1. Introduction
Theobroma cocoa is a plant native to the tropical regions of Central and South America. It thrives in equatorial climates and is cultivated in tropical regions across West and Central Africa, Latin America, and Southeast Asia [
1].
Theobroma cocoa produces cocoa pods which have an oval-shaped fruit of which the bark is about 80% and the rest, 20%, is made up of seeds known as cocoa beans and white pulp known as mucilage [
2]. Cocoa beans are processed into chocolate, cocoa powder, cocoa drinks, and other related products in the food industry, as well as products for the pharmaceutical and cosmetic industries [
3].
The African region is the world’s largest producer of cocoa, accounting for approximately 68% of production, followed by Asia/Oceania and Latin America with 14% and 18% [
4], respectively; also, approximately 80% of the world’s cocoa comes from smallholder farmers [
4,
5]. In Africa, Ivory Coast is the world’s leading producer of cocoa beans, followed by Ghana [
4]. In the Asia/Oceania region, Indonesia is the third largest producer of cocoa beans in the world [
4]. On the other hand, in Latin America, Ecuador is the sixth country in the world ranking, ahead of Brazil in 5th place, Peru in 8th place, and Colombia in 9th place [
4].
The production of cocoa and its products have a great demand in the food market, but the quality of cocoa cultivation depends on favorable climatic conditions and low incidence of pests and diseases [
6,
7]. Climate variations, such as changes in temperature or precipitation, can influence the cocoa pathogens, increasing the incidence of diseases that threaten their production [
7]. However, the environment in which cocoa plants grow is prone to various diseases that can cause damage to the fruit, resulting in losses in cocoa crop production [
8]. Witches’ broom caused by
Moniliophthora perniciosa, frosty pod caused by
Moniliphthora roreri, and black pod caused by
Phytophtora are the most common diseases in cocoa crops [
8,
9]. Frosty pod directly attacks the cocoa fruit and the losses can exceed 60% of its production; on the other hand, the losses due to black pod can exceed 40% [
10,
11].
Visual inspection, a task carried out by farmers, is the most common practice to control diseases in cocoa crops. Although the farmers have experience in this kind of task, visual inspection does not provide standardized results when diagnosing the health condition of cocoa pod [
12]. Other methods such as fungicides, pesticides, and other biological methods to control diseases in cocoa crops are costly and complex to apply [
13]. However, in the agricultural field, computer vision (CV) has surged to improve, facilitate, and reduce the complex tasks related to the evaluation of crops. Based in Machine Learning algorithms (MLA) and Deep Learning algorithms (DLA), CV has become a tool that allows farmers to make better decisions and is also used to identify and classify plant diseases automatically [
12,
13]. Thus, CV application is a technology that offers in a quick and accurate way the identification of plant diseases in agriculture.
To perform MLA, a large amount of image data are required to be trained and learn the patterns of data to perform a particular function. MLA are classified into supervised and unsupervised learning. In supervised learning, the training data include the output desire or the target, called labels. For example, in [
14], an open-source Kaggle dataset was used to identify healthy and damaged cocoa fruits affected by Moniliophthora and Phytophthora diseases. Thus, MLA was developed to detect healthy and unhealthy stages of cocoa fruits. Classification is a common task in supervised learning algorithms. On the other hand, in unsupervised learning, the data are not labeled and the system tries to learn to acquire patterns without prior knowledge [
15]. However, DLA uses an Artificial Neural Network (ANN) composed of multiples layers of neurons and weights connected between them trying to mimic how the human brain works [
16]. For example, for the DLA in [
17], an autoencoder network was implemented for unsupervised disease detection in cucumber leaf by means of clustering of features and anomaly detection.
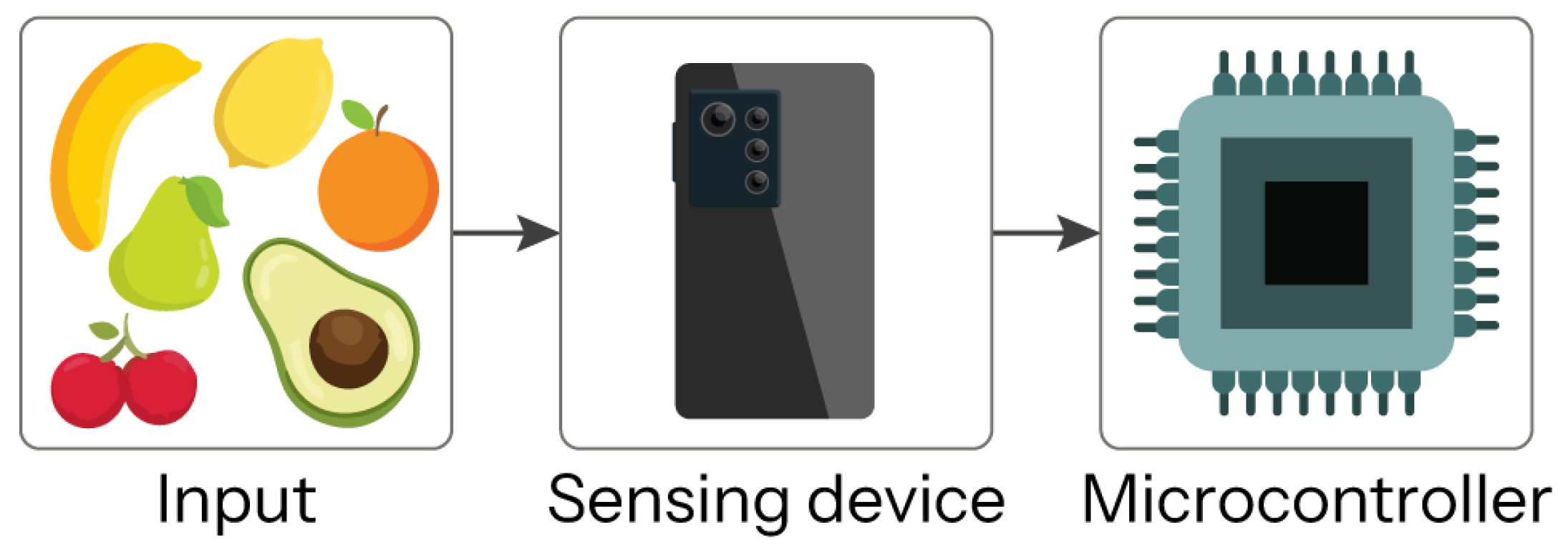
Computer vision systems (CVS) is the integration between software and hardware, which is made up of three elements, the input (captured image), the camera or sensor device that captures the image, and the microcontroller with its embedded algorithm to process the input data; then, the system provides an output where an object is classified or identified [
18].
Figure 1 shows the above-mentioned CVS process.
The incorporation of CV technologies, in farm applications, enables farmers to have a tool that allows them to make diagnoses that cannot be perceived with the naked eye; thus, the level of precision and accuracy provided by these applications helps to prevent production losses caused by unhealthy crops. Therefore, given the growth of Machine Learning (ML) in the farm field based on the detection and classification of plant diseases, the main objective of this document is to carry out a Systematic Literature Review (SLR) to explore the state of the art based on ML application to CV, mainly in cocoa crops. This study also covers the exploration of CV applications applied to the early detection of diseases in agriculture especially in Theobroma cacao (TC). Moreover, this paper includes different methods and algorithms used to detect and identify diseases in the agricultural field. Therefore, the present SLR has considered the following questions:
Q1 What are the main diseases affecting cocoa crop production?
Q2 What are the main Machine Learning algorithms and techniques used to detect and classify diseases in cocoa?
Q3 What are the types of imaging technologies (e.g., RGB, hyperspectral, or multispectral cameras) commonly used in these applications?
Q4 What are the main Machine Learning algorithms used in mobile applications and other platforms for cocoa disease detection?
The present paper is organized as follows:
Section 2 explains the methodology used for this SLR.
Section 3 presents the related works based on CV techniques implemented to detect disease in crops other than cocoa.
Section 4 presents the answer to the research questions.
Section 5 discusses the limitations in this SLR, and finally,
Section 6 presents the conclusions and future work.
2. Methodology
In order to ensure the quality of the papers in the SLR, the articles that were selected to be part of the review process must meet the following criteria:
Publication type: Studies published in peer-reviewed journals.
