Non-Destructive Prediction of Carotenoids, Ascorbic Acid, and Total Phenols Contents in ‘Tommy Atkins’ Mangoes Using Absorption and Scattering Properties Measured by Time-Resolved Reflectance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruits
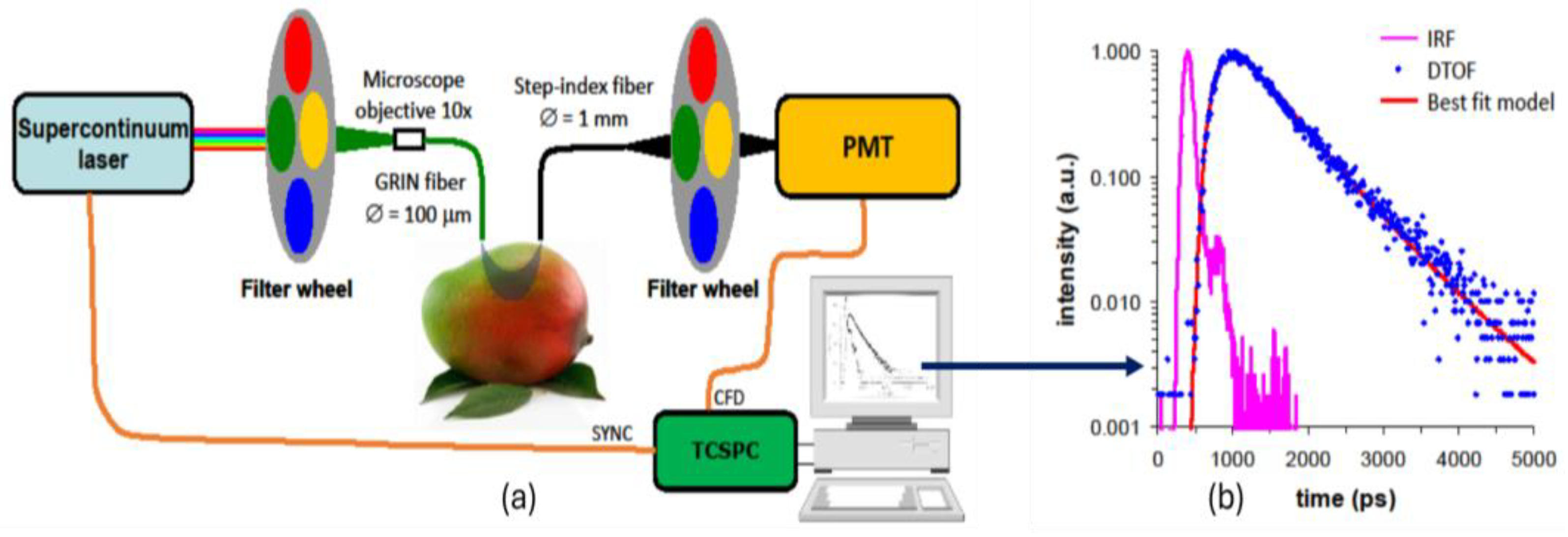
2.2. Time-Resolved Reflectance Spectroscopy
2.3. Pulp Color and Flesh Firmness
2.4. Determination of Carotenoids, Ascorbic Acid, Phenolic Compounds, and Total Antioxidant Capacity
2.4.1. Ascorbic Acid
2.4.2. Total Phenols Content
2.4.3. Total Antioxidant Capacity
2.4.4. Carotenoids
2.5. Statistics
2.5.1. Analysis of Variance
2.5.2. Predictive Models
3. Results
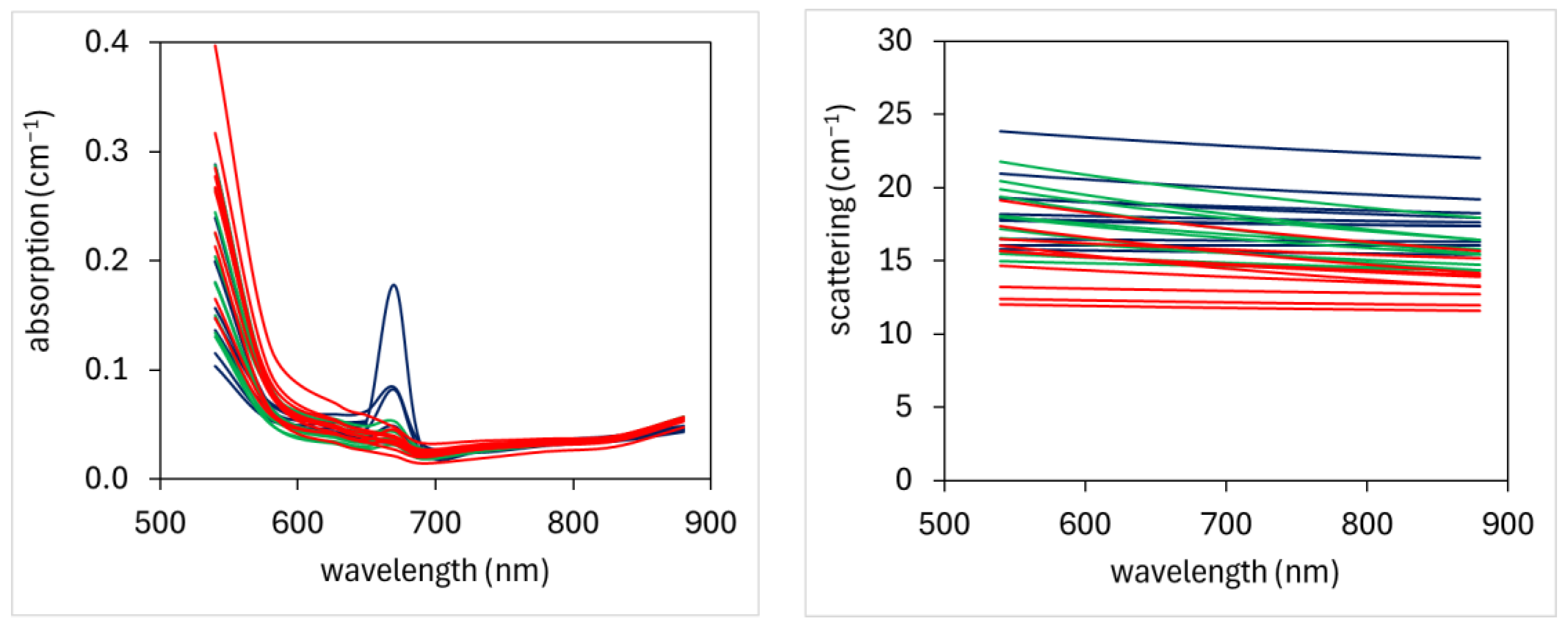
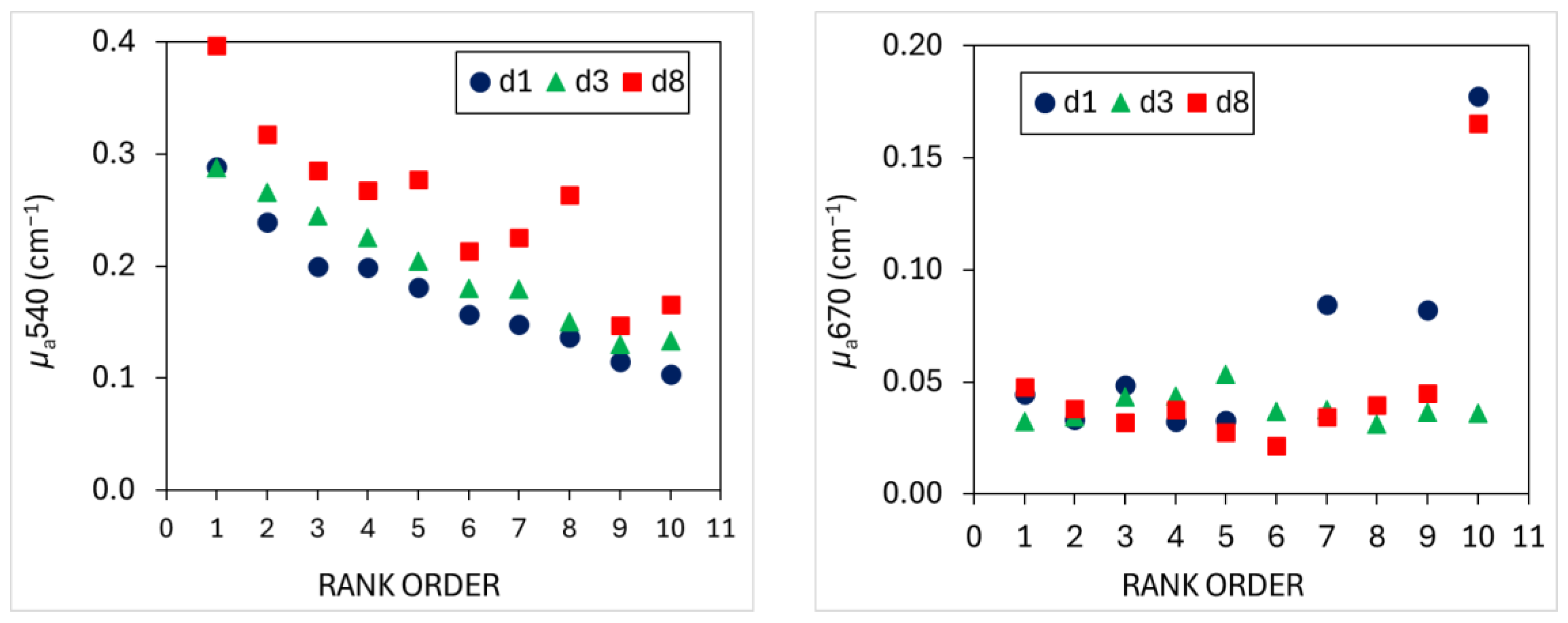
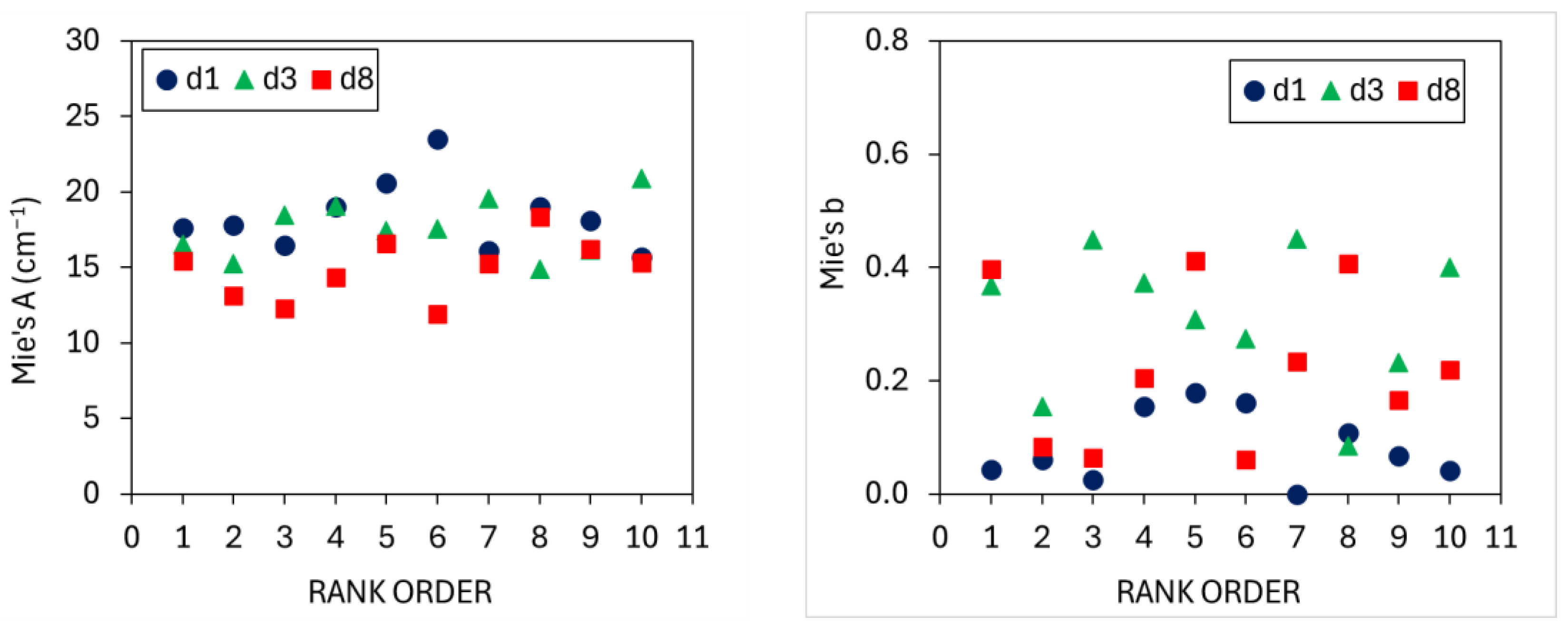
3.1. TRS Optical Properties
3.2. Quality Parameters
3.3. Ascorbic Acid, Total Phenolic Content, and Total Antioxidant Capacity
3.4. Carotenoids
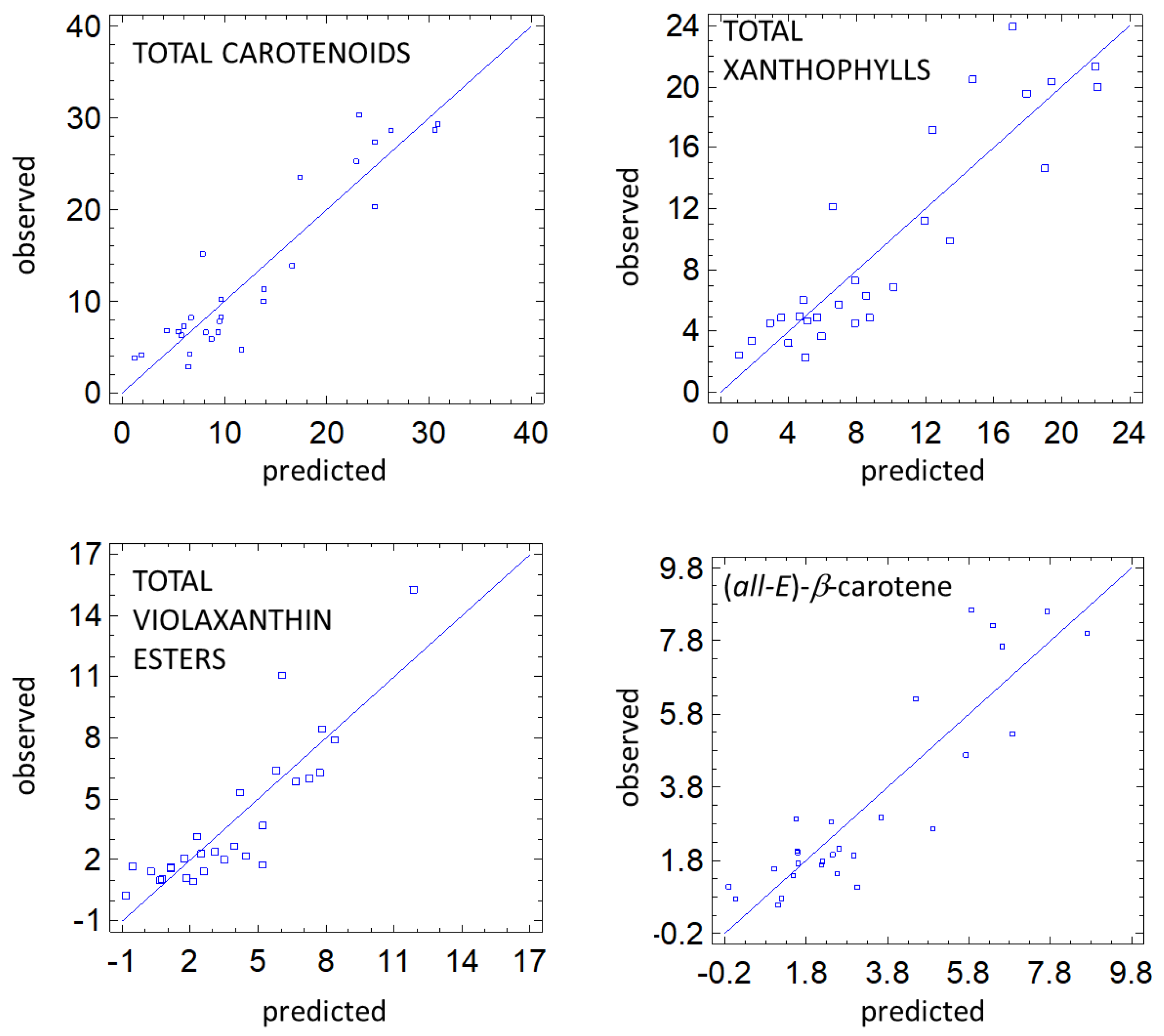
3.5. Predictive Models
4. Discussion
4.1. Optical Properties
4.2. Ascorbic Acid, Total Phenolic Content, and Total Antioxidant Capacity
4.3. Carotenoids
4.4. Predictive Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gentile, C.; Di Gregorio, E.; Di Stefano, V.; Mannino, G.; Perrone, A.; Avellone, G.; Sortino, G.; Inglese, P.; Farina, V. Food quality and nutraceutical value of nine cultivars of mango (Mangifera indica L.) fruits grown in Mediterranean subtropical environment. Food Chem. 2019, 277, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Liguori, G.; Gentile, C.; Sortino, G.; Inglese, P.; Farina, V. Food quality, sensory attributes and nutraceutical value of fresh “Osteen” mango fruit grown under mediterranean subtropical climate compared to imported fruit. Agriculture 2020, 10, 103. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.J.; Yahia, E.M.; Gardea, A.A. Identification and quantification of xanthophyll esters, carotenes, and tocopherols in the fruit of seven Mexican mango cultivars by liquid chromatography-atmospheric pressure chemical ionization-time-of-flight mass spectrometry [LC-(Apcl+)-MS]. J. Agric. Food Chem. 2007, 55, 6628–6635. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.J.; Yahia, E.M.; Gardea, A.A. Changes in external and internal color during postharvest ripening of ‘Manila’ and ‘Ataulfo’ mango fruit and relationship with carotenoid content determined by liquid chromatography-Apcl+-time-of-flight mass spectrometry. Postharvest Biol. Technol. 2008, 50, 145–152. [Google Scholar] [CrossRef]
- Manthey, J.A.; Perkins-Veazie, P. Influences of harvest date and location on the levels of β-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L.). J. Agric. Food Chem. 2009, 57, 10825–10830. [Google Scholar] [CrossRef]
- Liu, F.X.; Fu, S.F.; Bi, X.F.; Chen, F.; Liao, X.J.; Hu, X.S.; Wu, J.H. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef]
- Vásquez-Caicedo, A.L.; Heller, A.; Neidhart, S.; Carle, R. Chromoplast morphology and β-carotene accumulation during postharvest ripening of Mango cv. ‘Tommy Atkins’. J. Agric. Food Chem. 2006, 54, 5769–5776. [Google Scholar] [CrossRef]
- Vanoli, M.; Rizzolo, A.; Spinelli, L.; Azzollini, S.; Torricelli, A. Carotenoid content and pulp colour non-destructively measured by time-resolved reflectance spectroscopy in different cultivars of Brazilian mangoes. Acta Hortic. 2016, 1119, 305–312. [Google Scholar] [CrossRef]
