Potential of Cassava Clones for Iron, Zinc, and Selenium Biofortification
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Characterization of the Experimental Area
2.2. Experimental Setup
2.3. Determination of Chlorophyll Content
2.4. Digestion and Mineral Analysis
2.5. Gas Exchange Parameters
2.6. Statistical Analysis
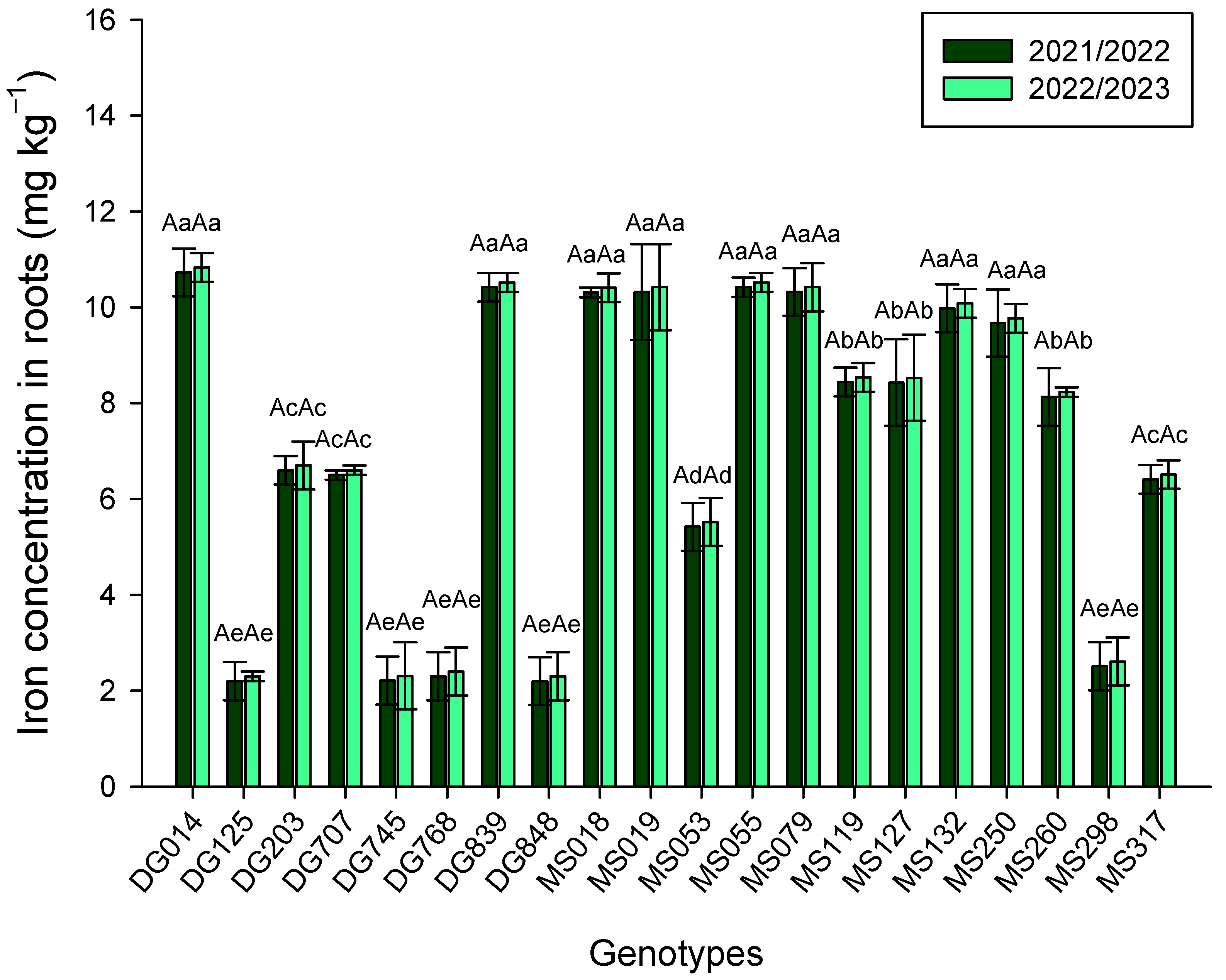
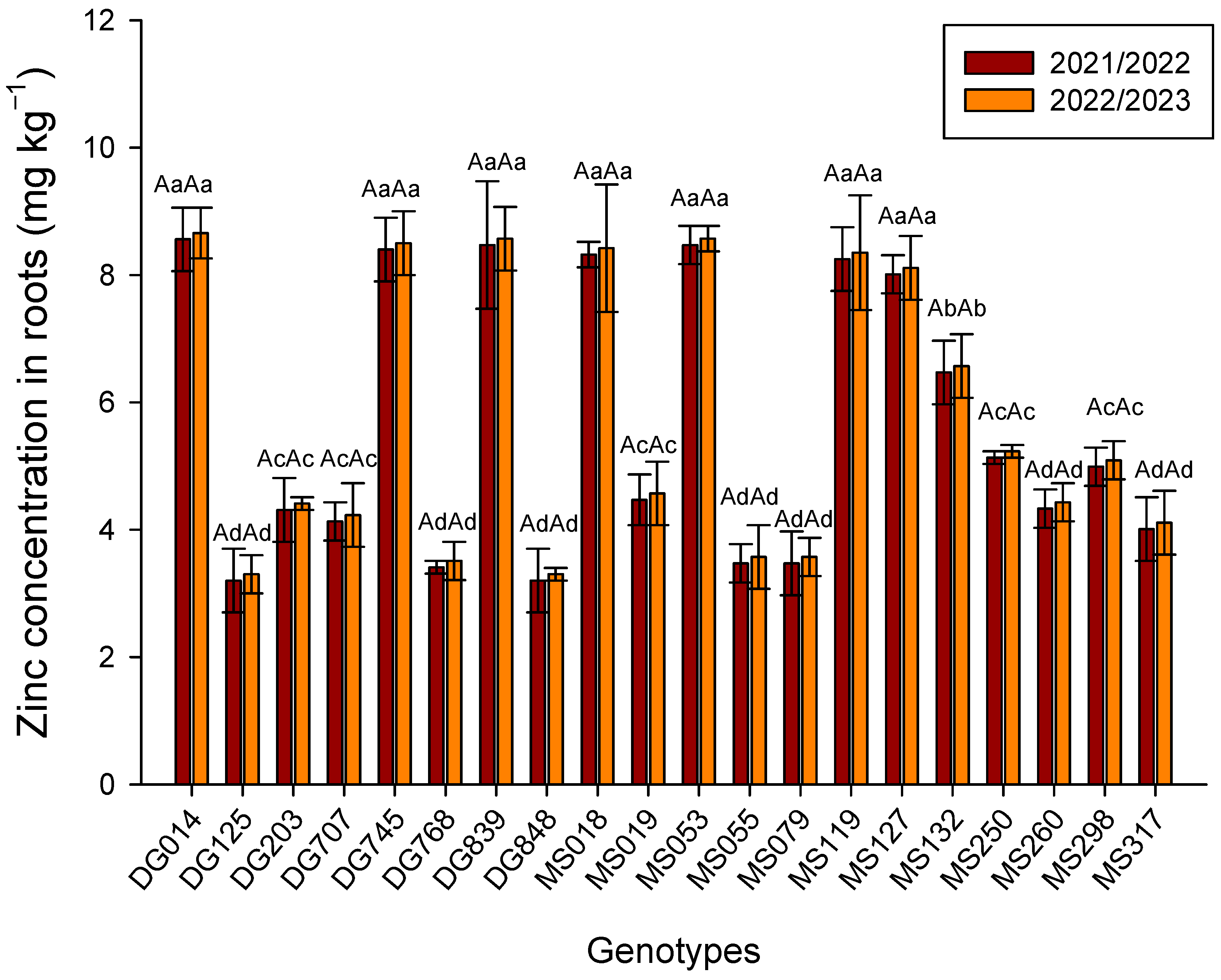
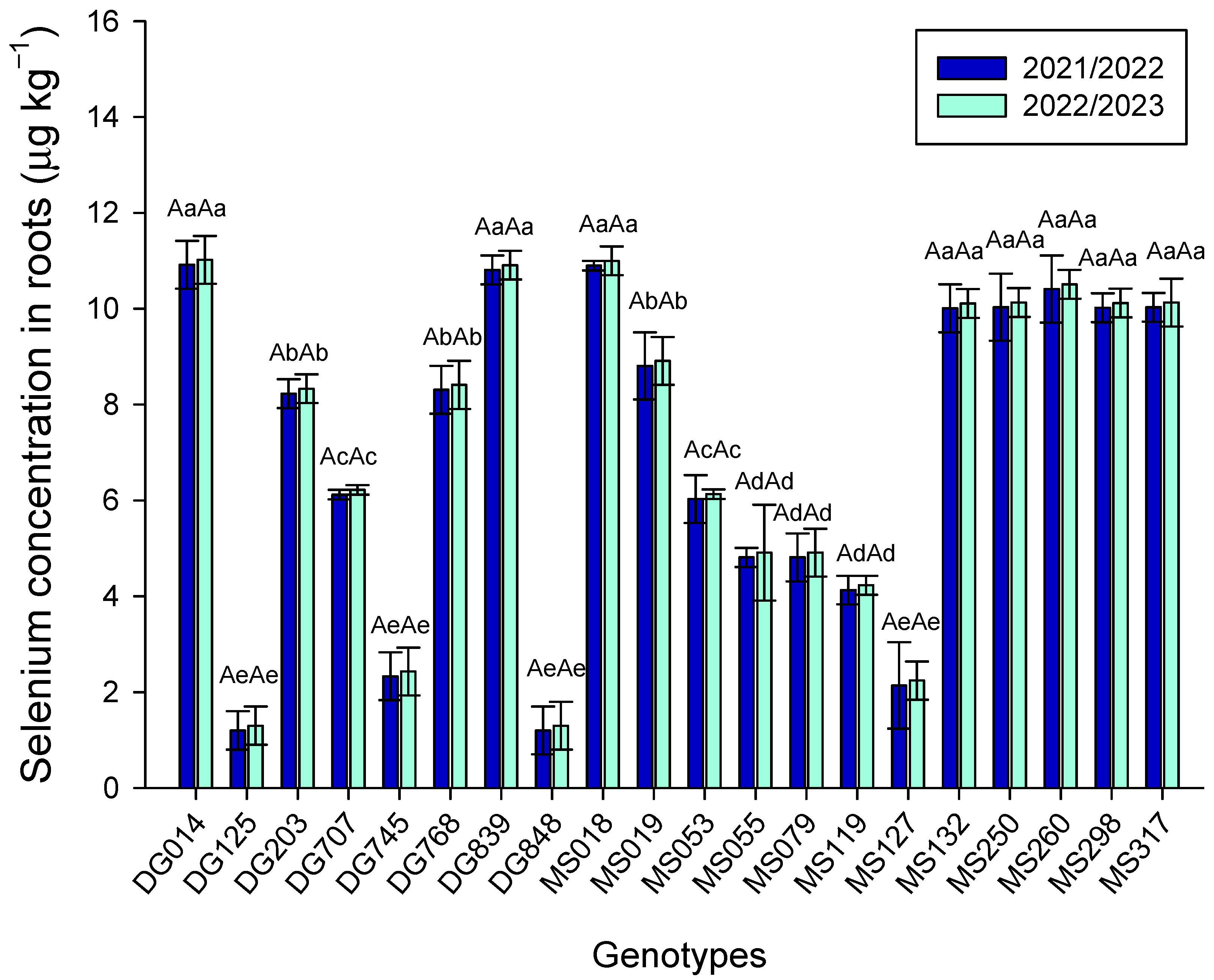
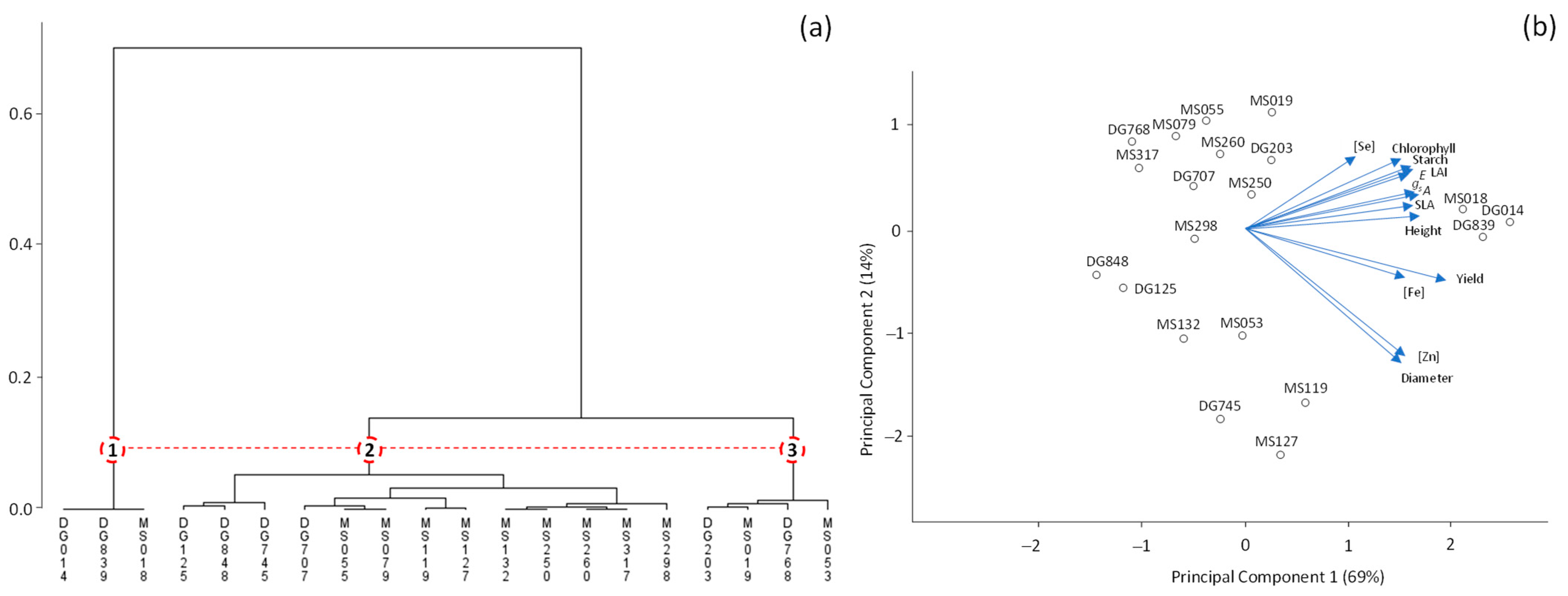
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of Food Security and Nutrition in the World: Safeguarding against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; ISBN 978-92-5-131570-5. [Google Scholar]
- Prom-u-thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guilherme, L.R.G.; Corguinha, A.P.B.; Guo, S.; Kaur, C.; Naeem, A.; Yamuangmorn, S.; et al. Simultaneous Biofortification of Rice With Zinc, Iodine, Iron and Selenium Through Foliar Treatment of a Micronutrient Cocktail in Five Countries. Front. Plant Sci. 2020, 11, 589835. [Google Scholar] [CrossRef]
- Swamy, B.P.M.; Marathi, B.; Ribeiro-Barros, A.I.F.; Calayugan, M.I.C.; Ricachenevsky, F.K. Iron Biofortification in Rice: An Update on Quantitative Trait Loci and Candidate Genes. Front. Plant Sci. 2021, 12, 647341. [Google Scholar] [CrossRef]
- Vélez-Bermúdez, I.C.; Schmidt, W. How Plants Recalibrate Cellular Iron Homeostasis. Plant Cell Physiol. 2022, 63, 154–162. [Google Scholar] [CrossRef]
- Yiannikourides, A.; Latunde-Dada, G.O. A Short Review of Iron Metabolism and Pathophysiology of Iron Disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef]
- Magee, P.J.; McCann, M.T. Micronutrient Deficiencies: Current Issues. Proc. Nutr. Soc. 2019, 78, 147–149. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Hacisalihoglu, G. Zinc (Zn): The Last Nutrient in the Alphabet and Shedding Light on Zn Efficiency for the Future of Crop Production under Suboptimal Zn. Plants 2020, 9, 1471. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.-S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent Aspects of the Effects of Zinc on Human Health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Rayman, M.P.; Blundell-Pound, G.; Pastor-Barriuso, R.; Guallar, E.; Steinbrenner, H.; Stranges, S. A Randomized Trial of Selenium Supplementation and Risk of Type-2 Diabetes, as Assessed by Plasma Adiponectin. PLoS ONE 2012, 7, e45269. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium Accumulation by Plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Figueiredo, C.; Rocha, M.; Lanza, M.; Martins Silva, V.; Reis, A. Phosphorus and Selenium Interaction Effects on Agronomic Biofortification of Cowpea Plants. J. Soil Sci. Plant Nutr. 2023, 23, 4385–4395. [Google Scholar] [CrossRef]
- Consentino, B.B.; Ciriello, M.; Sabatino, L.; Vultaggio, L.; Baldassano, S.; Vasto, S.; Rouphael, Y.; La Bella, S.; De Pascale, S. Current Acquaintance on Agronomic Biofortification to Modulate the Yield and Functional Value of Vegetable Crops: A Review. Horticulturae 2023, 9, 219. [Google Scholar] [CrossRef]
- Yabuta, S.; Fukuta, T.; Tamaru, S.; Goto, K.; Nakao, Y.; Khanthavong, P.; Ssenyonga, P.; Sakagami, J.-I. The Productivity of Cassava (Manihot Esculenta Crantz) in Kagoshima, Japan, Which Belongs to the Temperate Zone. Agronomy 2021, 11, 2021. [Google Scholar] [CrossRef]
- De Souza, A.P.; Wang, Y.; Orr, D.J.; Carmo-Silva, E.; Long, S.P. Photosynthesis across African Cassava Germplasm Is Limited by Rubisco and Mesophyll Conductance at Steady State, but by Stomatal Conductance in Fluctuating Light. New Phytol. 2020, 225, 2498–2512. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. International Research on Cassava Photosynthesis, Productivity, Eco-Physiology, and Responses to Environmental Stresses in the Tropics. Photosynthetica 2006, 44, 481–512. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Prospects of Photosynthetic Research for Increasing Agricultural Productivity, with Emphasis on the Tropical C4 Amaranthus and the Cassava C3-C4 Crops. Photosynthetica 2016, 54, 161–184. [Google Scholar] [CrossRef]
- Vieira, E.A.; Fialho, J.d.F.; de Oliveira, C.M.; Rinaldi, M.M.; Fernandes, F.D. New Cassava Cultivars for Starch and Flour Production in the Cerrado of Central Brazil. Crop Breed. Appl. Biotechnol. 2020, 20, e27362023. [Google Scholar] [CrossRef]
