Microbial Population in Curcuma Species at Different Growth Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Curcuma Species or Strains
2.2. Curcuma Cultivation
2.3. Sample Collection
2.4. Isolation and Enumeration of Endophytic Bacteria
2.5. Isolation and Enumeration of Rhizosphere Heterotrophic Bacteria
2.6. Data Analysis
3. Results
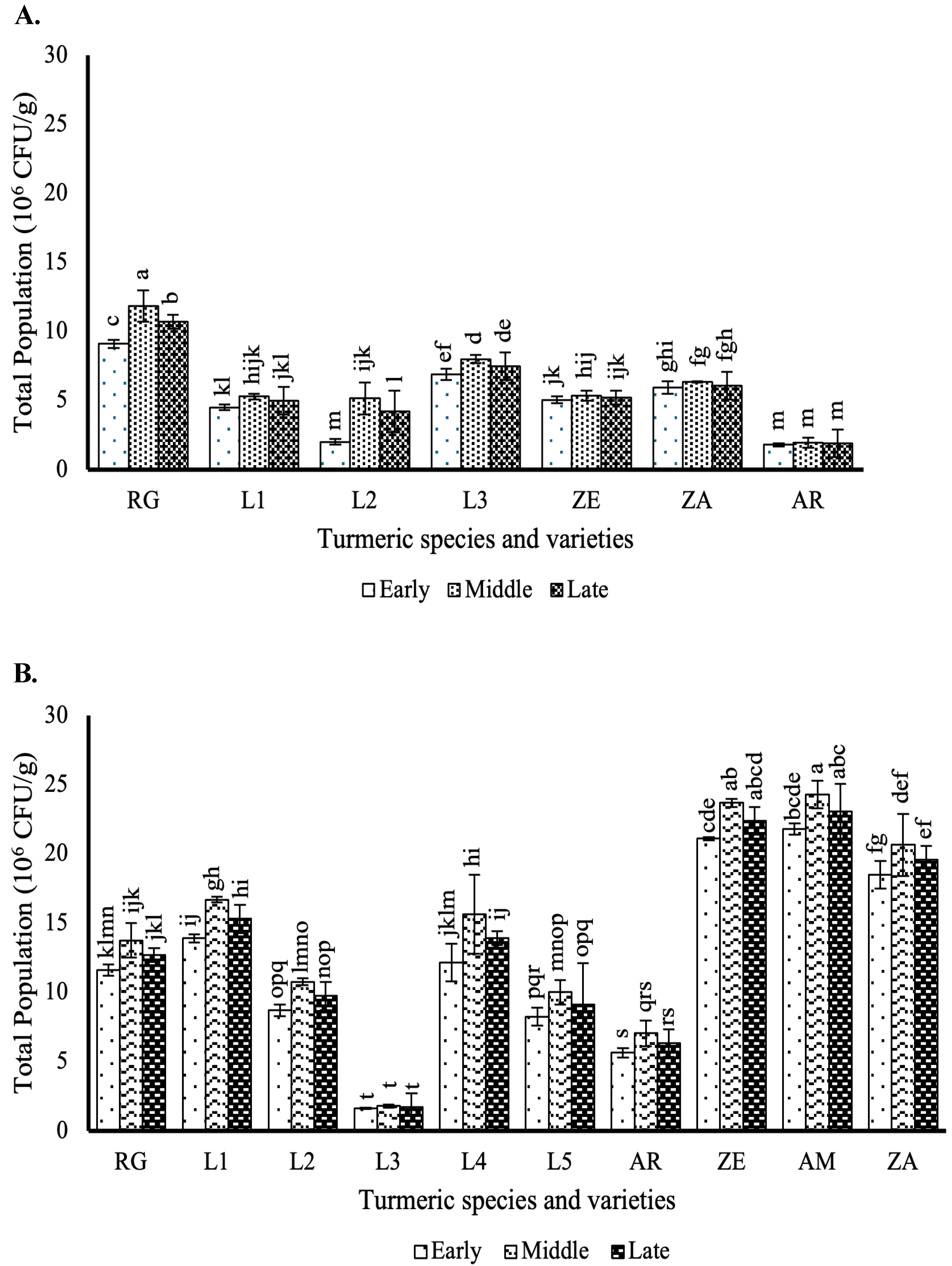
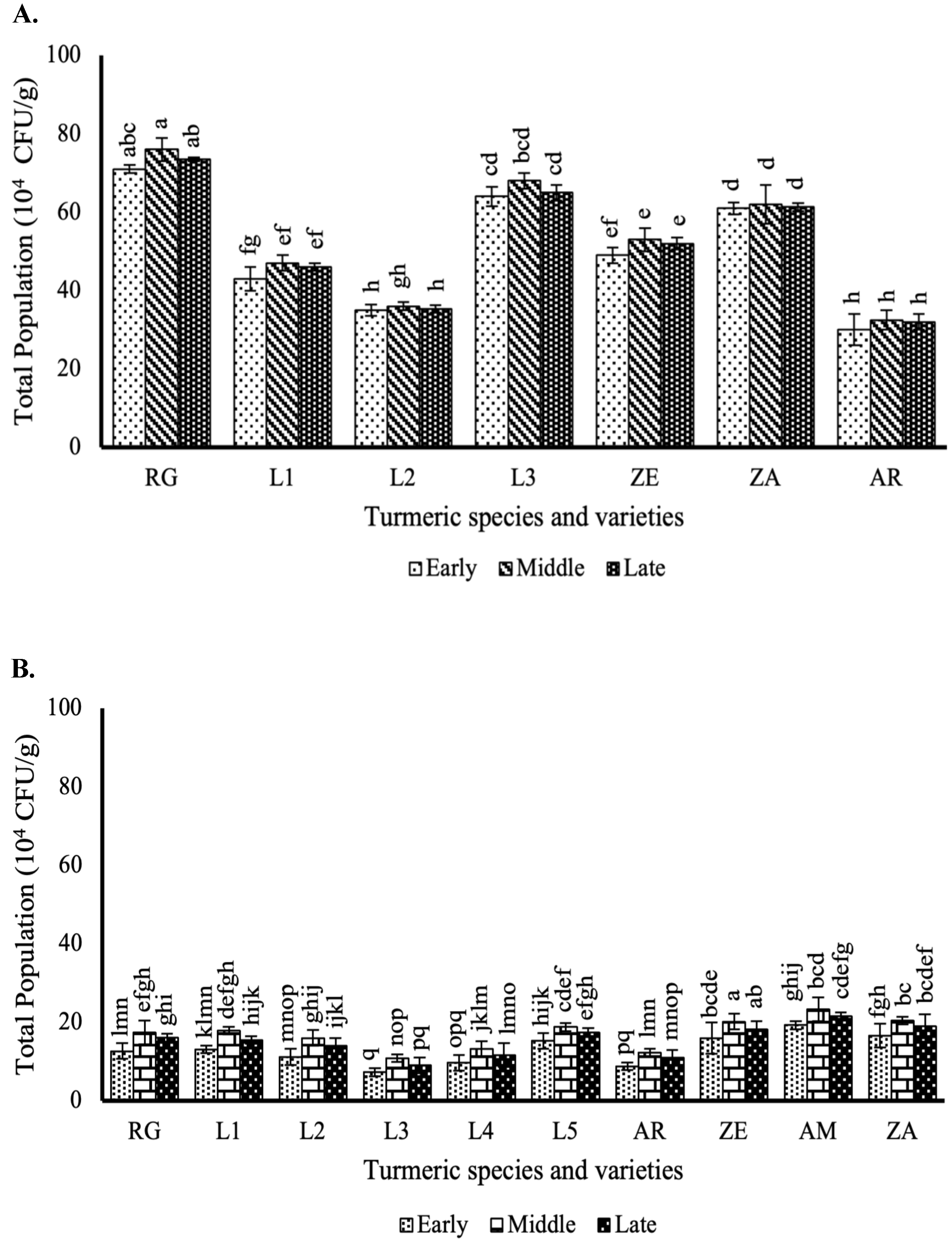
3.1. Effect of Different Curcuma Growth Stages on Rhizosphere Heterotrophic Bacteria Population
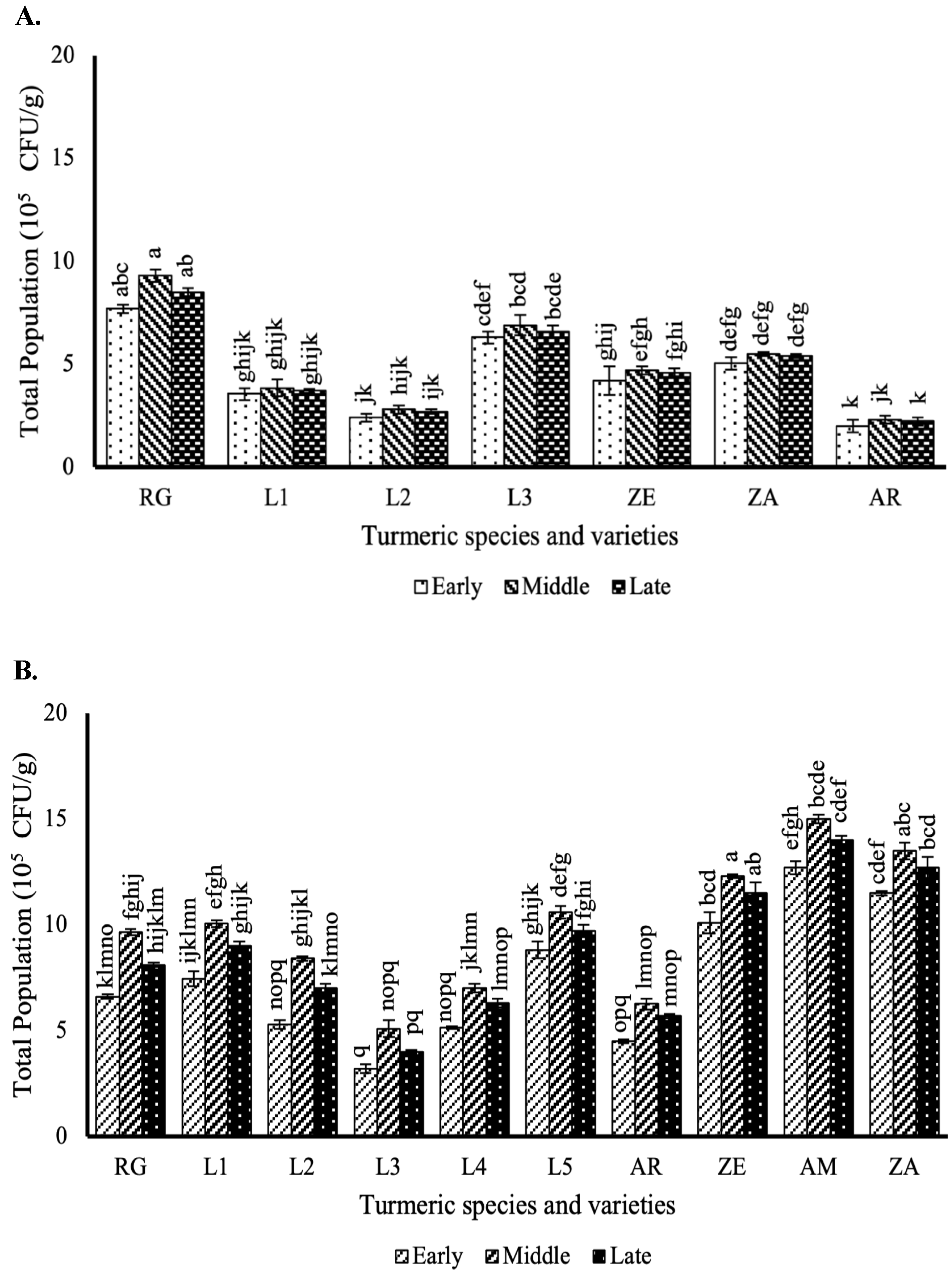
3.2. Effect of the Different Curcuma Growth Stages on Endophytic Bacteria Population in the Stems
3.3. Effect of the Different Curcuma Growth Stages on Endophytic Bacteria Population in the Leaves
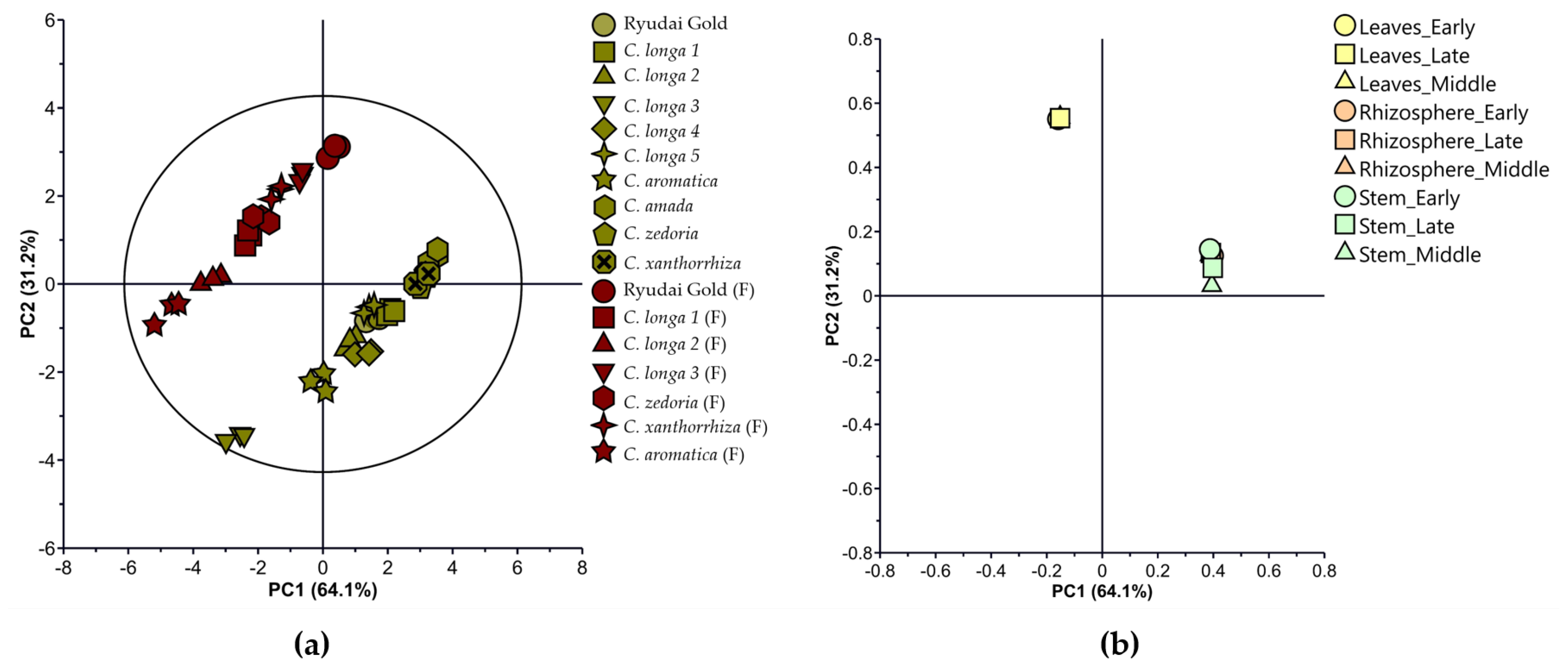
3.4. Multivariate Statistical Plot of the Bacterial Population in Curcuma Species Cultivated in the Field
3.5. Multivariate Statistical Plot of Bacterial Population of Curcuma Species Cultivated in the Plastic House
3.6. Multivariate Statistical Plot of Combined Data on Bacterial Population in Curcuma Species Cultivated in the Field and Plastic House
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaliyadasa, E.; Samarasinghe, B.A. A review on golden species of Zingiberaceae family around the world: Genus Curcuma. Afr. J. Agric. Res. 2019, 14, 519–531. [Google Scholar]
- Jyotirmayee, B.; Mahalik, G. A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.L.; dos Santos, D.C.; Egea, M.B. Bioactive compounds of turmeric (Curcuma longa L.). In Bioactive Compounds of Under-Utilized Vegetables and Legumes; Alexandru, M.G., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 297–318. [Google Scholar]
