. Reviewing the role of biochar in paddy soils: An agricultural and environmental perspective. Ecotoxicol. Environ. Saf. 2023, 263, 115228. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. J. Hazard. Mater. 2017, 322, 172–181. [Google Scholar] [CrossRef]
- Cuong, D.V.; Wu, P.-C.; Chen, L.-I.; Hou, C.-H. Active MnO2/biochar composite for efficient As (III) removal: Insight into redox transformation and adsorption mechanisms. Water Res. 2021, 188, 116495. [Google Scholar] [CrossRef]
- Luo, J.; Yi, Y.; Fang, Z. Effect of Mn-based magnetic biochar/PS reaction system on oxidation of metronidazole. Chemosphere 2023, 332, 138747. [Google Scholar] [CrossRef]
- Mabagala, F.S.; Zhang, T.; Zeng, X.; He, C.; Shan, H.; Qiu, C.; Gao, X.; Zhang, N.; Su, S. A review of amendments for simultaneously reducing Cd and As availability in paddy soils and rice grain based on a meta-analysis. J. Environ. Manag. 2024, 366, 121661. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lin, Q.; Cheng, S.; Guo, J.; Yao, X.; Liu, Q.; Yin, G.; Liu, D. Enhanced adsorption of Cd (II) from aqueous solution by a magnesium oxide–rice husk biochar composite. Environ. Sci. Pollut. Res. 2018, 25, 14032–14042. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-F.; Zhou, H.; Tan, W.