Abstract
New agronomic opportunities for more informed agricultural decisions and enhanced crop management have been made possible by drone-based near-ground remote sensing. Obtaining precise non-destructive information regarding crop biophysical characteristics at spatial and temporal scales is now possible. Drone-mounted multispectral and thermal sensors were used to assess crop phenology, condition, and stress by profiling spectral vegetation indices in crop fields. In this study, vegetation indices, viz., Atmospherically Resistant Vegetation Index (ARVI), Modified Chlorophyll Absorption Ratio Index (MCARI), Wide Dynamic Range Vegetation Index (WDRVI), Normalized Red–Green Difference Index (NGRDI), Excess Green Index (ExG), Red–Green Blue Vegetation Index (RGBVI), and Visible Atmospherically Resistant Index (VARI) were generated. Furthermore, Pearson correlation analysis showed a better correlation between WDRVI and VARI with LAI (R = 0.955 and R = 0.982) ground truth data. In contrast, a strong correlation (R = 0.931 and R = 0.844) was recorded with MCARI and NGRDI with SPAD chlorophyll ground truth data. Then, the best-performing indices, WDRVI and MCARI in cotton, and VARI and NGRDI in rice, were further used to generate the yield model. This study for determining LAI and chlorophyll shows that high spatial resolution drone imageries are accurate and fast. As a result, finding out the LAI and chlorophyll and how they affect crop yield at a regional scale is helpful. The widespread use of unmanned aerial vehicles (UAV) and yield prediction were technical components of large-scale precision agriculture.
1. Introduction
Agriculture has benefited from the advancement of remote sensing by collecting data at both temporal and spatial scales. However, the agricultural sector faces challenges as crop monitoring becomes difficult and ineffective across larger field areas. Dynamic Remotely Operated Navigation Equipment, [1], often known as a DRONE or Unmanned Aerial Vehicle (UAV), was initially developed for defense and civilian use. It might serve as a practical and affordable alternative to satellite remote sensing [2] for assessing the status of the vegetation in agricultural areas. Drones are more efficient for crop management and monitoring because, in a single flight, they can traverse hundreds of hectares [3]. Crop morphology, growth, health condition, and maturity were studied using RGB, multispectral, and thermal sensors mounted on the drone. An agricultural drone can use a variety of sensors based on the crop characteristics that need to be observed [4]. At different stages of crop development, these sensor data will be useful to evaluate vegetation health, soil moisture content, and other essential agricultural attributes [5]. They are also crucial for evaluating crop conditions, monitoring vegetation cover, nutrients, and water status, determining crop quality owing to weed/pest/disease infestations, crop yield, production forecasting, and so on [6].
UAVs are suitable for analyzing crop health, stress, and quick phenological changes in real time [7]. Recently, red-edge (RE) and near-infrared (NIR) band-integrated UAV platform sensors have become economically viable for agriculture uses, allowing for the benefit of vegetation indices (VIs) created for conventional remote sensing sensors with UAV data. For example, a crop’s absorption and reflectance properties could be used to determine its physiological characteristics [8]. In most cases, many VIs are extracted and used to make inferences. They can be calculated using data from individual pictures or entire crop ortho-images. Vegetation indices, including biophysical and biological vegetation parameters, can aid in identifying useful crop features. They can also provide exact spatial and temporal information on a farming area.
By combining the physiological characteristics of the plant with vegetative indices, the crop yield forecast can be performed. Yield estimation might be conducted quickly by combining these models and spectral data. In addition, they can facilitate a better understanding of the differences in vegetative development and yield potential [9]. UAVs are widely employed to estimate yields and monitor vegetation growth. However, the lack of technologies for systematically tracking crop growth is one of the main impediments to improving agricultural production and quality [10]. Crop biomass, nitrogen status, and yield prediction have been the primary areas of recent research. Crop condition assessment and real-time canopy monitoring can help develop precise management strategies to increase yields. Crop yield predictions using remote sensing data have expanded the possibilities for monitoring and analyzing crop yields.
