Arbuscular Mycorrhizal Fungi Inoculation and Water Regime Effects on Seedling P Uptake by Rice and Pearl Millet
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.2.1. Three Inoculant Types (Exp. 1)
2.2.2. Indigenous AMF in Two Soil Types (Exp. 2)
2.2.3. Water Regimes (Exp. 3 and Exp. 4)
2.3. Measurements
2.3.1. Shoot Growth
2.3.2. Shoot Phosphorus Concentration and Uptake
2.3.3. Root Growth
2.3.4. Root AMF Infection
2.4. Statistical Analysis
3. Results
3.1. Exp. 1 Inoculation Types
3.2. Exp. 2 Soil Types
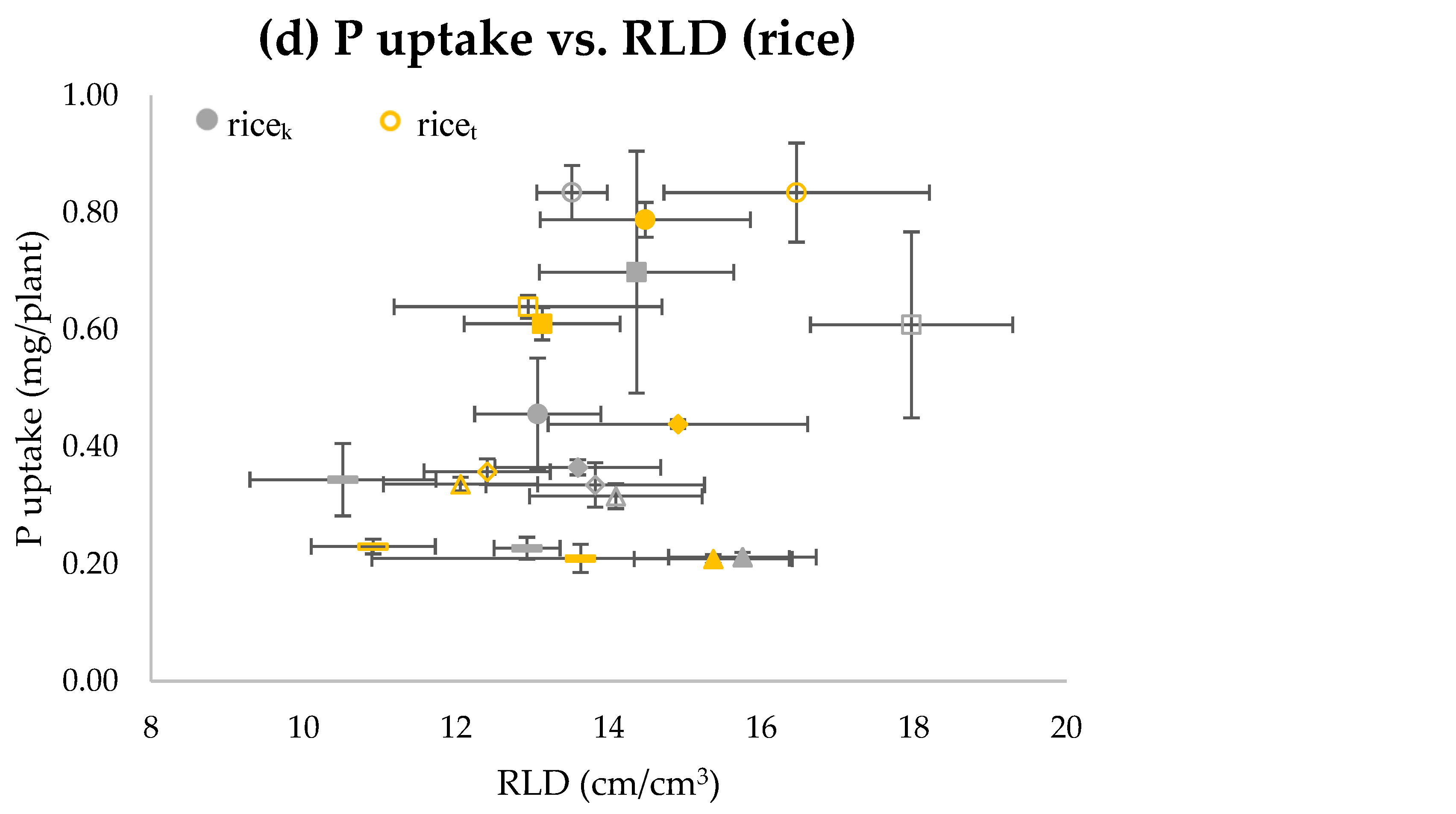
3.3. Exp. 3 and Exp. 4 Water Regimes
4. Discussion
4.1. Inoculation Types and Indigenous AMF
4.2. Inoculation and Water Regime Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mbodj, D.; Effa-Effa, B.; Kane, A.; Manneh, B.; Gantet, P.; Laplaze, L.; Diedhiou, A.G.; Grondin, A. Arbuscular mycorrhizal symbiosis in rice: Establishment, environmental control and impact on plant growth and resistance to abiotic stresses. Rhizosphere 2018, 8, 12–26. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture; ACIAR: Canberra, Australia, 1996; Available online: https://www.researchgate.net/publication/227365112 (accessed on 30 December 2024).