- Gaiero, J.R.; McCall, C.A.; Thompson, K.A.; Day, N.J.; Best, A.S.; Dunfield, K.E. Inside the root microbiome: Bacterial root endophytes and plant growth promotion. Am. J. Bot. 2013, 100, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; White, J.F. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis 2016, 68, 87–98. [Google Scholar] [CrossRef]
- Khan, S.; Ambika Rani, K.; Sharma, S.; Kumar, A.; Singh, S.; Singh, Y. Rhizobacterial-mediated interactions in Curcuma longa for plant growth and enhanced crop productivity: A systematic review. Front. Plant Sci. 2023, 14, 1231676. [Google Scholar] [CrossRef]
- Schrey, D.; Hartmann, A.; Hampp, R. Rhizosphere interactions. In Ecological Biochemistry: Environmental and Interspecies Interactions, 2nd ed.; Wink, M., Ed.; Wiley-Blackwell: Weinheim, Germany, 2014; pp. 292–311. [Google Scholar]
- Song, Q.; Deng, X.; Song, R.; Song, X. Plant growth-promoting rhizobacteria promote growth of seedlings, regulate soil microbial community, and alleviate damping-off disease caused by Rhizoctonia solani on Pinus sylvestris var. mongolica. Plant Dis. 2022, 106, 2730–2740. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The role of plant growth-promoting rhizobacteria (PGPR) in mitigating plant environmental stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth-promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world—Bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Sontsa-Donhoung, A.M.; Bahdjolbe, M.; Hawaou; Nwaga, D. Selecting endophytes for rhizome production, curcumin content, biocontrol potential, and antioxidant activities of turmeric (Curcuma longa). Biomed. Res. Int. 2022, 2022, 8321734. [Google Scholar] [CrossRef]
