Cultivation of Black Soldier Fly (Hermetia illucens) Larvae for the Valorization of Spent Coffee Ground: A Systematic Review and Bibliometric Study
Abstract
1. Introduction
1.1. Black Soldier Fly (BSF)
1.2. Spent Coffee Grounds (SCG) as a Potential Organic Substrate
1.3. The Effects of Feeding SCG to BSF
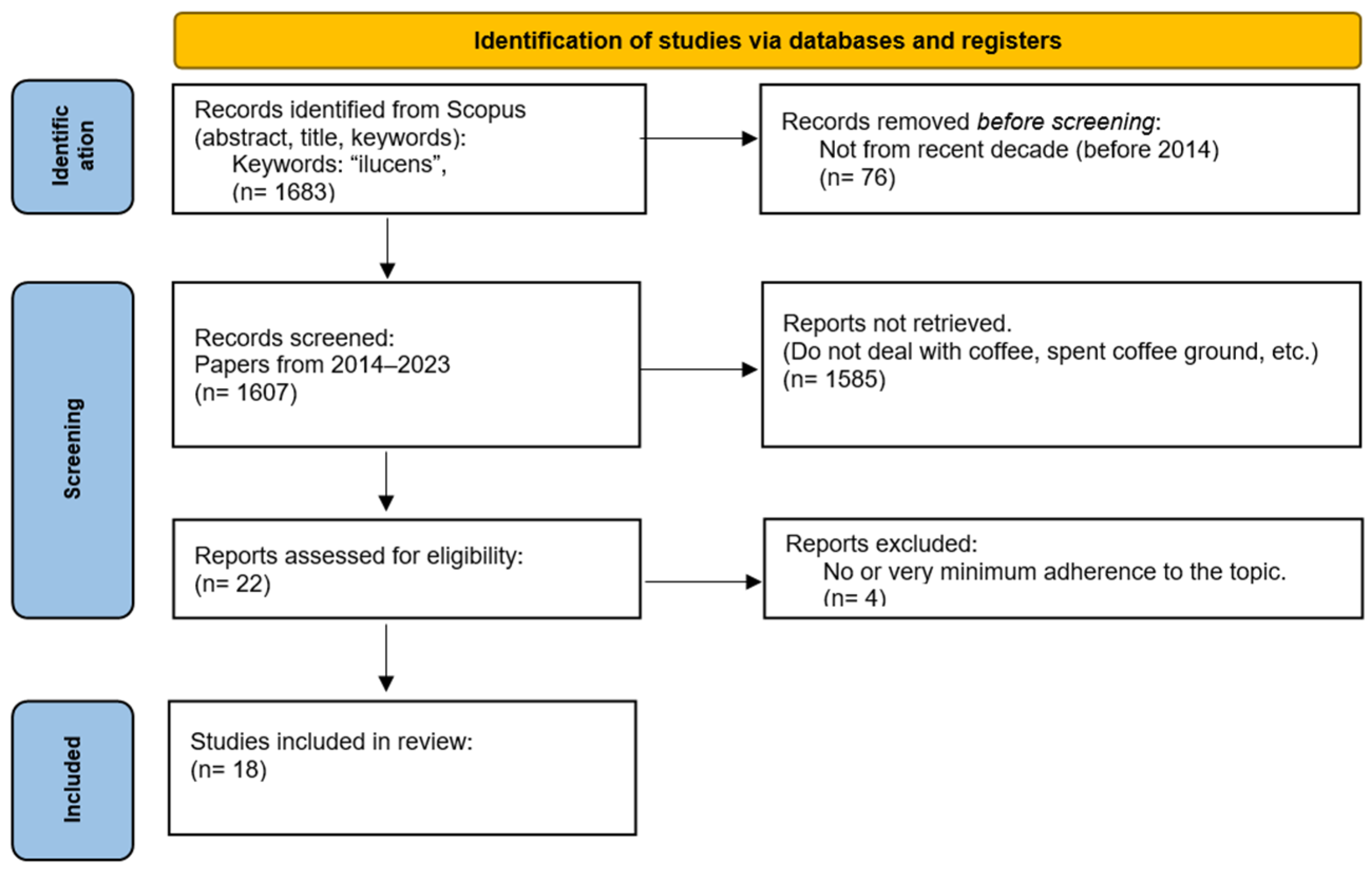
2. Materials and Methods
3. Results
3.1. Cultivation of BSF Larvae Using SCG and/or Coffee Parts
| No. | Name of Rearing Substrate | Rearing Conditions | Results | Reference |
|---|---|---|---|---|
| 1. | SCG | BSF larvae, 7 days old, feeding rate of 200, 100, 50, 25, and 12.5 mg/larvae/day | Best results for feeding with 200 mg/larvae/day
| [52] |
| 2. | Coffea arabica (Castillo variety (0.5% caffeine)) | BSF larvae, 100 mg/larvae/day | Reduction percentage of coffee pulp = 62.88% (wet basis) Efficiency of conversion of ingested food: 7.89% | [53] |
| BSF larvae, 160 mg/larvae/day | Highest weight reduction index (wet basis) is 0.85% | |||
| BSF larvae, 200 mg/larvae/day | Highest weight = 115.9 mg. Shortest average development time = 38.65 days. | |||
| 3. | Coffee silverskin (100%) | BSF larvae of 6 days old, in 9 groups × 5 replicates × 150 larvae = 6750 larvae. Feeding rate = 100 mg/day. Chamber temperature = 27 ± 1 °C, relative humidity (RH) = 65 ± 5%, 24 h darkness in plastic boxes 28 × 19 × 14 cm3. Note: Weight evolution of BSF larvae is not available | Best inclusion rate = Schizochytrium sp. 10%. The prepupae of BSF show increased lipid and protein content in prepupae, and high amounts of unsaturated fatty acids, especially omega-3. There is no information on the weight evolution of BSF. The BSF larvae were harvested when the tegument color changed from white to black. | [54] |
| Coffee silverskin with algae (Schizochytrium sp., 0, 5, 10, 20, 25%) | ||||
| Coffee silverskin with algae (Isochrysis galbana, 0, 5, 10, 20, 25%) | ||||
| 4. | Coffee silverskin (100%) | BSF larvae of 6 days old, in 9 groups × 5 replicates × 150 larvae = 6750 larvae. Feeding rate = 100 mg/day. Note: Weight evolution of BSF larvae is not available | High prevalence of tetracycline resistance genes. No significant effect on AR gene distribution in larvae. | [56] |
| Coffee silverskin enriched with Schizochytrium limacinum, (5, 10, 20, 25%) | ||||
| Coffee silverskin with Isochrysis galbana (5, 10, 20, 25%) | High prevalence of tetracycline resistance genes. Significant accumulation of AR genes in frass samples, especially at high percentages (>20%) of I. galbana. | |||
| 5. | Coffee silverskin (100%) | BSF larvae of 6 days old, in 9 groups × 5 replicates × 150 larvae = 6750 larvae. Feeding rate = 100 mg/day. Chamber temperature = 27 ± 1 °C, 65 ± 5% RH, 24 h dark photoperiod, in plastic boxes 28 × 19 × 14 cm3. Note: Weight evolution of BSF larvae is not available | Dominance of Paenibacillus in the larvae’s microbiota | [57] |
| Coffee silverskin enriched with Schizochytrium limacinum, (5, 10, 20, 25%) | Presence of Enterococcus, Lysinibacillus, Morganella, and Paenibacillus in the larvae’s microbiota. Dominance of Brevundimonas and Alcaligenes in frass. | |||
| Coffee silverskin with Isochrysis galbana, (5, 10, 20, 25%) | High relative abundances of Brevundimonas, Enterococcus, Paracoccus, and Paenibacillus in larvae. Predominance of Brevundimonas in frass. | |||
| 6. | Brewery spent grains, tomato peels and seeds, cows’ milk, whey, grape stalks, bread dough, and SCG. | Chamber (32.5 × 32.5 × 32.5 cm3) temperature = 27 ± 0.5 °C, 60–70% RH, with light:dark = 16:8 h, with 400–500 larvae per chamber. |
| [59] |