- Corguinha, A.P.B.; Carvalho, C.A.; de Souza, G.A.; de Carvalho, T.S.; Vieira, E.A.; Fialho, J.F.; Guilherme, L.R.G. Potential of Cassava Clones Enriched with β-Carotene and Lycopene for Zinc Biofortification under Different Soil Zn Conditions. J. Sci. Food Agric. 2019, 99, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Department of Agriculture, Natural Resources and Conservation Services. Soil Taxonomy: Keys to Soil Taxonomy, 11th ed.; Department of Agriculture, Natural Resources and Conservation Services: Washington, DC, USA, 2010; pp. 123–257. [Google Scholar]
- Van Raij, B. Fertilidade do Solo e Manejo de Nutrientes, 1st ed.; IPNI: Piracicaba, Brazi, 2011; ISBN 978-85-98519-07-4. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Wongnoi, S.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P. Physiology, Growth and Yield of Different Cassava Genotypes Planted in Upland with Dry Environment during High Storage Root Accumulation Stage. Agronomy 2020, 10, 576. [Google Scholar] [CrossRef]
- Wholey, D.W.; Booth, R.H. A Comparison of Simple Methods for Estimating Starch Content of Cassava Roots. J. Sci. Food Agric. 1979, 30, 158–164. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. ISBN 978-0-12-182048-0. [Google Scholar]
- Santos, E.F.; Pongrac, P.; Reis, A.R.; Rabêlo, F.H.S.; Azevedo, R.A.; White, P.J.; Lavres, J. Unravelling Homeostasis Effects of Phosphorus and Zinc Nutrition by Leaf Photochemistry and Metabolic Adjustment in Cotton Plants. Sci. Rep. 2021, 11, 13746. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation For Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- SAS Institute. SAS/STAT Software, Release 8.2; SAS Institute: Cary, NC, USA, 2001. [Google Scholar]
- Connorton, J.M.; Balk, J. Iron Biofortification of Staple Crops: Lessons and Challenges in Plant Genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef]
- Vilpoux, O.; Sharbel, T.; Posso-Terranova, A.; Hoogerheide, E.; Cereda, M. Use of Infrared Analysis to Identify Genetic Resources from Isolated Producers in Brazil as a Tool to Improve Cassava Competitiveness in the Starch Market. Int. J. Food Sci. Technol. 2020, 56, 1354–1361. [Google Scholar] [CrossRef]
- Silva, J.A.D.; Leonel, M.; Fernandes, A.M.; Garreto, F.G.D.S.; Nunes, J.G.D.S.; Tajra, R.F. Crescimento, produtividade e nutrientes da mandioca adubada com zinco. Cienc. Rural 2023, 53, e20220064. [Google Scholar] [CrossRef]
- de Onis, M.; Branca, F. Childhood Stunting: A Global Perspective. Matern. Child. Nutr. 2016, 12 (Suppl. 1), 12–26. [Google Scholar] [CrossRef]
