Synthesis of Highly Intercalated Urea–Clay Nanocomposite via Pomegranate Peel Waste as Eco-Friendly Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fertilizer Production
2.3. Characterization of Nanocomposites
2.4. Release Pattern
2.5. Statistical Analysis
3. Results
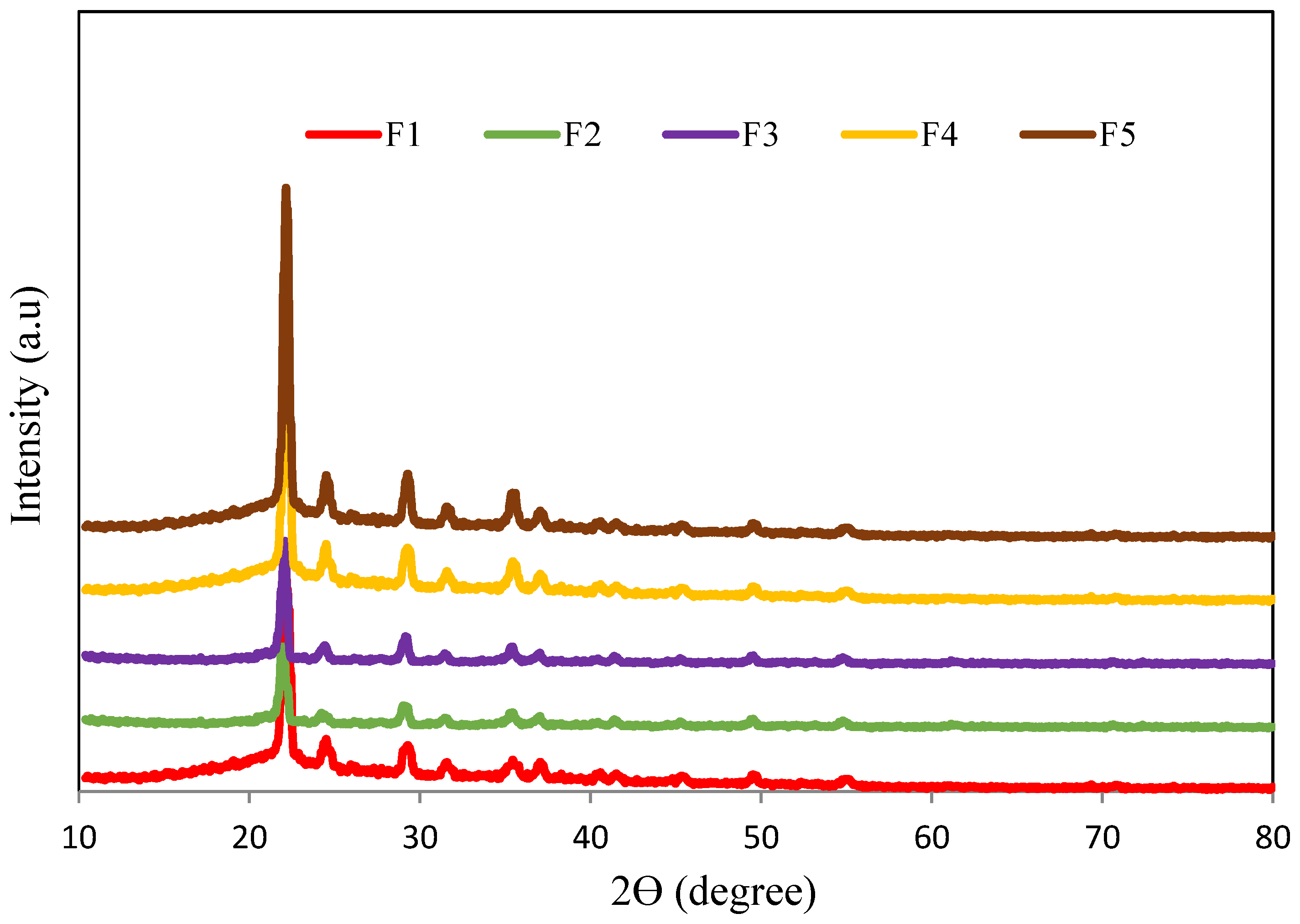
3.1. XRD Analysis (Low-Angle)
3.2. XRD Analysis (Normal-Angle)
3.3. FTIR Spectra
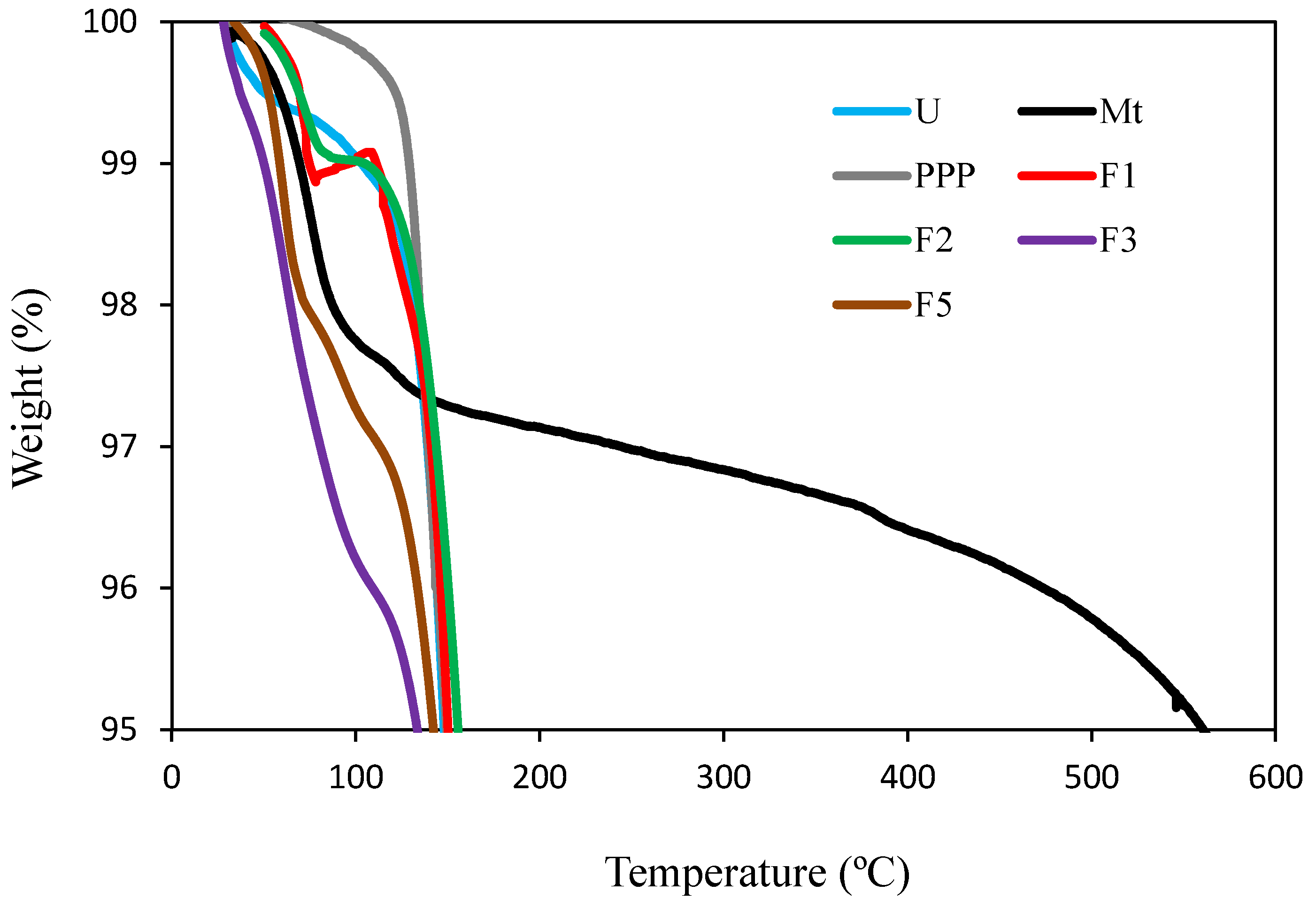
3.4. TGA
3.5. SEM Images
3.6. Release Pattern
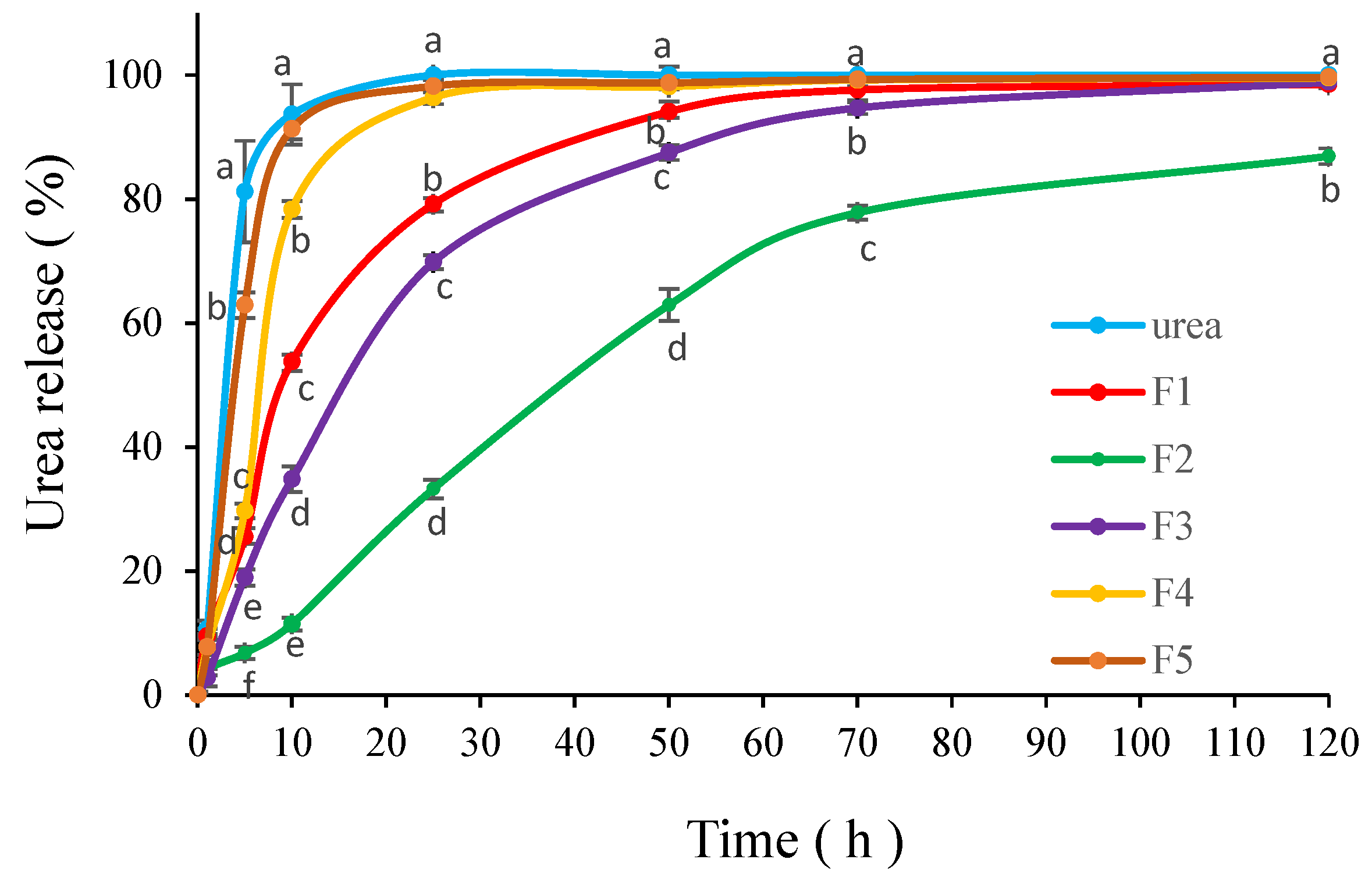
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borlaug, N.E. The Green Revolution Revisited and the Road Ahead; 1970 Nobel peace prize laureate. Anniversary lecture; The Norwegian Nobel Institute: Oslo, Norway, 2000. [Google Scholar]
- El-Razik, A.; Eman, M. Reducing nirate leaching through the soil profile using some nano-sized organic residues. J. Soil Sci. Agric. Eng. 2020, 11, 757–764. [Google Scholar]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W. Impacts of nitrogen emissions on ecosystems and human health: A mini review. Curr. Opin. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Xie, T.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. Effects of biological nitrification inhibitors on nitrogen use efficiency and greenhouse gas emissions in agricultural soils: A review. Ecotoxicol. Environ. Saf. 2021, 220, 112338. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ma, Q.; Luo, J.; Xie, Y.; Pan, W.; Zheng, N.; Liu, M.; Wu, L. The inhibition effect of tea polyphenols on soil nitrification is greater than denitrification in tea garden soil. Sci. Total Environ. 2021, 778, 146328. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, Y.; Li, X.; Li, W.; Huang, S. Lignin improves release behavior of slow-release fertilizers with high content of urea. J. Appl. Polym. Sci. 2019, 136, 48238. [Google Scholar] [CrossRef]
- Pereira, E.I.; da Cruz, C.C.T.; Solomon, A.; Le, A.; Cavigelli, M.A.; Ribeiro, C. Novel slow-release nanocomposite nitrogen fertilizers: The impact of polymers on nanocomposite properties and function. Ind. Eng. Chem. Res. 2015, 54, 3717–3725. [Google Scholar] [CrossRef]