| 7. | SCG | BSF larvae and prepupae fed for 35 days. Feeding of 6.8 kg feed in container 0.9 × 1.2 × 1.5 m3. | Survival: 45%. Longer and heavier BSFP from dough. Fatty acids higher in BSFL. Frass: Higher potassium (~1.00% dry matter (DM)), lowest phosphorus (<0.30% DM), moderate nitrogen content (~3.25% DM) Larval length = 16.86 ± 0.29 mm Larval weight = 0.11 ± 0.01 g Net production = 0.75 ± 0.58 g/day/m3 | [45] |
| Donut dough | Survival: 24%. Longer and heavier BSFP from dough. Lowest nitrogen content (~2.75% DM), lowest potassium (<0.25% DM), lowest calcium (<0.1% DM). Higher amino acid composition in BSFL. Larval length = 21.44 ± 0.59 mm Larval weight = 0.23 ± 0.01 g Net production = 0.60 ± 1.01 g/day/m3 | |||
| Blend (coffee and dough, 1:1) | Survival: 81%. Stage and food affected protein, lipid, glycogen content. Frass: Highest nitrogen (~4.20% DM). Comparable to soybean meal and organic fertilizers. Larval length = 19.12 ± 0.72 mm Larval weight = 0.18 ± 0.01 g Net production = 4.42 ± 1.02 g/day/m3 | |||
| 8. | SCG | BSF rearing with controlled climate chamber (14.5 × 9.5 × 9.5 cm3, 30 °C, 70% RH, with 24 h dark photoperiod. Total amount of feed per larva = 240 mg in DM. Variation: 200 and 300 larvae per container (1.45 and 2.17 larvae per cm2, respectively. | Larvae underperformed. Development time = 35 days. Larval yield 0.61–0.77 g in DM. | [61] |
| Brewer’s spent grain (BSG) | Better results than those solely reared with SCG. Development time = 9 days. Larval yield 6.02–8.53 g in DM (for 200 and 300 larvae per container, respectively). | |||
| SCG and/or BSG and/or brewers’ yeast (BY) | Acceptable values in substrate mass reduction (18.48–44.80%, SCG + BY and BSG + BY, respectively), protein conversion rate (17.55–30.15%, SCG + BY and BSG + BY, respectively), and bioconversion rate (6.25–17.22%, SCG + BY and BSG + BY, respectively). Development time = 8–17 days (for BSG + BY, and SCG + BY, respectively). Larval yields 2.99–12.72 g in DM (for SCG + BY 200 and BSG + BY 300, respectively). For SCG + BSG + BY, the development time is 12 days, with larval yield of 6.93–10.53 g in DM (for 200 and 300 larvae, respectively). | |||
| Reference feed (layer feed BΩ-321 (Viozois S.A., Athens, Greece)) | Highest values in substrate mass reduction (67.38%), protein conversion rate (45.77%), and bioconversion rate (23.28%) among all feeds. Development time = 11 days. Larval yield 11.32–16.78 g in DM (for 200 and 300 larvae, respectively). | |||
| 9. | Mixture of sludge-containing media (S), with brewery spent grains (BSG), coffee waste (C), and whey (W). | Fed to BSF with composition of S:BSG:C:W = 50:10:10:30, reared for 23 days. | Larvae did not complete their development, with very poor performance of: Final height = ~6 mm Final weight ≤ 10 mg | [46] |
| Control (feed from Entocycle.com, with unknown composition) | Final height = ~15–18 mm Final weight = 94.5 ± 7.2 mg | |||
| S: BSG:C:W = 50:10:30:10 | Final height = ~15–18 mm Final weight = 81.9 ± 5.3 mg | |||
| S: BSG:C:W = 50:50:0:0 | Final height = ~15–18 mm Final weight = 91.0 ± 0.1 mg | |||
| S: BSG:C:W = 50:30:10:10 | Final height = ~15–18 mm Final weight = 84.0 ± 6.0 mg | |||
| 10. | Sweet potato, 2 weeks | BSF larvae cultivated for 2 weeks. Temperature controlled room of 3.6 × 3.6 m2, 30 °C, 60–64% RH. |
| [47] |
| SCG, 2 weeks |
| |||
| Dough, 2 weeks |
| |||
| 11. | Fruit and vegetable pulp residue (apple 12%, pineapple 12%, carrot 25%, tomato 44%, guava 5%, beetroot 1.5%, celery 0.5%) mixed with 0% fermented SCG | Larvae kept in plastic containers with diameter 24.5 cm × height 12.5 cm, with 28–34 °C, 70–90% RH, light:dark = 13 h: 11 h. Feeding rate = 200 mg/larvae/day. |
| [62] |
| Pulp with 20% fermented SCG |
| |||
| Pulp with 40% fermented SCG |
| |||
| Pulp with 60% fermented SCG |
| |||
| Pulp with 80% fermented SCG |
| |||
| Pulp with 100% fermented SCG |