Therefore, it is necessary for farmers who grow crops to develop efficient methods for accurate prediction of yield prior to harvest. Compared to satellites, drones can take pictures with a far greater spatial resolution. Crop growth conditions [11,12] and crop yield [13,14] were monitored using UAV-based imaging systems. Improved crop monitoring is necessary for accurate crop development tracking and prediction in the agricultural sector. Some crop characteristics that can be assessed are leaf area index and chlorophyll [15,16,17]. Many researchers have found significant correlations between crop yield and VI for different types of crops, and some of these studies have even demonstrated that remote sensing data may be used to evaluate crop conditions and precisely estimate yield [18]. This study aimed (i) to use drone-derived multispectral vegetation indices to measure LAI and SPAD chlorophyll at a spatial level and (ii) to validate calculated spectral indices with observations of ground truth data (iii) to compare observations of ground truth data with calculated spectral indices.
2. Materials and Methods
2.1. Study Area
The experiment was conducted in the cotton and rice fields of the Department of Cotton and Wetland farm at Tamil Nadu Agricultural University, Coimbatore. The experimental sites and other field details are illustrated in Table 1.

Table 1.
Details of the study area.
2.2. Data Collection
2.2.1. Image Acquisition
For this research, a MicaSense RedEdge multispectral camera payload (Table 2) was mounted on a quadcopter drone (Figure 1a). The main benefit of this equipment is that it has a vertical takeoff and landing capability that can be used in confined spaces. By providing waypoints, this flight will also be automated. For planning the flight mission (waypoints, altitude, heading direction, and speed), ground control station software (UgCS version:4.11.0 build:3056) was utilized.

Table 2.
UAV flight information and sensor specifications.
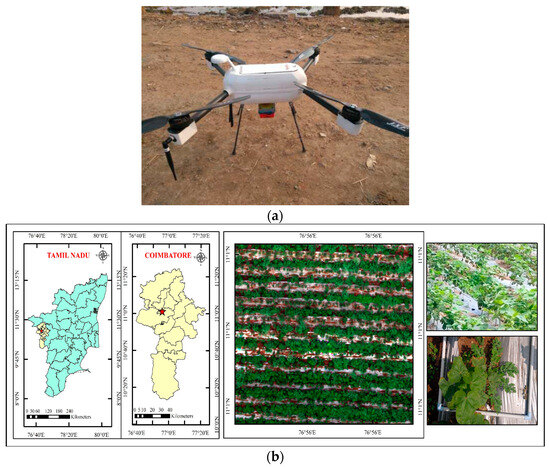
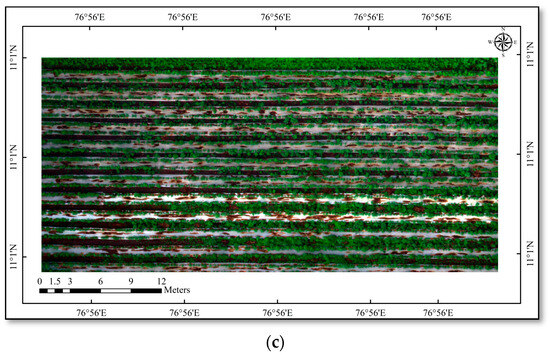
Figure 1.
(a) Quadcopter with MicaSense RedEdge Sensor; (b) study area—cotton; (c) drone-derived orthomosaic image of cotton field.
A flight mission was carried out between 11 AM and 12 PM under a clear sky to collect multispectral images (Figure 2b,c and Figure 3a,b). A ground reference point was used for the georeferencing process (GCP). The image is captured by the camera, stored in the memory, and transmitted via telemetry to the ground station. A calibrated reflectance panel (CRP) was also used to calibrate the MicaSense RedEdge. Before each flight, the calibration was immediately performed as per the calibration manual.
