Seed Dormancy Dynamics and Germination Characteristics of Malva parviflora L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Seeds Source
2.2. Seed-Dormancy-Breaking Treatments
2.3. Seed Germination Procedure
2.4. Water Uptake Studies
2.5. Hard Seeds
2.6. Determination of 1000-Seed Weight
2.7. Determination of Seed Physical Characteristics
2.8. Seed Vigor Indices
2.9. Tetrazolium Staining (TZ)
2.10. Statistical Analysis
3. Results
3.1. Effect of Seed Treatments on Germination Percent
3.2. Effect of Seed Treatments on Seedling Dry Weight
3.3. Effect of Seed Treatments on Vigor Index
3.4. Effect of Seed Treatment on Water Absorption %
3.5. Effect of Seed Treatments on Hard Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maity, S.K.; Patra, A.; Pal, B.; Malakar, A. Palyno-taxonomic study of some members of Malvaceae in Purba Medinipur, West Bengal, India. Ann. Plant Sci. 2018, 7, 2069–2072. [Google Scholar] [CrossRef]
- Punia, S.; Singh, S.; Yadav, D.B.; Sindhu, V.; Duhan, A. Abundance, distribution and diversity of weeds in wheat in Haryana. Indian J. Weed Sci. 2017, 49, 187–190. [Google Scholar] [CrossRef]
- Akbar, S.; Hanif, U.; Ali, J.; Ishtiaq, S. Pharmacognostic studies of stem, roots and leaves of Malva parviflora L. Asian Pac. J. Trop. Biomed. 2014, 4, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.G.; Baskin, C.C.; Baskin, J.M.; Auld, J.R.; Venable, D.L.; Cavender-Bares, J.; Donohue, K.; de Casas, R.R.; The NESCent Germination Working Group. The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol. 2014, 203, 300–309. [Google Scholar] [CrossRef]
- Egley, G.H.; Paul Jr, R.N.; Duke, S.O.; Vaughn, K.C. Peroxidase involvement in lignification in water-impermeable seed coats of weedy leguminous and malvaceous species. Plant Cell Environ. 1985, 8, 253–260. [Google Scholar]
- Corner, E.J.H. The Seeds of Dicotyledons; University Press: Cambridge, UK, 1976. [Google Scholar]
- El Balla, M.M.A.; Saidahmed, A.I.; Makkawi, M. Effect of moisture content and maturity on hardseededness and germination in okra (Abelmoschus esculentus L. Moench). Int. J. Plant Physiol. Biochem. 2011, 3, 102–107. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Michael, P.J.; Steadman, K.J.; Plummer, J.A. Climatic regulation of seed dormancy and emergence of diverse Malva parviflora populations from a Mediterranean-type environment. Seed Sci. Res. 2006, 16, 273–281. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Chauhan, B.S.; Gill, G.; Preston, C. Factors affecting seed germination of little mallow (Malva parviflora) in southern Australia. Weed Sci. 2006, 54, 1045–1050. [Google Scholar] [CrossRef]