| |||
| 12. | Fresh SCG (aged < 1 month, 50% water, incorporated into soil) | BSF larvae grown in the first chamber (275 × 250 × 210 cm3, 25–28 °C, 60% RH) for 3–5 days. After that, they will be grown in the second chamber (275 × 80 × 250 cm3, 28 ± 1.5 °C, 40% RH, 24 h dark photoperiod) for 12 days. Growing of radish and tomato = 30 days | Inhibited plant growth (radish and tomato, 30 days) and development; reduced slug herbivory (Arion atar, Deroceras laeve, Derocerus reticulatum, and Lehmannia marginata). Height of radish plant = ~9 cm Radish leaf consumed by slug ≤ 5% Height of tomato plant = ~7 cm Area of tomato leaf consumed by slug (SCG mixed with soil or layered on top of the soil) = 0 mm2, <1 mm2 | [37] |
| SCG (aged 7 months, 1 cm top dressing) | Promoted growth, while simultaneously reduced slug herbivory through repellent and host quality effects. Height of radish plant = ~10 cm Radish leaf consumed by slug ≤ 5% Height of tomato plant = ~8 cm Area of tomato leaf consumed by slug (SCG mixed with soil or layered on top of the soil) = 0 mm2, <3 mm2 | |||
| SCG (aged 14 months, incorporated into soil) | Promoted plant growth, while having no effect on slug herbivory. Height of radish plant = ~13 cm Radish leaf consumed by slug (SCG mixed with soil, or layered on top of the soil) = 10–80% Height of tomato plant = ~15 cm Area of tomato leaf consumed by slug (SCG mixed with soil or layered on top of the soil) = 10 mm2, 35 mm2 | |||
| SCG-derived BSF frass (incorporated into soil, or 1 cm top dressing) | Reduced development of plants, yellowing, reduced height. Height of radish plant = ~5 cm Radish leaf consumed by slug (SCG mixed with soil or layered on top of the soil) = not available Height of tomato plant = ~2.5 cm Area of tomato leaf consumed by slug (SCG mixed with soil or layered on top of the soil) = not available |
3.2. Utilization of Coffee-Reared BSF Larvae in Fisheries
| No. | Name of Rearing Substrate | Rearing Conditions | Produced BSF Fed to | Results | Reference |
|---|---|---|---|---|---|
| 1. | BSF reared on coffee silverskin enriched with 10% Schizochytrium sp. | BSF as substitution of fish meal (0, 25, 50, and 75%) | Zebrafish (Danio rerio) | At 0% (baseline) and 25%, zebrafishes show standard responses based on the control diet. The 50% substitution gives the best compromise between sustainability and proper fish growth. However, at 75% and 100% substitution, severe hepatic steatosis was observed, along with microbiota modification, increased lipid content, fatty acid modification, and higher expression of stress and immune response markers. | [65] |
| 2. | BSF reared on coffee silverskin with 10% Schizochytrium sp. microalgae | BSF as substitution of fish meal (0, 25, 50, 75, and 100%) for 6 months | Zebrafish (Danio rerio) | This study employed qPCR to assess the dynamics of antibiotic resistance (AR) genes in fish feed, including those with insect meal ingredients. Resistant genes studied: macrolide-lincosamide-streptogramin B (MLSB) [erm(A), erm(B), erm(C)], vancomycin (vanA, vanB), tetracyclines [tet(M), tet(O), tet(S), tet(K)], β-lactams (mecA, blaZ), and aminoglycosides [aac-aph]. Findings in diet samples: Detected: erm(B), tet(K), tet(M), tet(O), and tet(S). Not Detected: mecA, vanA, vanB, and aac-aph. Findings in zebrafish (juvenile and reproductive stages): Never Detected: erm(A), erm(C), vanB, and aac-aph. Widespread: erm(B), tet(M), and tet(S). | [66] |
| 0% BSF larvae | erm(A), erm(C), vanB, aac-aph not detected in zebrafish at any stage. | ||||
| 3. | Marine-based substrates | Zebrafish |
| [67] | |
| Fish discards |
| ||||
| Fish discards with coffee silverskin with Schyzochytrium sp. |
| ||||