-T.; Huang, J.-G.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Adsorption characteristics and mechanisms of Fe-Mn oxide modified biochar for Pb (II) in wastewater. Int. J. Environ. Res. Public Health 2022, 19, 8420. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, L.; Li, Z.; Tang, X.; He, M.; Yang, S.; Liu, X.; Xu, J. Simultaneous adsorption of Cd (II) and As (III) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems. Sci. Total Environ. 2020, 708, 134823. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Chen, P.; Zhu, Y.-G.; Cen, K.; Sun, G.-X. Simultaneous adsorption and immobilization of As and Cd by birnessite-loaded biochar in water and soil. Environ. Sci. Pollut. Res. 2019, 26, 8575–8584. [Google Scholar] [CrossRef] [PubMed]
- Suda, A.; Makino, T. Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: A review. Geoderma 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Amstaetter, K.; Borch, T.; Larese-Casanova, P.; Kappler, A. Redox transformation of arsenic by Fe (II)-activated goethite (α-FeOOH). Environ. Sci. Technol. 2010, 44, 102–108. [Google Scholar] [CrossRef]
- Huang, Q.; Tong, F.; Gao, Y.; Chen, J.; Zhou, D.; Qu, Z.; Fan, G.; Chen, W.; Shi, G. Enhanced simultaneous arsenite oxidation and sorption by Mn-modified biochar: Insight into the mechanisms under optimal modification condition. J. Environ. Chem. Eng. 2023, 11, 109612. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Chin, B.L.F.; Lock, S.S.M.; Yee, C.Y.; Yiin, C.L.; Peng, W.; Lam, S.S. Production of biochar from rice straw and its application for wastewater remediation—An overview. Bioresour. Technol. 2022, 360, 127588. [Google Scholar] [CrossRef]
- Sun, Z.; Qian, X.; Shaaban, M.; Wu, L.; Hu, J.; Hu, R. Effects of iron (III) reduction on organic carbon decomposition in two paddy soils under flooding conditions. Environ. Sci. Pollut. Res. 2019, 26, 12481–12490. [Google Scholar] [CrossRef]
- Giannetta, B.; Zaccone, C.; Plaza, C.; Siebecker, M.G.; Rovira, P.; Vischetti, C.; Sparks, D.L. The role of Fe (III) in soil organic matter stabilization in two size fractions having opposite features. Sci. Total Environ. 2019, 653, 667–674. [Google Scholar] [CrossRef]
- Patel, K.F.; Myers-Pigg, A.; Bond-Lamberty, B.; Fansler, S.J.; Norris, C.G.; McKever, S.A.; Zheng, J.; Rod, K.A.; Bailey, V.L. Soil carbon dynamics during drying vs. rewetting: Importance of antecedent moisture conditions. Soil Biol. Biochem. 2021, 156, 108165. [Google Scholar] [CrossRef]