- Yamamoto, C.F.; Pereira, E.I.; Mattoso, L.H.C.; Matsunaka, T.; Ribeiro, C. Slow release fertilizers based on urea/urea–formaldehyde polymer nanocomposites. Chem. Eng. J. 2016, 287, 390–397. [Google Scholar] [CrossRef]
- Pereira, E.I.; Minussi, F.B.; da Cruz, C.C.T.; Bernardi, A.C.C.; Ribeiro, C. Urea–montmorillonite-extruded nanocomposites: A novel slow-release material. J. Agric. Food Chem. 2012, 60, 5267–5272. [Google Scholar] [CrossRef]
- Xia, Y.; Kwon, H.; Wander, M. Developing county-level data of nitrogen fertilizer and manure inputs for corn production in the united states. J. Clean. Prod. 2021, 309, 126957. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef]
- Xiaoyu, N.; Yuejin, W.; Zhengyan, W.; Lin, W.; Guannan, Q.; Lixiang, Y. A novel slow-release urea fertilizer: Physical and chemical analysis of its structure and study of its release mechanism. Biosyst. Eng. 2013, 115, 274–282. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Jarosiewicz, A. Use of polysulfone in controlled-release NPK fertilizer formulations. J. Agric. Food Chem. 2002, 50, 4634–4639. [Google Scholar] [CrossRef] [PubMed]
- Baldanza, V.A.R.; Souza, F.G., Jr.; Filho, S.T.; Franco, H.A.; Oliveira, G.E.; Caetano, R.M.J.; Hernandez, J.A.R.; Ferreira Leite, S.G.; Furtado Sousa, A.M.; Nazareth Silva, A.L. Controlled-release fertilizer based on poly (butylene succinate)/urea/clay and its effect on lettuce growth. J. Appl. Polym. Sci. 2018, 135, 46858. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, M.; Choi, C.L.; Lee, D.H.; Seo, Y.J.; Kim, C.Y.; Kim, J.S.; Yun, S.-I.; Ro, H.-M.; Komarneni, S. Suppression of NH3 and N2O emissions by massive urea intercalation in montmorillonite. J. Soils Sediments 2011, 11, 416–422. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, M.-T.; Ryu, J.-H.; Choi, J.-S.; Park, K.D.; Kang, H.-W.; Park, M. Metal-urea-montmorillonite hybrid incorporated with citric acid. Korean J. Soil Sci. Fertil. 2013, 46, 610–614. [Google Scholar] [CrossRef]