| Coffee silverskin enriched with 10% of Schizochytrium sp. | 100% fish meal replacement | Affected fish stress response, oocytes maturation stages, spawning, and hatching success. | |||
| 4. | Coffee silverskin enriched with 10% of Schizochytrium sp. | 0% fish meal replacement | Zebrafish | No impairment observed in zebrafish physiological responses | [48] |
| 20% fish meal replacement | |||||
| 50% fish meal replacement | |||||
| 75% fish meal replacement | Affected fish stress response, oocytes maturation stages, spawning, and hatching success. | ||||
| 100% fish meal replacement | |||||
| 5. | Coffee by-products | BSF larvae and frass | Zebrafish (Danio rerio) | Zebrafishes fed with BSF larvae reared on coffee by products show:
| [68] |
| Mixture of vegetables | Zebrafishes fed by BSF larvae reared on mixture of vegetable show:
| ||||
| 6. | BSF meal (control) | Fed 6 weeks | Oncorhynchus mykiss | Well accepted; no impairment in fish growth, gut and liver health, or marketable characteristics. | [49] |
| 3% BSF prepupae meal | Fed 6 weeks | ||||
| 20% BSF prepupae meal | Fed 6 weeks | Well accepted; increased immuno-related gene expression and slight reduction of fillet redness and yellowness. |
3.3. Bibliometric Analysis of the Effects of Feeding SCG to BSF
- BSF reared with SCG for aquaculture (red)
- (Bio)chemical analysis related to BSF and SCG (green)
- BSF and bioconversion (blue)
- SCG and other substrates (yellow)

3.4. Bibliometric Analysis of a Single Keyword: “Black Soldier Fly”
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, R.J.; Nielsen, S.H.M.; Johansen, M.; Nielsen, F.K.N.; Dragsbæk, F.B.; Sørensen, O.S.B.; Eriksen, N.T. Metabolic performance of black soldier fly larvae during entomoremediation of brewery waste. J. Appl. Entomol. 2023, 147, 423–431. [Google Scholar] [CrossRef]
- Bekker, N.S.; Heidelbach, S.; Vestergaard, S.Z.; Nielsen, M.E.; Riisgaard-Jensen, M.; Zeuner, E.J.; Bahrndorff, S.; Eriksen, N.T. Impact of substrate moisture content on growth and metabolic performance of black soldier fly larvae. Waste Manag. 2021, 127, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T. Dynamic modelling of feed assimilation, growth, lipid accumulation, and CO2 production in black soldier fly larvae. PLoS ONE 2022, 17, e0276605. [Google Scholar] [CrossRef]
- Laganaro, M.; Bahrndorff, S.; Eriksen, N.T. Growth and metabolic performance of black soldier fly larvae grown on low and high-quality substrates. Waste Manag. 2021, 121, 198–205. [Google Scholar] [CrossRef]
- Mangindaan, D.; Kaburuan, E.; Meindrawan, B. Black Soldier Fly Larvae (Hermetia illucens) for Biodiesel and/or Animal Feed as a Solution for Waste-Food-Energy Nexus: Bibliometric Analysis. Sustainability 2022, 14, 13993. [Google Scholar] [CrossRef]
- Banks, I.J.; Gibson, W.T.; Cameron, M.M. Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Trop. Med. Int. Health 2014, 19, 14–22. [Google Scholar] [CrossRef]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrügg, C.; Lindström, A.; Vinnerås, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458–460, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sanchez-Muros, M.-J.; Venegas, E.; Martinez-Sanchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422–423, 193–201. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Elhag, O.; Zhou, D.; Song, Q.; Soomro, A.A.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Screening, expression, purification and functional characterization of novel antimicrobial peptide genes from Hermetia illucens (L.). PLoS ONE 2017, 12, e0169582. [Google Scholar] [CrossRef] [PubMed]
- Badwan, A.A.; Rashid, I.; Al Omari, M.M.H.; Darras, F.H. Chitin and Chitosan as Direct Compression Excipients in Pharmaceutical Applications. Mar. Drugs 2015, 13, 1519–1547. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing methods for the black soldier fly (diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef]
