Abstract
Amidst the escalating global demand for protein-rich livestock feed, there’s an urgent call to explore innovative alternatives. Insects, renowned for their rich protein, lipid, and nutrient profiles, offer a sustainable solution. Integrating agricultural waste into insect diets emerges as a promising strategy to alleviate rearing costs. However, large-scale investigations into by-product valorization remain limited. Thus, our study aims to evaluate Greek agricultural by-products—brewer’s spent grains, rice bran, oat and maize by-products, and animal feed mill leftovers—as potential feedstock for Tenebrio molitor larvae, an insect species that is authorized by the European Union for both livestock feed and human consumption. In this study, the larval growth and body composition were assessed in commercial trays, unveiling optimal performance with rice bran and brewer’s spent grains. Conversely, larvae fed with animal feed mill leftovers and maize by-products displayed suboptimal outcomes. These findings underscore the potential efficacy of integrating locally produced agricultural by-products into T. molitor commercial production. Such an approach not only addresses the growing demand for protein-rich livestock feed but also offers a sustainable solution to agricultural waste management. In conclusion, our research contributes valuable insights towards developing economically viable insect farming.
1. Introduction
Nowadays, the ongoing expansion of a human population urges for novel, nutritious and sustainable alternative protein sources for both human consumption and animal livestock nutrition [1,2,3,4,5,6]. Among the proposed alternative protein sources, insects emerge as a highly promising sustainable choice [4,5]. Briefly, insects have a high protein content, whereas their modest land and water requirements, coupled with their low greenhouse gas emissions and efficient feed conversion, enhance the sustainability of insect rearing compared to the traditional livestock production [4,7,8]. Along the same lines, due to their ability to biodegrade waste generated in the food industry, insect production is fully aligned with circular economy strategies that are currently promoted in the EU [9,10]. However, the complexities of their commercial production still remain a significant challenge [11].
The biggest hurdle that the insect production industry has to overcome in order to develop further is the high insect feed cost, which notably contributes to the overall production cost [12,13]. In this framework, the integration of agricultural by-products, generated during various agricultural production processes, into insect diets has been extensively examined for various edible insect species commercially produced for food and feed applications [14,15,16,17,18,19]. Extensive research has been conducted on the growth and performance of the larvae of the yellow mealworm, Tenebrio molitor L. (Coleoptera: Tenebrionidae) grown on a variety of agro-industrial by-products and former foodstuff products (FFPs) [14,20,21,22,23,24]. These included malt residual pellets, corn germ meal, sweet chestnuts, bread remains, soybeans, sweet potatoes, wheat germs, wheat, rye bran, rapeseed meal, rapeseed cake, flax, milk thistle cakes, brewery spent grains, bread and cookie leftovers, and mixes of brewer’s spent grains or bread with cookies. Among these, certain substrates emerged as particularly promising based on their impact on larval growth performance, feed conversion efficiency, nutrient composition, and other parameters. Notably, malt residual pellets, corn germ meal, sweet chestnuts, bread remains, soybeans, sweet potatoes, wheat germs, and brewery spent grains demonstrated favorable outcomes in terms of larval development, biomass production, and nutrient content. These substrates offer potential as sustainable and cost-effective alternatives for the mass rearing of mealworm larvae.
Fruits and vegetables have also been utilized in T. molitor diets aiming to enhance insects’ growth performance and nutritional value [18,25,26,27,28,29,30]. Various agri-food wastes, such as olive pomace, grape pomace, grape marcs, winery waste sludge, cucumber wastes, and tomato wastes, along with FFPs like orange peel waste and carrot pomace, have been evaluated as potential feed sources for the yellow mealworm larvae. Particularly, the inclusion of wastes like olive pomace in larval diets presented promising outcomes in terms of growth performance and nutritional properties. Similarly, the supplementation of T. molitor diets with vegetable and fruit wastes, such as albedo orange peel waste, carrots, and red cabbage, resulted in larvae with enhanced nutritional profiles, including increased protein content, vitamins, antioxidants, and fatty acid composition. These findings collectively underscore the feasibility and benefits of repurposing agricultural by-products and food wastes as substrates for T. molitor mass rearing, offering a sustainable approach to both waste management and protein production. Additionally, it is worth noting that this insect species is currently authorized within the EU not only as an ingredient of aquafeeds [31], poultry, and swine feeds [32], but also for human consumption [33].
The insect’s body composition is inextricably related to the nutrients and compounds present in the consumed feed [34,35,36,37]. For instance, Ramos-Elorduy et al. [37] tested 35 dried waste organic materials as a substrate for the development of T. molitor larvae and suggested that the larval amino acid and nutrient content depends not only on the size and weight of the larvae, but also on the substrate’s composition. Similar results were reported by Rumbos et al. [16] who evaluated the growth and body composition of the yellow mealworm larvae fed by-product-based isonitrogenous diets.