- Maiti, R.K.; Bidinger, F.R. Growth and Development of the Pearl Millet Plant; Research Bulletin; ICRISAT: Hyderabad, India, 1981; Volume 6, p. 14. [Google Scholar]
- Solaiman, M.Z.; Hirata, H. Effect of Arbuscular mycorrhizal fungi inoculation of rice seedlings at the nursery stage upon performance in the paddy field and greenhouse. Plant Soil 1997, 191, 1–12. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-Arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Salomon, M.J.; Demarmels, R.; Watts-Williams, S.J.; McLaughlin, M.J.; Kafle, A.; Ketelsen, C.; Soupir, A.; Bucking, H.; Cavagnaro, T.R.; van der Heijden, M.G.A. Global evaluation of commercial Arbuscular mycorrhizal inoculants under greenhouse and field conditions. Appl. Soil Ecol. 2022, 169, 104225. [Google Scholar] [CrossRef]
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of Arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Smith, F.A.; Smith, S.E.; Jakobsen, I. Functional diversity in Arbuscular mycorrhizas: Exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant Cell Environ. 2005, 28, 642–650. [Google Scholar] [CrossRef]
- Solaiman, Z.; Senoo, K. Interactions between Lotus japonicus genotypes and Arbuscular mycorrhizal fungi. J. Plant Interact. 2005, 1, 179–186. [Google Scholar] [CrossRef]
- Tawaraya, K.; Hirose, R.; Wagatsuma, T. Inoculation of Arbuscular mycorrhizal fungi can substantially reduce phosphate fertilizer application to Allium fistulosum L. and achieve marketable yield under field condition. Biol. Fertil. 2012, 48, 839–843. [Google Scholar] [CrossRef]
- Hayashi, M.; Niwa, R.; Urashima, Y.; Suga, Y.; Sato, S.; Hirakawa, H.; Yoshida, S.; Ezawa, T.; Karasawa, T. Inoculum effect of Arbuscular mycorrhizal fungi on soybeans grown in long-term bare-fallowed field with low phosphate availability. Soil Sci. Plant Nutr. 2018, 64, 306–311. [Google Scholar] [CrossRef]