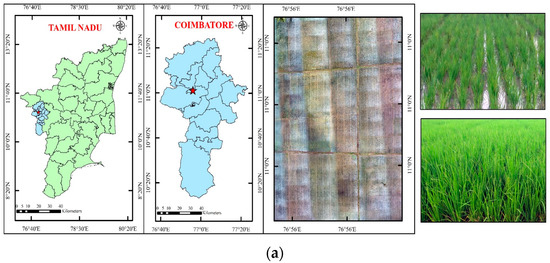
Figure 2.
(a) Study area—rice; (b) drone-derived orthomosaic image of rice field.
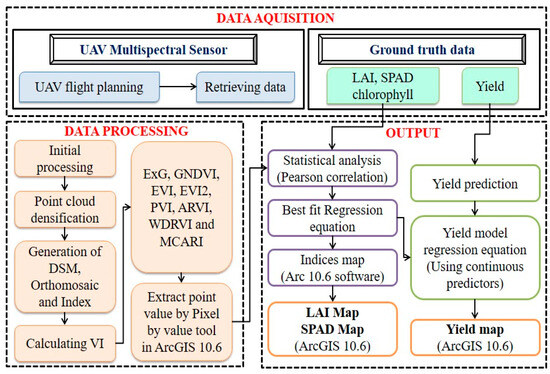
Figure 3.
Flowchart depicts the methodology.
2.2.2. Ground Data Collection
In this study, the most sensitive stages of cotton and rice crop, viz., boll formation and grain-filling stages were chosen for data collection. The ground truth for LAI and SPAD chlorophyll was collected as well when drone pictures were taken to validate the vegetation index. Therefore, ten ground data were collected at each field to validate the vegetation index.
Leaf Area Index (LAI)
By measuring the leaf length, breadth of the third open leaf from the top, and number of leaves/plants, the leaf area index was calculated [19].
where L = mean leaf length (cm), W = mean leaf breadth (cm), N = average number of leaves per plant, k = constant factor.
SPAD Chlorophyll
The SPAD-502 (Soil Plant Analysis Development-502) Minolta meter was used to measure the light transmittance ratio at wavelengths of 650 nm and 940 nm at three places of the third leaf employing non-destructive chlorophyll readings. To produce an accurate result, the readings were averaged. In addition, ten geotagged ground data were randomly selected to verify the vegetation index.
Yield (g/plant)
The yield was recorded during the harvest stage and expressed as g/plant.
2.3. Data Processing
2.3.1. Image Processing
Pix4D mapper software was used to analyze (Figure 3) the multispectral images that were collected. An orthomosaic was created using the original data after being georeferenced, processed, and examined. Additionally, many collected overlapped images were stitched together to create a large map with accurate georeferencing.
2.3.2. Vegetation Index Processing
The fundamental and essential tool for assessing aerial photographs was the vegetation indices [20]. First, ArcGIS 10.6 software was used to create maps of the vegetation indices from post-processed images. The processed data were then utilized to extract information using the vegetation indices formula. The NDVI, the most widespread vegetation index, is essential for determining a crop growth’s vigor, yield, and status. The ARVI, MCARI WDRVI, NGRDI, ExG, RGBVI, and VARI vegetation indices (Table 3) were also very helpful in forecasting the LAI and chlorophyll. Using the coordinates from the ground data, the pixel-by-value tool in ArcGIS 10.6 was then used to obtain the spectral data from the various vegetation indices. Finally, statistical analysis is performed on the retrieved spectral data.

Table 3.
Vegetation indices used in this study.
2.4. Statistical Analysis
SPSS 22 software was used for the statistical results’ validation. First, using Pearson correlation analysis, the best-performing vegetation index with a strong correlation to the ground truth chlorophyll data was identified. The correlation coefficient (R) number was very helpful in figuring out how strongly the two datasets were correlated. Equation (1) contains the formula used to calculate the R-value. The accuracy of the model was then determined by computing the coefficient of determination (R2) and RMSE values. In order to find the best line of fit, regression (R2) values for the vegetation indices (independent variable) and the ground truth chlorophyll data (dependent variable) were computed. If the R2 and RMSE values were higher, the independent variable was highly predictable from the dependent variable. Therefore, Equations (2) and (3) were used to calculate R2 and RMSE, respectively.
where xi and yi are the estimated and measured values, respectively; and are the average estimated and measured values, respectively, and n is the sample number.