- Ntsoane, M.L.; Zude-Sasse, M.; Mahajan, P.; Sivakumar, D. Quality assessment and postharvest technology of mango: A review of its current status and future perspectives. Sci. Hortic. 2019, 249, 77–85. [Google Scholar] [CrossRef]
- Ibarra-Garza, I.P.; Ramos-Parra, P.A.; Hernández-Brenes, C.; Jacobo-Velázquez, D.A. Effect of postharvest ripening on the nutraceutical and physico-chemical properties of mango (Mangifera indica L. cv. Keitt). Postharvest Biol. Technol. 2015, 103, 45–54. [Google Scholar] [CrossRef]
- Hu, K.; Peng, D.; Wang, L.; Liu, H.; Xie, B.; Sun, Z. Effect of mild high hydrostatic pressure treatments on physiological and physicochemical characteristics and carotenoid biosynthesis in postharvest mango. Postharvest Biol. Technol. 2021, 172, 111381. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, B.; Ma, Y.; Xu, W.; Wu, H.; Wang, S. Carotenoid accumulation and expression of carotenoid biosynthesis genes in mango flesh during fruit development and ripening. Sci. Hortic. 2018, 237, 201–206. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Tornese, R.; Mita, G.; Durante, M. Bioactive Compounds and Antioxidant Activities in Different Fractions of Mango Fruits (Mangifera indica L., Cultivar Tommy Atkins and Keitt). Antioxidants 2022, 11, 484. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodriguez-Amaya, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid esters in foods—A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of new carotenoid esters in mango and citrus. J. Agric. Food Chem. 2016, 64, 8207–8224. [Google Scholar] [CrossRef]
- Vanoli, M.; Grassi, M.; Spinelli, L.; Torricelli, A.; Rizzolo, A. Quality and nutraceutical properties of mango fruit: Influence of cultivar and biological age assessed by Time-resolved Reflectance Spectroscopy. Adv. Hortic. Sci. 2018, 32, 407–420. [Google Scholar] [CrossRef]
- Rocha Ribeiro, S.M.; Queiroz, J.H.; Lopes Ribeiro de Queiroz, M.E.; Campos, F.M.; Pinheiro Sant’Ana, H.M. Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Hum. Nutr. 2007, 62, 13–17. [Google Scholar] [CrossRef]
- Hu, K.; Ghani Dars, A.; Liu, Q.; Xie, B.; Sun, Z. Phytochemical profiling of the ripening of Chinese mango (Mangifera indica L.) cultivars by real-time monitoring using UPLC-ESI-QTOF-MS and its potential benefits as prebiotic ingredients. Food Chem. 2018, 256, 171–180. [Google Scholar] [CrossRef]
- da Silva Sauthier, M.C.; da Silva, E.G.P.; da Silva Santos, B.R.; Requião Silva, E.F.; da Cruz Caldas, J.; Minho, L.A.C.; dos Santos, A.M.P.; dos Santos, W.N.L. Screening of Mangifera indica L. functional content using PCA and neural networks (ANN). Food Chem. 2019, 273, 115–123. [Google Scholar] [CrossRef]
- Nassur, R.C.M.R.; Gonzales-Moscoso, S.; Crisosto, G.M.; Lima, L.C.O.; Vilas Boas, E.V.B.; Crisosto, C.H. Describing quality and sensory attributes of 3 mango (Mangifera indica L.) cultivars at 3 ripeness stages based on firmness. J. Food Sci. 2015, 80, S2055–S2063. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Internat. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Kienzle, S.; Sruamsiri, P.; Carle, R.; Sirisakulwat, S.; Spreer, W.; Neidhart, S. Harvest maturity specification for mango fruit (Mangifera indica L. ‘Chok Anan’) in regard to long supply chains. Postharvest Biol. Technol. 2011, 61, 41–55. [Google Scholar] [CrossRef]