Most experiments related to T. molitor growth have been carried out at laboratory scale, while there are disproportionally less data about the factors that affect the performance of T. molitor at a large scale. Recently, Deruytter et al. [38] tested the effect of crate size, oviposition time, number of adults, and cannibalism on the reproduction of T. molitor in terms of the number of adults produced, oviposition time, cannibalism, and tray size at pilot scale. Similarly, two studies of Adamaki-Sotiraki et al. [39,40] conducted both at pilot scale, highlight the importance of conducting experiments at a larger scale. In this regard, the objective of the present study was to valorize at large scale Greek, locally produced, agricultural organic by-products for the rearing of T. molitor larvae.
2. Materials and Methods
2.1. Insect Rearing and Experimental Design
Plastic insect breeding trays (60 × 40 × 14.5 cm) (Beekenkamp Verpakkingen BV, Maasdijk, The Netherlands) were used for the stock colonies rearing, in the pilot-scale insect rearing unit of the Laboratory of Entomology and Agricultural Zoology at the University of Thessaly, in Volos, Greece, under constant conditions, i.e., continuous darkness, 60 ± 5% relative humidity and 27 ± 0.5 °C. The substrate in which larvae were reared was wheat bran, while agar cubes (20 g/L) were provided 3 times per week, as a larval moisture source.
In order to acquire newly emerged larvae, adults were allowed to oviposit for 4 d on white wheat flour. Then, the young larvae were left to feed undisturbed for 10 d on the flour. After this interval, larvae were separated from the white wheat flour by sieving. To ensure that the selected larvae had a similar age and size, only larvae that passed through a 850 μm sieve and were kept by a 600 μm sieve were used for experimentation, corresponding, according to their larval head capsule size, to 6th- and 7th-instar larvae [41]. Plastic insect breeding trays were used as experimental units and wheat bran served as control. To estimate the number of larvae, 3 subsamples, each containing at least 100 larvae, were taken and weighed, in order to estimate the weight of 10,000 larvae. Then, approximately 10,000 larvae were inserted to each tray, along with 2.1 kg of each by-product. Larvae were left to feed undisturbed for two weeks. After this interval, the content of each tray was homogenized, and a subsample was taken from each tray to determine the number of larvae per tray and the average larval weight. Attention was given, so that each subsample contained at least 100 larvae. This procedure was repeated weekly until 2 or more replicates run out of feed or more than 10% of larvae pupate. During harvesting, larvae were separated from the substrate, using 2 mm sieve. Afterward, the total amount of larvae was weighted (total harvest). The remaining substrate was also sieved, using 500 μm sieve, in order to separate the produced frass from the leftover feed, which was also weighted and recorded. Frass that was produced by T. molitor larvae passed through the 500 μm sieve, while the leftover feed was kept by the 500 μm sieve. After harvesting, larvae were fasted for 24 h and frozen (−20 °C) for further analysis. During larval development, agar was provided to larvae three times per week as a moisture source, increasing the amount of agar provided over time, always ensuring the agar availability for larvae. There were four tray replicates for each by-product treatment (oat by-product, brewer’s spent grains, maize by-product, animal feed mill leftover, rice bran, and wheat bran—control).
2.2. By-Products
By-products locally produced in Greece and arising from the production processes of oats, beer, maize, rice and animal feed milling were subjected to evaluation for their suitability as T. molitor feed (Table 1). Specifically, the evaluation encompassed oat by-products (comprising oat straw, husks, and small oat seeds), brewer’s spent grains (BSGs; in pellet form), maize by-product (resulting from the seed cleaning process), rice bran, and animal feed mill leftover (AFML), which was the residue of the animal feed grinding process (composed of 75% maize, 15% barley, 5% soft wheat, and 5% other grains). All by-products were ground using a thermomix (TM31-96 1C, Vorwerk Elektrowerke GmbH & Co. K, Wuppertal, Germany), with the exception of rice bran and the AFML, which were already in powder form. All by-products passed through a 2 mm sieve.

Table 1.
Proximate composition, i.e., dry matter (%), protein (% DM), and ash (% DM) content of five agricultural by-products and wheat bran (control). For all treatments, 4 replicates were conducted (n = 4).