- Rummel, P.S.; Beule, L.; Hemkemeyer, M.; Schwalb, S.A.; Wichern, F. Black Soldier Fly Diet Impacts Soil Greenhouse Gas Emissions from Frass Applied as Fertilizer. Front. Sustain. Food Syst. 2021, 5, 709993. [Google Scholar] [CrossRef]
- Rehman, K.U.; Cai, M.; Xiao, X.; Zheng, L.; Wang, H.; Soomro, A.A.; Zhou, Y.; Li, W.; Yu, Z.; Zhang, J. Cellulose decomposition and larval biomass production from the co-digestion of dairy manure and chicken manure by mini-livestock (Hermetia illucens L.). J. Environ. Manag. 2017, 196, 458–465. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans—A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Pina, G.; Vergara-Castaneda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Chen, X.-E.; Mangindaan, D.; Chien, H.-W. Green sustainable photothermal materials by spent coffee grounds. J. Taiwan Inst. Chem. Eng. 2022, 137, 104259. [Google Scholar] [CrossRef]
- Chien, H.-W.; Kuo, C.-J.; Kao, L.-H.; Lin, G.-Y.; Chen, P.-Y. Polysaccharidic spent coffee grounds for silver nanoparticle immobilization as a green and highly efficient biocide. Int. J. Biol. Macromol. 2019, 140, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.-C.; Chen, Y.-C.; Zheng, N.-C.; Mangindaan, D.; Chien, H.-W. A low-cost and environmentally-friendly chitosan/spent coffee grounds composite with high photothermal properties for interfacial water evaporation. J. Ind. Eng. Chem. 2023, 126, 283–291. [Google Scholar] [CrossRef]
- Mangindaan, D.; Lin, G.-Y.; Kuo, C.-J.; Chien, H.-W. Biosynthesis of silver nanoparticles as catalyst by spent coffee ground/recycled poly(ethylene terephthalate) composites. Food Bioprod. Process. 2020, 121, 193–201. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent Coffee Grounds as a Versatile Source of Green Energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.M.; Melo, A.C.; Martins, A.A.; Mata, T.M. Spent coffee grounds for biodiesel production and other applications. Clean Technol. Environ. Policy 2014, 16, 1423–1430. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Romero Garcia, L.I.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Ayodele, O.O.; Adekunle, A.E.; Adesina, A.O.; Pourianejad, S.; Zentner, A.; Dornack, C. Stabilization of anaerobic co-digestion of biowaste using activated carbon of coffee ground biomass. Bioresour. Technol. 2021, 319, 124247. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Rios, M.B.; Herrera, T.; Rodriguez-Bertos, A.; Nuñez, F.; San Andres, M.I.; Sanchez-Fortun, S.; Del Castillo, M.D. Coffee Silverskin Extract: Nutritional Value, Safety and Effect on Key Biological Functions. Nutrients 2019, 7, 2693. [Google Scholar] [CrossRef] [PubMed]
- Mitraka, G.-C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability 2021, 13, 8818. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a new potential functional ingredient: Coffee silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Horgan, F.G.; Floyd, D.; Mundaca, E.A.; Crisol-Martínez, E. Spent Coffee Grounds Applied as a Top-Dressing or Incorporated into the Soil Can Improve Plant Growth While Reducing Slug Herbivory. Agriculture 2023, 13, 257. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Howieson, J.; Foysal, M.J.; Fotedar, R. Transformation of fish waste protein to Hermetia illucens protein improves the efficacy of poultry by-products in the culture of juvenile barramundi, Lates calcarifer. Sci. Total Environ. 2021, 796, 149045. [Google Scholar] [CrossRef]