- Khalofah, A. The impact of different seed dormancy release treatments on seed germination of juniper (Juniperus procera). J. King Saud Univ. Sci. 2022, 34, 102307. [Google Scholar] [CrossRef]
- Wang, Y.; Hanson, J.; Mariam, Y. Breaking hard seed dormancy in diverse accessions of five wild Vigna species by hot water and mechanical scarification. Seed Sci. Technol. 2011, 39, 12–20. [Google Scholar] [CrossRef]
- Ansari, O.; Gherekhloo, J.; Kamkar, B.; Ghaderi-Far, F. Breaking seed dormancy and determining cardinal temperatures for Malva sylvestris using nonlinear regression. Seed Sci. Technol. 2016, 44, 447–460. [Google Scholar] [CrossRef]
- Tadros, M.J.; Samarah, N.H.; Alqudah, A.M. Effect of different pre-sowing seed treatments on the germination of Leucaena leucocephala (Lam.) and Acacia farnesiana (L.). New For. 2011, 42, 397–407. [Google Scholar] [CrossRef]
- Zare, S.; Tavili, A.; Darini, M.J. Effects of different treatments on seed germination and breaking seed dormancy of Prosopis koelziana and Prosopis Juliflora. J. For. Res. 2011, 22, 35–38. [Google Scholar] [CrossRef]
- McDonnell, A.; Grant, M.; Coons, J.M. Effects of hot water on breaking seed dormancy of the endangered Kankakee mallow (Iliamna remota Greene (Malvaceae)). Erigenia 2012, 25, 8–13. [Google Scholar]
- Geneve, R.L. Impact of tempreture on seed dormancy. Hort. Sci. 2003, 3, 38. [Google Scholar]
- ISTA. International Rules for Seed Testing, 2021 ed.; International Seed Testing Association: Zurich, Switzerland, 2021. [Google Scholar]
- Koul, A.K.; Jacob, S.R. Seed viability testing: Principles and practices. In Training Manual International Training Programme on Management of Plant Genetic Resources for Officers from Directorate of Seed Testing and Certification; Ministry of Agriculture: Baghdad, Iraq; NBPGR: New Delhi, India, 2019; pp. 57–64. [Google Scholar]
- Botsheleng, B.; Mathowa, T.; Mojeremane, W. Effects of pre-treatments methods on the germination of Pod mahogany (Afzelia quanzensis) and mukusi (Baikiaea plurijuga) seeds. Int. J. Innov. Res. Technol. Sci. Eng. 2014, 3, 8108–8113. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop. Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing, 2003 ed.; International Seed Testing Association: Zurich, Switzerland, 2003. [Google Scholar]
- Gopinath, P.P.; Parsad, R.; Joseph, B.; Adarsh, V.S. GRAPES: General Rshiny Based Analysis Platform Empowered by Statistics. 2020. Version 1.0.0. Available online: https://www.kaugrapes.com/home (accessed on 16 August 2023).
