Ecotoxicological Effects of Commercial Microplastics on Earthworm Eisenia fetida (Savigny, 1826) (Clitellata; Lumbricidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Earthworm Cultivation and Artificial Soil Medium
2.2. Microplastics and Experimental Design
2.3. Microplastic Ingestion
2.4. Statistical Analysis
3. Results and Discussion
3.1. Mortality, Growth, and Reproduction
3.2. Avoidance Behavior
3.3. MPs in the Digestive System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista Research Department. Global Plastic Production—Results for the Period 1950–2021; Statista Research Department: New York, NY, USA, 2023. [Google Scholar]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Plastic Production, Waste and Legislation & The interactions of microplastics and chemical pollutants. In Microplastic Pollutants; Crawford, C.B., Quinn, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2017; pp. 131–157. [Google Scholar]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurence, Effects, and Fate of Microplastic Marine Debris, University of Washington Tacoma, Tacoma, WA, USA, 9–11 September 2008; Technical Memorandum NOSOR&R-30; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2009. [Google Scholar]
- Lots, F.A.E.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A largescale investigation of microplastic contamination: Abundance and characteristics of microplastics in European beach sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Fendall, L.S.; Sewell, M.A. Contributing to marine pollution by washing your face: Microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sul, J.A.I.D. Is this your glitter? An overlooked but potentially environmentally-valuable microplastic. Mar. Pollut. Bull. 2019, 146, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, M. Glitters as a source of primary microplastics: An approach to environmental responsibility and ethics. J. Agric. Environ. Ethics 2019, 32, 459–478. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef]
- Qiang, L.Y.; Cheng, J.P. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Wurl, O.; Obbard, J.P. A review of pollutants in the sea-surface microlayer (SML): A unique habitat for marine organisms. Mar. Pollut. Bull. 2004, 48, 1016–1030. [Google Scholar] [CrossRef]
- Baeza, C.; Cifuentes, C.; González, P.; Araneda, A.; Barra, R. Experimental exposure of Lumbricus terrestris to microplastics. Water Air Soil Pollut. 2020, 231, 308. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salanki, T.; Van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252 Pt B, 1246–1256. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total. Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Song, Y.; Cao, C.J.; Qiu, R.; Hu, J.N.; Liu, M.T.; Lu, S.B.; Shi, H.H.; Raley-Susman, K.M.; He, D.F. Uptake and adverse effects of polyethylene terephthalate microplastics fibers on terrestrial snails (Achatina fulica) after soil exposure. Environ. Pollut. 2019, 250, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A.J.; Horvat, P.; Skalar, T.; Kržan, A. Plastic bag and facial cleanser derived microplastic do not affect feeding behaviour and energy reserves of terrestrial isopods. Sci. Total. Environ. 2018, 615, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salanki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast Miller, M.T.; Thorpe, K.L. Plastic bag derived microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Seijo, A.; Lourenço, J.; Rocha-Santos, T.A.P.; da Costa, J.; Duarte, A.C.; Vala, H.; Pereira, R. Histopathological and molecular effects of microplastics in Eisenia andrei Bouche. Environ. Pollut. 2017, 220, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Prendergast-Miller, M.T.; Katsiamides, A.; Abbass, M.; Sturzenbaum, S.R.; Thorpe, K.L.; Hodson, M.E. Polyester-derived microfibre impacts on the soil dwelling earthworm Lumbricus terrestris. Environ. Pollut. 2019, 251, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Khaldoon, S.; Lalung, J.; Maheer, U.; Kamaruddin, M.A.; Yhaya, M.F.; Alsolami, E.S.; Alorfi, H.S.; Hussein, M.A.; Rafatullah, M.A. A Review on the Role of Earthworms in Plastics Degradation: Issues and Challenges. Polymers 2022, 14, 4770. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Academic Publishers: New York, NY, USA, 2001. [Google Scholar]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as Ecosystem Engineers. Oikos 1994, 69, 373. [Google Scholar] [CrossRef]
- Capowiez, Y.; Dittbrenner, N.; Rault, M.; Triebskorn, R.; Hedde, M.; Mazzia, C. Earthworm cast production as a new behavioural biomarker for toxicity testing. Environ. Pollut. 2010, 158, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Hallam, J.; Hodson, M.E. Impact of different earthworm ecotypes on water stable aggregates and soil water holding capacity. Biol. Fertil. Soils 2020, 56, 607–617. [Google Scholar] [CrossRef]
- Sobhani, Z.; Panneerselvan, L.; Fang, C.; Naidu, R.; Megharaj, M. Chronic and transgenerational effects of polystyrene microplastics at environmentally relevant concentrations in earthworms (Eisenia fetida). Environ. Toxicol. Chem. 2021, 40, 2240–2246. [Google Scholar] [CrossRef]
- Cao, D.; Wang, X.; Luo, X.; Liu, G.; Zheng, H. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 012148. [Google Scholar] [CrossRef]
- Kwak, J.I.; An, Y.J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard. Mater. 2021, 402, 124034. [Google Scholar] [CrossRef]
- Drake, H.L.; Horn, M.A. As the worm turns: The earthworm gut as a transient habitat for soil microbial biomes. Annu. Rev. Microbiol. 2007, 61, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Shaifuddin, S.N.M.; Akizuki, S. Exploration of microplastics from personal care and cosmetic products and its estimated emissions to marine environment: An evidence from Malaysia. Mar. Pollut. Bull. 2018, 136, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, A. Soil microplastics—Current research trends and challenges: Preliminary results of the earthworm Eisenia fetida impact on glitters. Acta Hort. Regiotec. 2022, 25, 141–150. [Google Scholar] [CrossRef]
- OECD. Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- ISO 17512-1:2008; Soil Quality—Avoidance Tests for Determining the Quality of Soils and Effects of Chemicals on Behaviour—Part 1: Test with Earthworms (Eisenia fetida and Eisenia andrei). International Organization for Standardization: Geneva, Switzerland, 2008; pp. 17512–17521.