Language: Studies written and published in English.
Research focus: Studies that directly address the research questions related to cocoa disease detection and plant disease detection based on images, as well as the early detection of cocoa diseases using computer-vision techniques.
Publication date: Studies published between 2019 and 2024 were considered.
To address the research questions, studies were considered according to their titles and keywords. The first selection included papers that aimed to address the problem of cocoa disease detection based on images and computer-vision techniques for early disease detection in cocoa. Finally, studies that did not include methods based on images and computer-vision techniques for cocoa and plant disease detection were excluded from this SLR from further analysis.
2.1. Information Sources
An initial search was conducted in the most recognized scientific databases to identify those that recovered the largest amount of articles with the most relevance for this study. After this approach, the digital databases selected for this SLR are listed in
Table 1.
2.2. Search Strategy
To ensure a structured search strategy, the search terms were selected and categorized into three specialized domains. The first domain refers to cocoa diseases, crop diseases, and plant disease detection. The second refers to CV and ML in the field of agriculture. Finally, the last domain refers to technologies used to detect diseases in the agriculture field. The keywords used for the search query are detailed in
Table 2.
The search query was performed by combining keywords from the groups mentioned in the
Table 2. Then, Boolean operators, such as
AND,
OR, and
NOT were implemented to maximize the retrieval of relevant articles. Lastly, the search query structure, shown in
Table 3, was applied to the information source.
In addition, after applying the search query to each scientific database, the search results were delimited by year (2019–2024), review articles, research articles, and conference papers. Also, the selection of some subject areas, such as Engineering, Computer Science, Agricultural and Biological Science, Artificial Intelligence in Agriculture, Sensors, Smart Agriculture Technology, and Computers and Electronics in Agriculture, helps to delimit and improve the search. Finally, to cover the search, Artificial and Computer Intelligence were subdisciplines included in Group 2 to focus on the specific topics within a larger field.
2.3. Selection and Collection Process
The selection process was implemented to ensure the inclusion of relevant studies. The search was performed using a combination of keywords from the thematic groups. The initial search was conducted by one researcher using digital databases and the retrieved articles were reviewed by two researchers. In this first phase, the titles, abstracts, and keywords were analyzed to figure out the relevance to the research objectives.
To fit the selection, researchers assessed whether the studies are related to the field of agricultural precision, specifically in the use of computer-vision techniques to detect disease in cocoa using MLA. Also, to extend this SLR, the studies based on plant disease detection (PDD) and plant disease classification (PDC) were included in this paper, and the use of MLA to detect disease through mobile applications.
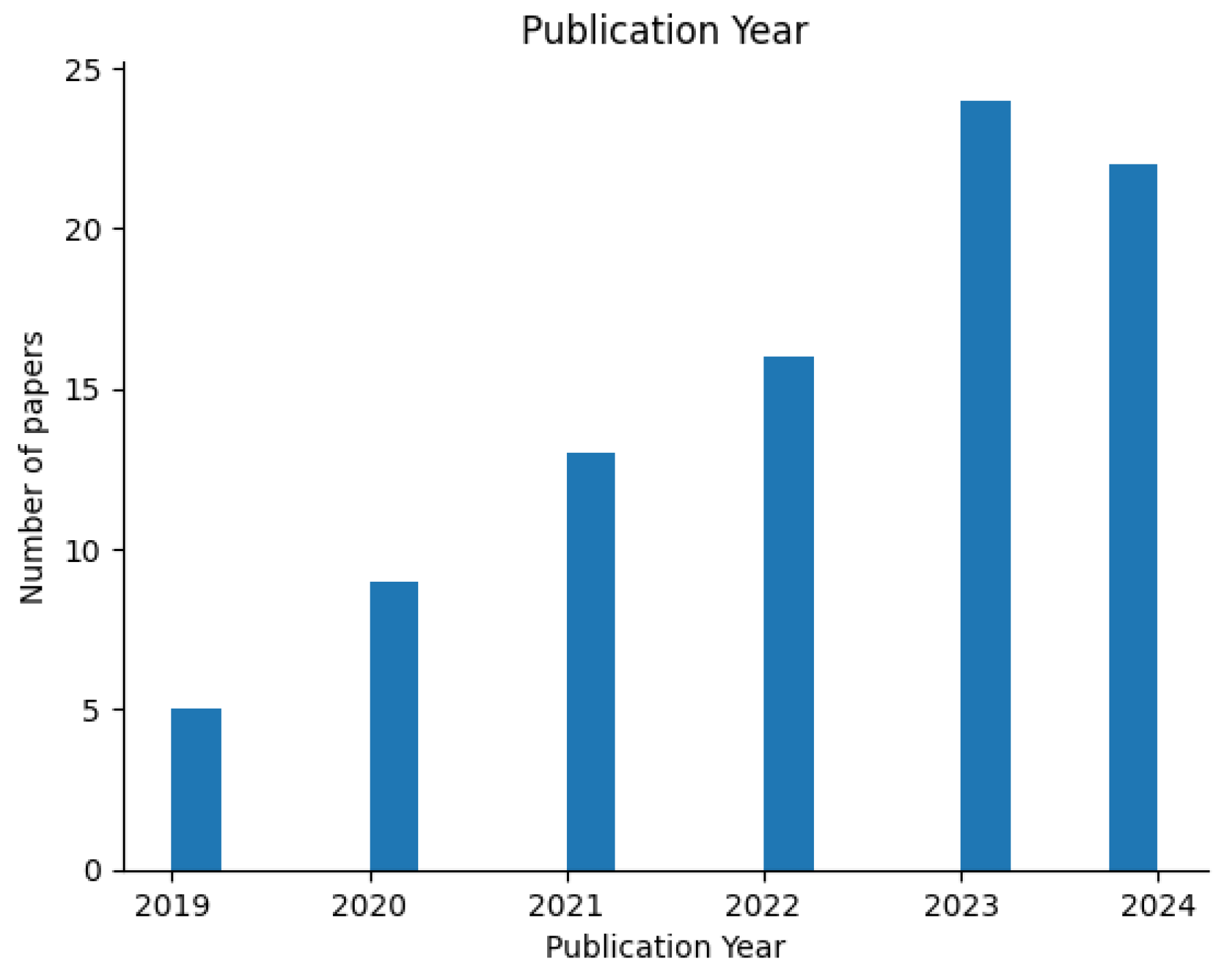
The PRISMA 2020 protocol [
19] was carried out to ensure traceability, allowing for the selection of the papers in a methodological way. Therefore, the final selection resulted in 88 studies, which were subsequently analyzed to identify research trends depicted in
Figure 2.