- Baskin, J.M.; Baskin, C.C. Evolutionary considerations of claims for physical dormancy-break by microbial action and abrasion by soil particles. Seed Sci. Res. 2000, 10, 409–413. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Onofri, A.; Taab, A.; Loddo, D.; Necajeva, J.; Uludag, A.; Synowiec, A.; Calha, I.M.; Lars, A.; Jensen, P.K.; et al. Comparing the emergence of Echinochloa crus-galli populations in different locations. Part II: Similarities and threshold parameters. Weed Res. 2022, 62, 203–214. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Yalamalle, V.R.; Dunna, V.; Chawla, G.; Mishra, G.P.; Vijayakumar, P.H.; Ahmad, D.; Lal, S.K.; Joshi, D.C.; Meena, R.P. Overcoming physiological dormancy in common buckwheat (Fagopyrum esculentum Moench). Genet. Resour. Crop. Evol. 2021. [Google Scholar] [CrossRef]
- Assche, J.A.; Vandelook, F.E. Germination ecology of eleven species of Geraniaceae and Malvaceae, with special reference to the effects of drying seeds. Seed Sci. Res. 2006, 16, 283–290. [Google Scholar] [CrossRef]
- Makowski, R.M.; Morrison, I.N. The Biology of Canadian Weeds: 91. Malva pusilla Sm.(=M. rotundifolia L.). Can. J. Plant Sci. 1989, 69, 861–879. [Google Scholar] [CrossRef]
- Azad, S.; Manik, M.R.; Hasan, S.; Matin, A. Effect of different pre-sowing treatments on seed germination percentage and growth performance of Acacia auriculiformis. J. For. Res. 2011, 22, 183. [Google Scholar] [CrossRef]
- Olatunji, D.; Maku, J.O.; Odumefun, O.P. The effect of pre-treatments on the germination and early seedlings growth of Acacia auriculiformis Cunn. Ex. Benth. Afr. J. Biotechnol. 2013, 7, 325–330. [Google Scholar]
- Aliero, B.L. Effects of sulphuric acid, mechanical scarification and wet heat treatments on germination of seeds of African locust bean tree, Parkia biglobosa. Afr. J. Biotechnol. 2004, 3, 179–181. [Google Scholar]
- Amusa, T.O. Effects of three pre-treatment techniques on dormancy and germination of seeds of Afzelia africana (Sm. Ex pers). J. Hortic. For. 2011, 3, 96–103. [Google Scholar]
- Dada, C.A.; Kayode, J.; Arowosegbe, S.; Ayeni, M.J. Effect of scarification on breaking seed dormancy and germination enhancement in Annona muricata L. World Sci. News 2019, 126, 136–147. [Google Scholar]
- Rincon, R.; Culebro, N.; Gutierrez, F.A.; Dendooven, L. Scarification of seeds of Acacia angustissima Mill Kuntze and its effect on germination. Seed Sci. Technol. 2003, 31, 301–307. [Google Scholar] [CrossRef]
- Elmore, C.L. Weed Control by Solarization: Soil Solarization; CRC Press: Boca Raton, FL, USA, 1991; pp. 61–72. [Google Scholar]
- Li, X.; Baskin, J.M.; Baskin, C.C. Physiological dormancy and germination requirements of seeds of several North American Rhus species (Anacardiaceae). Seed Sci. Res. 1999, 9, 237–245. [Google Scholar] [CrossRef]