- Gavina, A.; Bouguerra, S.; Lopes, I.; Marques, C.R.; Rasteiro, M.G.; Antunes, F.; Rocha-Santos, T.; Pereira, R. Impact of organic nano-vesicles in soil: The case of sodium dodecyl sulphate/didodecyl dimethylammonium bromide. Sci. Total Environ. 2016, 547, 413–421. [Google Scholar] [CrossRef] [PubMed]
- ISO 11268-2:2012; Soil Quality-Effects of Pollutants on Earthworms (Eisenia fetida)—Part 2: Determination of Effects on Reproduction. International Organization for Standardization: Geneva, Switzerland, 1998; pp. 11268–11272.
- IBM Corp Released. IBM SPSS Statistics for Windows, Version 20.0; IBM Corp.: Armonk, NY, USA, 2011. [Google Scholar]
- Ji, Z.; Huang, Y.; Feng, Y.; Johansen, A.; Xue, J.; Tremblay, L.A.; Li, Z. Effects of pristine microplastics and nanoplastics on soil invertebrates: A systematic review and meta-analysis of available data. Sci. Total Environ. 2021, 788, 147784. [Google Scholar] [CrossRef]
- Huang, K.; Xia, H. Role of Earthworms’ Mucus in Vermicomposting System: Biodegradation Tests Based on Humification and Microbial Activity. Sci. Total Environ. 2018, 610, 703–708. [Google Scholar] [CrossRef]
- Ding, W.; Li, Z.; Qi, R.; Jones, D.L.; Liu, Q.; Liu, Q.; Yan, C. Effect thresholds for the earthworm Eisenia fetida: Toxicity comparison between conventional and biodegradable microplastics. Sci. Total Environ. 2021, 781, 146884. [Google Scholar] [CrossRef]
- Butt, K.R.; Nuutinen, V. Reproduction of the earthworm Lumbricus terrestris Linnéafter the first mating. Can. J. Zool. 1998, 76, 104–109. [Google Scholar] [CrossRef]
- Lackmann, C.; Velki, M.; Šimić, A.; Müller, A.; Braun, U.; Ečimović, S.; Hollert, H. Two types of microplastics (polystyrene-HBCD and car tire abrasion) affect oxidative stress-related biomarkers in earthworm Eisenia andrei in a time-dependent manner. Environ. Int. 2022, 163, 107190. [Google Scholar] [CrossRef]
- Lattaud, C.; Locati, S.; Mora, P.; Rouland, C.; Lavelle, P. The Diversity of Digestive Systems in Tropical Geophagous Earthworms. Appl. Soil Ecol. 1998, 9, 189–195. [Google Scholar] [CrossRef]
- Kiyasudeen, K.S.; Ibrahim, M.H.; Quaik, S.I.S. Prospects of Organic Waste Management and the Significance of Earthworms; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Chen, Y.; Liu, X.; Leng, Y.; Wang, J. Defense responses in earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics in soils. Ecotoxicol. Environ. Safety 2020, 187, 109788. [Google Scholar] [CrossRef] [PubMed]
- Lahive, E.; Cross, R.; Saarloos, A.I.; Horton, A.A.; Svendsen, C.; Hufenus, R.; Mitrano, D.M. Earthworms ingest microplastic fibres and nanoplastics with effects on egestion rate and long-term retention. Sci. Total Environ. 2022, 807, 151022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Chen, M.; Zhao, Y. Effects of microplastics on the innate immunity and intestinal microflora of juvenile Eriocheir sinensis. Sci. Total Environ. 2019, 685, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, H.; Cui, H.; Chen, B.; Li, L.; Wu, F. Impacts of polystyrene microplastics on the behavior and metabolism 511 in a marine demersal teleost, black rockfish (Sebastes schlegelii). J. Hazard. Mater. 2019, 380, 120861. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/2055 of 25 September 2023 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Synthetic Polymer Microparticles. Available online: https://eur-lex.europa.eu/eli/reg/2023/2055/oj (accessed on 20 November 2023).