- Solaiman, M.Z.; Hirata, H. Effects of indigenous Arbuscular mycorrhizal fungi in paddy fields on rice growth and N, P, K nutrition under different water regimes. Soil Sci. Plant Nutr. 1995, 41, 505–514. [Google Scholar] [CrossRef]
- Marro, N.; Grilli, G.; Soteras, F.; Caccia, M.; Longo, S.; Cofré, N.; Borda, V.; Burni, M.; Janoušková, M.; Urcelay, C. The effects of Arbuscular mycorrhizal fungal species and taxonomic groups on stressed and unstressed plants: A global meta-analysis. New Phytol. 2022, 235, 320–332. [Google Scholar] [CrossRef]
- Salomon, M.J.; Watts-Williams, S.J.; McLaughlin, M.J.; Bücking, H.; Singh, B.K.; Hutter, I.; Schneider, C.; Martin, F.M.; Vosatka, M.; Guo, L.; et al. Establishing a quality management framework for commercial inoculants containing Arbuscular mycorrhizal fungi. iScience 2022, 25, 104636. [Google Scholar] [CrossRef] [PubMed]
- Niwa, R.; Koyama, T.; Sato, T.; Adachi, K.; Tawaraya, K.; Sato, S.; Hirakawa, H.; Yoshida, S.; Ezawa, T. Dissection of niche competition between introduced and indigenous Arbuscular mycorrhizal fungi with respect to soybean yield responses. Sci. Rep. 2018, 8, 7419. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Kamoshita, A.; Ramalingam, P. Genetic analysis of root vascular traits in a population from two temperate japonica rice ecotypes. Plant Prod. Sci. 2022, 25, 320–336. [Google Scholar] [CrossRef]
- Phoura, Y.; Kamoshita, A.; Norisada, M.; Deshmukh, V. Eco-physiological evaluation of Stele Transversal Area 1 for rice root anatomy and shoot growth. Plant Prod. Sci. 2020, 23, 202–210. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-Arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-Arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Karasawa, T.; Ariharal, J.; Kasahara, Y. Effects of previous crops on Arbuscular mycorrhizal formation and growth of maize under various soil moisture conditions. Soil Sci. Plant Nutr. 2000, 46, 53–60. [Google Scholar] [CrossRef]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of silica nanoparticles in abiotic and biotic stress tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef]