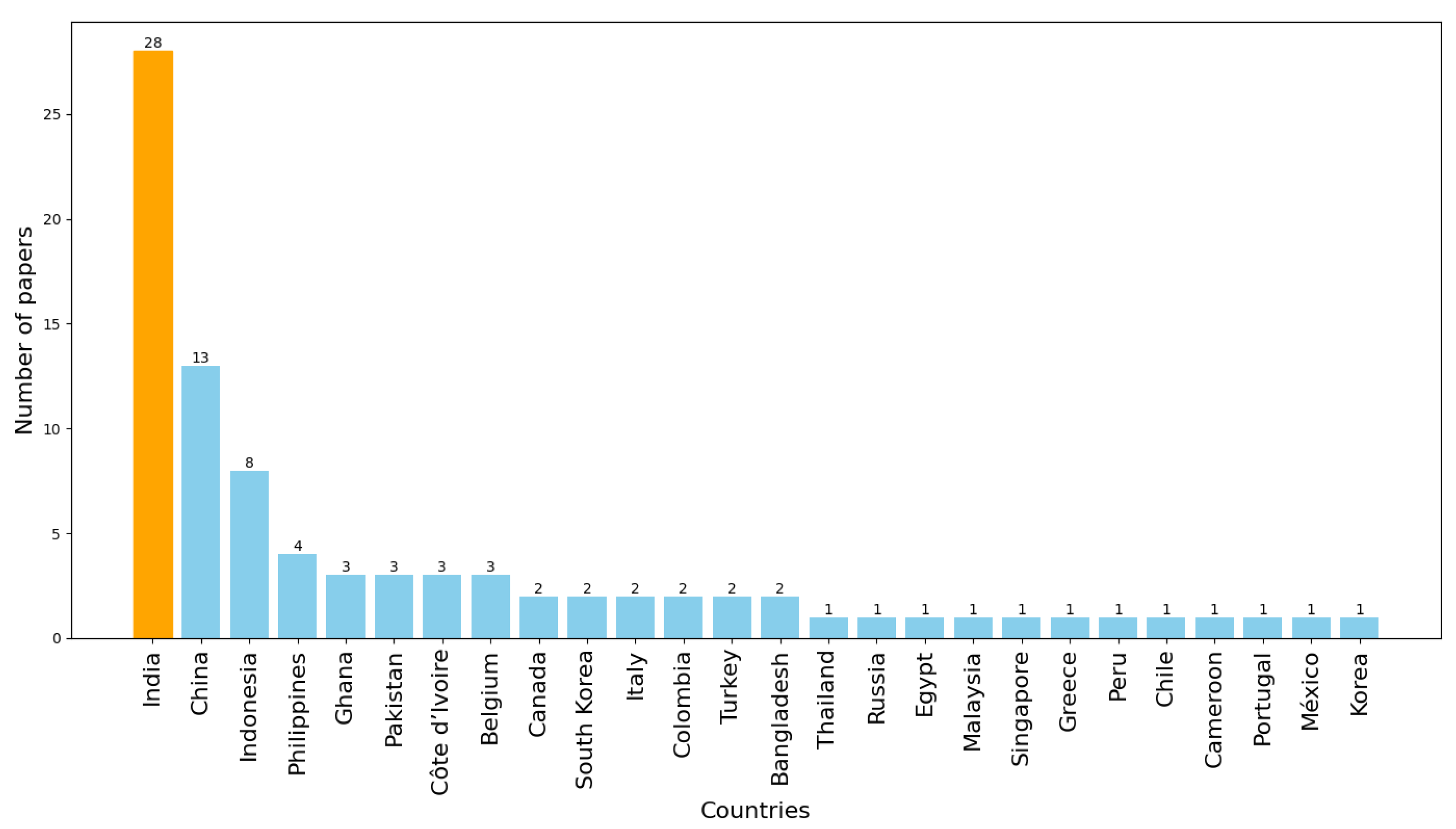
According to the PRISMA 2020 protocol [
19],
Figure 3 shows that most articles selected were developed in India, with a significant difference compared to other countries. This difference may be attributed to the fact that India, being one of the most populated countries in the world, has shown interest in applied research in agriculture. Additionally, India is among the countries with extensive agricultural activity [
4], and the corresponding demand for technological solutions to enhance crop health and productivity likely contributes to this trend [
20].
Finally, the titles and abstracts of the papers were identified and categorized to identify the approach in CV applications in the agriculture. The categories, depicted in
Figure 4a, were separated by crops, the use of hyperspectral images in agriculture, PDC, PDD and plant-leaf disease detection (PLDD) applications. On the other hand,
Figure 4b provides a detailed breakdown of papers focusing on individual crops.
As shown in
Figure 4b, most of the retrieved studies categorized by crop was the cocoa. However, it is important to clarify that due to the limited number of studies published in peer-reviewed journals, the majority of studies related to CV techniques to detect disease on cocoa crops were retrieved from conference proceedings. On the other hand,
Figure 4b indicates in this SLR greater interest in apple, tomato, rice, and strawberry crops compared to plantain, mango, and corn crops. This approach to the selection process ensures papers with the most relevant studies associated in plant disease detection, with a special emphasis on research addressing cocoa disease through MLA.
4. Results
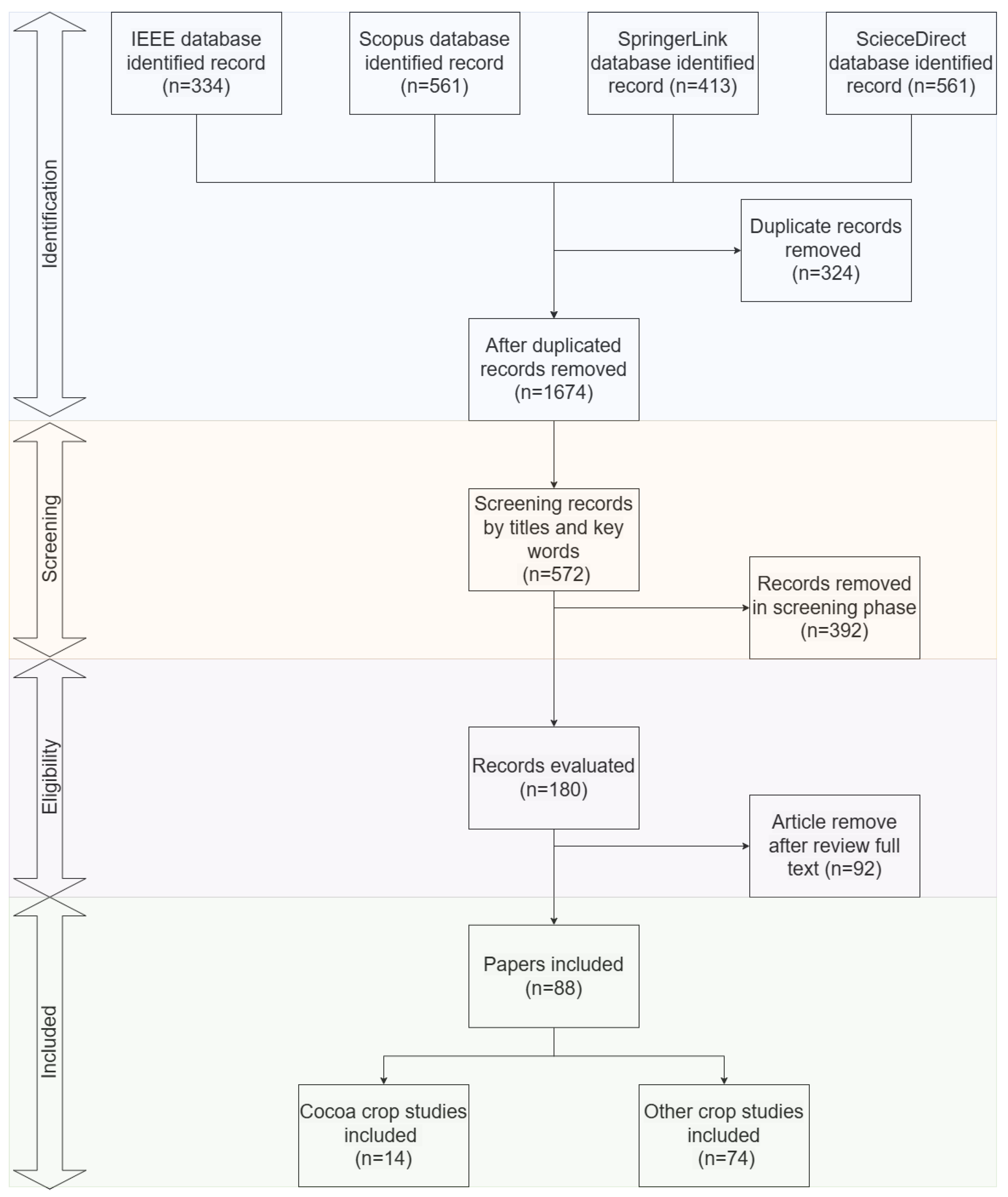
This phase presents the results of the SLR to address the research questions based on the information extracted from the selected primary studies. Initially, 1869 records were identified from the selected electronic databases, distributed as follows: 334 records from IEEE Explore, 561 from Scopus, 413 from SpringerLink, and 561 from ScienceDirect. In the first place, duplicate records were excluded, reducing the dataset to 1674 unique records. Subsequently, a screening was performed based on keywords and titles such as disease and detection, PDD, PDC, image processing, detection, and classifications were then performed, and 572 studies were considered relevant at this stage. A second screening, conducted on 180 studies, was carried out based on their applications looking at the title and the abstract tools and computer vision algorithms (CVA) used to detect or diagnose disease crops. In this phase, diseases in cocoa were explored, as well as MLA and DLA methods to detect and classify diseases in cocoa. After fully screening 180 studies, 92 articles were excluded, resulting in 88 articles that were given a full reading for this SLR. See
Figure 5.