To predict the yield model, the ground truth data (LAI and SPAD chlorophyll) were correlated with the yield. The yield was considered a dependent variable, whereas LAI and SPAD chlorophyll were regarded as independent variables. To obtain the best-fit regression equation for the yield model forecast, the dependent variable was regressed with the independent variables in different combinations.
3. Results and Discussion
LAI and chlorophyll were calculated using high-resolution multispectral images, and the results showed strong correlations between both positive and negative outcomes. The study area generated different vegetation indices maps, viz., ARVI, MCARI, WDRVI, NGRDI, ExG, RGBVI, and VARI (Figure 4 and Figure 5). The mean vegetation indices of different indices and ground truth data collected are given in Table 4 and Table 5. The vegetation indices generated different map outputs because each index uses a different set of wavebands. These indices can therefore be used to identify variations in the LAI and chlorophyll content of the crops. Ten points were chosen from the index map using the ground truth data coordinates in ArcGIS 10.6, and the extracted point values were used in the pixel-by-value tool.
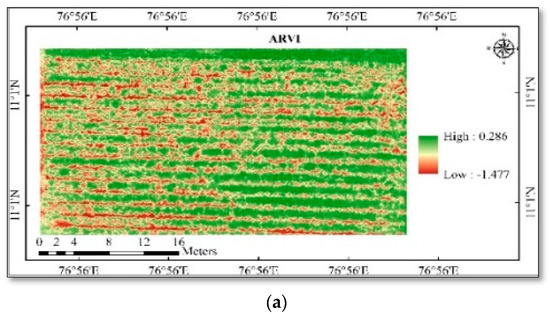
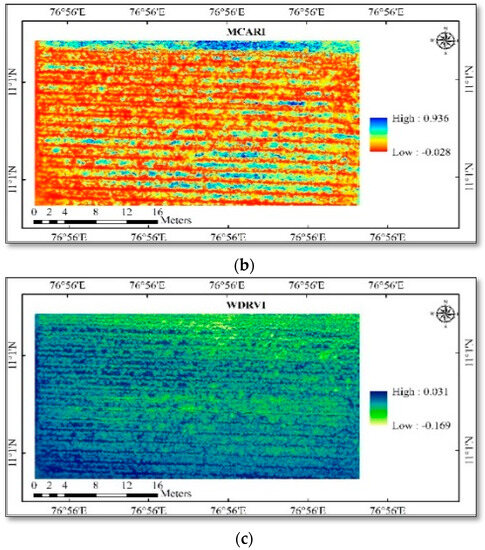
Figure 4.
(a) Spatial variability of vegetation indices in cotton—ARVI; (b) spatial variability of vegetation indices in cotton—MCARI; (c) spatial variability of vegetation indices in cotton—WDRVI.
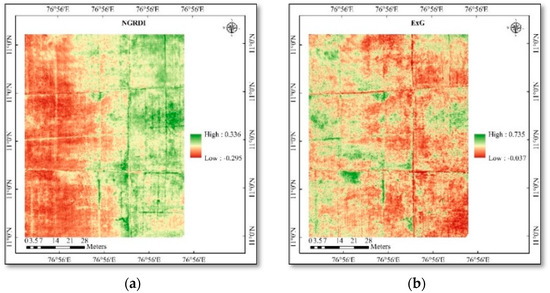
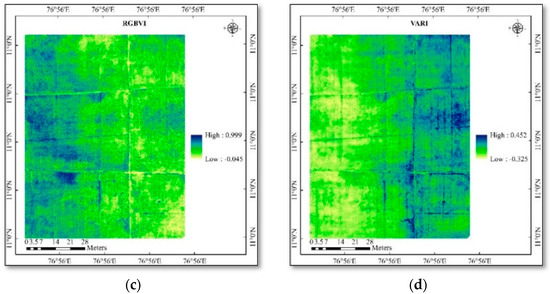
Figure 5.