- Thapa, S.; Prasanna, R. Prospecting the characteristics and significance of the phyllosphere microbiome. Ann. Microbiol. 2018, 68, 229–245. [Google Scholar] [CrossRef]
- Manikandan, A.; Anandham, R.; Madhan, S.; Raghu, R.; Krishnamoorthy, R.; Senthilkumar, M. Exploring the phyllosphere: Microbial diversity, interactions, and ecological significance in plant health. In Plant–Microbe Interactions in Stress Management; Giri, B., Varma, A., Eds.; Springer: Singapore, 2024; pp. 29–49. [Google Scholar]
- Sumathi, C.S.; Balasubramanian, V.; Ramesh, N.; Kannan, V.R. Influence of biotic and abiotic features on Curcuma longa L. plantation under tropical condition. Middle-East J. Sci. Res. 2008, 3, 171–178. [Google Scholar]
- Harish, B.S.; Umesha, K.; Venugopalan, R.; Prasad, B.M. Photo-selective nets influence physiology, growth, yield and quality of turmeric (Curcuma longa L.). Ind. Crops Prod. 2022, 186, 115202. [Google Scholar] [CrossRef]
- Ishimine, Y.; Hossain, M.; Murayama, S. Optimal planting depth for turmeric (Curcuma longa L.) cultivation in dark red soil in Okinawa Island, southern Japan. Plant Prod. Sci. 2003, 6, 83–89. [Google Scholar] [CrossRef]
- Hossain, M.A. Effect of harvest time on shoot biomass and yield of turmeric (Curcuma longa L.) in Okinawa, Japan. Plant Prod. Sci. 2010, 13, 97–103. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Yadav, A.; Giri, D.D.; Singh, P.K.; Pandey, K.D. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech 2016, 6, 60. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Yao, S.; Yang, X.; Jiang, X. Correlations between soil metabolomics and bacterial community structures in the pepper rhizosphere under plastic greenhouse cultivation. Sci. Total Environ. 2020, 728, 138439. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gomez-Cadenas, A.; Perez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Bünger, W.; Burbano, C.S.; Sabale, M.; Hurek, T. Roots shaping their microbiome: Global hotspots for microbial activity. Annu. Rev. Phytopathol. 2015, 53, 403–424. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Kaskoos, R.A.; Ahamad, J.; Mir, S.R.; Javed, S.A. Antimicrobial properties of curcumin and its potential in the treatment of infections. Pharm. Biol. 2013, 51, 607–611. [Google Scholar]
- Cavaglieri, L.; Orlando, J.; Etcheverry, M. Rhizosphere microbial community structure at different maize plant growth stages and root locations. Microbiol. Res. 2009, 164, 391–399. [Google Scholar] [CrossRef]
- Yuan, Z.S.; Liu, F.; He, S.B.; Zhou, L.I.; Pan, H. Community structure and diversity characteristics of rhizosphere and root endophytic bacterial community in different Acacia species. PLoS ONE 2022, 17, e0262909. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root exudates: Mechanistic insight of plant growth-promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the role of fungal endophytes in plant growth and stress tolerance. Plant Signal. Behav. 2020, 15, 1780043. [Google Scholar]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.C.; Zhou, Y.Q.; Ren, D.; Wang, R.; Wang, C.; Lin, L.G.; Zhang, X.Q.; Ye, W.C.; Zhang, Q.W. Turmeric: A review of its chemical composition, quality control, bioactivity, and pharmaceutical application. In Handbook of Food Bioengineering; Volume 7, Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 299–350. [Google Scholar]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Marag, P.S.; Suman, A. Growth stage and tissue-specific colonization of endophytic bacteria having plant growth-promoting traits in hybrid and composite maize (Zea mays L.). Microbiol. Res. 2018, 214, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Barka, E.A. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef]