According to
Figure 5, out of the 88 studies retrieved in this SLR, a total of 14 studies are focused on the applications of computer vision (CV) techniques in cocoa. The remaining documents are retrieved to analyze these applications in other agricultural contexts which could be of support for transferring them in the cocoa context.
4.1. Answer to the First Research Question
What are the main diseases that affect the production of cocoa crops?
In the context of CV, the most common reported cocoa diseases in this SLR, which researchers aim to detect are Moniliophthora pod rot or frosty pod rot (
Moniliophthora roreri), black pod disease (
Phytophthora palmivora), and witches’ broom disease (
Moniliophthora perniciosa) [
29,
30].
4.1.1. Frosty Pod Rot (Moniliophthora roreri)
Frosty pod rot (FPR) or
Moniliasis is a fungal disease caused by
Moniliophthora roreri, and it is the most common disease in Central and Latin America, causing economic losses of between 30% and 40% [
31]. This disease thrives in highly warm and humid environments, which creates an environment conducive to its propagation; in addition, farmers and growers can suffer crop losses of 40% to 80% of total annual production, and the Moniliasis affects not only the crop but also the raw material [
31,
32].
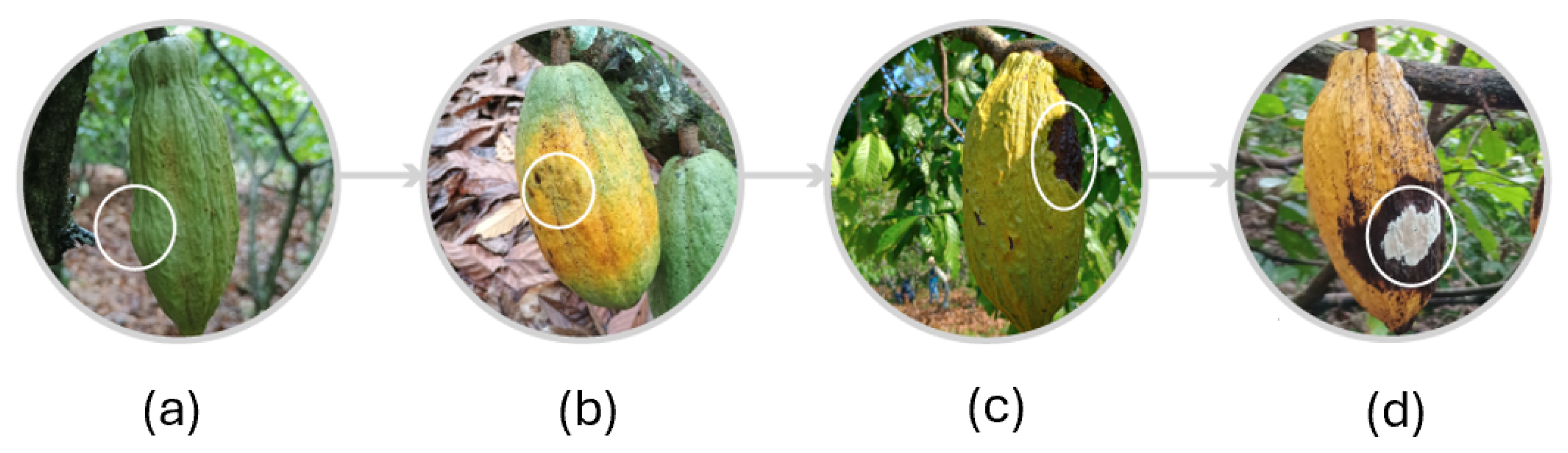
Figure 6 shows the Moniliasis disease in four cycles. First, it starts as yellowish-green spots on the surface of the cocoa fruit. Within three months, a hump light in color appears on the surface of the fruit. The second symptom, which appears after three months, is the appearance of oily spots [
6,
33]. One month later, the color of the surface turns brown, and as the infection progresses, the brown spot becomes more intense. By the end of the infection and after 8–10 days, the brown spot turns into a white powder known as spores, which are visible on the surface of the cocoa fruit [
33].
The cycle of the FPR, depicted in
Figure 6, is developed in four cycles. When the spores are visible on the surface, they spread to other fruits. The FPR attacks the cocoa fruit directly and therefore, is more dangerous and difficult to control compared to other diseases [
31,
34].
In Latin America, cocoa-producing countries such as Colombia and Ecuador report significant losses of up to 60% of cocoa production due to FPR diseases; by contrast, Peru reports lower losses of up to 16% [
32,
33].
4.1.2. Black Pod Disease (Phytophthora palmivora)
Black pod disease (BPD), caused by the
Phytophthora palmivora, is a similar fungal disease that can affect the cocoa fruit. Like FPR, BPD causes losses of up to 20–30% of the annual cocoa production in regions such as Latin America, Africa, and Asia [
7,
32,
33]. BPD thrives in tropical regions where the humidity is up to 100% and the temperature is up to 25 °C. See
Table 5. However, unlike FPR, BPD not only affects the cocoa fruit but also other parts of the tree such as leaves, branches, stems, and roots.
The disease starts with a small wound on the surface of the fruit, which gradually spreads throughout the fruit, turning it brown [
32,
35].
Figure 7 shows two examples of BPD. Example one shows the disease spreading from the stem. In contrast, example two shows the disease spreading from the apex.
Table 5.
Losses due to BPD by country.
Table 5.
Losses due to BPD by country.
| Country | Yield Loss (%) | Reference |
|---|
| Côte d’Ivoire | 40% | [32,33] |
| Ghana | 40% | [7,32] |
| Nigeria | 40% | [36] |
| Cameroon | 40% | [32,36] |
| Brazil | 20% | [33] |
| Indonesia | 20% | [33] |
| Ecuador | 20% | [32,33] |
| Mexico | 20% | [33] |
On the leaves, it causes a reduction in photosynthesis, thus weakening the plant [
31]. The infections in the branches cause cankers, further weakening the plant. Additionally, BPD affects the stems, which impact the nutrients and cause the lack of water to feed the tree. Finally,
Phytophthora palmivora infects the roots of the cocoa tree, causing health problems throughout the plant [
31].
4.1.3. Witches’ Broom Disease (Moniliophthora perniciosa)
Witches’ broom, a disease caused by the fungus
Moniliophthora perniciosa, is considered the second most threat to cocoa production after BPD. Witches’ broom disease (WBD) attacks the crops at different stages, causing significant yield and economic losses in cocoa production [
12,
31,
33]. South America, particularly Brazil, is the region most affected by WBD due to the production of cocoa; ten years after the appearance of the disease, the production dropped by 70% [
6]. The disease produces a series of broom-like sprouts, from which the name “Witches’ Broom” originated. When the broom forming on the tree is alive and green, it dies after the short time, leaving dry residues in the tree. The dry residues, WBD, adhere to the cocoa tree [
6,
35].
Moniliophthora perniciosa grows on the stem of the cocoa tree.
Figure 8 illustrates witches’ broom disease developed on the stem of the tree (1), and the example highlighting the disease area (2).
Cocoa production takes place in tropical areas, where the temperature and humidity levels range from 24 °C–30 °C and 70–90%, respectively, which assists in better production [
6]. However, these conditions allow the faster spread of diseases. Frosty pot rot, black pod disease and witches’ broom are fungal diseases that impact the cocoa production. Another disease that impacts the cocoa production is the Cocoa Swollen Shoot Virus Disease (CSSVD), which caused losses by 15% to 50% [
6,
31,
33]. This disease is transmitted to the cocoa tree by mealybugs, an insect that thrives in warm environments [
31].
There are regions where the cocoa diseases have a major impact according to
Figure 9. This figure shows the leading TC diseases and their impact by region. The FPR is a disease that takes precedence in Caribean, Central, and South America; BPD has a major impact in the West of Africa, Asia, Latin America, and Caribbean, WBD shows relevance in South America; and CSSVD in the West of Africa [
31].
4.2. Answer to the Second Research Question
What are the main Machine Learning algorithms and techniques used to detect and classify diseases in cocoa?