- Bortolin, A.; Aouada, F.A.; Mattoso, L.H.C.; Ribeiro, C. Nanocomposite paam/methyl cellulose/montmorillonite hydrogel: Evidence of synergistic effects for the slow release of fertilizers. J. Agric. Food Chem. 2013, 61, 7431–7439. [Google Scholar] [CrossRef]
- Madusanka, N.; Sandaruwan, C.; Kottegoda, N.; Sirisena, D.; Munaweera, I.; De Alwis, A.; Karunaratne, V.; Amaratunga, G.A.J. Urea–hydroxyapatite-montmorillonite nanohybrid composites as slow release nitrogen compositions. Appl. Clay Sci. 2017, 150, 303–308. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Valorization of pomegranate peels: A biorefinery approach. Waste Biomass Valorization 2017, 8, 1127–1137. [Google Scholar] [CrossRef]
- Shinde, P.N.; Mandavgane, S.A.; Karadbhajane, V. Process development and life cycle assessment of pomegranate biorefinery. Environ. Sci. Pollut. Res. 2020, 27, 25785–25793. [Google Scholar] [CrossRef] [PubMed]
- Czaban, W.; Rasmussen, J.; Laursen, B.B.; Vidkjaeer, N.H.; Sapkota, R.; Nicolaisen, M.; Fomsgaard, I.S. Multiple effects of secondary metabolites on amino acid cycling in white clover rhizosphere. Soil Biol. Biochem. 2018, 123, 54–63. [Google Scholar] [CrossRef]
- Peter Constabel, C.; Yoshida, K.; Walker, V. Diverse ecological roles of plant tannins: Plant defense and beyond. In Recent Advances in Polyphenol Research; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zolotareva, O.K.; Podorvanov, V.V.; Dubyna, D.V. Polyphenolic compounds of macrophytes and their ecological importance. Ukr. Bot. J. 2017, 74, 373–384. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef] [PubMed]
- Munakata, R.; Larbat, R.; Duriot, L.; Olry, A.; Gavira, C.; Mignard, B.; Hehn, A.; Bourgaud, F. Polyphenols from plant roots: An expanding biological frontier. Recent Adv. Polyphen. Res. 2019, 6, 207–236. [Google Scholar]
- Rabiei, M.; Sabahi, H.; Rezayan, A.H. Gallic acid-loaded montmorillonite nanostructure as a new controlled release system. Appl. Clay Sci. 2016, 119, 236–242. [Google Scholar] [CrossRef]