2.3. Proximate Composition
Proximate composition was conducted to determine the nutrient composition of the by-products and the produced larvae. Thermal drying to constant weight in an oven at 105 °C was applied to determine the moisture content. The dried larvae were ground, in order to form an insect flour and undergo further analysis. The crude protein content was determined by Kjeldahl analysis, (Behr Labor-Technik GmbH, Düsseldorf, Germany). Four samples of larvae (0.2 g insect meal) sourced from each of the by-products were transferred to digestion test cubes. To expedite the digestion reaction, two Kjeltabs catalyst tablets (5 g Potassium Sulphate and 5 g copper (II) Sulphate , ) were added. Subsequently, the samples were combined with 15 mL of concentrated sulfuric acid () and placed in the Kjeltec 2000 digestion apparatus. The digestion process occurred at 150 °C for a duration of 85 min. Upon completion of the digestion process, the samples were moved to the distillation apparatus. Here, 100 mL of distilled , 80 mL of NOH, and 50 mL of were added before distillation commenced for 6 min. Finally, the titration of the resulting ammonium borate solution was conducted using a dilute hydrochloric acid solution (0.1 N). The nitrogen content of the sample (N %) was then calculated using the formula:
The Jones’ default nitrogen-to-protein conversion factor (Kp) of 6.25 for the by-products and a specific nitrogen-to-protein conversion factor (Kp) of 4.76, for T. molitor larvae, were used as previously suggested [42].
The crude fat content was determined using the exhaustive Soxhlet extraction method, employing petroleum ether (40–60 °C, boiling point) in a Soxtherm Multistat/SX PC (Sox-416 Macro, Gerhard, Germany). In detail, the extraction process involved several steps. Firstly, two boiling stones were placed into extraction glass vessels, and their weights were recorded. Paper filters were then inserted into the extraction glass vessels, and 1 g of sample was weighed and deposited into the filter paper container. Next, 150 mL of petroleum ether was added to each extraction glass vessel, immersing the filter paper containers containing the sample. The extraction glass vessels, along with the filter paper, were transferred to a specialized fat extraction apparatus (Soxhlet apparatus). The extraction process proceeded in two steps: initially, the samples were heated at 150 °C in the presence of the organic solvent. Subsequently, the organic solvent was absorbed and washed into the sample for a period of 1.5 h. Afterward, the solvent was allowed to evaporate for 15 min, leaving the total lipids in the sample at the bottom of the glass extraction container. To remove any residual petroleum ether, the glass vessels were placed in an oven at 105 °C for 15 min (without the filter paper), followed by a 15 min period in a dehumidifier. Weight measurements were recorded throughout the process. The net weight of fat in the by-products was calculated using the formula:
Ash content was determined by dry ashing in porcelain crucibles in a muffle furnace (Nabertherm L9/12/C6, Lilienthal, Germany) at 600 °C for 5 h, and the gross energy content was determined adiabatically using an IKA oxygen bomb calorimeter (C5000; IKA Werke GmbH, Staufen, Germany) [43].
2.4. Calculations
The average individual larval weight was determined by dividing the total weight of the larvae with the number of counted larvae in each subsample. The Feed Conversion Ratio (FCR) was determined by dividing the weight of the consumed feed by the weight the larvae produced within each tray. In addition, the Efficiency of Conversion of Ingested Food (ECI) was calculated as the weight the larvae produced within each tray divided by the consumed feed and multiplied by 100%. Finally, the specific growth rate (SGR) was calculated according to the formula SGR = 100 × (lnFBW − lnIBW)/days, where FBW and IBW stand for the final and initial body weight. FCR and SGR were calculated on a wet weight basis, whereas ECI was calculated on a dry matter basis. The Economic Conversion Ratio (ECR) was calculated by multiplying the FCR with the cost of feed per ton, and it represents the feed costs per kilogram of live larvae. At the end of the experiment, the feed with a particle size under 500 μm was considered frass. Agar was not included in the calculations.
2.5. Statistical Analysis
Statistical analysis was conducted using SPSS 26.0 (IBM Corporation, Armonk, NY, USA) for the final individual larval weight, total harvest, FCR, ECI, SGR, dry matter, lipid, ash, and protein content. All data were tested for the homogeneity and normality of variances through the Shapiro–Wilk and Levene’s tests, respectively. As the by-products’ ash content, ECR, and ECI data displayed a normal distribution, a parametric analysis, i.e., an analysis of variance (ANOVA), which was performed to detect the differences among treatment. The Bonferroni correction for the multiple comparisons test was performed for post hoc analysis. For the rest of the variables that did not follow a normal distribution, i.e., final individual larval weight, total harvest, FCR, SGR, larval dry matter, lipid, ash, and protein content, as well as by-products’ dry matter, protein content data were subjected to non-parametric analysis. Particularly, the Kruskal–Wallis H test was employed to detect whether there were significant differences (p < 0.05) among the treatments, followed by Dunn multiple comparisons for post hoc testing.
3. Results
3.1. By-Products’ Proximate Composition
The ash content varied significantly among the different by-products (df = (5,12), p < 0.001, F = 1787.6). Wheat bran (control) exhibited an ash content of 4.6%, AFML had the lowest ash content at 1.0%. Rice bran showed the highest ash content (7.6%), followed by BSG (7.2%). The oat by-product demonstrated an ash content of 6.8%, whereas the maize by-product had an ash content of 3.4%. The dry matter content differed significantly between the different by-products (df = 5, p = 0.006, test statistic: 16.336). Dry matter ranged between 85.8% and 97.1%, with the highest dry matter observed in the oat by-product, while the lowest was observed in what bran (control). Protein content did not present statistically significant differences (p = 0.11) and it was above 5.6% for all by-products.