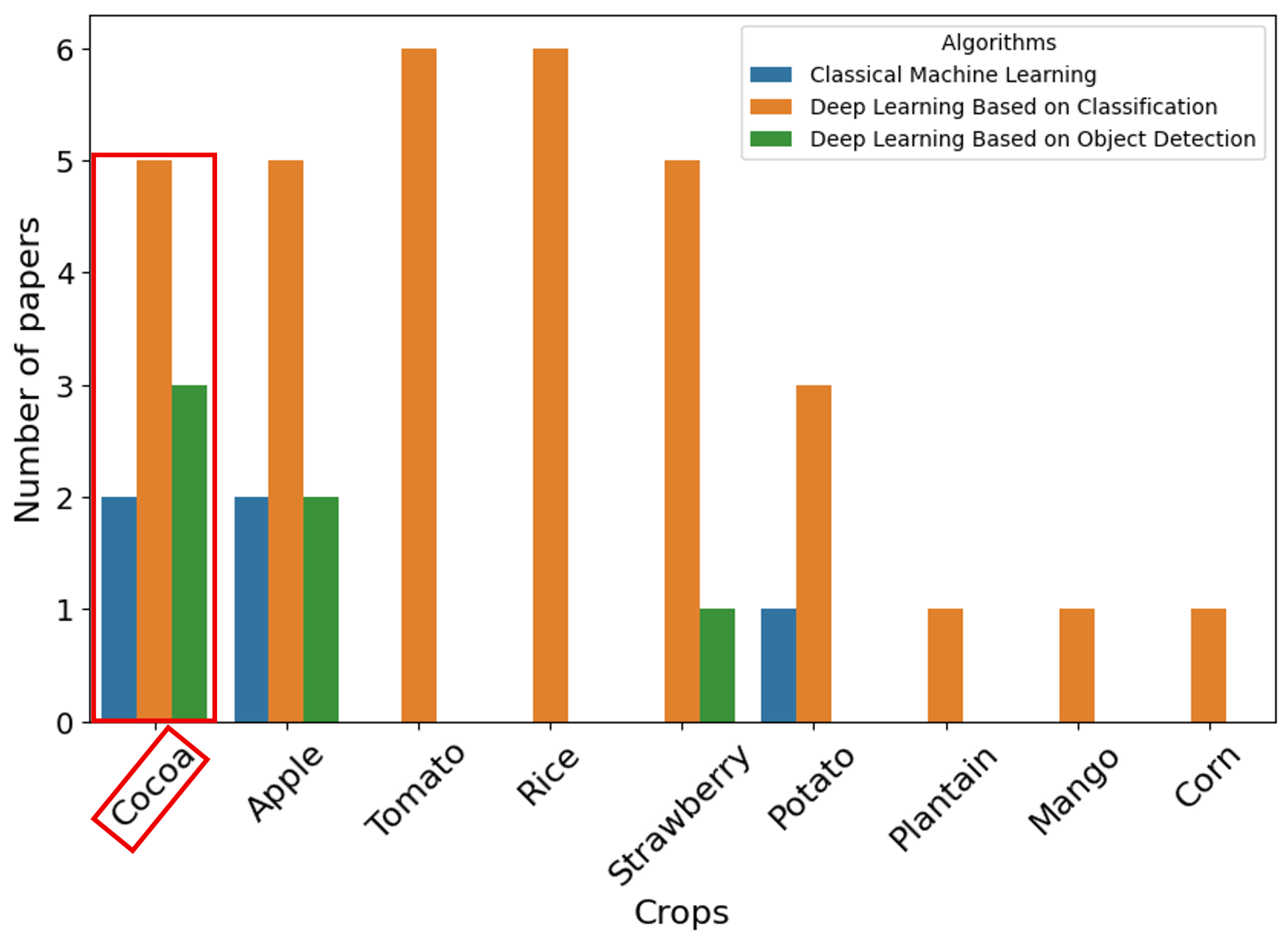
The application of MLAs for disease detection in crops has emerged as a valuable tool that provides solutions for precision agriculture and crop management. Nevertheless, research specifically focused on ML-based disease detection in cocoa remains limited. According to this SLR, 14 studies in the context of CV on cocoa were identified, of which only 10 are addressed in the detection of disease. In this context, MLA has been applied to disease identification, anomaly detection, ripeness assessment, and pod counting. To address Q2, the retrieved studies were separated by algorithms employed in CV to detect and classify diseases in cocoa. The studies were grouped into three categories corresponding to (1) classical ML, (2) DL based on classification and (3) DL based on object detection. These categories are illustrated in
Figure 10. As shown in
Figure 10, DL based on classification is the predominant approach for disease detection in cocoa pods, followed by DL based on object detection and, finally, classical ML. This trend indicates a strong preference for deep neural networks in cocoa disease detection.
Disease Detection in Cocoa Crops with CV Techniques
DL based on classification in cocoa crops: DL architectures, such as EfficientNetB0, SMulti-Layer Perceptron (MLP), Extreme Learning Machine (ELM), MobileNet and ResNet have been employed to classify and diagnose disease cocoa pods [
37,
38,
39,
40,
41]. As a result, several studies have explored Convolutional Neural Networks (CNNs) for cocoa classification tasks. Villamonte et al. [
37] used DL frameworks such as VGG16 and MobileNet to classify defects in cocoa beans. On the other hand, Godmalin et al. [
39] demonstrated the effectiveness of a MobileNet-based CNN architecture for classifying infection levels in cocoa pods. Similarly, Godmalin et al. [
38] applied EfficientNet to categorize cocoa pods into three states: healthy, pest-infested, and affected by BPD. In another study, Achmad et al. [
42] employed ELM and MLP to identify pest attacks in cocoa pods. To assess ripeness in cocoa, Ayikpa et al. [
43] incorporated a Convolutional Block Attention Module (CBAM) integrated into CNN. Additionally, Ayikpa et al. [
43] and Galindo et al. [
30] combined image-processing techniques, specifically the Gray-Level Co-occurrence Matrix (GLCM) for feature extraction with CNN for classification. Furthermore, Ayikpa et al. [
43] and Galindo et al. [
30] incorporated Support Vector Machine (SVM) for ripeness assessment.
DL based on object detection in cocoa crops: Beyond classification tasks, object detection frameworks have been employed to detect and classify cocoa diseases. Object detection algorithms such as You Only Look Once (YOLO) and Single-Shot MultiBox Detector (SSD) have been used to identify diseases in cocoa [
14,
41]. Ferraris et al. [
14] developed an automatic object detection method using YOLO for detecting cacao disease, including FPR and BPD. Similarly, Ayubi et al. [
44] developed an efficient YOLO-based model for detecting cocoa ripeness. Kumi et al. [
41] developed a smartphone application to support farmers in detecting BPD and Swollen Shoot diseases in cocoa pods. Their approach utilized SSD MobileNetV2, which integrates SSD (Single-Shot Multibox Detector) for object detection with MobileNetV2 for classification.
Classical ML in cocoa crops: Traditional ML classifiers, such as SVM, Random Forest (RF), and K-nearest neighbor (KNN), have also been employed in cacao disease classification, evaluation of cocoa bean quality and ripeness assessment. Nasution et al. [
29] introduced an automatic approach for classifying BPD using RF. Similarly, Acharya et al. [
42] used Local Binary Pattern (LBP) for feature extraction, followed by SVM for the early detection of pest-induced damage in cocoa pods. Likewise, Jintawatsakoon et al. [
45] and Appiah et al. [
46] have proposed methods to classify and assess cocoa beans using traditional classifiers such as SVM, RF, and KNN. Moreover, Zainuddin et al. [
40] and Ekawaty et al. [
47] employed an Unmanned Aerial Vehicle (UAV) to recognize and count cocoa pods on trees using image processing. However, their study did not include methods to detect or classify cocoa diseases.
Table 6 presents a summary of the traditional ML and DL architectures employed for detecting diseases in cocoa pods. According to the evidence retrieved in this SLR, DL models are primarily used to classify BPD. Additionally,
Table 6 highlights the implementation of DL methods to classify pest-induced damage. On the other hand, traditional ML classifiers have been applied to detect different diseases than previously mentioned. However, retrieved documents based on ML and DL approaches to detect or classify FPR or
Moniliasis were not found in this SLR, indicating that research in this area remains scarce and requires further exploration to leverage the potential of ML and DL to detect FPR or
Moniliasis.