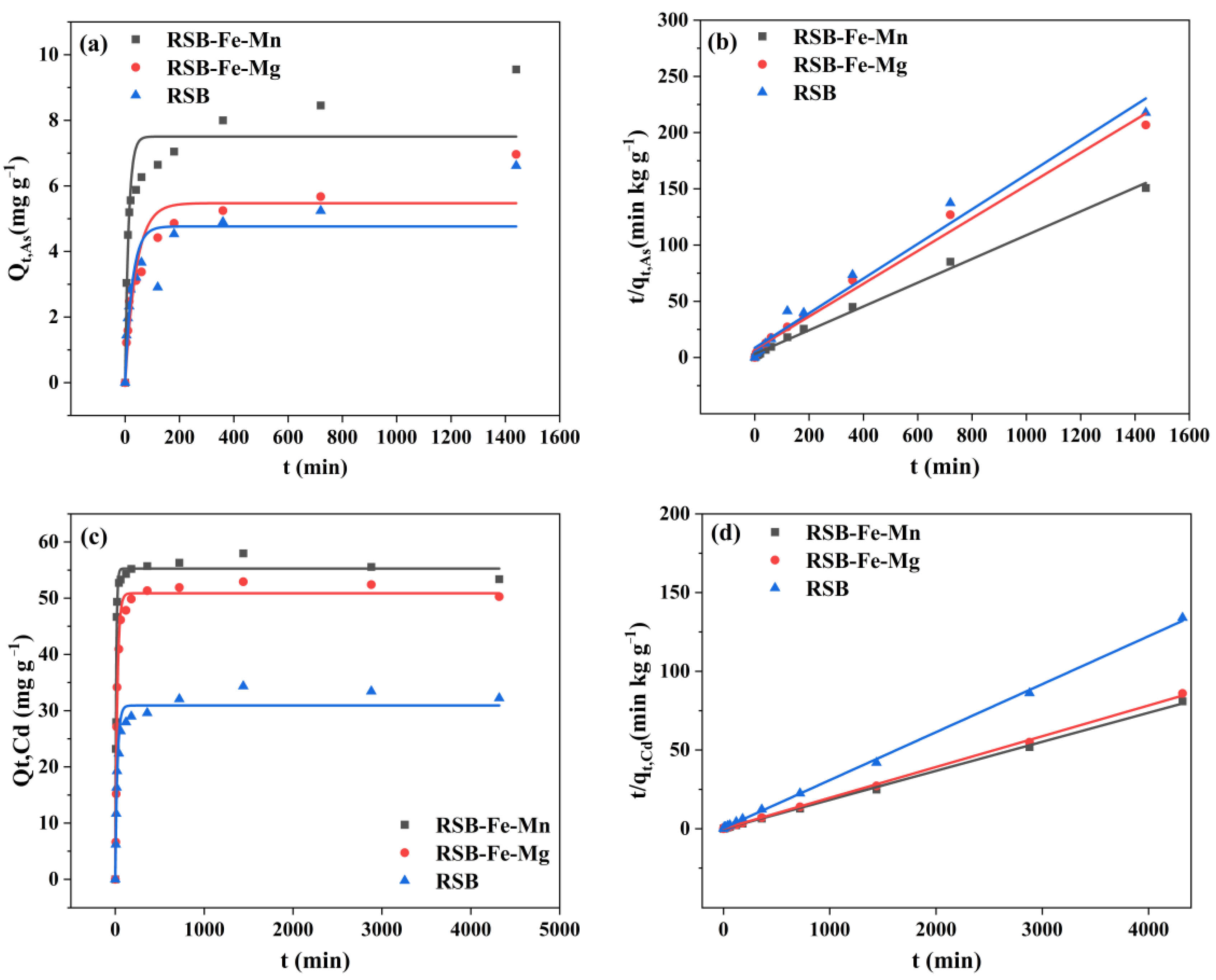

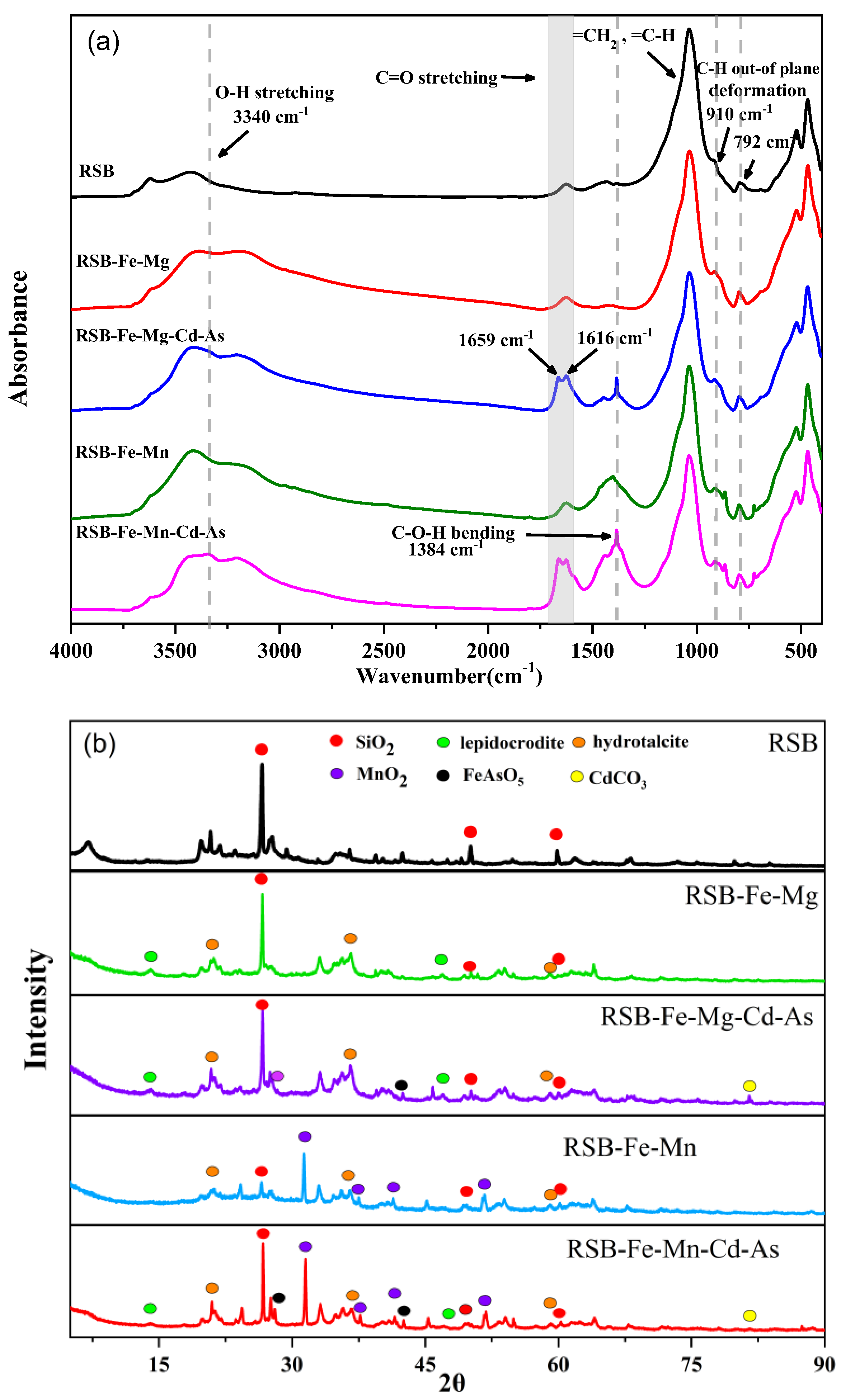
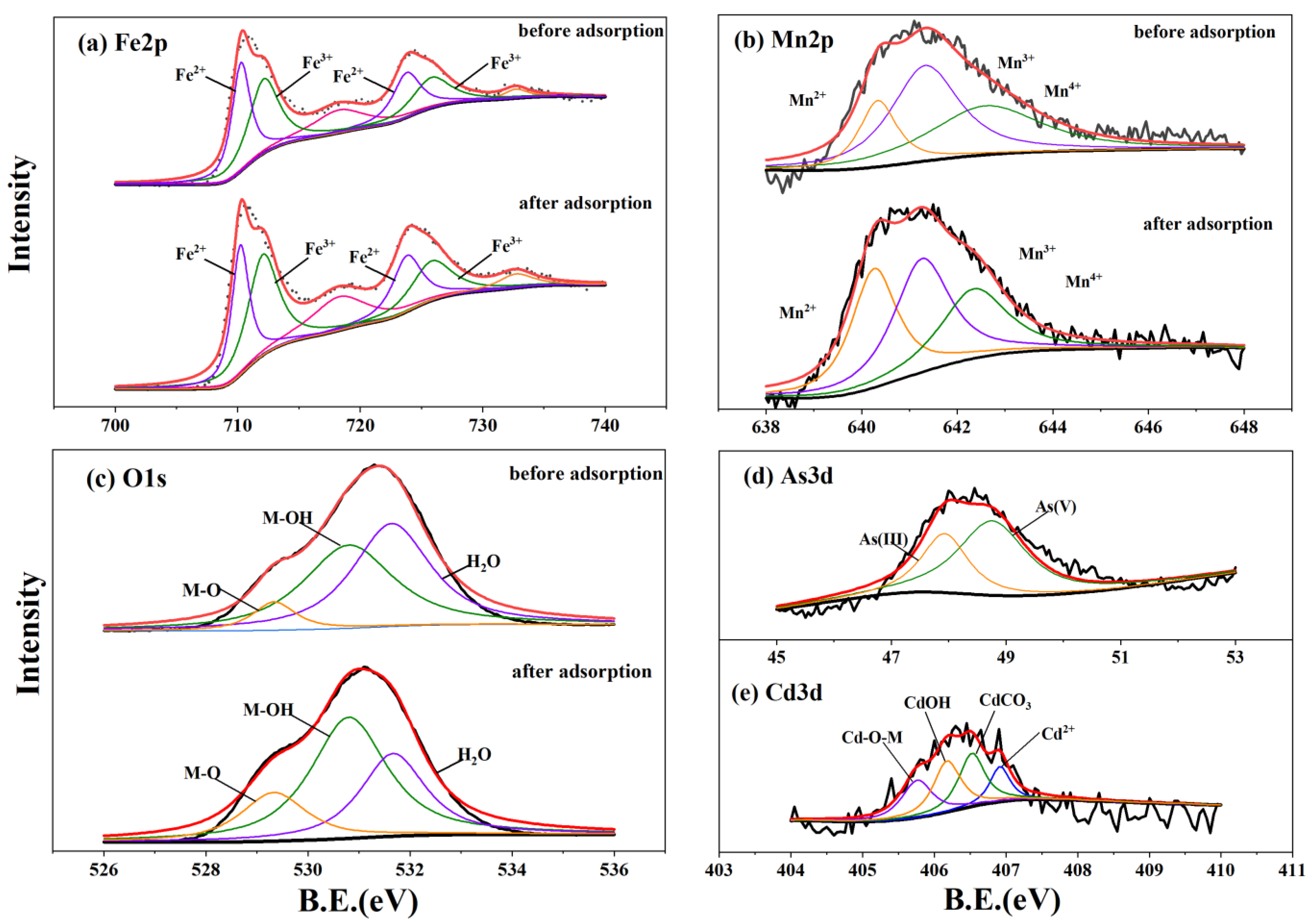
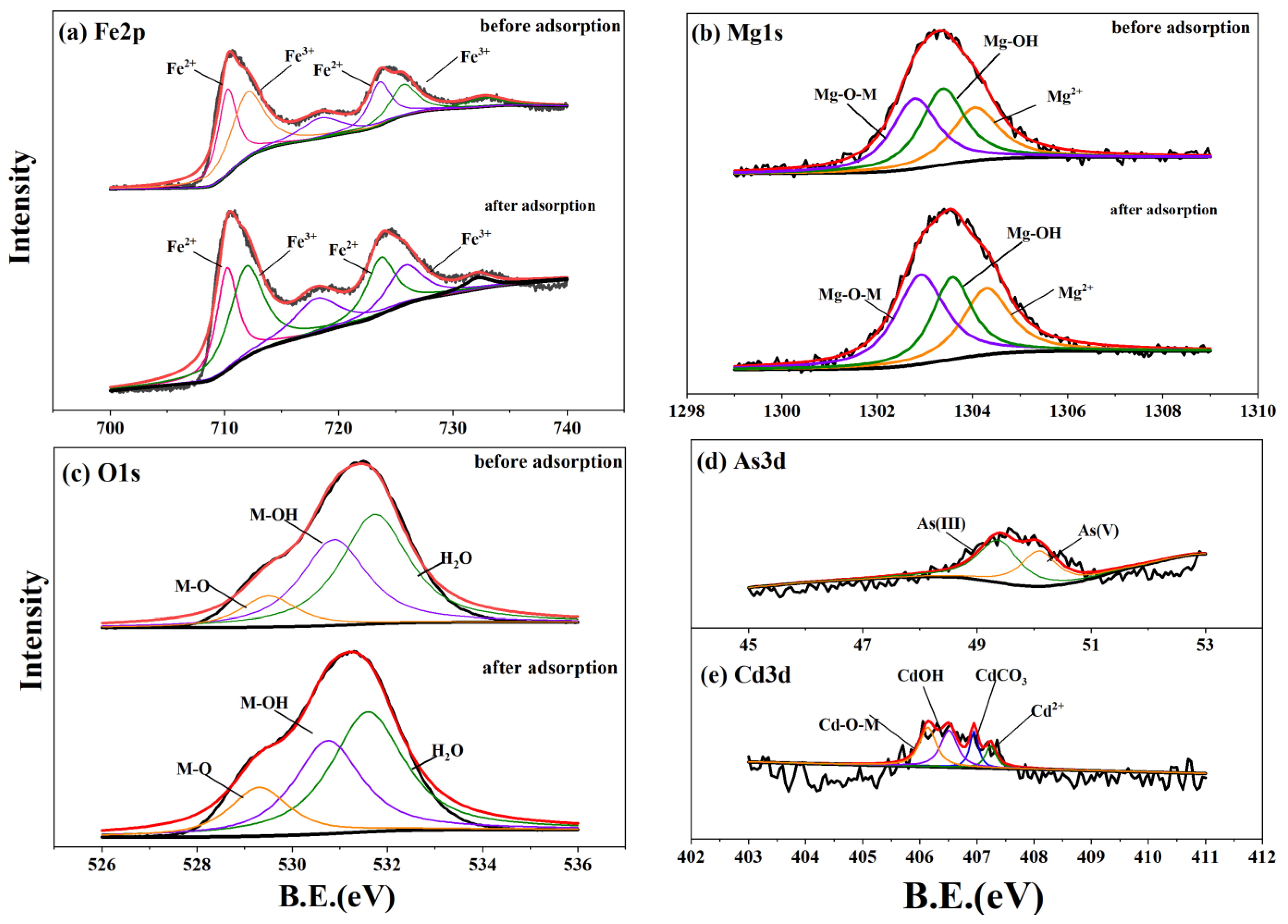

| Pseudo-First Order qe (mg·g−1) | K1 (h−1) | R2 | Pseudo-Second Order qe (mg·g−1) | K2 (g·mg−1·h−1) | h (mg·g−1·h−1) | R2 | ||
|---|---|---|---|---|---|---|---|---|
| As | RSB-Fe/Mn | 7.504 | 0.0774 | 0.846 | 9.446 | 0.01116 | 0.320 | 0.995 |
| RSB-Fe/Mg | 5.473 | 0.0247 | 0.863 | 6.869 | 0.02119 | 0.136 | 0.987 | |
| RSB | 4.764 | 0.0381 | 0.747 | 6.493 | 0.02372 | 0.116 | 0.978 | |
| Cd | RSB-Fe/Mn | 55.268 | 0.0976 | 0.977 | 54.171 | 0.00034 | 6.347 | 0.999 |
| RSB-Fe/Mg | 50.879 | 0.0447 | 0.985 | 51.125 | 0.00038 | 13.988 | 0.999 | |
| RSB | 30.917 | 0.0427 | 0.965 | 32.798 | 0.00093 | 2.870 | 0.999 |
| Heavy Metal | Adsorbent | Model Type | (Mean Absolute Error) MAE | Root Mean Square Error (RMSE) | R² |
|---|---|---|---|---|---|
| Cd | Mn | PSO | 0.44 | 0.44 | 0.66 |
| Cd | Mn | PFO | 1.79 | 6.09 | 2.46 |
| Cd | Mg | PSO | 0.41 | 0.40 | 0.63 |
| Cd | Mg | PFO | 1.80 | 4.65 | 2.15 |
| Cd | RSB | PSO | 0.66 | 1.04 | 1.02 |
| Cd | RSB | PFO | 1.71 | 3.83 | 1.95 |
| As | Mn | PSO | 2.60 | 9.28 | 3.04 |