- Padda, M.S.; do Amarante, C.V.T.; Garcia, R.M.; Slaughter, D.C.; Mitcham, E.J. Methods to analyze physico-chemical changes during mango ripening: A multivariate approach. Postharvest Biol. Technol. 2011, 62, 267–274. [Google Scholar] [CrossRef]
- Subedi, P.P.; Walsh, K.B.; Owens, G. Prediction of mango eating quality at harvest using short-wave near infrared spectrometry. Postharvest Biol. Technol. 2007, 43, 326–334. [Google Scholar] [CrossRef]
- O’Brien, C.; Falagan, N.; Kourmpetli, S.; Landahl, S.; Terry, L.A.; Alamar, M.C. Non-destructive methods for mango ripening prediction: Visible and near-infrared spectroscopy (visNIRS) and laser Doppler vibrometry (LDV). Postharvest Biol. Technol. 2024, 212, 112878. [Google Scholar] [CrossRef]
- Lu, R.; Van Beers, R.; Saeys, W.; Li, C.; Cen, H. Measurement of optical properties of fruits and vegetables: A review. Postharvest Biol. Technol. 2020, 159, 111003. [Google Scholar] [CrossRef]
- Lammertyn, J.; Peirs, A.; De Baerdemaeker, J.; Nicolaï, B.M. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Dover, C.; Johnson, D.; Ruiz-Altisent, M.; Valero, C. Nondestructive quantification of chemical and physical properties of fruits by time-resolved reflectance spectroscopy in the wavelength range 650–1000 nm. Appl. Opt. 2001, 40, 538–543. [Google Scholar] [CrossRef]
- Torricelli, A.; Spinelli, L.; Contini, D.; Vanoli, M.; Rizzolo, A.; Eccher Zerbini, P. Time-resolved reflectance spectroscopy for nondestructive assessment of food quality. Sens. Instrumen. Food Qual. 2008, 2, 82–89. [Google Scholar] [CrossRef]
- Rizzolo, A.; Vanoli, M. Time-resolved technique for measuring optical properties and quality of food. In Light Scattering Technology for Food Property, Quality and Safety Assessment; Lu, R., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 187–224. [Google Scholar]
- Eccher Zerbini, P.; Vanoli, M.; Rizzolo, A.; Jacob, S.; Torricelli, A.; Spinelli, L.; Schouten, R.E. Time-resolved Reflectance Spectroscopy as a management tool in the fruit supply chain: An export trial with nectarines. Biosyst. Eng. 2009, 102, 360–363. [Google Scholar] [CrossRef]
- Rizzolo, A.; Vanoli, M.; Torricelli, A.; Spinelli, L.; Sadar, N.; Zanella, A. Modeling optical properties of Braeburn apples during fruit maturation on the tree. Acta Hortic. 2021, 1311, 113–121. [Google Scholar] [CrossRef]
- Pereira, T.; Tijskens, L.M.M.; Vanoli, M.; Rizzolo, A.; Eccher Zerbini, P.; Torricelli, A.; Spinelli, L.; Filgueiras, H. Assessing the harvest maturity of Brazilian mangoes. Acta Hortic. 2010, 880, 269–276. [Google Scholar] [CrossRef]
- Eccher Zerbini, P.; Vanoli, M.; Rizzolo, A.; Grassi, M.; Pimentel, R.M.A.; Spinelli, L.; Torricelli, A. Optical properties, ethylene production and softening in mango fruit. Postharvest Biol. Technol. 2015, 101, 58–65. [Google Scholar] [CrossRef]
- Vanoli, M.; Rizzolo, A.; Grassi, M.; Spinelli, L.; Torricelli, A. Modeling mango ripening during shelf life based on pulp color nondestructively measured by time-resolved reflectance spectroscopy. Sci. Hortic. 2023, 310, 111714. [Google Scholar] [CrossRef]
- Martelli, F.; Del Bianco, S.; Ismaelli, A.; Zaccanti, G. Light Propagation Through Biological Tissue and Other Diffusive Media: Theory, Solution, and Software; SPIE Press: Washington, DC, USA, 2009. [Google Scholar]