3.2. Final Individual Larval Weight
As indicated by the individual larval weights recorded, variation in the larval growth was observed among the different dietary treatments tested (df = 5, p < 0.001, test statistic: 20.320). More specifically, the final individual larval weight spanned from 35.6 mg (AFML) to 98.4 mg (wheat bran) (Figure 1; Table 2). Larvae raised on wheat bran (control), BSG, and rice bran had the highest final individual larval weights. In contrast, larvae fed on the maize and the oat by-product displayed comparatively smaller growth, with the final weight remaining below 82.6 mg.
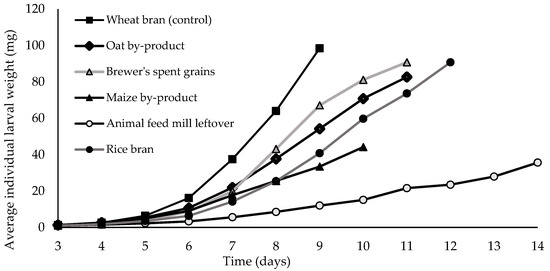
Figure 1.
Average individual larval weight (mg) of Tenebrio molitor larvae reared on five agricultural by-products and wheat bran (control). For all treatments, 4 replicates were conducted (n = 4).

Table 2.
Final individual larval weight (FILW, mg ± SEM), total harvest (g ± SEM), feed conversion ratio (FCR, ±SEM), efficiency of conversion of ingested food (ECI, % ±SEM), specific growth rate (SGR, %/day ± SEM) of Tenebrio molitor larvae reared on five agricultural by-products and wheat bran (control), at the end of the experiment. For all treatments, 4 replicates were conducted (n = 4).
3.3. Total Harvest
The total harvest of larvae varied among the different substrates from 335.3 g to 914.4 g, with the significant differences being observed among the treatments (df = 5, p = 0.001, test statistic: 20.110) (Table 2). Total harvest was high for wheat bran (control) (872.3 g), BSG (914.4 g), rice bran (748.0 g), and oat by-product (708.9 g), while the lowest total harvest was recorded for AFML (335.3 g).
3.4. Feed Conversion Ratio, Efficiency of Conversion of Ingested Food, Specific Growth Rate, Economic Conversion Ratio
FCR (df = 5, p < 0.001, test statistic: 21.080) and ECI (df = (5, 18) p < 0.001, F = 41.141) varied among treatments, ranging from 2.0 to 4.9 and from 20.4 to 50.9%, respectively (Table 2). Low FCRs and high ECIs suggest the efficient utilization of the feed by the larvae. Larvae reared on BSG and oat by-product exhibited the lowest FCR and the highest ECI values (2.0 and 50.9%, 2.0 and 50.8%, respectively). Similar values were noted for larvae fed on wheat bran (control) (2.3 and 43.8%, respectively). Conversely, larvae reared on AFML resulted in high FCR (4.9) and low ECI values (20.4%), indicating the inefficient bioconversion of this by-product into body mass. SGR varied between 5.9 and 10.7%/day (df = 5, p < 0.001, test statistic: 21.088) (Table 2). Larvae reared on BSG (8.8%/day) and maize by-product (8.6%/day) displayed high SGR values, similar to the control (10.7%/day), while those reared on AFML, rice bran, and oat by-product exhibited lower SGR values (<8.5%/day). The economic conversion rate (ECR) of larvae varied significantly across different by-products used as feedstock (df = (5, 18), p < 0.001, F = 87.500) (Table 3). Larvae fed with wheat bran (control) exhibited an ECR of 342.5 EUR/ton larvae, while larvae fed with oat by-product had an ECR at 138.0 EUR/ton larvae. Treatments were grouped based on Bonferroni post hoc comparisons, with larvae reared on BSG (555.9 EUR/ton larvae) and rice bran (558.3 EUR/ton larvae) showing the highest ECR, followed by larvae reared on maize by-product (275.1 EUR/ton larvae). Larvae fed with AFML (73.6 EUR/ton larvae) demonstrated the lowest ECR among all treatments.

Table 3.
Economic conversion ratio (ECR, EUR/ton larvae ± SEM) of Tenebrio molitor larvae reared on five agricultural by-products and wheat bran (control), at the end of the experiment and cost of by-products (price, EUR/ton). For all treatments, 4 replicates were conducted (n = 4).