The studies retrieved on object detection architectures are listed in
Table 7, indicating that research based on object detection remains limited. As shown in
Table 7, YOLO architectures have been utilized to classify cocoa ripeness classification and have also been applied to detect diseases in cocoa pods. Similarly, Single-Shot Multibox Detector (SSD) has also been employed to detect black pod disease (BPD) and Cocoa Swollen Shoot Virus Disease (CSSVD). Although cocoa ripeness is not the main focus of this SLR, it has been considered as a complementary method for trait detection, as a complementary approach for disease detection, and as an approach to assess disease across different ripening stages.
Studies employing CV techniques to address Q2 have been analyzed in this SLR. These studies were categorized into three groups: (1) classical ML; (2) DL based on classification; and (3) DL based on object detection. Among these, DL architectures based on classification and object detection are the most employed to identify BPD. Additionally, these architectures mentioned have been utilized in research to classify and identify BPD as well as pest-induced damages (pest attack and CSSVD). However, within these categories, methods to detect and identify Moniliasis disease remain scarce. According to this SLR, further research is needed to leverage the potential of ML and DL to detect Moniliasis disease.
Studies based on disease detection in cocoa crops using CV techniques remain limited in the literature. However, to obtain a background on how these solutions have been applied in other crops, this SLR also explores CV techniques in different agricultural contexts. The objective is to harness the potential of classical ML and DL approaches for detecting disease in crops other than cocoa. These crops include apples, tomatoes, rice, strawberries, and potatoes, among others. As shown in
Figure 10, the use of DL algorithms to detect diseases in crops other than cocoa outperformed classical ML algorithms.
4.3. Disease Detection Based on CV Techniques in Other Types of Crops
4.3.1. Disease Detection in Apple Crops
According to this SLR, there is significant interest in the implementation of CV techniques to diagnose disease in apple crops. However, in apple crops, disease diagnosis is primarily based on the condition of their leaves.
DL based on classification of apple crops: According to this SLR, There is significant interest in the use of DL architectures to disease classification in apple crops [
50,
51,
52]. Apples are among the most widely cultivated crops globally and represent an important source of income for many farmers around the world. However, in this crop, disease diagnosis is primarily based on the condition of the leaves.
DL based on object detection in apple crops: This SLR has identified the use of DL architectures based on object detection to detect apple-leaf disease in real time. Khan et al. [
53] and Liu et al. [
54] employed object detection models such as YOLOV4, Faster R-CNN, and RetinaNet to develop applications for detecting apple-leaf diseases. These studies evidence the importance of these architectures in real environments.
Classical ML to classify disease in apple crops: According to the evidence found in this SLR, Random Forest (RF) is the most commonly used traditional ML classifier to detect diseases in apple leaves [
55,
56].
The studies retrieved on disease detection in apple crops provide evidence solutions through ML and DL techniques for image classification. However, it is worth mentioning that most of the studies were focused on apple leaves [
50,
51,
53,
54,
55,
56], with no evidence found in this SLR especially addressing disease detection in apple fruits. In addition, most of the data used to carry out these algorithms are based on RBG images, which are often unbalanced. To solve these problems, data augmentation techniques are commonly applied to enhance the performance of the models. On the other hand, two of the studies retrieved were based on hyperspectral images (HSI), demonstrating that some diseases can be detected at an early stage in a spectral range different from RGB [
52,
55].
4.3.2. Disease Detection in Tomato and Rice Crops
Among the studies retrieved, this SLR highlights a strong interest in the use of CV techniques to detect disease in tomato and rice crops [
57,
58,
59,
60,
61,
62,
63,
64]. However, no studies using classical ML or DL architectures based on object detection to identify diseases in tomato and rice crops were found in this SLR [
57,
58,
59]. Like apple crops, tomato crops are not only a key source of income for farmers worldwide but also one of the most widely cultivated crops, occupying an essential place in cuisines worldwide. On the other hand, rice is another important crop. Researchers consider it a vital food source, as it is a staple food for a significant part of the world’s population [
60,
61,
62,
63,
64]. However, similar to tomato crops, no studies using classical ML or DL approach based on object detection to identify diseases in this crop were found in this SLR. As shown in
Figure 10.
DL based on classification in tomato and rice crops: According to
Figure 10, Deep Learning (DL) architectures based on classification have been widely used to detect disease in tomato crops [
57,
65,
66]. Similarly, DL approaches based on image classification have been extensively employed to identify diseases in rice crops [
60,
61,
62,
63,
64].
The retrieved studies in tomato and rice crops indicate that the input images used to train these architectures were obtained from public image datasets, suggesting that most of these studies were conducted in controlled environments rather than under real-world conditions. Also, the input data used to carry out these algorithms primarily consist of RBG images. Finally, it is worth highlighting that most of the approaches have been focused on tomato and rice leaves [
57,
58,
59,
62,
63,
64].
4.3.3. Disease Detection in Strawberry and Potato Crops
Among the studies retrieved, this SLR highlights research efforts focused on disease detection in strawberry and potato crops using CV techniques [
67,
68,
69,
70,
71,
72]. As shown in
Figure 10, most of the studies employ DL based on classification, while studies that explore classical ML or DL architectures based on object detection are still scarce. This suggests that disease detection in these crops is relatively underexplored compared to other crops such as tomato, rice, and apple. The impact of diseases on strawberry quality and yield is of considerable importance, which has led researchers to explore effective CV methodologies to detect diseases in strawberry. On the other hand, potatoes, a staple vegetable essential for daily nutrition, play an important role for human beings.
DL based on classification in strawberry and potato crops: In both crops, acResNet architecture based on classification is the most frequently used approach to detect disease [
67,
68,
69,
71]. In contrast to the studies reported in previous sections, strawberry diseases can affect both the fruit and the leaves [
67], whereas potato diseases primarily appear in their leaves [
71,
73].
DL based on object detection in strawberry crops: In the context of DL architectures based on object detection, Faster R-CNNs is the architecture used to detect disease in strawberry leaves and fruits [
69]. This research highlights the uses of hyperspectral images for this approach. In addition, the deployment of the model was conducted in both under controlled environments and real-world conditions [
69].
Classical ML in potato crops: Studies retrieved in this SLR demonstrate that classical ML approaches have been used to detect and classify diseases in potato crops [
70]. Partial Least-Squares Discriminant Analysis (PLS-DA) and Support Vector Machines (SVM) based on Principal Component Analysis (PCA) scores are supervised classification algorithms employed to detect disease in potato leaves [
70]. This approach also highlights the application of these methods under real-world conditions, as well as the incorporation of hyperspectral cameras to detect diseases.
According to this Systematic Literature Review (SLR), the evidence indicates that studies employing DL architectures based on object detection and Machine Learning (ML) classifiers in strawberry and potato crops were conducted under real-world conditions [
69,
70]. In addition, these studies suggest that input images used to train these algorithms were not obtained from a public dataset due to the use of hyperspectral imaging (HSI). In contrast to studies in DL based on classification studies, the findings indicate that these architectures were trained using public image datasets, suggesting that most of these studies were not conducted under real-world conditions.
Finally,
Table 8 presents the most common machine learning algorithms (MLA) used to classify diseases in the crops mentioned above. As evidenced in
Table 8, there is a clear predominance of Deep Learning (DL) models, particularly on Convolutional Neural Networks (CNN)-based architectures, through crops other than cocoa. According to
Table 8, models such as ResNet, custom CNN, and more advanced frameworks like Fully Convolutional–Switchable Normalization Dual-Path Networks (FCSNDPN) and Multi-head Attention Mechanism Depthwise Separable Convolution Inception Reduction (MDSCIRNet) have evidenced strong performance, achieving accuracies above 95%. These results highlight the powerful capabilities of deep convolutional networks in learning hierarchical features from images, which are fundamentals for disease classification.