- Jha, S.N.; Kingsly, A.R.P.; Chopra, S. Non-destructive determination of firmness and yellowness of mango during growth and storage using visual spectroscopy. Biosyst. Eng. 2006, 94, 397–402. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Rojas-Graü, M.A.; Odriozola-Serrano, I.; González-Aguilar, G.A.; Martin-Belloso, O. Effect of minimal processing on bioactive compounds and antioxidant activity of fresh-cut ‘Kent’ mango (Mangifera indica L.). Postharvest Biol. Technol. 2009, 51, 384–390. [Google Scholar] [CrossRef]
- Rizzolo, A.; Brambilla, A.; Valsecchi, S.; Eccher Zerbini, P. Evaluation of sampling and extraction procedures for the analysis of ascorbic acid from pear fruit tissue. Food Chem. 2002, 77, 257–262. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methodol. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Confirmation of the identity of the carotenoids of tropical fruits by HPLC-DAD and HPLC-MS. J. Food Compos. Anal. 2004, 17, 385–396. [Google Scholar] [CrossRef]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and quantitative differences in the carotenoid composition of yellow and red peppers determined by HPLC-DAD-MS. J. Sep. Sci. 2009, 32, 3652–3658. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Ahmad, S.; Tang, A.; Wang, J.; Cheng, T.; Pan, H.; Zhan, O. Flavonols and carotenoids in yellow petals of rose cultivar (Rosa ‘Sun City’): A possible rich source of bioactive compounds. J. Agric. Food Chem. 2018, 66, 4171–4181. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The carotenoid pigment Zaexanthin—A Review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; HarvestPlus Technical Monograph 2, HarvestPlus, c/o; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2004. [Google Scholar]
- Mercadante, A.Z.; Rodriguez-Amaya, D.B.; George Britton, G. HPLC and Mass Spectrometric Analysis of Carotenoids from Mango. J. Agric. Food Chem. 1997, 45, 120–123. [Google Scholar] [CrossRef]
- Rizzolo, A.; Vanoli, M.; Spinelli, L.; Torricelli, A. Non-destructive assessment of pulp colour in mangoes by time-resolved reflectance spectroscopy: Problems and solutions. Acta Hortic. 2016, 1119, 147–154. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef]
- Kienzle, S.; Sruamsiri, P.; Carle, R.; Sirisakulwat, S.; Spreer, W.; Neidhart, S. Harvest maturity detection for ‘Nam Dokmai #4’ mango fruit (Mangifera indica L.) in consideration of long supply chains. Postharvest Biol. Technol. 2012, 72, 64–75. [Google Scholar] [CrossRef]
- De Morais, P.L.D.; Filgueiras, H.A.C.; De Pinho, J.L.N.; Alves, R.E. Ponto de colheita ideal de mangas ‘Tommy Atkins’ destinadas ao mercado europeu. Rev. Bras. Frutic. 2002, 24, 671–675. [Google Scholar] [CrossRef]
- Sabato, S.F.; da Silva, J.M.; da Cruz, J.N.; Salmieri, S.; Rela, P.R.; Lacroix, M. Study of physical–chemical and sensorial properties of irradiated Tommy Atkins mangoes (Mangifera indica L.) in an international consignment. Food Control 2009, 20, 284–288. [Google Scholar] [CrossRef]
- de Mello Vasconcelos, O.C.; Duarte, D.; de Castro Silva, J.; Oliveros Mesa, N.F.; Teruel Mederos, B.J.; de Freitas, T.S. Modeling ‘Tommy Atkins’ mango cooling time based on fruit physicochemical quality. Sci. Hortic. 2019, 244, 413–420. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Lea, J.M. Volatile and quality changes in fresh-cut mangos prepared from firm-ripe and soft-ripe fruit, stored in clamshell containers and passive MAP. Postharvest Biol. Technol. 2003, 30, 15–28. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Chanona-Pérez, J.J.; Güemes-Vera, N.; Cybulska, J.; Szymanska-Chargot, M.; Chylinska, M.; Kozioł, A.; Gawkowska, D.; Pieczywek, P.M.; Zdunek, A. Structural, mechanical and enzymatic study of pectin and cellulose during mango ripening. Carbohydr. Polym. 2018, 196, 313–321. [Google Scholar] [CrossRef]