3.5. Insects’ Body Proximate Composition (Dry Matter, Lipid, Ash, and Protein Content)
The composition of larvae reared on the various by-products tested reflects significant differences in key nutritional components (dry matter content: df = 5, p = 0.001, test statistic: 15.700; lipid content: df = 5, p = 0.001, test statistic 18.748; ash content: df = 5, p = 0.002, test statistic: 18.676; protein content: df = 5, p = 0.001, test statistic: 10.057) (Table 4). Notably, larvae reared on BSG exhibited the lowest dry matter content (23.9%), similarly to the DM content of larvae fed wheat bran (33.8%). In contrast, larvae reared on AFML had the highest dry matter content (39.1%), which was not significantly different from the DM content of larvae reared on maize by-product (36.6%), rice bran (35.8%), and oat by-product (35.3%). The lipid content fluctuated between 9 and 44.3%. Larvae grown on AFML, maize, and oat by-product had a high lipid content (>39%). Conversely, BSG, wheat bran, and rice bran had the lowest lipid content (<35.7%). The ash content took its highest values for larvae raised on BSG (5.5%) and wheat bran (4.4%). Larval protein content ranged from 29.2 to 49%. Larvae raised on wheat bran and rice bran had a high protein content (37.7% and 36.7%, respectively) similarly to those raised on BSG (49%), while the lowest protein content was observed in the larvae reared on maize by-product and oat by-product (32.0% and 34.4%, respectively).

Table 4.
Insects’ body composition: dry matter, lipid, ash, and protein content (% DM ± SEM) of Tenebrio molitor larvae reared on five agricultural by-products and wheat bran (control). For all treatments, 4 replicates were conducted (n = 4), unless indicated with * (n = 3).
4. Discussion
The upcycling of low economic value side-streams and by-products of the agri-food sector through insect bioconversion can substantially enhance the sustainable profile of the insect sector, whereas it can further contribute to the reduction in the insect production costs, improving the economic feasibility of commercial insect production. In this regard, a considerable amount of recent research works has been dedicated to the valorization of agricultural by-products as insect feedstocks [16,17,18,20,21,27,44]. Although of high significance, most of these studies present results generated at lab scale, which may not be directly applicable to industrial scale production systems. For instance, Yang and Tomberlin [45] reported significant differences in the larval survival, weight, and biomass conversion of larvae of the black soldier fly, Hermetia illucens (L.) (Diptera: Stratiomyidae), between the benchtop and industrial treatments and suggested that the application of laboratory data to an industrial production system could lead to the underestimation of biomass production and conversion rate. Accordingly, Adamaki-Sotiraki et al. [40] evaluated the larval performance and growth of T. molitor grown on diets, composed of agricultural by-products, at both the laboratory and industrial scales. Although, at the laboratory scale, two diets seemed to efficiently support the larval development, only one of them resulted in a high total harvest when they were evaluated at the industrial scale. Therefore, there is a necessity for experimentation at a larger scale in order to generate data that are closer to commercial settings and could be more easily adopted by the industry. Along these lines, in the present study, we examined at large scale the potential of five agricultural by-products as single feeding substrates for T. molitor larvae.
Based on our results, several of the by-products tested efficiently supported the growth of T. molitor larvae and could be used for its rearing at commercial scale. In terms of the final individual larval weight and SGR, larvae reared on rice bran and BSG grew the best compared to larvae reared on the rest of the by-products tested and similarly to larvae reared on wheat bran, a commonly used substrate for commercial mealworm rearing. Those results are in accordance with the studies of Morales-Ramos et al. [44] and Lienhard et al. [14] that both suggested these by-products as suitable components for T. molitor diets. Specifically, the BSG is a by-product that has been extensively studied for its potential to be utilized as feed for T. molitor [23,24,26,46]. Along with the aforementioned by-products, the oat by-product gave good results regarding to the total harvest, FCR, and ECI, being in accordance with the results of previous works [16,17,44,47].
Although larvae fed the maize by-product had a high SGR, close to the SGR of larvae fed the BSG and rice bran, they had lower final individual larval weight, total harvest, and ECI, as well as a higher FCR compared to those bred in BSG and rice bran. Interestingly, our results do not coincide with the results of Lienhard et al. [14], who reported that a maize by-product, originating from the seed cleaning process, efficiently supported the development of T. molitor larvae, when provided as a single substrate. This may be attributed to the different experimental protocol followed in the two studies. In our study, the experiment was terminated when two or more replicates run out of feed or more than 10% pupation occurred. Regarding the maize by-product, the experiment was terminated on the grounds of feed depletion, and as a result, the larvae did not reach their optimal final weight. Among the five by-products evaluated as feedstock for T. molitor larvae, the worst results in terms of final individual larval weight, total harvest, FCR, ECI, and SGR were attained with the AFML. Similar results were also reported by Riudavets et al. [48] who also suggested that the T. molitor larvae reared on feed mill by-products performed deficiently.