- Jalil, N.A.A.; Abdullah, S.H.; Ahmad, I.K.; Basri, N.E.A.; Mohamed, Z.S. Decomposition of food waste from protein and carbohydrate sources by black soldier fly larvae, Hermetia illucens L. J. Environ. Biol. 2021, 42, 756–761. [Google Scholar] [CrossRef]
- Gold, M.; Ireri, D.; Zurbrügg, C.; Fowles, T.; Mathys, A. Efficient and safe substrates for black soldier fly biowaste treatment along circular economy principles. Detritus 2021, 16, 31–40. [Google Scholar] [CrossRef]
- Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of black soldier fly larvae reared on organic side-streams. Sustainability 2021, 13, 12953. [Google Scholar] [CrossRef]
- Veldkamp, T.; van Rozen, K.; Elissen, H.; van Wikselaar, P.; van der Weide, R. Bioconversion of digestate, pig manure and vegetal residue-based waste operated by black soldier fly larvae, Hermetia illucens L. (diptera: Stratiomyidae). Animals 2021, 11, 3082. [Google Scholar] [CrossRef]
- Addeo, N.F.; Vozzo, S.; Secci, G.; Mastellone, V.; Piccolo, G.; Lombardi, P.; Parisi, G.; Asiry, K.A.; Attia, Y.A.; Bovera, F. Different combinations of butchery and vegetable wastes on growth performance, chemical-nutritional characteristics and oxidative status of black soldier fly growing larvae. Animals 2021, 11, 3515. [Google Scholar] [CrossRef]
- Borel, P.; Hammaz, F.; Morand-Laffargue, L.; Creton, B.; Halimi, C.; Sabatier, D.; Desmarchelier, C. Using black soldier fly larvae reared on fruits and vegetables waste as a sustainable dietary source of provitamin a carotenoids. Food Chem. 2021, 359, 129911. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N.; Sinha, A.K. Conversion of spent coffee and donuts by black soldier fly (Hermetia illucens) larvae into potential resources for animal and plant farming. Insects 2021, 12, 332. [Google Scholar] [CrossRef]
- Villa, R.; Muñoz, H.M.B.; Jawiarczyk, N.; Vaya, A.M. Black soldier fly biorefinery: A novel upcycling route for municipal biosolids. In Clean Energy and Resources Recovery: Biomass Waste Based Biorefineries; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 487–500. [Google Scholar]
- Romano, N.; Fischer, H.; Kumar, V.; Francis, S.A.; Sinha, A.K. Productivity, conversion ability, and biochemical composition of black soldier fly (Hermetia illucens) larvae fed with sweet potato, spent coffee or dough. Int. J. Trop. Insect Sci. 2022, 42, 183–190. [Google Scholar] [CrossRef]
- Chemello, G.; Zarantoniello, M.; Randazzo, B.; Gioacchini, G.; Truzzi, C.; Cardinaletti, G.; Riolo, P.; Olivotto, I. Effects of black soldier fly (Hermetia illucens) enriched with Schizochytrium sp. on zebrafish (Danio rerio) reproductive performances. Aquaculture 2022, 550, 737853. [Google Scholar] [CrossRef]
- Ratti, S.; Zarantoniello, M.; Chemello, G.; Giammarino, M.; Palermo, F.A.; Cocci, P.; Mosconi, G.; Tignani, M.V.; Pascon, G.; Cardinaletti, G.; et al. Spirulina-enriched Substrate to Rear Black Soldier Fly (Hermetia illucens) Prepupae as Alternative Aquafeed Ingredient for Rainbow Trout (Oncorhynchus mykiss) Diets: Possible Effects on Zootechnical Performances, Gut and Liver Health Status, and Fillet Quality. Animals 2023, 13, 173. [Google Scholar] [CrossRef]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant. Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Mangindaan, D.; Adib, A.; Febrianta, H.; Hutabarat, D.J.C. Systematic Literature Review and Bibliometric Study of Waste Management in Indonesia in the COVID-19 Pandemic Era. Sustainability 2022, 14, 2556. [Google Scholar] [CrossRef]
- Permana, A.D.; Ramadhani Eka Putra, J.E.N. Growth of Black Soldier Fly (Hermetia illucens) Larvae Fed on Spent Coffee Ground. IOP Conf. Ser. Earth Environ. Sci. 2018, 187, 012070. [Google Scholar] [CrossRef]
- Ospina-Granobles, K.; Carrejo-Gironza, N. Efficiency of bioconversion of coffee pulp using Hermetia illucens (Diptera: Stratiomyidae) Larvae. Pertanika J. Trop. Agric. Sci. 2021, 44, 237–254. [Google Scholar] [CrossRef]