- Narayanan, N.; Beyene, G.; Chauhan, R.D.; Gaitán-Solís, E.; Gehan, J.; Butts, P.; Siritunga, D.; Okwuonu, I.; Woll, A.; Jiménez-Aguilar, D.M.; et al. Biofortification of Field-Grown Cassava by Engineering Expression of an Iron Transporter and Ferritin. Nat. Biotechnol. 2019, 37, 144–151. [Google Scholar] [CrossRef]
- Lahai, M.; Ekanayake, I.J. Accumulation and Distribution of Dry Matter in Relation to Root Yield of Cassava under a Fluctuating Water Table in Inland Valley Ecology. Afr. J. Biotechnol. 2009, 8, 4895–4905. [Google Scholar]
- Howeler, R.H.; Cadavid, L.F. Accumulation and Distribution of Dry Matter and Nutrients during a 12-Month Growth Cycle of Cassava. Field Crops Res. 1983, 7, 123–139. [Google Scholar] [CrossRef]
- Ezui, K.S.; Franke, A.C.; Mando, A.; Ahiabor, B.D.K.; Tetteh, F.M.; Sogbedji, J.; Janssen, B.H.; Giller, K.E. Fertiliser Requirements for Balanced Nutrition of Cassava across Eight Locations in West Africa. Field Crops Res. 2016, 185, 69–78. [Google Scholar] [CrossRef]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P. Determination of Cassava Leaf Area for Breeding Programs. Agronomy 2022, 12, 3013. [Google Scholar] [CrossRef]
- Katono, K.; Macfadyen, S.; Omongo, C.A.; Odong, T.L.; Colvin, J.; Karungi, J.; Otim, M.H. Influence of Cassava Morphological Traits and Environmental Conditions on Field Populations of Bemisia Tabaci. Insects 2021, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Mwamba, S.; Kaluba, P.; Moualeu-Ngangue, D.; Winter, E.; Chiona, M.; Chishala, B.H.; Munyinda, K.; Stützel, H. Physiological and Morphological Responses of Cassava Genotypes to Fertilization Regimes in Chromi-Haplic Acrisols Soils. Agronomy 2021, 11, 1757. [Google Scholar] [CrossRef]
- Tappiban, P.; Smith, D.R.; Triwitayakorn, K.; Bao, J. Recent Understanding of Starch Biosynthesis in Cassava for Quality Improvement: A Review. Trends Food Sci. Technol. 2019, 83, 167–180. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Ort, D.R.; Whitmarsh, J.; Long, S.P. The Slow Reversibility of Photosystem II Thermal Energy Dissipation on Transfer from High to Low Light May Cause Large Losses in Carbon Gain by Crop Canopies: A Theoretical Analysis. J. Exp. Bot. 2004, 55, 1167–1175. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.