The economic effectiveness of employing a specific feeding substrate is paramount, as it plays a crucial role in determining the overall financial success of insect farming. The lower ECR between the three most promising by-products of our research (rice bran, BSG, oat by-product) was emerged by the larvae reared on oat by-product (138.0 EUR/ton larvae) and it is even lower than the ECR emerged by the larvae reared on wheat bran (342.5 EUR/ton larvae)—which is widely used as T. molitor feed. On the other hand, the utilization of the other two promising by-products (rice bran and BSG) on which T. molitor larvae thrived, resulted in higher ECR (558.3 EUR/ton larvae and 555.9 EUR/ton larvae, respectively) than the ECR that occurred from wheat bran (control) utilization (342.5 EUR/ton larvae). Larvae fed with the maize by-product had a higher ECR (275.1 EUR/ton larvae) than the larvae fed with oat by-product (138.0 EUR/ton larvae), even though those substrates had the same price (70 EUR/ton). This attributes these different FCRs (FCR of larvae reared on oat by-product: 2.0, FCR of larvae reared on maize by-product: 3.9).
The chemical composition of the consumed feed is not only affecting the insects’ growth, but also their body composition [22,34,35,36,37]. The highest percentage of crude protein content was found for larvae fed BSG (49.0%) and it was similar to the T. molitor larval protein content reported by Mancini et al. [22] and Oonincx et al. [24]. Interestingly, the BSG substrate had the highest protein content (21.3%) among the by-products tested, as well as wheat bran that was used as control (18.8%). Overall, the protein content of the larvae ranged between 29.2% and 49.0%, while it was observed that the highest the nitrogen percentage in the rearing substrate, the highest the larval protein content, as also suggested by previous studies [21,49,50]. Notably, in the case of the protein-poor AFML that only contained 5% protein, larvae fed with it had a protein content of 29.2%, which could be explained by the insects’ capability to regulate their bodies’ protein content, regardless of the protein content of the feed [18,36].
With respect to the larval lipid content, in most cases it was high (>28.2%), being in accordance with previous studies [16,24,50]. Conversely, larvae fed the BSG substrate had a significantly lower lipid content (9.0%), compared to the larvae reared on wheat bran (control) and the rest of the by-products. Melis et al. [23] also pointed out a noticeably lower lipid content for the larvae reared on BSG (6.39% FW), compared to the larvae reared on wheat bran (control) (12.42% FW). Finally, with regard to the dry matter content of T. molitor larvae, this was comparable to previously published results [16,49,50], with the relative humidity, the moisture source and feeding substrate being the major factors that affect the larval dry matter content [50].
5. Conclusions
Our investigation highlights the pragmatic feasibility of integrating oat by-product, brewery spent grains (BSG), and rice bran into the commercial production of T. molitor (yellow mealworm) larvae. By utilizing locally abundant agricultural residues, we not only streamline the economics of insect farming but also mitigate pressure on the agri-food sector, offering a sustainable solution for managing its by-products. This dual benefit shows the potential for synergistic relationships between the insect farming and agri-food industry, fostering a more circular and resilient approach to resource utilization. However, it is not feasible to directly translate our protocol and results to other agricultural by-products due to the inherent variability and unique characteristics of each substrate. Managing different by-products requires distinct approaches, as factors such as particle size play a crucial role. The further processing of the by-product may be necessary to ensure it is suitable for the consumption by the insects. Additionally, the efficiency of conversion from consumed feed to body mass varies depending on the specific properties of the by-product. For instance, in our experiment, we encountered challenges with the quantity of maize by-product provided to the insects, leading to a suboptimal larval weight.
In conclusion, our study emphasizes the critical importance of conducting large-scale experiments to ensure the practical applicability of research findings to industrial insect production. By bridging the gap between laboratory insights and real-world implementation, we facilitate the seamless integration of insect bioconversion technologies into commercial operations. By valorizing the agricultural by-products as valuable insect feedstocks, we not only improve the environmental footprint of food production but also create opportunities for enhanced nutritional and economic outcomes. This underscores the need for collaborative efforts between stakeholders to explore innovative solutions for sustainable resource management and pave the way for a more resilient food system.
Author Contributions
Conceptualization, C.I.R., C.G.A. and A.A.; methodology, C.A.-S. and M.V.; validation, C.G.A. and C.I.R.; formal analysis, M.V.; investigation, M.V. and C.A.-S.; resources, C.G.A. and A.A.; data curation, M.V.; writing—original draft preparation, M.V.; writing—review and editing, C.G.A., C.I.R., C.A.-S. and A.A.; visualization, C.I.R. and C.G.A.; supervision, C.I.R. and C.G.A.; project administration, C.I.R. and C.G.A.; funding acquisition, C.G.A. and C.I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of the project (ΚΜΡ6-0077802) entitled “EntoFEED” that is co-funded by Greece and European Union by the Action “Investment Plans of Innovation” in Central Macedonia under the framework of the Operational Program “Central Macedonia 2014–2020”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be available upon request.