(a) Spatial variability of vegetation indices in rice—NGRDI; (b) spatial variability of vegetation indices in rice—ExG; (c) spatial variability of vegetation indices in rice—RGBVI; (d) spatial variability of vegetation indices in rice—VARI.

Table 4.
Vegetation indices value and ground truth data for LAI and SPAD chlorophyll for cotton.

Table 5.
Vegetation indices value and ground truth data for LAI and SPAD chlorophyll for rice.
From the (Table 4 and Table 5) values, it is identified that there was a difference among the index’s values, LAI, SPAD chlorophyll, and yield values. The LAI values range from 0.7 to 1.7 in cotton and 2.5 to 3.3 in rice. The SPAD chlorophyll values range from 38.1 to 60.5 in cotton and 34.2 to 59.7 in rice. The range of values for different indices, viz., ARVI from 0.581 to 0.845, MCARI from 0.175 to 0.505, WDRVI from −0.375 to 0.058, NGRDI from −0.092 to 0.052, ExG from 0.076 to 0.222, RGBVI from 0.113 to 0.41, and VARI from −0.118 to 0.082. Some indices’ very lowest and negative values denote that it is sensitive to non-photosynthetic materials and background soil properties in some pixel parts. The seed cotton yield value ranges from 67 to 76 g/plant, and rice yield ranges from 86 to 134 g/plant.
A correlation between ground data and vegetation indices collected from UAVs was found through statistical analysis. Additionally, Pearson correlation coefficient analysis (Table 6) was performed to determine the vegetation index that performed the best with regard to the LAI and SPAD chlorophyll.

Table 6.
Relationship between vegetation indices against SPAD and LAI of cotton and rice crop.
Among the vegetation indices, the WDRVI had a higher positive correlation coefficient (R = 0.955) with the LAI ground truth data. WDRVI recorded an R2 value of 0.911 and an RMSE of 0.097 in cotton. And in the rice crop, VARI recorded a higher positive correlation coefficient (R = 0.982) with an R2 value of 0.965 and an RMSE of 0.051. This demonstrates that WDRVI and VARI were more accurate than other vegetation indices at predicting the LAI. Higher correlation coefficients describe vegetation that is healthy and dense and has a higher LAI, whereas lower values depict stressed and sparse vegetation that has a lower LAI. It is well known that the red wavelength helps assess the level of chlorophyll and the leaf area index [30]. In addition, the red edge, or the slope between the spectrum’s red and near-infrared regions at 700 nm, was a significant area of the spectral structure. Furthermore, this spectral region was associated with the leaf area index (LAI) [31], which measures the amount of leaf’s greenness per unit of ground area and the chlorophyll concentration. NDVI is the most often used VI to investigate the impact of hail protection and serves as the reference index for accuracy and sensitivity to LAI compared to other VIs [31]. Taking into account the NDVI’s drawbacks or limitations, such as its saturation in densely vegetated areas and sensitivity to meteorological impacts and soil influence, indices derived from ARVI and WDRVI have been provided for comparison to address both of its alleged drawbacks, susceptibility to soil noise, and lack of sensitivity at high LAI [32]. Canopy cover across the cotton growing season was found using a model that integrated RGB-based vegetation indices [33].
Among the vegetation indices, the MCARI (R = 0.931) and NGRDI (R = 0.844) had a higher positive correlation coefficient with the SPAD chlorophyll ground truth data in cotton and rice crops, respectively. MCARI recorded an R2 value of 0.866, an RMSE of 2.851, and NGRDI recorded an R2 value of 0.712 and an RMSE of 6.034. This shows that MCARI and NGRDI had higher accuracy for predicting the SPAD chlorophyll in cotton and rice crops. Both the indices had a very strong positive linear correlation with SPAD chlorophyll from vegetative to boll formation stages in cotton and from flowering to grain-filling stages in rice. This was because the chlorophyll-sensitive vegetation indices, which employ red and red-edge wavelengths, perform better than other indices based on the green and red wavelengths. Between 660 and 680 nm, there is a peak in the red region’s absorption where the chlorophylls have strong red absorption and near-infrared reflectance peaks [34]. Hence NGRDI (red wavelength) and MCARI (red and red-edge wavelength) correlate significantly to crop chlorophyll content than other indices. But in comparison, multispectral-based MCARI performed much better than other indices with higher correlation. These indices act as direct proxies to crop biochemistry because the crop leaves are more translucent to the red edge wavelength, which can penetrate more deeply into leaf cells than red and blue [35]. This also confirms the findings [36], that chlorophyll-specific VIs are more suitable for predicting chlorophyll content.