- Cantre, D.; Herremans, E.; Verboven, P.; Ampofo-Asiama, J.; Nicolaï, B. Characterization of the 3-D microstructure of mango (Mangifera indica L. cv. Carabao) during ripening using X-ray computed microtomography. IFSET 2014, 24, 28–39. [Google Scholar] [CrossRef]
- Herremans, E.; Bongaers, E.; Estrade, P.; Gondek, E.; Hertog, M.; Jakubczyk, E.; Do Trong, N.N.; Rizzolo, A.; Saeys, W.; Spinelli, L.; et al. Microstructure–texture relationships of aerated sugar gels: Novel measurement techniques for analysis and control. IFSET 2013, 18, 202–211. [Google Scholar] [CrossRef]
- Modesto, J.H.; Leonel, S.; Segantini, D.M.; Azèvedo Souza, J.M.; Ferraz, R.A. Qualitative attributes of some mango cultivars fruits. Aust. J. Crop Sci. 2016, 10, 565–570. [Google Scholar] [CrossRef]
- da Silva Oliveira, D.; Lobato, A.L.; Machado Rocha Ribeiro, S.; Campos Santana, Â.M.; Paes Chaves, J.B.; Pinheiro-Sant’Ana, H.M. Carotenoids and vitamin C during handling and distribution of guava (Psidium guajava L.), mango (Mangifera indica L.), and papaya (Carica papaya L.) at commercial restaurants. J. Agric. Food Chem. 2010, 58, 6166–6172. [Google Scholar] [CrossRef]
- Barbosa Gámez, I.; Caballero Montoya, K.P.; Ledesma, N.; Sáyago Ayerdi, S.G.; García Magaña, M.d.L.; Bishop von Wettberg, E.J.; Montalvo-González, E. Changes in the nutritional quality of five Mangifera species harvested at two maturity stages. J. Sci. Food Agric. 2017, 97, 4987–4994. [Google Scholar] [CrossRef]
- Kim, Y.; Brecht, J.K.; Talcott, S.T. Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007, 105, 1327–1334. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.M.; Islas-Osuna, M.A.; Astiazarán-García, H.; Vázquez-Ortiz, F.A.; Martín-Belloso, O.; Gorinstein, S.; González-Aguilar, G.A. Quality index, consumer acceptability, bioactive compounds, and antioxidant activity of fresh-cut “Ataulfo” mangoes (Mangifera indica L.) as affected by low-temperature storage. J. Food Sci. 2009, 74, S126–S134. [Google Scholar] [CrossRef]
- Kim, Y.; Lounds-Singleton, A.J.; Talcott, S.T. Antioxidant phytochemical and quality changes associated with hot water immersion treatment of mango (Mangifera indica L.). Food Chem. 2009, 115, 989–993. [Google Scholar] [CrossRef]
- Silva, K.D.R.R.; Sirasa, M.S.F. Antioxidant properties of selected fruit cultivars grown in Sri Lanka. Food Chem. 2018, 238, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Corral-Aguayo, R.D.; Yahia, E.M.; Carrillo-Lopez, A.; González- Aguilar, G. Correlation between some nutritional components and the total antioxidant capacity measured with six different assays in eight horticultural crops. J. Agric. Food Chem. 2008, 56, 10498–10504. [Google Scholar] [CrossRef]
- Pott, I.; Breithaupt, D.E.; Carle, R. Detection of unusual carotenoid esters in fresh mango (Mangifera indica L. cv. ‘Kent’). Phytochemistry 2003, 64, 825–829. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodriguez-Amaya, D.B. Effects of Ripening, Cultivar Differences, and Processing on the Carotenoid Composition of Mango. J. Agric. Food Chem. 1998, 46, 128–130. [Google Scholar] [CrossRef]