- Ortas, I.; Akpinar, Ç. Response of maize genotypes to several Mycorrhizal inoculums in terms of plant growth, nutrient uptake and spore production. J. Plant Nutr. 2011, 34, 970–987. [Google Scholar] [CrossRef]
- Phoura, Y. Effects of Water Regime and Inoculation with Arbuscular mycorrhizal Fungi on Growth and Mycorrhizal Communities of Rice and Pearl Millet. Ph.D. Thesis, University of Tokyo, Tokyo, Japan, 2024. [Google Scholar]
- Ohtomo, R.; Kamoshita, A. Effects of water regime and inoculation with Arbuscular mycorrhizal fungi on mycorrhizal communities of roots of rice and pearl millet in upland and lowland fields. Plant Root 2024, 18, 10–21. [Google Scholar] [CrossRef]
- Phoura, Y.; Ohtomo, R.; Nakanishi, H.; Kamoshita, A. Effects of Arbuscular mycorrhizal fungi inoculation on infection and growth of rice and pearl millet in upland fields with three water regimes. Plant Prod. Sci. 2023, 26, 350–363. [Google Scholar] [CrossRef]
- Vallino, M.; Fiorilli, V.; Bonfante, P. Rice flooding negatively impacts root branching and Arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ. 2014, 37, 557–572. [Google Scholar] [CrossRef]
- Corkidi, L.; Allen, E.B.; Merhaut, D.; Allen, M.F.; Downer, J.; Bohn, J.; Evans, M. Assessing the infectivity of commercial mycorrhizal inoculants in plant nursery conditions. J. Environ. Hortic. 2004, 22, 149–154. [Google Scholar] [CrossRef]

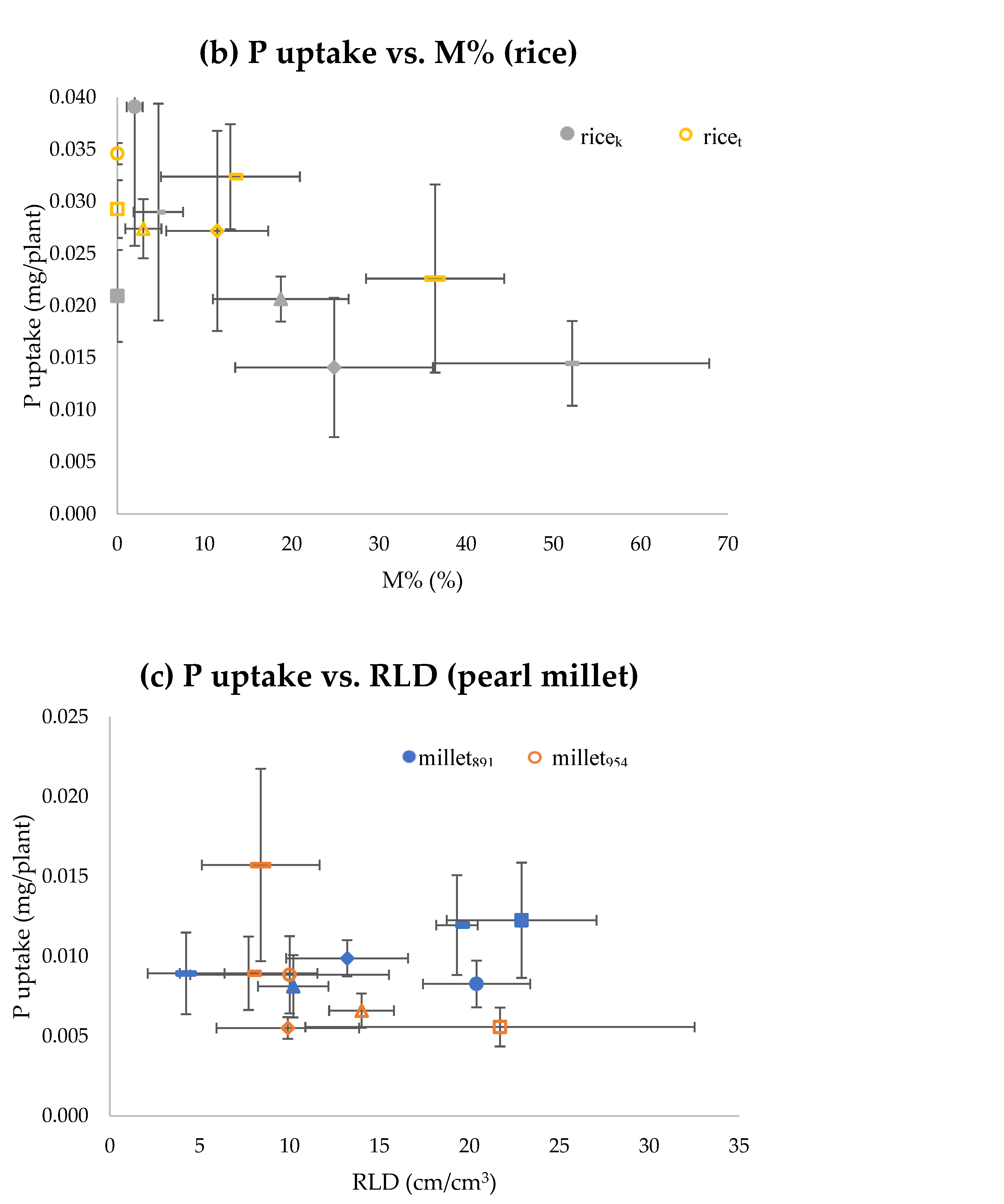
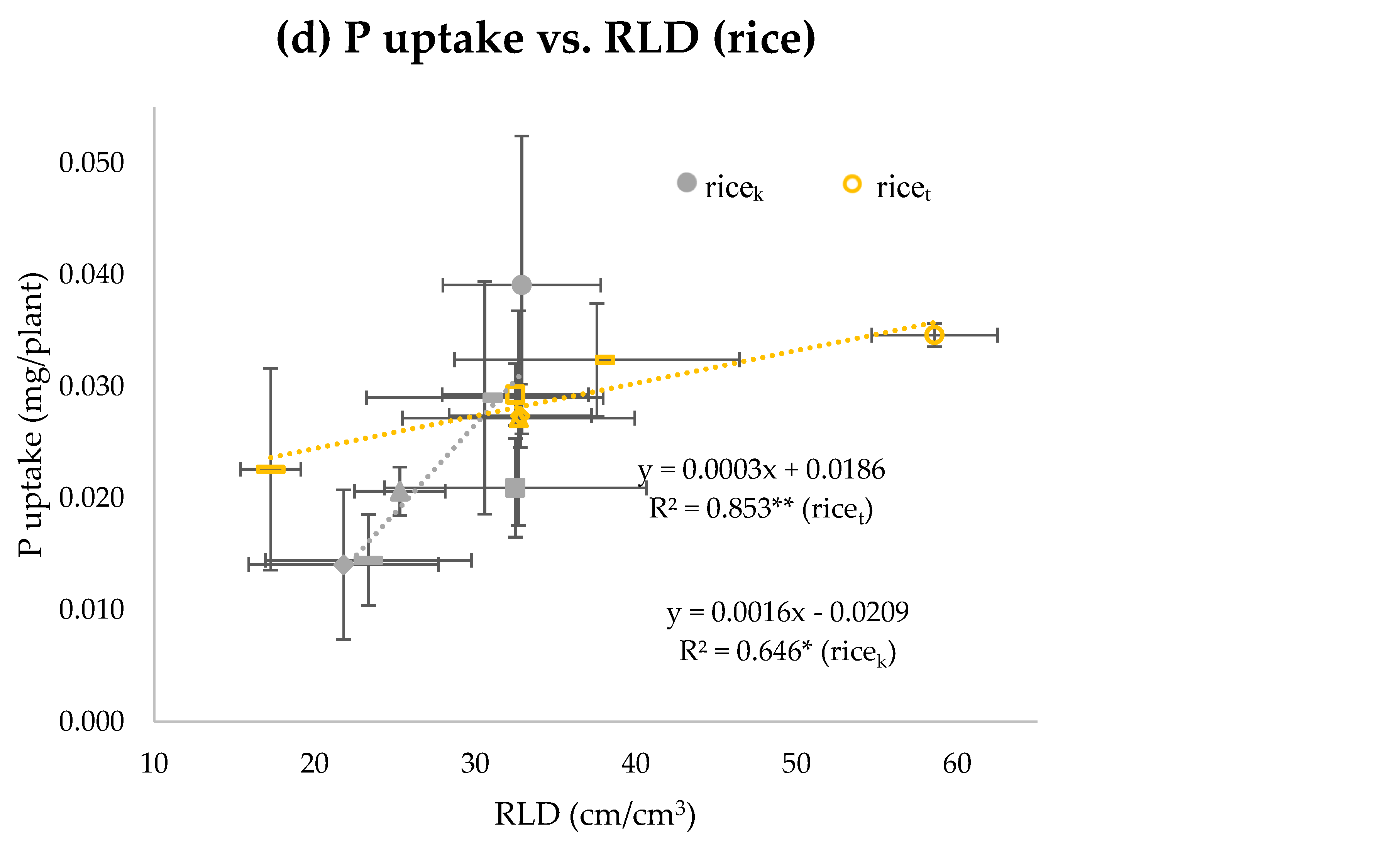
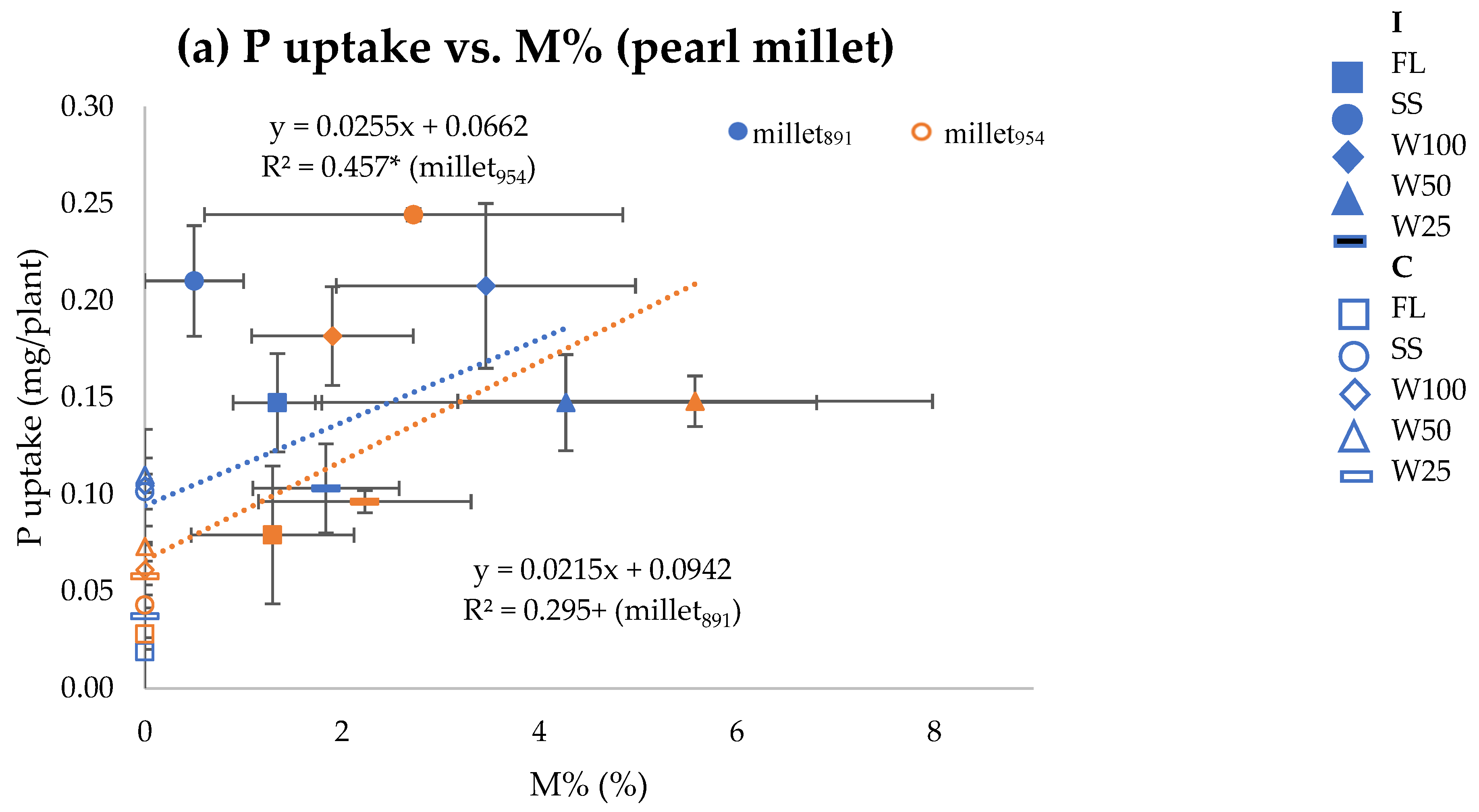
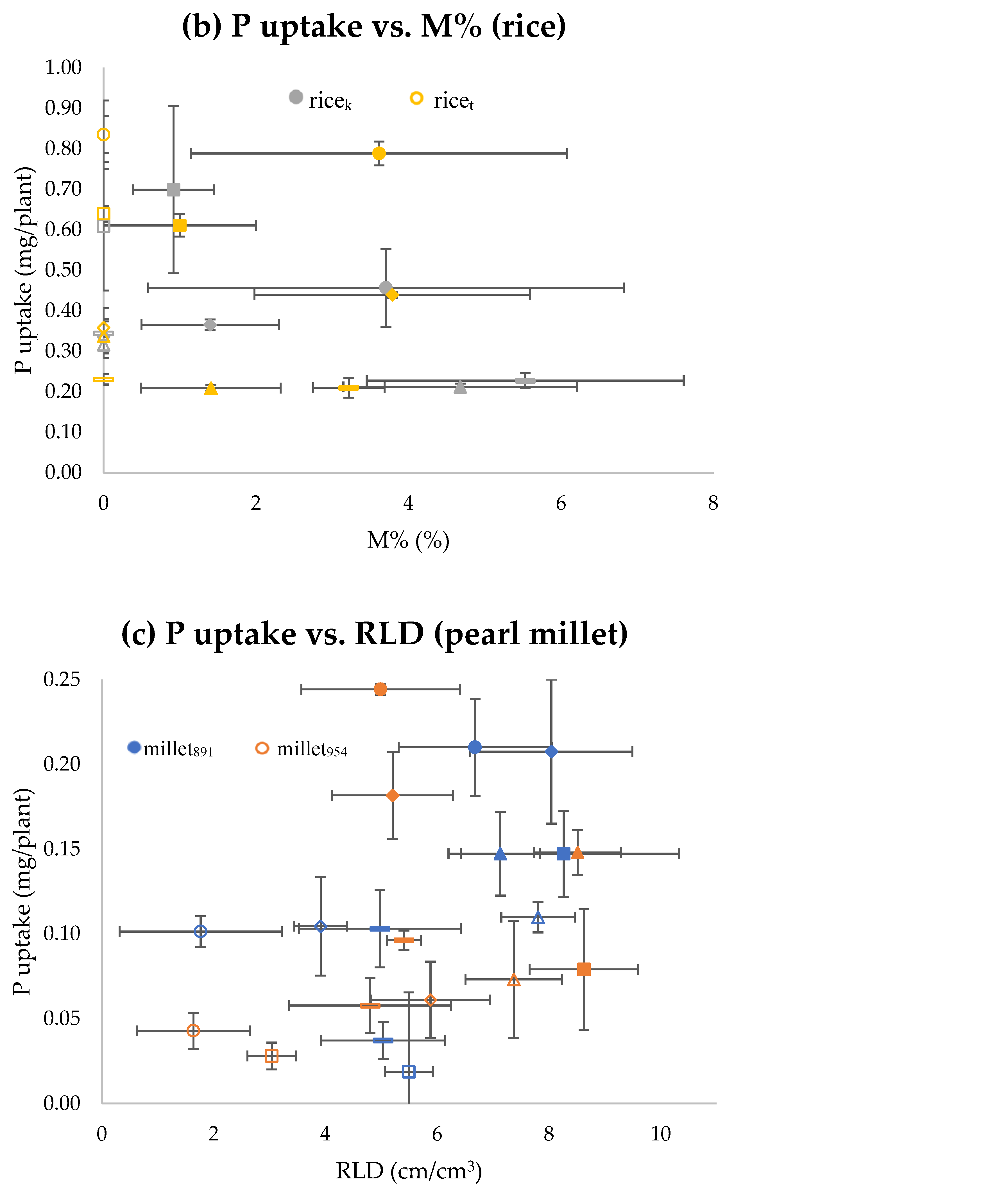
| Parameters | M% | A% | RLD | RTA | P% | P Uptake | SDW | PH | LA |
|---|---|---|---|---|---|---|---|---|---|
| Inoculation (I) | *** | NS | *** | * | NS | NS | *** | NS | *** |
| C | 0.0 ± 0.0 a | 0.0 ± 0.0 | 27 ± 3 ab | 9.6 ± 0.9 ab | 0.146 ± 0.018 | 0.017 ± 0.003 | 0.011 ± 0.000 ab | 11.4 ± 0.2 | 3.8 ± 0.0 b |