-G.; Naidu, S.L.; Ort, D.R. Can Improvement in Photosynthesis Increase Crop Yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving Photosynthesis and Crop Productivity by Accelerating Recovery from Photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Arrivault, S.; Alexandre Moraes, T.; Obata, T.; Medeiros, D.B.; Fernie, A.R.; Boulouis, A.; Ludwig, M.; Lunn, J.E.; Borghi, G.L.; Schlereth, A.; et al. Metabolite Profiles Reveal Interspecific Variation in Operation of the Calvin-Benson Cycle in Both C4 and C3 Plants. J. Exp. Bot. 2019, 70, 1843–1858. [Google Scholar] [CrossRef]
- Sonnewald, U.; Fernie, A.R.; Gruissem, W.; Schläpfer, P.; Anjanappa, R.B.; Chang, S.-H.; Ludewig, F.; Rascher, U.; Muller, O.; van Doorn, A.M.; et al. The Cassava Source-Sink Project: Opportunities and Challenges for Crop Improvement by Metabolic Engineering. Plant J. 2020, 103, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, R.; Lamm, C.E.; Bodampalli Anjanappa, R.; Müdsam, C.; Saeed, M.; Klima, J.; Kraner, M.E.; Ludewig, F.; Knoblauch, M.; Gruissem, W.; et al. Symplasmic Phloem Unloading and Radial Post-Phloem Transport via Vascular Rays in Tuberous Roots of Manihot Esculenta. J. Exp. Bot. 2019, 70, 5559–5573. [Google Scholar] [CrossRef]
- Sayre, R.; Beeching, J.R.; Cahoon, E.B.; Egesi, C.; Fauquet, C.; Fellman, J.; Fregene, M.; Gruissem, W.; Mallowa, S.; Manary, M.; et al. The BioCassava plus Program: Biofortification of Cassava for Sub-Saharan Africa. Annu. Rev. Plant Biol. 2011, 62, 251–272. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets--Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, B.L.; Zanon, A.J.; Tironi, L.F.; Streck, N.A.; Richards, N.S.P.D.S. Qualidade nutricional e aceitação sensorial da mandioca biofortificada. Braz. J. Food Technol. 2021, 24, e2020247. [Google Scholar] [CrossRef]
- White, P.J. Improving Potassium Acquisition and Utilisation by Crop Plants. J. Plant Nutr. Soil Sci. 2013, 176, 305–316. [Google Scholar] [CrossRef]
- Mayer, J.E.; Pfeiffer, W.H.; Beyer, P. Biofortified Crops to Alleviate Micronutrient Malnutrition. Curr. Opin. Plant Biol. 2008, 11, 166–170. [Google Scholar] [CrossRef]