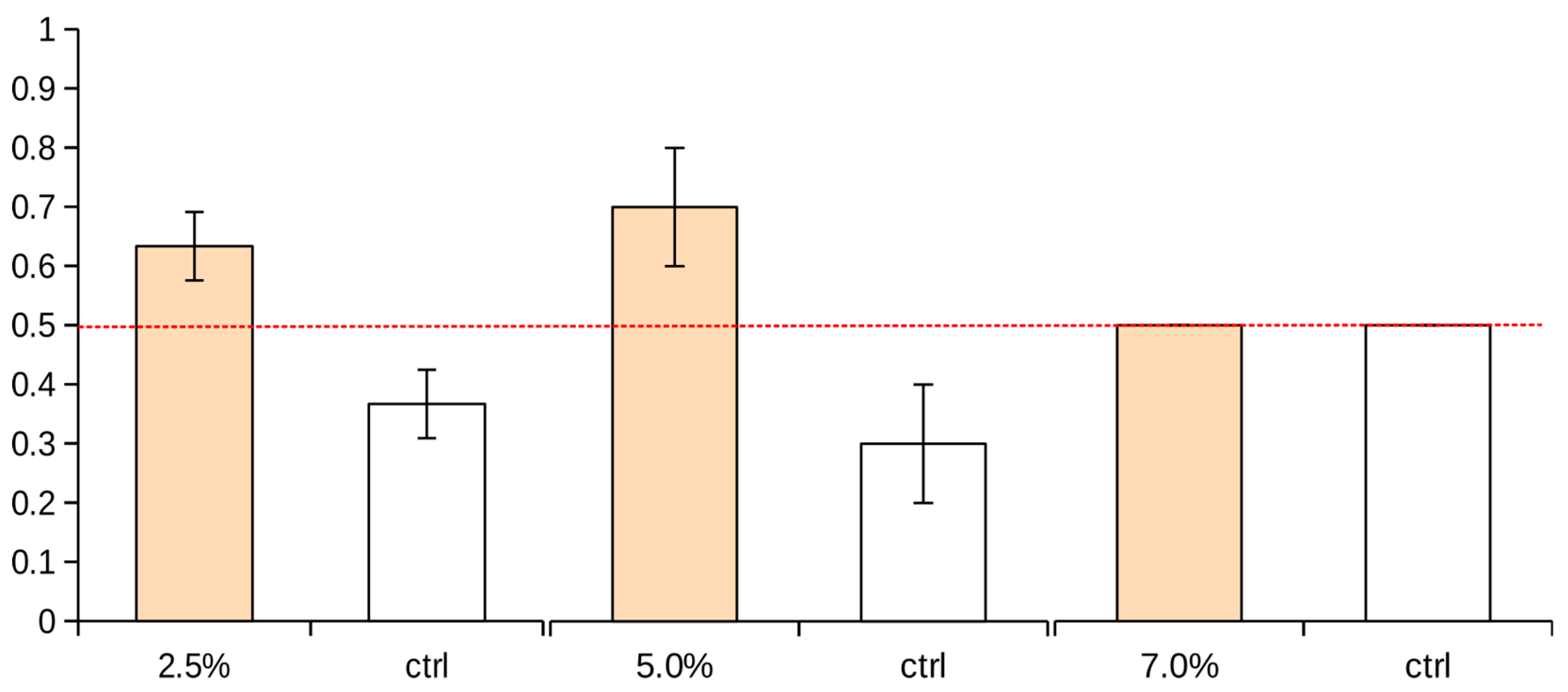
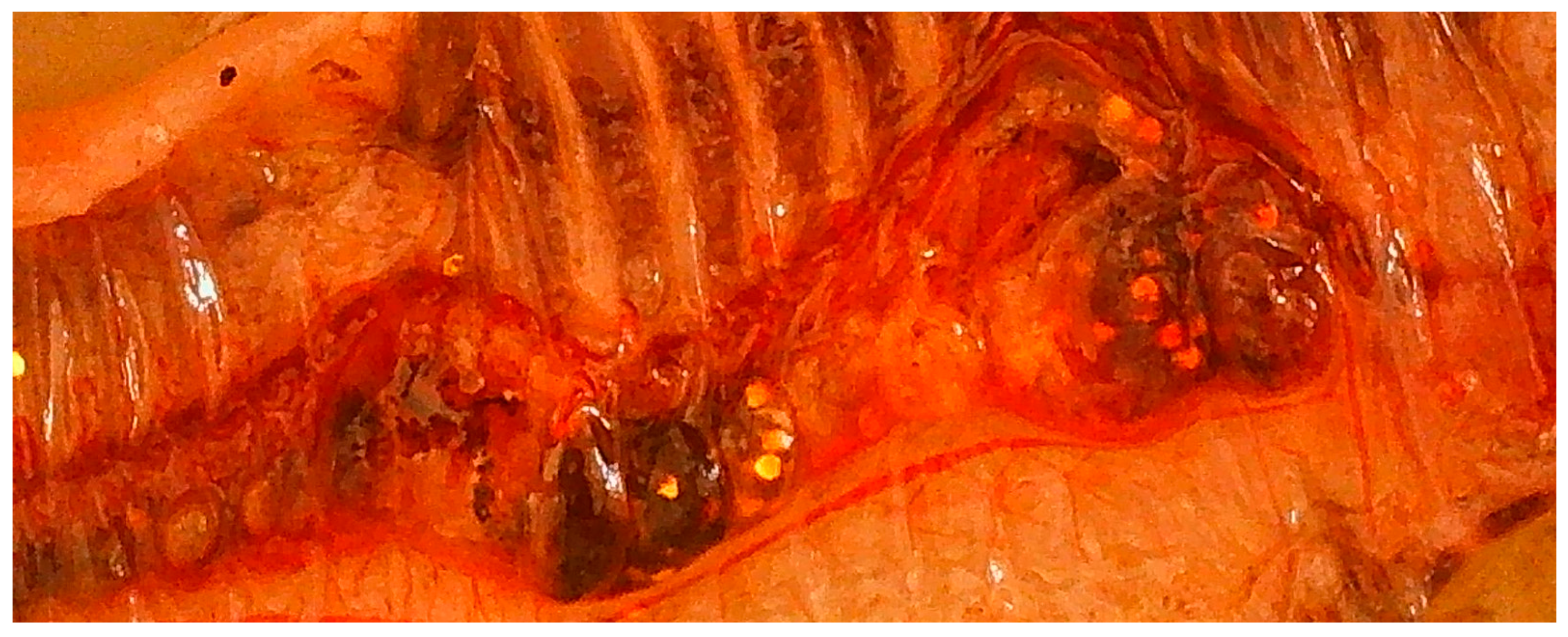


| Test Boxes | Left Zone (LZ) | Right Zone (RZ) |
|---|---|---|
| Control | 300 g of soil | 300 g of soil |
| 2.5% | 300 g of soil | 7.5 g of MPs, 292.5 g of soil |
| 5% | 300 g of soil | 15 g of MPs, 285 g of soil |
| 7% | 300 g of soil | 21 g of MPs, 279 g of soil |
| Treatment | ||||
|---|---|---|---|---|
| MPs | 0% | 2.50% | 5% | 7% |
| Growth rate | 3.8 ± 0.10 | 2.93 ± 0.06 | 2.70 ± 0.05 * | 21.70 ± 0.08 * |
| Cocoon production | 3.32 ± 7.42 | 2.26 ± 5.05 | 1.92 ± 0.89 * | 1.85 ± 0.58 * |
| Mortality | 0 ± 0 | 0 ± 0 | 0.2 ± 0.44 | 0.6 ± 1.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trakić, T.; Popović, F.; Sekulić, J.; Hackenberger, D.K. Ecotoxicological Effects of Commercial Microplastics on Earthworm Eisenia fetida (Savigny, 1826) (Clitellata; Lumbricidae). Agriculture 2024, 14, 267. https://doi.org/10.3390/agriculture14020267
Trakić T, Popović F, Sekulić J, Hackenberger DK. Ecotoxicological Effects of Commercial Microplastics on Earthworm Eisenia fetida (Savigny, 1826) (Clitellata; Lumbricidae). Agriculture. 2024; 14(2):267. https://doi.org/10.3390/agriculture14020267
Chicago/Turabian StyleTrakić, Tanja, Filip Popović, Jovana Sekulić, and Davorka K. Hackenberger. 2024. "Ecotoxicological Effects of Commercial Microplastics on Earthworm Eisenia fetida (Savigny, 1826) (Clitellata; Lumbricidae)" Agriculture 14, no. 2: 267. https://doi.org/10.3390/agriculture14020267
APA StyleTrakić, T., Popović, F., Sekulić, J., & Hackenberger, D. K. (2024). Ecotoxicological Effects of Commercial Microplastics on Earthworm Eisenia fetida (Savigny, 1826) (Clitellata; Lumbricidae). Agriculture, 14(2), 267. https://doi.org/10.3390/agriculture14020267