- Truzzi, C.; Giorgini, E.; Annibaldi, A.; Antonucci, M.; Illuminati, S.; Scarponi, G.; Riolo, P.; Isidoro, N.; Conti, C.; Zarantoniello, M.; et al. Fatty acids profile of black soldier fly (Hermetia illucens): Influence of feeding substrate based on coffee-waste silverskin enriched with microalgae. Anim. Feed Sci. Technol. 2020, 259, 114309. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The Effects of Diet Formulation on the Yield, Proximate Composition, and Fatty Acid Profile of the Black Soldier Fly (Hermetia illucens L.) Prepupae Intended for Animal Feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef]
- Milanovic, V.; Roncolini, A.; Cardinali, F.; Garofalo, C.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Corsi, L.; Isidoro, N.; Zarantoniello, M.; et al. Occurrence of antibiotic resistance genes in Hermetia illucens larvae fed coffee silverskin enriched with Schizochytrium limacinum or Isochrysis galbana microalgae. Genes 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Ferrocino, I.; Corvaglia, M.R.; Roncolini, A.; Milanović, V.; Garofalo, C.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Jamshidi, E.; et al. Microbial dynamics in rearing trials of Hermetia illucens larvae fed coffee silverskin and microalgae. Food Res. Int. 2021, 140, 110028. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; De Vuyst, L.; Zhang, S.J.; De Bruyn, F.; Verce, M.; Torres, J.; Callanan, M.; Moccand, C.; Weckx, S. Temporal shotgun metagenomics of an Ecuadorian coffee fermentation process highlights the predominance of lactic acid bacteria. Curr. Res. Biotechnol. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Montevecchi, G.; Zanasi, L.; Bortolini, S.; Macavei, L.I.; Masino, F.; Maistrello, L.; Antonelli, A. Lipid profile and growth of black soldier flies (Hermetia illucens, Stratiomyidae) reared on by-products from different food chains. J. Sci. Food Agric. 2020, 100, 3648–3657. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Sideris, V.; Georgiadou, M.; Papadoulis, G.; Mountzouris, K.; Tsagkarakis, A. Effect of processed beverage by-product-based diets on biological parameters, conversion efficiency and body composition of Hermetia illucens (L) (diptera: Stratiomyidae). Insects 2021, 12, 475. [Google Scholar] [CrossRef]
- Khaekratoke, K.; Laksanawimol, P.; Thancharoen, A. Use of fermented spent coffee grounds as a substrate supplement for rearing black soldier fly larvae, Hermetia illucens (L), (Diptera: Stratiomyidae). PeerJ 2022, 10, e14340. [Google Scholar] [CrossRef]
- Lopes, I.G.; Yong, J.W.H.; Lalander, C. Frass derived from black soldier fly larvae treatment of biodegradable wastes. A critical review and future perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutiérrez-Ginés, M.J. Black Soldier Fly-based bioconversion of biosolids creates high-value products with low heavy metal concentrations. Resour. Conserv. Recycl. 2022, 180, 106149. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Zimbelli, A.; Randazzo, B.; Compagni, M.D.; Truzzi, C.; Antonucci, M.; Riolo, P.; Loreto, N.; Osimani, A.; Milanović, V.; et al. Black Soldier Fly (Hermetia illucens) reared on roasted coffee by-product and Schizochytrium sp. as a sustainable terrestrial ingredient for aquafeeds production. Aquaculture 2020, 518, 734659. [Google Scholar] [CrossRef]
- Milanović, V.; Cardinali, F.; Aquilanti, L.; Maoloni, A.; Garofalo, C.; Zarantoniello, M.; Olivotto, I.; Riolo, P.; Ruschioni, S.; Isidoro, N.; et al. Quantitative assessment of transferable antibiotic resistance genes in zebrafish (Danio rerio) fed Hermetia illucens-based feed. Anim. Feed Sci. Technol. 2021, 277, 114978. [Google Scholar] [CrossRef]