- With, T.K.; Petersen, T.D.; Petersen, B. A simple spectrophotometric method for the determination of urea in blood and urine. J. Clin. Pathol. 1961, 14, 202–204. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Newton, A.G.; Lee, J.-Y.; Kwon, K.D. Na-montmorillonite edge structure and surface complexes: An atomistic perspective. Minerals 2017, 7, 78. [Google Scholar] [CrossRef]
- Kastner, H.; Einhorn-Stoll, U.; Fatouros, A.; Drusch, S. Impact of sodium ions on material properties, gelation and storage stability of citrus pectin. Food Hydrocoll. 2020, 104, 105750. [Google Scholar] [CrossRef]
- Owens, H.S.; Swenson, H.A.; Schultz, T.H. Factors influencing gelation with pectin. In Natural Plant Hydrocolloids; ACS Publications: Washington, DC, USA, 1954. [Google Scholar]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin hydrogels: Gel-forming behaviors, mechanisms, and food applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Mougán, M.; Meijide, F.; Jover, A.; Rodríguez-Núñez, E.; Vázquez-Tato, J. Rheological behaviour of an amide pectin. J. Food Eng. 2002, 55, 123–129. [Google Scholar] [CrossRef]
- Balooch, M.R.; Sabahi, H.; Aminian, H.; Hosseini, M. Intercalation technique can turn pomegranate industrial waste into a valuable by-product. LWT 2018, 98, 99–105. [Google Scholar] [CrossRef]
- Elshayb, O.M.; Nada, A.M.; Farroh, K.Y.; Al-Huqail, A.A.; Aljabri, M.; Binothman, N.; Seleiman, M.F. Utilizing urea–chitosan nanohybrid for minimizing synthetic urea application and maximizing oryza sativa l. productivity and N uptake. Agriculture 2022, 12, 944. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Lubis, M.A.R.; Park, B.-D.; Kim, J.S.; Causin, V. Converting crystalline thermosetting urea–formaldehyde resins to amorphous polymer using modified nanoclay. J. Ind. Eng. Chem. 2020, 87, 78–89. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.-D. Crystalline lamellar structure of thermosetting urea–formaldehyde resins at a low molar ratio. Macromolecules 2021, 54, 2366–2375. [Google Scholar] [CrossRef]