| I1 | 2.4 ± 1.1 a | 0.0 ± 0.0 | 31 ± 5 b | 12.5 ± 1.4 b | 0.173 ± 0.027 | 0.023 ± 0.005 | 0.013 ± 0.000 b | 12.9 ± 0.3 | 3.6 ± 0.1 ab |
| I2 | 13.1 ± 3.7 b | 0.0 ± 0.1 | 19 ± 3 a | 10.5 ± 1.3 ab | 0.149 ± 0.009 | 0.014 ± 0.004 | 0.008 ± 0.001 a | 12.2 ± 0.7 | 3.4 ± 0.1 a |
| I3 | 7.5 ± 2.6 ab | 0.1 ± 0.0 | 21 ± 2 a | 8.4 ± 0.8 a | 0.126 ± 0.008 | 0.016 ± 0.003 | 0.012 ± 0.002 b | 12.2 ± 0.8 | 3.5 ± 0.1 a |
| Genotype (G) | * | NS | ** | ** | NS | *** | ** | *** | *** |
| millet891 | 2.2 ± 0.9 a | 0.0 ± 0.0 | 17 ± 2 a | 9.1 ± 1.3 a | 0.135 ± 0.018 | 0.010 ± 0.001 a | 0.006 ± 0.002 a | 6.2 ± 1.3 a | 3.3 ± 0.1 a |
| millet954 | 5.8 ± 1.9 ab | 0.1 ± 0.1 | 14 ± 3 a | 7.9 ± 0.8 a | 0.140 ± 0.012 | 0.007 ± 0.001 a | 0.004 ± 0.002 a | 5.2 ± 1.7 a | 3.3 ± 0.1 a |
| ricek | 11.4 ± 4.1 b | 0.0 ± 0.0 | 28 ± 2 b | 10.5 ± 0.9 ab | 0.173 ± 0.027 | 0.024 ± 0.004 b | 0.013 ± 0.002 b | 17.2 ± 1.6 b | 3.9 ± 0.1 b |
| ricet | 3.6 ± 1.8 a | 0.0 ± 0.0 | 39 ± 4 c | 13.6 ± 1.2 c | 0.147 ± 0.006 | 0.030 ± 0.002 b | 0.016 ± 0.001 c | 17.9 ± 1.5 b | 4.0 ± 0.1 b |
| I×G | NS | NS | * | NS | NS | NS | * | * | NS |
| Parameters | RLD | SDW | ||||||
|---|---|---|---|---|---|---|---|---|
| C | I1 | I2 | I3 | C | I1 | I2 | I3 | |
| millet891 | 23 ± 4 abc | 21 ± 3 abc | 13 ± 3 ab | 10 ± 2 a | 0.007 ± 0.001 a | 0.007 ± 0.001 a | 0.007 ± 0.000 a | 0.007 ± 0.001 a |
| millet954 | 22 ± 11 abc | 10 ± 6 a | 10 ± 4 a | 14 ± 2 ab | 0.004 ± 0.001 a | 0.003 ± 0.001 a | 0.004 ± 0.001 a | 0.006 ± 0.00 a |
| ricek | 33 ± 5 c | 33 ± 5 c | 22 ± 6 bc | 25 ± 3 bc | 0.016 ± 0.003 bcd | 0.016 ± 0.001 bcd | 0.007 ± 0.002 a | 0.014 ± 0.001 b |
| ricet | 33 ± 5 c | 59 ± 4 d | 33 ± 7 c | 33 ± 4 c | 0.018 ± 0.003 bcd | 0.024 ± 0.002 e | 0.015 ± 0.005 bc | 0.020 ± 0.001 cde |
| Parameters | M% | A% | RLD | RTA | P% | P Uptake | SDW | PH | LA |
|---|---|---|---|---|---|---|---|---|---|
| Soil (S) | ** | NS | NS | NS | ** | NS | *** | *** | *** |
| UP | 32.9 ± 4 b | 2.5 ± 0.4 | 13 ± 4 | 6.9 ± 0.7 | 0.417 ± 0.020 b | 0.015 ± 0.004 | 0.004 ± 0.002 a | 7.4 ± 1.5 a | 2.9 ± 0.1 a |