- Argel, P.J.; Paton, C.J. Overcoming legume hardseededness. In Forage Seed Production Volume 2: Tropical and Sub-Tropical Species; Loch, D.S., Ferguson, J.E., Eds.; CABI Publishing: New York, NY, USA, 1999; pp. 247–266. [Google Scholar]
- Barbosa, V.L.; Ruiz, C.; Correa, E.C.; Pérez-García, F. Dormancy imposed by a tough seed coat in Malvella sherardiana (Malvaceae), a highly threatened species of Spain. Bot. Lett. 2016, 163, 321–327. [Google Scholar] [CrossRef]
- Gupta, R.K.; Das, S.K. Fracture resistance of sunflower seed and kernel to compressive loading. J. Food Eng. 2000, 46, 1–8. [Google Scholar] [CrossRef]
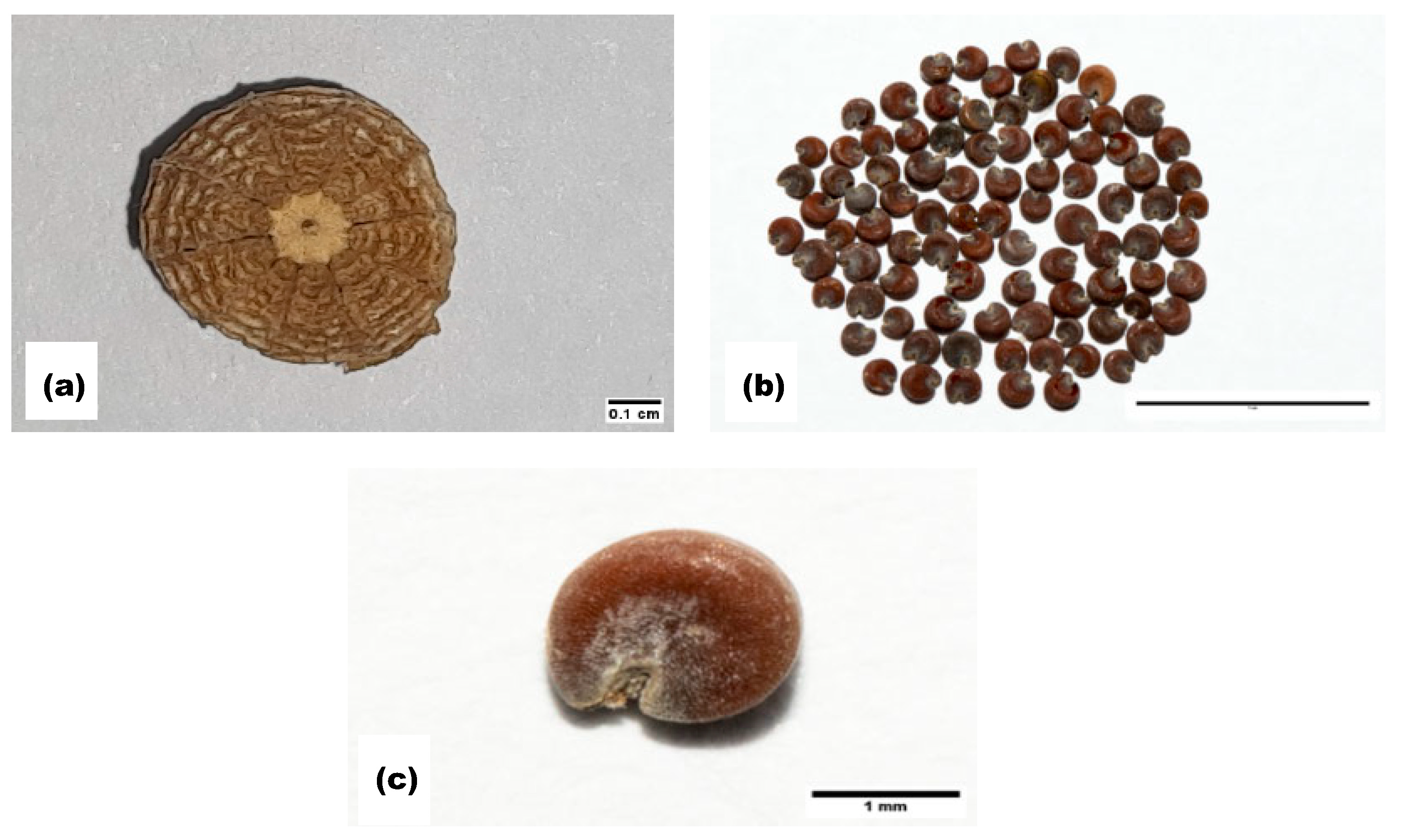


| 1000-Seed Weight (g) | Length (mm) | Width (mm) | Length-to-Width Ratio | |
|---|---|---|---|---|
| Average | 0.83 ± 0.02 | 0.156 ± 0.014 | 0.138 ± 0.016 | 1.144 ± 0.168 |
| Range | 0.73 | 0.064 | 0.072 | 0.80 |
| Treatments | Note |
|---|---|
| Mechanical scarification | # 80 wood sandpaper |
| Acid treatment 5 min | 95% sulfuric acid (H2SO4) |
| Acid treatment 10 min | |
| Acid treatment 15 min | |
| Hot water treatment for 30 min | Seeds immersed in distilled water at 80 °C |
| Hot water treatment 60 min | |
| Intact seed |
| Treatments | Germination (%) | |||
|---|---|---|---|---|
| 15 °C | 15–20 °C | 20 °C | Mean Treatments | |
| Mechanical scarification | 40.67 (39.52) ab # | 58.67 (50.01) a | 32.00 (34.40) bc | 43.78 (41.31) A |
| Acid 5 min | 23.33 (28.83) cde | 28.67 (32.35) bcd | 21.33 (27.44) cde | 24.44 (29.54) B |
| Acid 10 min | 27.33 (31.51) bcd | 37.33 (37.63) bc | 22.67 (28.29) cde | 29.11 (32.48) B |
| Acid 15 min | 30.67 (33.58) bc | 44.67 (41.93) ab | 37.33 (37.65) bc | 37.56 (37.72) A |
| HW 30 min | 0.00 (0.02) h | 1.33 (3.86) gh | 0.00 (0.02) h | 0.44 (1.30) F |
| HW 60 min | 0.00 (0.02) h | 0.00 (0.02) h | 0.00 (0.02) h | 0.00 (0.02) F |
| Intact seed | 14.00 (21.94) def | 12.67 (20.48) ef | 6.00 (13.84) fg | 10.89 (18.75) E |
| Mean temperature | 19.43 (22.20) B | 26.19 (26.61) A | 17.05 (20.24) B | |
| Factor | ||||
| Factor A (Seed treatment) | 4.49 *** | |||
| Factor B (Temperature) | 2.94 *** | |||
| Factor (A × B) | 7.78 ** | |||
| Treatment | Seedling Dry Weight (g) | Seed Vigor Index | ||||||
|---|---|---|---|---|---|---|---|---|
| 15 °C | 15–20 °C | 20 °C | Mean Treatments | 15 °C | 15–20 °C | 20 °C | Mean Treatments | |
| Mechanical scarification | 0.34 # | 0.35 | 0.31 | 0.33 A | 13.82 ab | 20.60 a | 9.79 bc | 14.74 A |
| Acid 5 min | 0.34 | 0.23 | 0.31 | 0.29 A | 7.76 bcde | 6.81 bcde | 6.70 bcde | 7.09 B |
| Acid 10 min | 0.36 | 0.35 | 0.32 | 0.34 A | 9.72 bcd | 13.24 ab | 7.54 bcde | 10.17 B |
| Acid 15 min | 0.36 | 0.24 | 0.34 | 0.31 A | 10.99 b | 10.24 bc | 12.54 ab | 11.26 AB |
| HW 30 min | NA | 0.07 | NA | 0.02 B | NA | 0.26 | NA | 0.09 C |