- Reis, H.P.G.; de Queiroz Barcelos, J.P.; Silva, V.M.; Santos, E.F.; Tavanti, R.F.R.; Putti, F.F.; Young, S.D.; Broadley, M.R.; White, P.J.; dos Reis, A.R. Agronomic Biofortification with Selenium Impacts Storage Proteins in Grains of Upland Rice. J. Sci. Food Agric. 2020, 100, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Nardeli, A.J.; de Carvalho Mendes, N.A.; de Moura Rocha, M.; Wilson, L.; Young, S.D.; Broadley, M.R.; White, P.J.; dos Reis, A.R. Agronomic Biofortification of Cowpea with Zinc: Variation in Primary Metabolism Responses and Grain Nutritional Quality among 29 Diverse Genotypes. Plant Physiol. Biochem. 2021, 162, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Ghislain, M.; Muzhingi, T.; Low, J.W. Zinc and Iron Fortification in Cassava. Nat. Biotechnol. 2019, 37, 130–132. [Google Scholar] [CrossRef]
- Okwuonu, I.C.; Narayanan, N.N.; Egesi, C.N.; Taylor, N.J. Opportunities and Challenges for Biofortification of Cassava to Address Iron and Zinc Deficiency in Nigeria. Glob. Food Secur. 2021, 28, 100478. [Google Scholar] [CrossRef]
| Identification | State of Origin in Brazil | Region or City Collected | Classification |
|---|---|---|---|
| DG014 | São Paulo | Iguape | Brave |
| DG125 | São Paulo | Iguape | Brave |
| DG203 | Pará | Belém/Embrapa | Sweet |
| DG707 | Amazonas | Boa Esperança | Brave |
| DG745 | Minas Gerais | Conceição dos Ouros/Ouros Velho | Brave |
| DG768 | Minas Gerais | Conceição dos Ouros/Ouros Velho | Sweet |
| DG839 | Minas Gerais | Conceição dos Ouros/Pintos | Sweet |
| DG848 | Minas Gerais | Conceição dos Ouros/Sertãozinho | Sweet |
| MS018 | Mato Grosso do Sul | Bodoquena | Sweet |
| MS019 | Mato Grosso do Sul | Miranda | Sweet |
| MS053 | Mato Grosso do Sul | Bela Vista | Sweet |
| MS055 | Mato Grosso do Sul | Rio Verde | Sweet |
| MS077 | Mato Grosso do Sul | Chapadão do Sul | Sweet |
| MS119 | Mato Grosso do Sul | Cassilândia | Sweet |
| MS127 | Mato Grosso do Sul | Bonito | Sweet |
| MS132 | Mato Grosso do Sul | Bonito | Sweet |
| MS250 | Mato Grosso do Sul | Novo Horizonte do Sul | Sweet |
| MS260 | Mato Grosso do Sul | Antônio João | Sweet |
| MS298 | Mato Grosso do Sul | Ponta Porã | Sweet |
| MS317 | Mato Grosso do Sul | Ponta Porã | Sweet |
| Genotypes | H 1 (m) | H 2 (m) | D 1 (mm) | D 2 (mm) | CHL 1 (µg kg−1) | CHL 2 (µg kg−1) |
|---|---|---|---|---|---|---|
| DG014 | 2.73 Aa | 2.76 Aa | 8.56 Aa | 8.43 Aa | 9.85 Ba | 9.92 Aa |
| DG125 | 1.20 Ab | 1.24 Ab | 3.20 Bc | 3.94 Ac | 1.24 Ae | 1.20 Ae |