| PD | 10.2 ± 6 a | 0.4 ± 1.1 | 24 ± 2 | 8.4 ± 0.4 | 0.199 ± 0.085 a | 0.021 ± 0.003 | 0.010 ± 0.000 b | 11.5 ± 1.2 b | 3.7 ± 0.1 b |
| Genotype (G) | *** | NS | NS | NS | NS | * | *** | *** | *** |
| millet891 | 4.2 ± 1.8 a | 0.2 ± 0.2 | 12 ± 3 | 7.3 ± 0.9 | 0.249 ± 0.062 | 0.010 ± 0.002 a | 0.005 ± 0.001 a | 4.9 ± 0.8 a | 3.0 ± 0.2 a |
| millet954 | 28.9 ± 8.1 b | 2.6 ± 1.5 | 8 ± 2 | 8.2 ± 0.6 | 0.454 ± 0.136 | 0.012 ± 0.003 a | 0.003 ± 0.000 a | 3.5 ± 0.3 a | 3.0 ± 0.1 a |
| ricek | 28.4 ± 10.9 b | 2.8 ± 1.9 | 27 ± 5 | 5.6 ± 0.6 | 0.22 ± 0.020 | 0.022 ± 0.006 ab | 0.009 ± 0.002 b | 15.1 ± 1.2 b | 3.5 ± 0.2 b |
| ricet | 24.7 ± 6.6 b | 0.2 ± 0.2 | 27 ± 5 | 9.4 ± 0.9 | 0.31 ± 0.117 | 0.028 ± 0.005 b | 0.011 ± 0.003 b | 14.4 ± 1.6 b | 3.7 ± 0.2 b |
| SxG | NS | NS | NS | NS | NS | NS | * | NS | NS |
| Parameters | M% | A% | RLD | RTA | P% | P Uptake | SDW | PH |
|---|---|---|---|---|---|---|---|---|
| Water (W) | NS | NS | *** | *** | NS | ** | *** | *** |
| FL | 1.6 ± 0.9 | 0.0 ± 0.0 | 27 ± 3 b | 12.5 ± 1.2 b | 0.153 ± 0.008 | 0.023 ± 0.004 b | 0.015 ± 0.002 c | 13.9 ± 1.8 b |
| W100 | 2.4 ± 1.1 | 0.0 ± 0.0 | 30 ± 5 b | 12.5 ± 1.4 b | 0.173 ± 0.027 | 0.023 ± 0.005 b | 0.013 ± 0.002 bc | 12.9 ± 1.7 ab |
| W50 | 0.5 ± 1.0 | 0.0 ± 0.6 | 30 ± 2 b | 10.5 ± 0.6 b | 0.166 ± 0.023 | 0.022 ± 0.003 b | 0.011 ± 0.001 ab | 12.6 ± 1.2 ab |
| W25 | 2.5 ± 0.4 | 0.5 ± 0.0 | 19 ± 3 a | 6.2 ± 1.0 a | 0.166 ± 0.011 | 0.011 ± 0.004 a | 0.006 ± 0.001 a | 10.7 ± 1.5 a |
| Genotype (G) | NS | NS | *** | NS | NS | *** | *** | *** |
| millet891 | 0.8 ± 0.5 | 0.0 ± 0.0 | 15 ± 2 a | 10.1 ± 1.5 | 0.128 ± 0.016 | 0.008 ± 0.001 a | 0.006 ± 0.001 a | 6.4 ± 0.2 a |
| millet954 | 1.7 ± 1.2 | 0.0 ± 0.0 | 17 ± 2 a | 9.1 ± 1.2 | 0.168 ± 0.015 | 0.009 ± 0.001 a | 0.005 ± 0.000 a | 5.7 ± 0.3 a |
| ricek | 1.9 ± 0.8 | 0.5 ± 0.6 | 35 ± 2 b | 10.3 ± 1.0 | 0.184 ± 0.025 | 0.031 ± 0.004 b | 0.016 ± 0.001 b | 18.3 ± 0.7 b |