| HW 60 min | NA | NA | NA | 0.00 B | NA | NA | NA | 0.00 C |
| Intact seed | 0.36 | 0.21 | 0.17 | 0.24 A | 4.98 bcde | 1.87 cde | 0.67 de | 2.51 C |
| Mean temperature | 0.25 A | 0.21 A | 0.21 A | 6.75 A | 7.57 A | 5.32 A | ||
| Factor | ||||||||
| Factor A (Seed treatment) | 0.08 *** | 2.74 *** | ||||||
| Factor B (Temperature) | NS | NS | ||||||
| Factor (A × B) | NS | 4.80 ** | ||||||
| Treatments | Percent Hard Seeds | |||
|---|---|---|---|---|
| 15 °C | 15–20 °C | 20 °C | Mean Treatments | |
| Mechanical scarification | 28.00 (31.79) abcd # | 12.00 (20.15) d | 30.67 (33.55) abcd | 23.56 (28.50) C |
| Acid 5 min | 40.00 (39.32) abcd | 48.66 (44.23) abc | 44.00 (41.51) abcd | 44.22 (41.66) AB |
| Acid 10 min | 54.00 (47.67) abc | 32.67 (34.68) abcd | 30.67 (33.44) abcd | 39.11 (38.49) BC |
| Acid 15 min | 40.67 (39.32) abcd | 21.33 (27.41) cd | 22.67 (28.30) bcd | 28.22 (31.68) BC |
| HW 30 min | 29.33 (32.72) abcd | 35.33 (36.40) abcd | 38.00 (37.99) abcd | 34.22 (35.71) BC |
| HW 60 min | 24.67 (29.58) abcd | 35.33 (36.46) abcd | 32.00 (34.37) abcd | 30.67 (33.47) BC |
| Intact seed | 58.00 (49.60) ab | 54.67 (48.56) abc | 60.00 (50.94) a | 57.56 (49.70) A |
| Mean temperature | 39.24 (38.52) A | 34.28 (37.16) A | 36.86 35.41) A | |
| Factor | ||||
| Factor A (Seed treatment) | 6.58 *** | |||
| Factor B (Temperature) | NS | |||
| Factor (A × B) | NS | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawar, R.; Yalamalle, V.R.; Bana, R.S.; Kaur, R.; Shivay, Y.S.; Choudhary, A.K.; Singh, T.; Meena, S.L.; Vijay, D.; Vijayakumar, H.P.; et al. Seed Dormancy Dynamics and Germination Characteristics of Malva parviflora L. Agriculture 2024, 14, 266. https://doi.org/10.3390/agriculture14020266
Dawar R, Yalamalle VR, Bana RS, Kaur R, Shivay YS, Choudhary AK, Singh T, Meena SL, Vijay D, Vijayakumar HP, et al. Seed Dormancy Dynamics and Germination Characteristics of Malva parviflora L. Agriculture. 2024; 14(2):266. https://doi.org/10.3390/agriculture14020266
Chicago/Turabian StyleDawar, Rakesh, Vishwanath Rohidas Yalamalle, Ram Swaroop Bana, Ramanjit Kaur, Yashbir Singh Shivay, Anil K. Choudhary, Teekam Singh, Samrath Lal Meena, Dunna Vijay, H. P. Vijayakumar, and et al. 2024. "Seed Dormancy Dynamics and Germination Characteristics of Malva parviflora L." Agriculture 14, no. 2: 266. https://doi.org/10.3390/agriculture14020266
APA StyleDawar, R., Yalamalle, V. R., Bana, R. S., Kaur, R., Shivay, Y. S., Choudhary, A. K., Singh, T., Meena, S. L., Vijay, D., Vijayakumar, H. P., Kumar, V., & Yadav, A. (2024). Seed Dormancy Dynamics and Germination Characteristics of Malva parviflora L. Agriculture, 14(2), 266. https://doi.org/10.3390/agriculture14020266