| DG203 | 1.60 Ab | 1.62 Ab | 4.31 Ac | 4.13 Bc | 7.25 Ab | 7.23 Ab |
| DG707 | 1.50 Bb | 1.63 Ab | 4.13 Ac | 4.06 Ac | 6.15 Ac | 6.12 Ac |
| DG745 | 1.21 Bb | 1.43 Ab | 8.40 Aa | 8.02 Ba | 2.35 Ae | 2.33 Ae |
| DG768 | 1.30 Bb | 1.55 Ab | 3.41 Bc | 3.74 Ac | 8.34 Ab | 8.31 Ab |
| DG839 | 2.42 Ba | 2.67 Aa | 8.47 Aa | 8.49 Aa | 9.83 Aa | 9.81 Aa |
| DG848 | 1.20 Bb | 1.31 Ab | 3.20 Ac | 3.22 Ac | 1.22 Ae | 1.20 Ae |
| MS018 | 2.31 Ba | 2.41 Aa | 8.32 Aa | 8.36 Aa | 9.94 Aa | 9.90 Aa |
| MS019 | 1.32 Bb | 1.47Ab | 4.47 Ac | 4.52 Ac | 7.86 Ab | 7.81 Ab |
| MS053 | 1.42 Ab | 1.47 Ab | 8.47 Aa | 8.41 Aa | 4.86 Bd | 5.03 Ad |
| MS055 | 1.42 Ab | 1.36 Ab | 3.47 Bc | 4.01 Ac | 4.91 Ad | 4.81 Ad |
| MS079 | 1.32 Ab | 1.21 Bb | 3.47 Bc | 3.91 Ac | 4.93 Ad | 4.81 Ad |
| MS119 | 1.44 Ab | 1.26 Bb | 8.25 Aa | 8.17 Aa | 4.94 Ad | 4.13 Bd |
| MS127 | 1.43 Ab | 1.26 Bb | 8.01 Aa | 8.03 Aa | 2.17 Ae | 2.14 Ae |
| MS132 | 1.08 Bb | 1.39 Ab | 6.47 Ab | 6.31 Bb | 2.22 Ae | 2.01 Ae |
| MS250 | 1.07 Bb | 1.29Ab | 5.13 Ab | 5.03 Bb | 2.06 Ae | 2.03 Ae |
| MS260 | 1.13 Ab | 1.14 Ab | 4.33 Ad | 4.34 Ad | 2.42 Ae | 2.41 Ae |
| MS298 | 1.51 Ab | 1.56 Ab | 5.09 Ab | 5.04 Ab | 2.07 Ae | 2.02 Ae |
| MS317 | 1.41 Ab | 1.46 Ab | 4.01 Ac | 3.96 Ac | 2.08 Ae | 2.03 Ae |
| Genotypes | Yield 1 (t ha−1) | Yield 2 (t ha−1) | LAI 1 (cm2) | LAI 2 (cm2) | SLA 1 (cm2 g−1) | SLA 2 (cm2 g−1) | Starch 1 (t ha−1) | Starch 2 (t ha−1) |
|---|---|---|---|---|---|---|---|---|
| DG014 | 21.15 Aa | 22.33 Aa | 1.43 Aa | 1.40 Aa | 185.25 Aa | 184.45 Aa | 14.91 Aa | 14.20 Aa |
| DG125 | 19.05 Bd | 19.55 Ad | 0.81 Bc | 0.75 Ac | 156.05 Bc | 155.65 Ac | 6.33 Ad | 6.20 Ac |
| DG203 | 20.05 Ab | 20.25 Ab | 1.19 Ab | 1.10 Ab | 164.95 Ab | 164.45 Ab | 11.05 Ab | 11.45 Ab |
| DG707 | 20.15 Ab | 20.55 Ab | 0.83 Ac | 0.90 Ac | 153.25 Ac | 152.45 Bc | 10.56 Ab | 10.96 Ab |
| DG745 | 19.47 Bc | 20.37 Ab | 0.77 Ac | 0.72 Bc | 151.31 Ac | 150.91 Bc | 6.81 Ad | 6.01 Ac |
| DG768 | 19.42 Bd | 19.92 Ac | 1.17 Ab | 1.12 Bb | 165.85 Ab | 165.05 Ab | 11.33 Ab | 10.63 Ab |
| DG839 | 21.11 Ba | 21.91 Aa | 1.45 Aa | 1.41 Aa | 184.23 Aa | 184.13 Aa | 14.82 Aa | 14.02 Aa |
| DG848 | 19.05 Ad | 19.25 Ac | 0.78 Ac | 0.74 Ac | 150.21 Ab | 150.10 Ac | 6.71 Ad | 6.31 Ac |
| MS018 | 21.10 Aa | 21.31 Aa | 1.48 Aa | 1.39 Aa | 184.05 Aa | 183.15 Ba | 14.33 Aa | 14.10 Aa |