- Rodrigues, D.P.; Ameixa, O.M.C.C.; Vázquez, J.A.; Calado, R. Improving the Lipid Profile of Black Soldier Fly (Hermetia illucens) Larvae for Marine Aquafeeds: Current State of Knowledge. Sustainability 2022, 14, 6472. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Roncolini, A.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; Franciosi, E.; Tuohy, K.; Olivotto, I.; et al. Hermetia illucens in diets for zebrafish (Danio rerio): A study of bacterial diversity by using PCR-DGGE and metagenomic sequencing. PLoS ONE 2019, 14, e0225956. [Google Scholar] [CrossRef]
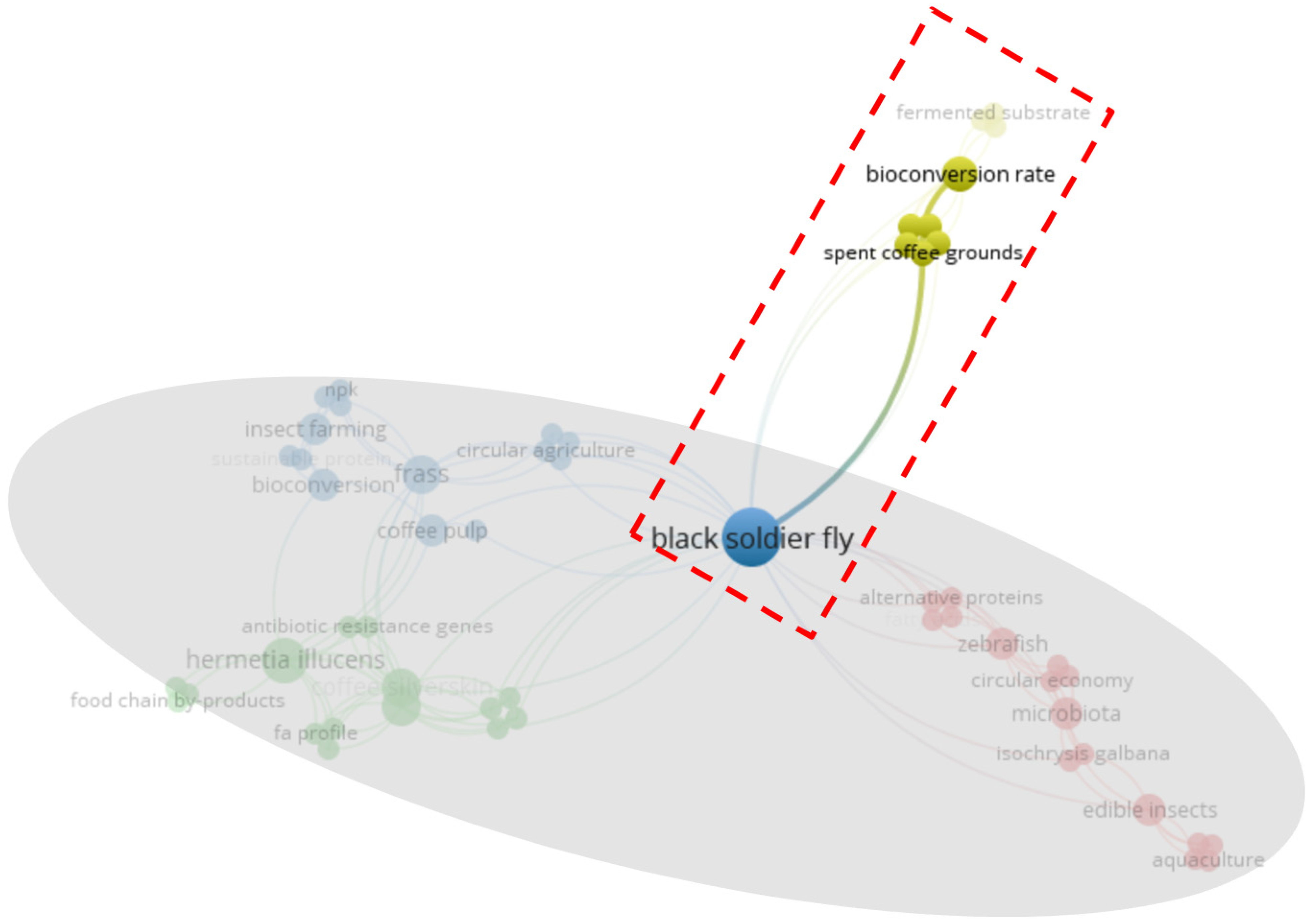
| Cluster 1 | Weight | Cluster 2 | Weight | Cluster 3 | Weight | Cluster 4 | Weight |
|---|---|---|---|---|---|---|---|
| zebrafish | 9 | coffee silverskin | 13 | black soldier fly | 28 | bioconversion rate | 9 |
| edible insects | 8 | Hermetia illucens | 13 | frass | 14 | brewer’s spent grains | 6 |
| microbiota | 8 | microalgae | 13 | insect farming | 7 | protein conversion | 6 |
| alternative proteins | 5 | bioaccumulation | 6 | bioconversion | 5 | spent coffee grounds | 6 |
| fatty acids | 5 | chemical hazard | 6 | circular agriculture | 5 | substrate mass reduction | 6 |
| reproduction | 5 | Hermetia illucens prepupae | 6 | integrated pest management | 5 | upcycling | 6 |
| Schizochytrium sp. | 5 | potentially toxic elements | 6 | repellence | 5 | fermented substrate | 3 |
| aquaculture | 4 | antibiotic resistance genes | 5 | systemic defenses | 5 | growth performances | 3 |
| circular economy | 4 | fa profile | 5 | coffee pulp | 4 | SCG | 3 |
| fish feed | 4 | principal component analysis | 5 | npk | 4 | ||
| insect meal | 4 | rearing substrates | 5 | prepupae | 4 | ||
| Isochrysis galbana | 4 | relative macromolecular composition | 5 | spent coffee | 4 | ||
| polyunsaturated fatty acids | 4 | food chain by-products | 3 | sustainable protein | 3 | ||
| qPCR | 4 | prepupal fatty acids profile | 3 | waste management | 3 | ||
| Schizochytrium limacinum | 4 | waste valorization | 3 | caffeine | 2 | ||
| tetracyclines | 4 |
| No. | First Node | Second Node | Cluster for the First Node | Cluster for the Second Node | Link Strength |
|---|---|---|---|---|---|
| 1. | spent coffee grounds | substrate mass reduction | 4 | 4 | 1 |
| 2. | spent coffee grounds | upcycling | 4 | 4 | 1 |
| 3. | black soldier fly | spent coffee grounds | 3 | 4 | 1 |
| 4. | bioconversion rate | spent coffee grounds | 4 | 4 | 1 |
| 5. | brewer’s spent grains | spent coffee grounds | 4 | 4 | 1 |
| 6. | protein conversion | spent coffee grounds | 4 | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutabarat, D.J.C.; Mangindaan, D. Cultivation of Black Soldier Fly (Hermetia illucens) Larvae for the Valorization of Spent Coffee Ground: A Systematic Review and Bibliometric Study. Agriculture 2024, 14, 205. https://doi.org/10.3390/agriculture14020205
Hutabarat DJC, Mangindaan D. Cultivation of Black Soldier Fly (Hermetia illucens) Larvae for the Valorization of Spent Coffee Ground: A Systematic Review and Bibliometric Study. Agriculture. 2024; 14(2):205. https://doi.org/10.3390/agriculture14020205
Chicago/Turabian StyleHutabarat, Donald John Calvien, and Dave Mangindaan. 2024. "Cultivation of Black Soldier Fly (Hermetia illucens) Larvae for the Valorization of Spent Coffee Ground: A Systematic Review and Bibliometric Study" Agriculture 14, no. 2: 205. https://doi.org/10.3390/agriculture14020205
APA StyleHutabarat, D. J. C., & Mangindaan, D. (2024). Cultivation of Black Soldier Fly (Hermetia illucens) Larvae for the Valorization of Spent Coffee Ground: A Systematic Review and Bibliometric Study. Agriculture, 14(2), 205. https://doi.org/10.3390/agriculture14020205