| ricet | 2.6 ± 1.0 | 0.0 ± 0.0 | 40 ± 3 c | 12.2 ± 1.2 | 0.180 ± 0.013 | 0.032 ± 0.002 b | 0.018 ± 0.001 b | 19.7 ± 0.6 c |
| WxG | * | NS | *** | NS | NS | NS | *** | *** |
| Parameters | M% | A% | V% | RLD | RTA | P% | P Uptake | SDW | PH | TN |
|---|---|---|---|---|---|---|---|---|---|---|
| Water (W) | NS | NS | NS | *** | *** | ** | *** | *** | *** | *** |
| FL | 0.6 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 9 ± 1 a | 87.4 ± 6.4 d | 0.127 ± 0.007 ab | 0.354 ± 0.062 c | 0.252 ± 0.037 d | 30.5 ± 2.8 d | 2.3 ± 0.2 ab |
| SS | 1.3 ± 0.6 | 0.1 ± 0.1 | 0.1 ± 0.1 | 10 ± 1 ab | 101.8 ± 5.7 e | 0.118 ± 0.008 ab | 0.439 ± 0.072 d | 0.336 ± 0.043 e | 30.8 ± 2.7 d | 2.5 ± 0.3 b |
| W100 | 1.3 ± 0.4 | 0.1 ± 0.1 | 0.0 ± 0.0 | 10 ± 1 ab | 77.0 ± 3.3 c | 0.136 ± 0.01 ab | 0.257 ± 0.028 b | 0.206 ± 0.021 c | 27.5 ± 1.9 c | 2.0 ± 0.2 ab |
| W50 | 2.0 ± 0.4 | 0.1 ± 0.0 | 0.1 ± 0.1 | 11 ± 1 b | 55.8 ± 2.7 b | 0.112 ± 0.01 a | 0.194 ± 0.022 ab | 0.163 ± 0.010 b | 24.8 ± 1.4 b | 1.7 ± 0.1 ab |
| W25 | 1.6 ± 0.6 | 0.0 ± 0.1 | 0.1 ± 0.1 | 9 ± 1 a | 40.5 ± 3.1 a | 0.142 ± 0.005 b | 0.163 ± 0.019 a | 0.108 ± 0.012 a | 19.1 ± 1.4 a | 1.4 ± 0.1 a |
| Inoculation (I) | *** | * | NS | *** | *** | NS | NS | *** | NS | *** |
| C | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 | 9 ± 1 a | 67.4 ± 3.8 a | 0.124 ± 0.005 | 0.274 ± 0.035 | 0.198 ± 0.020 a | 26.5 ± 1.4 | 1.9 ± 0.1 a |
| I | 2.7 ± 0.4 b | 0.1 ± 0.0 b | 0.1 ± 0.0 | 10 ± 1 b | 77.6 ± 3.6 b | 0.130 ± 0.005 | 0.289 ± 0.028 | 0.228 ± 0.019 b | 26.6 ± 1.4 | 2.0 ± 0.1 b |
| Genotype (G) | NS | NS | NS | *** | *** | NS | *** | *** | *** | *** |
| millet891 | 1.1 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 6 ± 0 a | 59.9 ± 3.9 a | 0.122 ± 0.009 | 0.119 ± 0.013 b | 0.096 ± 0.009 a | 15.4 ± 0.5 a | 2.0 ± 0.0 a |
| millet954 | 1.4 ± 0.4 | 0.1 ± 0.1 | 0.0 ± 0.0 | 6 ± 0 a | 76.5 ± 5.4 b | 0.120 ± 0.007 | 0.101 ± 0.013 a | 0.079 ± 0.008 a | 15.6 ± 0.5 a | 2.0 ± 0.0 a |
| ricek | 1.6 ± 0.5 | 0.1 ± 0.0 | 0.1 ± 0.0 | 14 ± 0 b | 62.5 ± 3.3 a | 0.133 ± 0.009 | 0.440 ± 0.044 c | 0.339 ± 0.025 b | 38.1 ± 1.2 b | 3.0 ± 0.1 b |