| As | Mn | PFO | 0.72 | 0.83 | 0.91 |
| As | Mg | PSO | 5.18 | 43.28 | 6.57 |
| As | Mg | PFO | 0.57 | 0.46 | 0.68 |
| As | RSB | PSO | 7.49 | 80.56 | 8.97 |
| As | RSB | PFO | 0.60 | 0.69 | 0.83 |
| Spectra | Material | Attribution | RSB-Fe/Mg | RSB-Fe/Mn | ||||
|---|---|---|---|---|---|---|---|---|
| B.E. (eV) | Area | Ratio % | B.E. (eV) | Area | Ratio % | |||
| Fe2p | Before adsorption | 2p3/2, Fe2+ | 710.27 | 43,520.25 | 46.13 | 710.22 | 55,730.69 | 45.70 |
| 2p1/2, Fe2+ | 723.6 | 25,427.42 | 723.82 | 40,918.89 | ||||
| 2p3/2, Fe3+ | 712.01 | 58,345.59 | 53.87 | 712.06 | 75,086.89 | 54.30 | ||
| 2p1/2, Fe3+ | 725.68 | 22,181.62 | 725.83 | 39,745.8 | ||||
| After adsorption | 2p3/2, Fe2+ | 710.23 | 57,029.4 | 45.76 | 710.27 | 49,853.2 | 49.38 | |
| 2p1/2, Fe2+ | 723.7 | 43,518.42 | 723.8 | 31,340.03 | ||||
| 2p3/2, Fe3+ | 711.93 | 81,603.43 | 54.24 | 712.13 | 54,753.88 | 50.62 | ||
| 2p1/2, Fe3+ | 725.82 | 37,599.7 | 725.82 | 28,484.61 | ||||
| Mg1s | Before adsorption | Mg-O-M | 1302.79 | 12,785.66 | 35.65 | |||
| Mg-OH | 1303.38 | 13,111 | 36.56 | |||||
| Mg2+ | 1304.05 | 9965.49 | 27.79 | |||||
| After adsorption | Mg-O-M | 1302.91 | 13,855.32 | 40.86 | ||||
| Mg-OH | 1303.58 | 10,220.45 | 30.14 | |||||