Conflicts of Interest
Author Anastasios Anastasiadis was employed by the company Animal Feed Anastasiadi Single Member P.C., Akropotamia Kilkis Greece. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, X.; Zhang, T.; Zhao, Y.; Jiang, L.; Sui, X. Structural, extraction and safety aspects of novel alternative proteins from different sources. Food Chem. 2023, 436, 137712. [Google Scholar] [CrossRef]
- Mancini, M.C.; Antonioli, F. Italian consumers standing at the crossroads of alternative protein sources: Cultivated meat, insect-based and novel plant-based foods. Meat Sci. 2022, 193, 108942. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.M.; Lopez-Viso, C. Role of novel protein sources in sustainably meeting future global requirements. Proc. Nutr. Soc. 2021, 80, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Gasco, L. Insects as feed for livestock production. Science 2023, 379, 138–139. [Google Scholar] [CrossRef] [PubMed]
- FAO. How to Feed the World in 2050, Expert Meeting on How to Feed the World in 2050; FAO Headquarters: Rome, Italy, 2009; Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 10 February 2024).
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel protein sources for applications in meat-alternative products—Insight and challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef]
- Smetana, S.; Spykman, R.; Heinz, V. Environmental aspects of insect mass production. J. Insects Food Feed 2021, 7, 553–571. [Google Scholar] [CrossRef]
- Oonincx, D.G.; De Boer, I.J. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Rumpold, B.A.; Van der Fels-Klerx, H.J.; Tomberlin, J.K. Advancing edible insects as food and feed in a circular economy. J. Insects Food Feed 2021, 7, 935–948. [Google Scholar] [CrossRef]
- Frasnetti, E.; Sadeqi, H.; Lamastra, L. Integrating insects into the agri-food system of northern Italy as a circular economy strategy. Sustain. Prod. Consum. 2023, 43, 181–193. [Google Scholar] [CrossRef]
- Bermúdez-Serrano, I.M. Challenges and opportunities for the development of an edible insect food industry in Latin America. J. Insects Food Feed 2020, 6, 537–556. [Google Scholar] [CrossRef]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Niyonsaba, H.H.; Höhler, J.; Kooistra, J.; Van der Fels-Klerx, H.J.; Meuwissen, M.P.M. Profitability of insect farms. J. Insects Food Feed 2021, 7, 923–934. [Google Scholar] [CrossRef]
- Lienhard, A.; Rehorska, R.; Pöllinger-Zierler, B.; Mayer, C.; Grasser, M.; Berner, S. Future Proteins: Sustainable Diets for Tenebrio molitor Rearing Composed of Food By-Products. Foods 2023, 12, 4092. [Google Scholar] [CrossRef] [PubMed]
- Gourgouta, M.; Rumbos, C.I.; Michail, V.; Athanassiou, C.G. Valorization of agricultural side-streams for the rearing of larvae of the lesser mealworm, Alphitobius diaperinus (Panzer). Sustainability 2022, 14, 7680. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Oonincx, D.G.A.B.; Karapanagiotidis, I.T.; Vrontaki, M.; Gourgouta, M.; Asimaki, A.; Mente, E.; Athanassiou, C.G. Agricultural by-products from Greece as feed for yellow mealworm larvae: Circular economy at a local level. J. Insects Food Feed 2022, 8, 9–22. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Bliamplias, D.; Gourgouta, M.; Michail, V.; Athanassiou, C.G. Rearing Tenebrio molitor and Alphitobius diaperinus larvae on seed cleaning process byproducts. Insects 2021, 12, 293. [Google Scholar] [CrossRef]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio molitor L.) larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Aljewicz, M.; Stolarski, M.J. Influence of different diets on growth and nutritional composition of yellow mealworm. Foods 2022, 11, 3075. [Google Scholar] [CrossRef]
- Montalbán, A.; Sánchez, C.J.; Hernández, F.; Schiavone, A.; Madrid, J.; Martínez-Miró, S. Effects of agro-industrial byproduct-based diets on the growth performance, digestibility, nutritional and microbiota composition of mealworm (Tenebrio molitor L.). Insects 2022, 13, 323. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Melis, R.; Braca, A.; Sanna, R.; Spada, S.; Mulas, G.; Fadda, M.L.; Sassu, M.M.; Serra, G.; Anedda, R. Metabolic response of yellow mealworm larvae to two alternative rearing substrates. Metabolomics 2019, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Adamaki-Sotiraki, C.; Rumbos, C.I.; Athanassiou, C.I.; Lalas, S.I. Waste Orange Peels as a Feed Additive for the Enhancement of the Nutritional Value of Tenebrio molitor. Foods 2023, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Fasce, B.; Ródenas, L.; López, M.C.; Moya, V.J.; Pascual, J.J.; Cambra-López, M. Nutritive value of wheat bran diets supplemented with fresh carrots and wet brewers’ grains in yellow mealworm. J Insect Sci. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Brai, A.; Vagaggini, C.; Pasqualini, C.; Poggialini, F.; Tarchi, F.; Francardi, V.; Dreassi, E. Use of distillery by-products as Tenebrio molitor mealworm feed supplement. J. Insects Food Feed 2023, 9, 611–623. [Google Scholar] [CrossRef]
- López-Gámez, G.; del Pino-García, R.; López-Bascón, M.A.; Verardo, V. Improving Tenebrio molitor Growth and Nutritional Value through Vegetable Waste Supplementation. Foods 2024, 13, 594. [Google Scholar] [CrossRef]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth performance and nutrient composition of mealworms (Tenebrio molitor) fed on fresh plant materials-supplemented diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef]
- Rovai, D.; Ortgies, M.; Amin, S.; Kuwahara, S.; Schwartz, G.; Lesniauskas, R.; Garza, J.; Lammert, A. Utilization of carrot pomace to grow mealworm larvae (Tenebrio Molitor). Sustainability 2021, 13, 9341. [Google Scholar] [CrossRef]
- European Commission (EC). EU Commission Regulation 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Off. J. Eur. Union L 2017, 138, 92–116. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0893&from=EN (accessed on 10 February 2024).