Despite the higher predominance of DL models, classical machine learning (ML) algorithms, such as Random Forest (RF) and Principal Component Analysis (PCA)-SVM combined with Partial Least-Squares Discriminant Analysis (PLS-DA) have achieved performance levels comparable to those of more complex DL models. In particular, the studies to detect disease on apples [
55,
56] report a 98.6% accuracy using Random Forest, slightly outperforming others complex DL models within the same domain. Likewise, the use of hybrid methods such as PCA-SVM+PLS-DA has evidenced an accuracy of 92%, indicating an acceptable performance to detect disease in potatoes. On the other hand, DL algorithms based on object detection, such as YOLO and RetinaNet, have been applied in real-world farming for detecting disease in apple leaves, demonstrating distinctive performance metrics [
53,
54]. Khan et al. [
53] employed YOLO for real-time disease detection, achieving mean Average Precision (mAP) of 41.1 and a high detection speed of approximately 47 frames per second (FPS), making their approach suitable for real-world and real-time applications [
53]. Similarly, Liu et al. [
54] achieved a mean Average Precision (mAP) of 79.6, demonstrating the capacity of RetinaNet for more accurate detection under real conditions. These findings probably evidence the challenges related to object detection tasks in agriculture. In the context of research question Q2, it should be noted that the number of studies based on CV techniques applied to cocoa remains limited compared to other crops. However, the algorithms shown in
Table 8 demonstrated the effectiveness of DL architectures and MLA, which provide an opportunity to be probably applied to cocoa crops. Given that these architectures can extract features such as leaf sport, deformation, and discoloration, these architectures may be suitably adapted to identify characteristic symptoms in cocoa pods.
4.4. Answer to the Third Research Question
What are the types of imaging technologies (e.g., RGB, hyperspectral, or multispectral cameras) commonly used in these applications?
To address Q3 in this Systematic Literature Review (SLR), the retrieved studies were grouped by technology into three categories corresponding to (1) technologies based on RGB cameras, (2) technologies based on hyperspectral cameras, and (3) multispectral cameras. Based on this SLR, RGB cameras (n = 6) are the most commonly used technology in agriculture for dataset creation or as sensors to capture plant information (6 studies), followed by hyperspectral (4 studies) and multispectral cameras (1 study).
RGB technologies: According to this SLR, RGB cameras in agriculture are the most widely used technological tool for CV due to its portability, accessibility, and cost effectiveness [
74,
77]. The images generated by these technologies are fundamental for creating datasets used to train ML models. Additionally, the integration of RGB cameras into mobile devices has facilitated and driven the development of computer-vision applications [
63].
Singh et al. [
77] employed a Sony HDR PJ540 RGB camera with a lens of 26.8 mm to generate a dataset with healthy leaves. Also, Shin et al. [
74] and Singh et al. [
77] have employed DSLR (Digital Single Lens Reflex) cameras such as EOS 1300D Canon and Nikon DSLRD5600 with an 18–55 mm lens to obtain information based on images of infected and healthy leaves. On the other hand, mobile devices equipped with advanced camera technology have also been used for dataset creation. Then, Nayak et al. [
63] used mobile devices such as the iPhone X (12 MP) and Xiaomi Redmi Note 8 (48 MP) to build a dataset of images with healthy and unhealthy rice leaves.
Most of the images used for training ML models have been created using RGB technologies, providing detailed visual information that supports large-scale analysis and has proven suitable for general disease classification and diagnosis in real applications.
Table 9 lists the RGB technologies identified in this SLR. According to the retrieved studies, the limited use of RGB technologies suggests that most of these studies rely on public datasets. However, the creation of our own datasets enables the training models with images captured under real-world conditions.
Hyperspectral and multispectral technologies: Unlike RGB cameras, hyperspectral cameras provide enhanced imaging capabilities by capturing data across multiple bands of the electromagnetic spectrum, extending beyond the visible range detected by RGB cameras. These bands include ultraviolet (UV), near-infrared (NIR), and other bands not visible to the human eye. As a result, the integration of hyperspectral cameras is complex because these technologies are more expensive than RGB technologies [
52,
54,
55]. Based on this SLR, the use of hyperspectral cameras in agriculture remains scarce; however, some studies have used these technologies to create image datasets and diagnose plant diseases in more detail. Cameras such as SOC 710VP, Corning MicroHSI 410 camera, Gaiasky mini2-VN, and Specim FX 10 were hyperspectral technologies employed for disease identification using wavelengths outside the visible RGB spectrum, covering a range between 700 nm and 1000 nm, relating to the near-infrared (NIR) [
52,
54,
55]. Another technology identified in this SLR is a multispectral MAPIR Survey3 used to detect disease in coffee crops [
16].
Although the use of hyperspectral cameras requires a greater investment due to their advanced technology, studies retrieved in this SLR have demonstrated that these cameras can identify patterns that RGB cameras are unable to detect due to their broad spectrum of information.
Table 9 lists the technologies identified in this SLR based on hyperspectral and multispectral cameras, which were employed to create datasets for training ML models, enabling the development of the models with hyperspectral images (HSI) captured under real-world conditions.
Finally,
Table 10 summarizes the main advantages and disadvantages of using RGB and hyperspectral cameras in crop disease detection. This comparison shows the differences such as spectral sensitivity, cost and applicability.
Datasets Used for Training ML Models
As mentioned above, the generation of image datasets to feed Machine Learning algorithms (MLAs) depends entirely on the technologies discussed above. However, several MLAs were developed using public datasets available in PlantVillage [
50,
56,
57,
58,
59,
65,
66]. To extend Q3 in this SLR, the retrieved studies related to plant disease detection (PLDD), plant disease detection (PDD), and plant disease classification (PDC) were analyzed to identify the dataset used for training ML models.
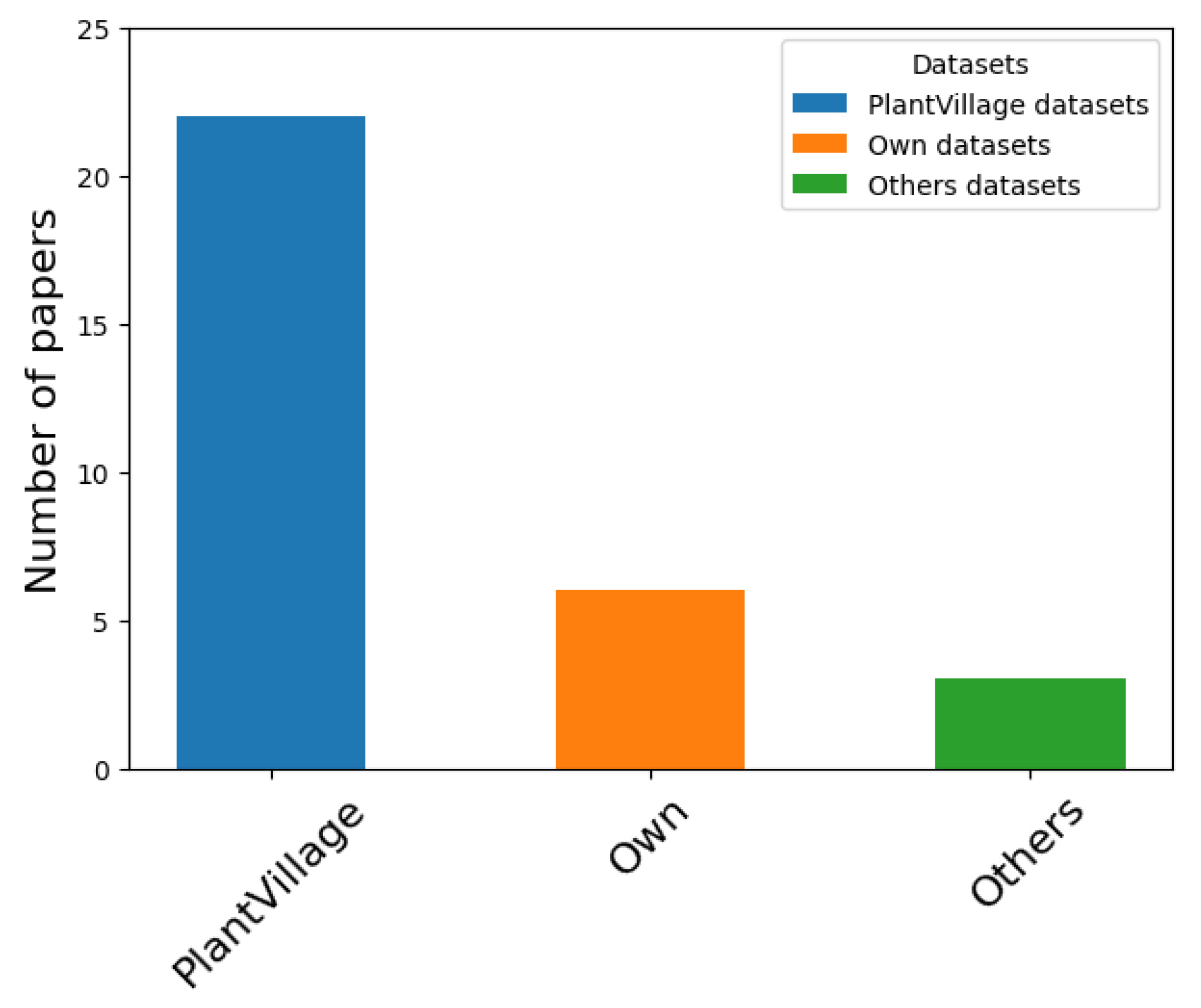
Figure 11 illustrates the papers based on the type of dataset used for training MLA. According to the evidence collected in this SLR,
Figure 11 indicates that PlantVillage is the most commonly used dataset for training classification models. However, PlantVillage datasets cover various crops and diseases without a specific focus on particular crops [
50,
56,
57,
58,
59,
65,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91,
92,
93]. In contrast, the use of proprietary datasets for training classification models is less. However, these datasets are focused on particular crops and specific diseases [
52,
53,
54,
55,
74]. Other datasets represent the smaller portion, with fewer than five studies. Nonetheless, these studies also employed public datasets from Kaggle [
62,
63,
64].