- Rungpichayapichet, P.; Chaiyarattanachote, N.; Khuwijitjaru, P.; Nakagawa, K.; Nagle, M.; Müller, J.; Mahayothee, B. Comparison of near-infrared spectroscopy and hyperspectral imaging for internal quality determination of ‘Nam Dok Mai’ mango during ripening. J. Food Meas. Charact. 2023, 17, 1501–1514. [Google Scholar] [CrossRef]
- Bicanic, D.; Dimitrovski, D.; Luterotti, S.; Twisk, C.; Buijnsters, J.G.; Dóka, O. Estimating rapidly and precisely the concentration of beta carotene in mango homogenates by measuring the amplitude of optothermal signals, chromaticity indices and the intensities of Raman peaks. Food Chem. 2010, 121, 832–838. [Google Scholar] [CrossRef]
- Vásquez-Caicedo, A.L.; Sruamsiri, P.; Heller, A.; Carle, R.; Neidhart, S. Accumulation of All-trans-β-carotene and Its 9-cis and 13-cis Stereoisomers during Postharvest Ripening of Nine Thai Mango Cultivars. J. Agric. Food Chem. 2005, 53, 4827–4835. [Google Scholar] [CrossRef]
- Kiran, P.R.; Parray, R.A. Non-destructive Prediction of Quality Parameters in Alphonso Mangoes Using Near-infrared Spectroscopy: A Comprehensive Study on Physicochemical Characteristics and Ripening Dynamics. J. Sci. Res. Rep. 2024, 30, 82–90. [Google Scholar] [CrossRef]
- Vanoli, M.; Spinelli, L.; Torricelli, A.; Ibrahim, A.; Parisi, B.; Lo Scalzo, R.; Rizzolo, A. Anthocyanin and carotenoid contents assessed by time-resolved reflectance spectroscopy in potato tubers (Solanum tuberosum L.) with different flesh colors. Adv. Hortic. Sci. 2020, 34, 71–80. [Google Scholar] [CrossRef]
- Munawar, A.A.; Kusumiyati; Wahyuni, D. Near infrared spectroscopic data for rapid and simultaneous prediction of quality attributes in intact mango fruits. Data Brief 2019, 27, 104789. [Google Scholar] [CrossRef] [PubMed]
- Hayati, R.; Munawar, A.A.; Fachruddin, F. Enhanced near infrared spectral data to improve prediction accuracy in determining quality parameters of intact mango. Data Brief 2020, 30, 105571. [Google Scholar] [CrossRef] [PubMed]
- Kusumiyati; Munawar, A.A.; Suhandy, D. Fast, simultaneous and contactless assessment of intact mango fruit by means of near infrared spectroscopy. AIMS Agric. Food 2021, 6, 172–184. [Google Scholar] [CrossRef]
| TRS Optical Properties | Day 1 | Day 3 | Day 8 | ANOVA |
|---|---|---|---|---|
| μa540 (cm−1) | 0.177 ± 0.018 b | 0.200 ± 0.017 ab | 0.256 ± 0.017 a | 4.27 * |
| μa630 (cm−1) | 0.048 ± 0.002 a | 0.043 ± 0.002 a | 0.047 ± 0.007 a | 1.11 ns |
| μa650 (cm−1) | 0.046 ± 0.003 a | 0.037 ± 0.002 a | 0.040 ± 0.003 a | 2.83 ns |
| μa670 (cm−1) | 0.067 ± 0.017 a | 0.038 ± 0.002 b | 0.036 ± 0.002 b | 3.56 * |
| μa690 (cm−1) | 0.026 ± 0.001 a | 0.023 ± 0.001 a | 0.024 ± 0.002 a | 1.16 ns |
| Mie’s A (cm−1) | 18.39 ± 0.74 a | 17.57 ± 0.61 a | 14.88 ± 0.63 b | 7.67 ** |
| Mie’s b (−) | 0.084 ± 0.020 b | 0.309 ± 0.039 a | 0.225 ± 0.044 a | 10.09 *** |
| Parameter | Day 1 | Day 3 | Day 88 | ANOVA |
|---|---|---|---|---|
| Firmness (N) | 39.4 ± 11.9 a | 1 5.0 ± 2.8 ab | 7.3 ± 0.9 b | 5.58 ** |
| L* | 82.2 ± 0.9 a | 80.3 ± 0.7 a | 76.9 ± 0.9 b | 10.20 *** |
| a* | 0.4 ± 1.2 b | 3.5 ± 0.9 ab | 6.2 ± 0.9 a | 8.20 ** |
| b* | 53.6 ± 2.2 b | 56.6 ± 1.8 ab | 60.9 ± 1.6 a | 3.77 * |
| C* | 53.7 ± 2.2 b | 56.8 ± 1.8 ab | 61.2 ± 1.7 a | 3.89 * |