- Whittig, L.D.; Allardice, W.R. X-ray diffraction techniques. Methods Soil Anal. Part 1 Phys. Mineral. Methods 1986, 5, 331–362. [Google Scholar]
- Stanev, V.; Vesselinov, V.V.; Kusne, A.G.; Antoszewski, G.; Takeuchi, I.; Alexandrov, B.S. Unsupervised phase mapping of x-ray diffraction data by nonnegative matrix factorization integrated with custom clustering. NPJ Comput. Mater. 2018, 4, 43. [Google Scholar] [CrossRef]
- He, X.; Liao, Z.; Huang, P.; Duan, J.; Ge, R.; Li, H.; Geng, Z. Characteristics and performance of novel water-absorbent slow release nitrogen fertilizers. Agric. Sci. China 2007, 6, 338–346. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ni, B.; Zhang, X.; Wang, Y. Slow-release nitrogen and boron fertilizer from a functional superabsorbent formulation based on wheat straw and attapulgite. Chem. Eng. J. 2011, 167, 342–348. [Google Scholar] [CrossRef]
- Gul, W.; Shah, S.R.A.; Khan, A.; Pruncu, C.I. Characterization of zinc oxide-urea formaldehyde nano resin and its impact on the physical performance of medium-density fiberboard. Polymers 2021, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Y.; Sabahi, H.; Rahaie, M. Stability and loading properties of curcumin encapsulated in Chlorella vulgaris. Food Chem. 2016, 211, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Ali, S.W. Leveraging flame retardant efficacy of pomegranate rind extract, a novel biomolecule, on ligno-cellulosic materials. Polym. Degrad. Stab. 2017, 144, 83–92. [Google Scholar] [CrossRef]
- Sabzevari, A.G.; Sabahi, H. Montmorillonite an efficient oral nanocarrier for punicalagin-rich pomegranate peel extract: An in vitro study. J. Drug Deliv. Sci. Technol. 2023, 86, 104713. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Elimelech, M. Surface element integration: A novel technique for evaluation of DLVO interaction between a particle and a flat plate. J. Colloid Interface Sci. 1997, 193, 273–285. [Google Scholar] [CrossRef]