- Awin, T.; Mediani, A.; Leong, S.W.; Faudzi, S.M.M.; Shaari, K.; Abas, F. Phytochemical and bioactivity alterations of Curcuma species harvested at different growth stages by NMR-based metabolomics. J. Food Compos. Anal. 2019, 77, 66–76. [Google Scholar] [CrossRef]
- Mengistu, A.A. Endophytes: Colonization, behaviour, and their role in defense mechanisms. Int. J. Microbiol. 2020, 2020, 6927219. [Google Scholar] [CrossRef]
- Graner, G.; Paula, P.; Johan, M.; Sadhna, A. Microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen Verticillium longisporum. FEMS Microbiol. Lett. 2003, 224, 269–276. [Google Scholar] [CrossRef]
- Monika, S.; Prem, P.S.; Arun, K.P.; Singh, P.K.; Pandey, K.D. Enumeration of culturable endophytic bacterial population of different Lycopersicon esculentum L. varieties. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3344–3352. [Google Scholar]
- Wheatley, R.M.; Poole, P.S. Mechanisms of bacterial attachment to roots. FEMS Microbiol. Rev. 2018, 42, 448–461. [Google Scholar] [CrossRef]
- Beattie, G.A.; Lindow, S.E. The secret life of foliar bacterial pathogens on leaves. Annu. Rev. Phytopathol. 1995, 33, 145–172. [Google Scholar] [CrossRef]
- Jin, H.; Yang, X.Y.; Yan, Z.Q.; Liu, Q.; Li, X.Z.; Chen, J.X.; Qin, B. Characterization of rhizosphere and endophytic bacterial communities from leaves, stems, and roots of medicinal Stellera chamaejasme L. Syst. Appl. Microbiol. 2014, 37, 376–385. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.J.; Hand, P.; Pink, D.; Whipps, J.M.; Bending, G.D. Both leaf properties and microbe–microbe interactions influence within-species variation in bacterial population diversity and structure in the lettuce phyllosphere. Appl. Environ. Microbiol. 2010, 76, 8117–8125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, R.; Yadav, A.; Giri, D.D.; Singh, P.K.; Pandey, K.D. Diversity of bacterial endophytes from Curcuma longa L. and their role in plant growth promotion and antifungal activity. J. Appl. Microbiol. 2016, 120, 1653–1665. [Google Scholar]
- Aswathy, R.G.; Joshi, S.R.; Jha, D.K. Paenibacillus sp.: A novel endophyte from Curcuma longa with potential plant growth-promoting properties. Res. J. Microbiol. 2013, 8, 115–123. [Google Scholar]
- Gumiere, T.; Durrer, A.; Bohnen, H.; Vargas, L.K. Plant growth stage drives temporal and spatial dynamics of the bacterial microbiome in the rhizosphere of Vigna subterranea. Front. Microbiol. 2022, 13, 825377. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raharja, N.I.; Hossain, M.A.; Akamine, H. Microbial Population in Curcuma Species at Different Growth Stages. Agriculture 2025, 15, 1092. https://doi.org/10.3390/agriculture15101092
Raharja NI, Hossain MA, Akamine H. Microbial Population in Curcuma Species at Different Growth Stages. Agriculture. 2025; 15(10):1092. https://doi.org/10.3390/agriculture15101092
Chicago/Turabian StyleRaharja, Neptu Islamy, Mohammad Amzad Hossain, and Hikaru Akamine. 2025. "Microbial Population in Curcuma Species at Different Growth Stages" Agriculture 15, no. 10: 1092. https://doi.org/10.3390/agriculture15101092
APA StyleRaharja, N. I., Hossain, M. A., & Akamine, H. (2025). Microbial Population in Curcuma Species at Different Growth Stages. Agriculture, 15(10), 1092. https://doi.org/10.3390/agriculture15101092