- European Commission (EC). Commission Regulation (EC) 2021/1372 of 17 August 2021 amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other Than Fur Animals, with Protein Derived from Animals. Off. J. Eur. Union L 2021, 295, 1–17. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L:2021:295:FULL&from=EN (accessed on 10 February 2024).
- European Food Safety Authority (EFSA) NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6343. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.; Subramanian, S.; Ekesi, E.; Van Huis, A.; Borgemeister, C. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef] [PubMed]
- Van Broekhoven, S.; Oonincx, D.G.; Van Huis, A.; Van Loon, J.J. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol 2002, 95, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Deruytter, D.; Coudron, C.L.; Teerlinck, S. Influence of crate size, oviposition time, number of adults and cannibalism on the reproduction of Tenebrio molitor. J. Insects Food Feed 2019, 5, 247–255. [Google Scholar] [CrossRef]
- Adamaki-Sotiraki, C.; Deruytter, D.; Rumbos, C.I.; Athanassiou, C.G. Cross-breeding of Tenebrio molitor strains from a large-scale perspective. J. Insects Food Feed 2023, 1, 1–10. [Google Scholar] [CrossRef]
- Adamaki-Sotiraki, C.; Choupi, D.; Vrontaki, M.; Rumbos, C.I.; Athanassiou, C.G. Go local: Enhancing sustainable production of Tenebrio molitor through valorization of locally available agricultural byproducts. J. Environ. Manag. 2024, 355, 120545. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ramos, J.A.; Kay, S.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Morphometric analysis of instar variation in Tenebrio molitor (Coleoptera: Tenebrionidae). Ann. Entomol. 2015, 108, 146–159. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Karapanagiotidis, I.T.; Psofakis, P.; Mente, E.; Malandrakis, E.; Golomazou, E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac. Nutr. 2018, 25, 3–14. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Kelstrup, H.C.; Emery, V. Self-selection of agricultural by-products and food ingredients by Tenebrio molitor (Coleoptera: Tenebrionidae) and impact on food utilization and nutrient intake. Insects 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tomberlin, J.K. Comparing selected life-history traits of black soldier fly (Diptera: Stratiomyidae) larvae produced in industrial and bench-top-sized containers. J. Insect Sci. 2020, 20, 25. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Lee, K.Y.; Yoon, H.J.; Kim, N.J. Effects of Brewer’s spent grain (BSG) on larval growth of mealworms, Tenebrio molitor (Coleoptera: Tenebrionidae). Int. J. Indust Entomol. IJIE 2016, 32, 41–48. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Self-selection of feeding substrates by Tenebrio molitor larvae of different ages to determine optimal macronutrient intake and the influence on larval growth and protein content. Insects 2022, 13, 657. [Google Scholar] [CrossRef]
- Riudavets, J.; Castañé, C.; Agustí, N.; Del Arco, L.; Diaz, I.; Castellari, M. Development and biomass composition of Ephestia kuehniella (Lepidoptera: Pyralidae), Tenebrio molitor (Coleoptera: Tenebrionidae), and Hermetia illucens (Diptera: Stratiomyidae) reared on different byproducts of the agri-food industry. J. Insect Sci. 2020, 20, 17. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, N.; Benning, R. Determination of moisture and protein content in living mealworm larvae (Tenebrio molitor L.) using near-infrared reflectance spectroscopy (NIRS). Insects 2022, 13, 560. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).