- Cai, D.; Wu, Z.; Jiang, J.; Ding, K.; Tong, L.; Chu, P.K.; Yu, Z. A unique technology to transform inorganic nanorods into nano-networks. Nanotechnology 2009, 20, 255302. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Zhuang, X.; Chen, X.; Jing, X. Controlled release of urea encapsulated by starch-g-poly (l-lactide). Carbohydr. Polym. 2008, 72, 342–348. [Google Scholar] [CrossRef]
- Costa, M.M.E.; Cabral-Albuquerque, E.C.M.; Alves, T.L.M.; Pinto, J.C.; Fialho, R.L. Use of polyhydroxybutyrate and ethyl cellulose for coating of urea granules. J. Agric. Food Chem. 2013, 61, 9984–9991. [Google Scholar] [CrossRef]
- Niu, Y.; Li, H. Controlled release of urea encapsulated by starch-g-poly (vinyl acetate). Ind. Eng. Chem. Res. 2012, 51, 12173–12177. [Google Scholar] [CrossRef]



| Treatments | Mass Ratio U/Mt (w/w) | Mass Ratio U/PPP (w/w) |
|---|---|---|
| U | - | - |
| F1 (U/Mt) | 4:1 | - |
| F2 (U/Mt/PPP) | 4:1 | 4:0.25 |
| F3 (U/Mt/PPP) | 4:1 | 4:0.5 |
| F4 (U/Mt/PPP) | 4:1 | 4:75 |
| F5 (U/PPP) | - | 4:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teimouri Yanehsari, A.; Sabahi, H.; Jahani, Y.; Mahmoodi, M.H.; Shalileh, F. Synthesis of Highly Intercalated Urea–Clay Nanocomposite via Pomegranate Peel Waste as Eco-Friendly Material. Agriculture 2024, 14, 2097. https://doi.org/10.3390/agriculture14122097
Teimouri Yanehsari A, Sabahi H, Jahani Y, Mahmoodi MH, Shalileh F. Synthesis of Highly Intercalated Urea–Clay Nanocomposite via Pomegranate Peel Waste as Eco-Friendly Material. Agriculture. 2024; 14(12):2097. https://doi.org/10.3390/agriculture14122097
Chicago/Turabian StyleTeimouri Yanehsari, Abolfazl, Hossein Sabahi, Yousef Jahani, Mohammad Hossein Mahmoodi, and Farzaneh Shalileh. 2024. "Synthesis of Highly Intercalated Urea–Clay Nanocomposite via Pomegranate Peel Waste as Eco-Friendly Material" Agriculture 14, no. 12: 2097. https://doi.org/10.3390/agriculture14122097
APA StyleTeimouri Yanehsari, A., Sabahi, H., Jahani, Y., Mahmoodi, M. H., & Shalileh, F. (2024). Synthesis of Highly Intercalated Urea–Clay Nanocomposite via Pomegranate Peel Waste as Eco-Friendly Material. Agriculture, 14(12), 2097. https://doi.org/10.3390/agriculture14122097