| Mg2+ | 1304.3 | 9837.08 | 29.01 | |||||
| Mn2p | Before adsorption | Mn2+ | 640.34 | 3193.84 | 17.28 | |||
| Mn3+ | 641.33 | 8293.13 | 44.86 | |||||
| Mn4+ | 642.59 | 6997.74 | 37.86 | |||||
| After adsorption | Mn2+ | 640.26 | 5759.72 | 33.27 | ||||
| Mn3+ | 641.26 | 6679.01 | 38.58 | |||||
| Mn4+ | 642.35 | 4874.38 | 28.15 | |||||
| O1s | Before adsorption | M-O | 529.5 | 41,920.48 | 11.72 | 529.34 | 27,138.22 | 7.30 |
| M-OH | 530.88 | 136,710.8 | 38.24 | 530.8 | 167,010.4 | 44.95 | ||
| H2O | 531.74 | 178,922.2 | 50.04 | 531.64 | 177,435 | 47.75 | ||
| After adsorption | M-O | 529.31 | 62,861.85 | 16.35 | 529.33 | 64,052.89 | 18.16 | |
| M-OH | 530.75 | 134,316 | 34.92 | 530.8 | 182,437.8 | 51.72 | ||
| H2O | 531.59 | 187,406.9 | 48.73 | 531.67 | 106,232.7 | 30.12 | ||
| Cd3d | After adsorption | Cd-OM | 406.14 | 385.761 | 36.10 | 405.76 | 398.66 | 24.81 |
| Cd-OH | 406.51 | 360.441 | 33.73 | 406.18 | 483.49 | 30.09 | ||
| Cd-CO3 | 406.95 | 183.721 | 17.19 | 406.52 | 483.51 | 30.09 | ||
| Cd2+ | 407.24 | 138.553 | 12.97 | 406.92 | 241.24 | 15.01 | ||
| As3d | After adsorption | As(III) | 49.38 | 707.847 | 57.30 | 47.93 | 1115.97 | 35.12 |