| h° | 90.0 ± 1.2 a | 86.7 ± 2.7 ab | 84.4 ± 0.8 b | 8.60 *** |
| IY | 117.4 ± 7.2 b | 129.3 ± 5.8 ab | 147.6 ± 5.9 a | 5.82 ** |
| Parameter | Day 1 | Day 3 | Day 8 | ANOVA |
|---|---|---|---|---|
| AA (mg kg FW−1) | 170.4 ± 11.3 a | 129.2 ± 11.7 ab | 90.2 ± 8.4 b | 10.11 *** |
| TPC (mg GAE kg FW−1) | 244.8 ± 21.7 a | 253.8 ± 26.2 a | 242.8 ± 24.4 a | 0.06 ns |
| TAC (μmol TE kg FW−1) | 748.6 ± 72.2 a | 641.9 ± 105.9 ab | 427.7 ± 76.3 b | 3.60 * |
| CAR (mg β-CARE kg FW−1) | 7.41 ± 1.32 b | 9.24 ± 1.65 b | 22.21 ± 2.94 a | 14.65 *** |
| Compound | Day 1 | Day 3 | Day 8 | ANOVA |
|---|---|---|---|---|
| 9-Viol | 0.34 ± 0.06 a | 0.42 ± 0.04 a | 0.43 ± 0.04 a | 0.96 ns |
| UNK | 0.06 ± 0.02 a | 0.07 ± 0.02 a | 0.14 ± 0.04 a | 2.53 ns |
| Viol | 0.04 ± 0.04 b | 0.23 ± 0.13 b | 0.63 ± 0.07 a | 10.52 *** |
| Crypt | 0.93 ± 0.15 b | 1.62 ± 0.24 a | 0.33 ± 0.11 b | 13.04 *** |
| ViolBut | 1.56 ± 0.39 a | 2.06 ± 0.66 a | 2.00 ± 0.31 a | 0.33 ns |
| NeoDibut | 0.59 ± 0.25 b | 0.32 ± 0.15 b | 3.23 ± 0.83 a | 10.00 *** |
| ViolDibut | 0.19 ± 0.09 ab | 0.06 ± 0.04 b | 2.02 ± 0.93 a | 4.11 * |
| 13-ViolDibut | 0.32 ± 0.17 b | 0.21 ± 0.11 b | 1.09 ± 0.29 a | 5.48 ** |
| 9-ViolBut | 0.39 ± 0.12 a | 0.38 ± 0.09 a | 1.09 ± 036 a | 3.15 ns |
| 9-NeoDibut | 0.18 ± 0.07 a | 0.08 ± 0.04 a | 0.64 ± 0.27 a | 3.32 * |
| ViolButCap | 0.44 ± 0.07 b | 0.48 ± 0.06 b | 1.66 ± 0.22 a | 24.66 *** |
| 9-ViolDibut | 0.16 ± 0.02 b | 0.26 ± 0.04 b | 0.73 ± 0.10 a | 20.32 *** |
| NeoButMyr | 0.31 ± 0.10 a | 0.22 ± 0.06 a | 0.57 ± 0.15 a | 2.69 ns |
| 9-ViolButLau | 0.16 ± 0.05 a | 0.10 ± 0.03 a | 0.23 ± 0.05 a | 2.12 ns |
| b-car | 1.44 ± 0.17 b | 2.45 ± 0.32 b | 5.77 ± 0.61 a | 30.49 *** |
| ViolEster1 | 0.08 ± 0.02 b | 0.05 ± 0.02 b | 0.57 ± 0.12 a | 15.55 *** |
| NeoEster1 | 0.09 ± 0.03 b | 0.09 ± 0.03 b | 0.54 ± 0.08 a | 22.71 *** |
| 9-ViolButPalm | 0.11 ± 0.03 b | 0.12 ± 0.04 b | 0.42 ± 0.10 a | 7.20 ** |
| ΣViol | 2.28 ± 0.38 b | 2.66 ± 0.67 b | 6.26 ± 0.85 a | 11.00 *** |
| Σ9-Viol | 0.82 ± 0.19 b | 0.85 ± 0.15 b | 2.47 ± 0.87 a | 7.75 *** |
| ΣNeo | 0.99 ± 0.33 b | 0.64 ± 0.16 b | 4.35 ± 0.87 a | 14.12 *** |
| ΣFree | 1.31 ± 0.18 b | 2.28 ± 0.23 a | 1.39 ± 0.11 b | 8.48 *** |
| TotalXant | 5.91 ± 0.80 b | 6.72 ± 0.84 b | 16.21 ± 1.49 a | 27.06 *** |
| Compound | L* | a* | b* | C* | h° | IY |
|---|---|---|---|---|---|---|
| 9-Viol | −0.637 | 0.620 | 0.577 | 0.577 | −0.582 | 0.612 |
| UNK | −0.048 | 0.040 | −0.066 | −0.066 | −0.039 | −0.013 |
| Viol | −0.479 | 0.452 | 0.392 | 0.392 | −0.474 | 0.435 |
| Crypt | 0.180 | −0.110 | −0.173 | −0.173 | 0.114 | −0.164 |
| ViolBut | −0.691 | 0.653 | 0.670 | 0.670 | −0.652 | 0.679 |
| NeoDibut | −0.587 | 0.604 | 0.503 | 0.503 | −0.602 | 0.571 |
| ViolDibut | −0.068 | 0.081 | 0.120 | 0.120 | −0.053 | 0.120 |
| 13-ViolDibut | −0.637 | 0.632 | 0.586 | 0.586 | −0.626 | 0.627 |
| 9-ViolBut | −0.210 | 0.192 | 0.220 | 0.220 | −0.190 | 0.215 |
| 9-NeoDibut | 0.276 | −0.301 | −0.385 | −0.385 | 0.289 | −0.354 |
| ViolButCap | −0.578 | 0.529 | 0.484 | 0.484 | −0.532 | 0.532 |
| 9-ViolDibut | −0.744 | 0.734 | 0.625 | 0.625 | −0.720 | 0.676 |
| NeoButMyr | −0.316 | 0.283 | 0.200 | 0.200 | −0.313 | 0.275 |
| 9-ViolButLau | −0.113 | 0.080 | 0.031 | 0.031 | −0.097 | 0.074 |
| b-Car | −0.745 | 0.777 | 0.663 | 0.663 | −0.791 | 0.723 |
| ViolEster1 | −0.187 | 0.169 | 0.126 | 0.126 | −0.161 | 0.160 |