| ricet | 1.3 ± 0.4 | 0.0 ± 0.0 | 0.1 ± 0.0 | 14 ± 0 b | 91.2 ± 6.5 c | 0.134 ± 0.005 | 0.465 ± 0.043 d | 0.339 ± 0.026 b | 37.2 ± 1.2 b | 3.0 ± 0.1 b |
| WxI | NS | NS | NS | NS | NS | *** | NS | NS | *** | NS |
| WxG | NS | NS | NS | * | *** | ** | *** | *** | *** | *** |
| IxG | NS | NS | NS | NS | NS | *** | *** | * | NS | * |
| WxIxG | NS | NS | NS | *** | NS | NS | * | NS | *** | NS |
| Parameters | P% | P uptake | SDW | |||
|---|---|---|---|---|---|---|
| C | I | C | I | C | I | |
| millet891 | 0.098 ± 0.008 a | 0.145 ± 0.014 c | 0.074 ± 0.014 a | 0.163 ± 0.016 b | 0.071 ± 0.012 a | 0.120 ± 0.011 b |
| millet954 | 0.100 ± 0.006 a | 0.140 ± 0.010 b | 0.053 ± 0.009 a | 0.150 ± 0.018 b | 0.047 ± 0007 a | 0.111 ± 0.009 b |
| ricek | 0.150 ± 0.013 c | 0.117 ± 0.009 ab | 0.488 ± 0.063 d | 0.392 ± 0.061 c | 0.339 ± 0.034 c | 0.339 ± 0.038 c |
| ricet | 0.145 ± 0.006 c | 0.120 ± 0.005 ab | 0.480 ± 0.062 d | 0.451 ± 0.061 cd | 0.334 ± 0.036 c | 0.343 ± 0.037 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Y, P.; Kamoshita, A. Arbuscular Mycorrhizal Fungi Inoculation and Water Regime Effects on Seedling P Uptake by Rice and Pearl Millet. Agriculture 2025, 15, 753. https://doi.org/10.3390/agriculture15070753
Y P, Kamoshita A. Arbuscular Mycorrhizal Fungi Inoculation and Water Regime Effects on Seedling P Uptake by Rice and Pearl Millet. Agriculture. 2025; 15(7):753. https://doi.org/10.3390/agriculture15070753
Chicago/Turabian StyleY, Phoura, and Akihiko Kamoshita. 2025. "Arbuscular Mycorrhizal Fungi Inoculation and Water Regime Effects on Seedling P Uptake by Rice and Pearl Millet" Agriculture 15, no. 7: 753. https://doi.org/10.3390/agriculture15070753
APA StyleY, P., & Kamoshita, A. (2025). Arbuscular Mycorrhizal Fungi Inoculation and Water Regime Effects on Seedling P Uptake by Rice and Pearl Millet. Agriculture, 15(7), 753. https://doi.org/10.3390/agriculture15070753