| As(V) | 50.08 | 527.435 | 42.70 | 48.76 | 2061.48 | 64.88 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabagala, F.S.; Wang, T.; Feng, Q.; Zeng, X.; He, C.; Wu, C.; Zhang, N.; Su, S. Application of Iron-Bimetal Biochar for As and Cd Reduction and Soil Organic Carbon Preservation Under Varying Moisture. Agriculture 2025, 15, 1114. https://doi.org/10.3390/agriculture15111114
Mabagala FS, Wang T, Feng Q, Zeng X, He C, Wu C, Zhang N, Su S. Application of Iron-Bimetal Biochar for As and Cd Reduction and Soil Organic Carbon Preservation Under Varying Moisture. Agriculture. 2025; 15(11):1114. https://doi.org/10.3390/agriculture15111114
Chicago/Turabian StyleMabagala, Frank Stephano, Tingjuan Wang, Qiufen Feng, Xibai Zeng, Chao He, Cuixia Wu, Nan Zhang, and Shiming Su. 2025. "Application of Iron-Bimetal Biochar for As and Cd Reduction and Soil Organic Carbon Preservation Under Varying Moisture" Agriculture 15, no. 11: 1114. https://doi.org/10.3390/agriculture15111114
APA StyleMabagala, F. S., Wang, T., Feng, Q., Zeng, X., He, C., Wu, C., Zhang, N., & Su, S. (2025). Application of Iron-Bimetal Biochar for As and Cd Reduction and Soil Organic Carbon Preservation Under Varying Moisture. Agriculture, 15(11), 1114. https://doi.org/10.3390/agriculture15111114