| MS019 | 19.51 Bc | 20.41 Ab | 1.17 Ab | 1.12 Ab | 162.55 Ab | 162.45 Ab | 10.74 Ab | 10.94 Ab |
| MS053 | 19.42 Ac | 19.92 Ab | 0.81 Ac | 0.82 Ac | 160.44 Ab | 160.14 Ab | 8.95 Ac | 8.45 Ac |
| MS055 | 20.51 Ab | 20.81 Ab | 0.84 Ac | 0.82 Ac | 159.15 Ab | 152.45 Bc | 8.75 Ac | 8.35 Ac |
| MS079 | 20.03 Ab | 20.43 Ab | 0.87 Ac | 0.80 Ac | 153.25 Ac | 152.45 Ac | 9.03 Ac | 8.23 Ac |
| MS119 | 20.21 Ab | 20.41 Ab | 0.86 Ac | 0.80 Ac | 151.66 Ac | 151.46 Ac | 8.81 Ac | 8.01 Ac |
| MS127 | 20.12 Ab | 20.52 Ab | 0.79 Ac | 0.70 Ac | 151.55 Ac | 151.45 Ac | 6.93 Ad | 6.13 Ad |
| MS132 | 20.51 Ab | 20.21 Ab | 0.71 Ac | 0.70 Ac | 151.66 Ac | 151.46 Ac | 7.11 Ad | 6.31 Ad |
| MS250 | 20.41 Ab | 20.21 Ab | 0.72 Ac | 0.70 Ac | 151.87 Ac | 151.37 Ac | 6.73 Ad | 6.03 Ad |
| MS260 | 19.51 Ac | 19.71 Ac | 0.78 Ac | 0.70 Ac | 152.11 Ac | 151.21 Ac | 7.61 Ad | 6.91 Ad |
| MS298 | 19.00 Ad | 18.93 Ad | 0.81 Ac | 0.71 Ac | 151.77 Ac | 151.37 Ac | 6.85 Ad | 6.05 Ad |
| MS317 | 19.01 Bd | 19.91 Ac | 0.78 Ac | 0.70 Ac | 151.73 Ac | 151.03 Ac | 6.58 Ad | 6.38 Ad |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inacio, K.A.M.; Farfan, N.C.; Azevedo, C.E.X.; Polatto, M.A.G.; Carrion, N.S.; Mendes, P.V.S.; Mateus, N.S.; Santos, E.F. Potential of Cassava Clones for Iron, Zinc, and Selenium Biofortification. Agriculture 2024, 14, 268. https://doi.org/10.3390/agriculture14020268
Inacio KAM, Farfan NC, Azevedo CEX, Polatto MAG, Carrion NS, Mendes PVS, Mateus NS, Santos EF. Potential of Cassava Clones for Iron, Zinc, and Selenium Biofortification. Agriculture. 2024; 14(2):268. https://doi.org/10.3390/agriculture14020268
Chicago/Turabian StyleInacio, Karini Aparecida Matos, Nancy Carrasco Farfan, Carlos Eduardo Xisto Azevedo, Marco Antônio Gomes Polatto, Natã Souza Carrion, Polliany Vitória Santos Mendes, Nikolas Souza Mateus, and Elcio Ferreira Santos. 2024. "Potential of Cassava Clones for Iron, Zinc, and Selenium Biofortification" Agriculture 14, no. 2: 268. https://doi.org/10.3390/agriculture14020268
APA StyleInacio, K. A. M., Farfan, N. C., Azevedo, C. E. X., Polatto, M. A. G., Carrion, N. S., Mendes, P. V. S., Mateus, N. S., & Santos, E. F. (2024). Potential of Cassava Clones for Iron, Zinc, and Selenium Biofortification. Agriculture, 14(2), 268. https://doi.org/10.3390/agriculture14020268