| NeoEster1 | −0.559 | 0.546 | 0.460 | 0.460 | −0.538 | 0.533 |
| 9-ViolButPalm | −0.336 | 0.317 | 0.205 | 0.205 | −0.341 | 0.280 |
| ΣViol | −0.827 | 0.792 | 0.818 | 0.818 | −0.766 | 0.825 |
| Σ9-Viol | −0.555 | 0.559 | 0.464 | 0.464 | −0.562 | 0.532 |
| ΣNeo | −0.505 | 0.486 | 0.430 | 0.430 | −0.493 | 0.471 |
| ΣFree | −0.316 | 0.370 | 0.286 | 0.286 | −0.375 | 0.313 |
| TotalXant | −0.866 | 0.857 | 0.780 | 0.780 | −0.850 | 0.837 |
| CAR | −0.851 | 0.845 | 0.766 | 0.766 | −0.838 | 0.821 |
| Dependent Variables | Coefficients of Independent Variables | Constant | ANOVA Model | R2 (%) | R2adj (%) | SEE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μa540SORT | μa540 | μa650 | μa630 | μa670 | μa690 | Mie’s A | Mie’s b | ||||||
| CAR | −165.15 p > 0.001 | 258.87 p < 0.001 | −1445.18 p = 0.030 | 1713.93 p = 0.003 | – | −1425.21 p = 0.056 | – | – | 16.01 p = 0.006 | <0.001 | 86.3 | 83.1 | 3.845 |
| TotalXant | −124.28 p > 0.001 | 200.12 p < 0.001 | −1493.34 p = 0.006 | 1122.76 p = 0.012 | – | – | – | – | 10.18 p = 0.025 | <0.001 | 81.7 | 78.5 | 3.224 |
| ΣViol | −30.49 p = 0.033 | 59.86 p < 0.001 | – | – | – | – | – | – | −0.392 p = 0.006 | <0.001 | 76.3 | 74.4 | 1.780 |
| b-Car | −44.36 p < 0.001 | 57.61 p < 0.001 | – | 217.55 p = 0.051 | – | −528.59 p = 0.025 | – | – | 2.33 p = 0.165 | <0.001 | 80.3 | 76.9 | 1.286 |
| 9-ViolDibut | −4.72 p = 0.013 | 5.57 p < 0.001 | – | – | – | −23.20 p = 0.043 | – | −0.06 p = 0.014 | 1.46 p = 0.003 | <0.001 | 68.5 | 63.0 | 0.22 |
| ViolButCap | – | – | 134.53 p = 0.002 | – | – | −285.93 p < 0.001 | – | −0.20 p < 0.001 | 4.98 p < 0.001 | <0.001 | 66.4 | 62.2 | 0.50 |
| NeoEster1 | −7.60 p < 0.001 | 6.15 p < 0.001 | −10.96 p = 0.046 | – | – | – | – | – | 0.70 p = 0.004 | <0.001 | 61.3 | 56.5 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanoli, M.; Rizzolo, A.; Lovati, F.; Spinelli, L.; Levoni, P.; Torricelli, A.; Cortellino, G. Non-Destructive Prediction of Carotenoids, Ascorbic Acid, and Total Phenols Contents in ‘Tommy Atkins’ Mangoes Using Absorption and Scattering Properties Measured by Time-Resolved Reflectance Spectroscopy. Agriculture 2024, 14, 1902. https://doi.org/10.3390/agriculture14111902
Vanoli M, Rizzolo A, Lovati F, Spinelli L, Levoni P, Torricelli A, Cortellino G. Non-Destructive Prediction of Carotenoids, Ascorbic Acid, and Total Phenols Contents in ‘Tommy Atkins’ Mangoes Using Absorption and Scattering Properties Measured by Time-Resolved Reflectance Spectroscopy. Agriculture. 2024; 14(11):1902. https://doi.org/10.3390/agriculture14111902
Chicago/Turabian StyleVanoli, Maristella, Anna Rizzolo, Fabio Lovati, Lorenzo Spinelli, Pietro Levoni, Alessandro Torricelli, and Giovanna Cortellino. 2024. "Non-Destructive Prediction of Carotenoids, Ascorbic Acid, and Total Phenols Contents in ‘Tommy Atkins’ Mangoes Using Absorption and Scattering Properties Measured by Time-Resolved Reflectance Spectroscopy" Agriculture 14, no. 11: 1902. https://doi.org/10.3390/agriculture14111902
APA StyleVanoli, M., Rizzolo, A., Lovati, F., Spinelli, L., Levoni, P., Torricelli, A., & Cortellino, G. (2024). Non-Destructive Prediction of Carotenoids, Ascorbic Acid, and Total Phenols Contents in ‘Tommy Atkins’ Mangoes Using Absorption and Scattering Properties Measured by Time-Resolved Reflectance Spectroscopy. Agriculture, 14(11), 1902. https://doi.org/10.3390/agriculture14111902