Table 11 presents the datasets used for training MLA in disease detection and classification, highlighting the data augmentation and image-processing techniques applied. As shown in
Table 11, PlantVillage is the most used dataset and is also the most frequently referenced in the studies retrieved in this SLR.
4.5. Answer to the Fourth Research Question
What are the main Machine Learning algorithms used in mobile applications and other platforms for cocoa disease detection?
Based on the studies found in this SLR, the execution of ML models has been carried out through processing units such as NVIDIA Tesla V100 GPU, NVIDIA RTX A4000 GPU, NVIDIA Tesla V100 GPU, Intel Core i5-7500 CPU y AMD Radeon R7 430 GPU and (Intel Core i5-7200U [
68,
73,
75,
77]. As shown in
Table 8, column four presents some ML models based on DL architectures, which are trained and deployed on this kind of unit. However, several ML models, listed in
Table 12, have been developed with optimized architectures and computational constraints, enabling their implementation on mobile applications and low-cost devices suitable for resource-restricted environments. It should be emphasized that, to address Question Q4 in this SLR, additional studies exploring MLA in mobile applications for crops other than cocoa were included. This inclusion was made due to the limited number of studies retrieved that focused on cocoa crops, with just two studies identified.
As presented in
Table 12, non-traditional MLA are employed to be deployed in mobile and edge devices in the agriculture field. As previously mentioned, there are processing units used to train and run ML models. However, there are other low-cost processing units that can perform the same solutions while providing a portable alternative. Therefore, the Raspberry Pi is the most common edge device to deploy and test MLA. Based on the Raspberry Pi device, Maurya et al. [
95] implemented a lightweight framework combining MLP and Long Short-Term Memory (LSTM), to reduce the computational overhead for disease detection in cotton and maize crops. Thus, Gonzalez et al. [
57], through CNNs architectures such as MobileNet, NasNetMobile, and Xception, developed a low-cost embedded system using a Raspberry Pi with a webcam to detect anomalies in tomato crops in an early way. In addition, Mishra et al. [
76] developed and deployed a custom Dual-Channel Convolutional Neural Network (DC-CNN) on a Raspberry Pi device to detect corn disease in real time.
On the other hand, smartphones are equipped with architectures specifically designed to run MLA. Optimized architectures have been developed to adapt to the computational and power constraints of this device to deploy these algorithms. Therefore, based on mobile application algorithms, Ayubi et al. [
44] developed an efficient model for cocoa ripeness detection using YOLO model, an object detection algorithms, that can work on mobile devices. Moreover, Godmalin et al. [
39] proposed a CNN architecture based on MobileNetV3Small that can run on mobile devices to classify cocoa pod-level infections. Finally, Kumi et al. [
41] developed a smartphone application to support farmers in detecting BPD and Swollen Shoot diseases in cocoa fruits using SSD MobileNet V2, which combines features from SSD (Single-Shot Multibox Detector) for object detection with MobileNet V2 for its classification.
The Systematic Literature Review (SLR) highlights specific models that are adaptable to the field of agriculture. However, the retrieved studies demonstrate a limited use of these models for crop disease detection. Moreover, although this SLR reports the use of these architectures, not all studies indicate that they have been implemented through mobile applications. Notably, among the studies retrieved in this SLR, MobileNet and its variants (MobileNetVx, MobileNetV3Small, and SSD MobileNet V2) are the most frequently used architectures for detecting disease in crops, as evidenced in
Table 12. The preference of these architectures suggests a focus on lightweight and computationally efficient Deep Learning models, which are especially suited for deployment in mobile or embedded systems.
Although the use of architectures designed for mobile devices is less frequent compared to those mentioned above in question Q2, these architectures offer significant advantages related to computational efficiency. The use of DL models for mobile applications is still under exploration in the context of cocoa compared with other crops. However, according to
Table 12, the use of lightweight models in mobile applications in agriculture demonstrates that it is possible to develop portable and cost-effective solutions for disease detection that can be transferred to the cocoa context.
6. Conclusions and Future Work
This work has carried out a SLR focused on the detection of diseases in cocoa crops using computer-vision techniques (CV). The protocol used was PRISMA 2020, which allowed a careful selection of the literature published to date (31 August 2024). According to each research question, this SLR provides insights that may contribute to future research works. The questions proposed in this SLR aim to identify the needs currently facing cocoa production and to explore how the use of ML and CV tools can contribute to providing solutions to these challenges. Therefore, according to the question Q1, the production of cocoa faces challenges due to the incidence of diseases caused by Phytophthora palmivora and Moniliophthora roreri, which can affect more than half of the production, bringing significant losses to farmers. Furthermore, it should be noted that there are diseases that have a great impact on specific regions due to environmental conditions. In the case of FPR caused by Moniliophthora roreri, it mainly affects Latin American countries. According to question Q2, the application of ML algorithms to detect and classify diseases in cocoa remains limited and require further development. Most of the CV techniques to date have focused on detecting BPD, caused by Phytophthora palmivora. Furthermore, existing approaches usually only distinguish between healthy and unhealthy cocoa pods, without providing more detailed insights into the specific stage of the Monilia disease cycle. Regarding research question Q3, it has been observed that RGB cameras are the most commonly used sensors in computer vision (CV) systems, while the use of hyperspectral and multispectral cameras remains limited, indicating that these technologies are still in the exploration phase. This predominance of RGB cameras is likely due to their portability and accessibility, particularly in devices such as smartphones, as well as the widespread availability of RGB image-based datasets, which are commonly used to train machine learning algorithms (MLA). However, a relevant finding of this SLR is the limited presence of proprietary datasets. Many of the retrieved papers rely on public datasets, such as PlantVillage and Kaggle. Although these datasets offer a large number of images, most of them have been focused on leaves and have been created in controlled environments, which limits their applicability in real field scenarios. In addition, there is a noticeable limitation of datasets related to cocoa pods especially in the Monilia disease cycle. On the other hand, multispectral and hyperspectral technologies have not been applied to the detection of diseases in cocoa crops, particularly in the context of Monilia. Finally, regarding research question Q4, the lack of mobile application development makes the limited implementation of these tools in uncontrolled environments clear, which limits their practical use in the diagnosis of diseases in the real world. For future work, it is recommended that the SLR be updated to include more scientific databases. Also, a SLR should be performed on each domain identified in this study; this will allow for a more detailed examination of the strategies used to address the problems focused on detecting disease in the agricultural field.