Then the regression equation (Table 7) of the highly correlated vegetation indices (WDRVI, VARI, MCARI, and NGRDI) were also used to create the LAI and SPAD chlorophyll map for the study area (Figure 6 and Figure 7). This will be important for calculating the canopy coverage and chlorophyll content of the crop. The higher SPAD chlorophyll content was recorded in the LAI area covered by areas with higher values. This demonstrates that increasing LAI will have an impact on increased SPAD chlorophyll.

Table 7.
Generated regression equation model for predicting the SPAD and LAI of cotton and rice.
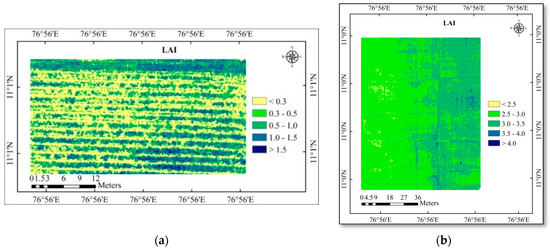
Figure 6.
(a) LAI map of the cotton study area; (b) LAI map of the rice study area.
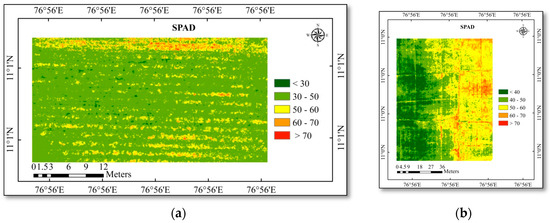
Figure 7.
(a) SPAD chlorophyll map of the cotton study area; (b) SPAD chlorophyll map of the rice study area.
Using correlation analysis (Table 8) between the actual observed value and the predicted value from the maps, the predicted maps (LAI and SPAD chlorophyll) were evaluated for accuracy. In cotton and rice, the predicted LAI positively correlated with the observed LAI with an R-value of 0.844 and 0.803, respectively. Likewise, the predicted SPAD chlorophyll positively correlated with observed SPAD chlorophyll with an R-value of 0.880 and 0.830 in cotton and rice crops.

Table 8.
Accuracy assessment between observed and predicted LAI and SPAD chlorophyll.
The yield was predicted using stepwise multiple linear regression between the independent variables (LAI and SPAD chlorophyll) and the dependent variable (yield) (Table 9). In cotton and rice, the equation generated based on LAI has an R2 value of 0.093 and 0.222 with RMSE 3.060 and 15.692, whereas the equation generated based on SPAD chlorophyll has an R2 value of 0.272 and 0.561 with RMSE 2.741 and 11.783. The combined regression model showed a higher R2 value of 0.387 and 0.635 with an RMSE of 2.690 and 11.485. This indicates that SPAD chlorophyll is highly correlated to yield compared to LAI. The area with higher LAI and SPAD chlorophyll levels generated more yield, a sign of the crop’s health. However, the region with lower LAI and SPAD chlorophyll indicates that the crop with lower yield and stressed.

Table 9.
Yield prediction equation using LAI and SPAD chlorophyll in cotton and rice.
The yield map for the study area was created using the yield equation model, which recorded a higher R2 value. The yield map developed based on LAI (Figure 8), SPAD chlorophyll (Figure 9), and a combination of LAI and SPAD chlorophyll (Figure 10) was given.
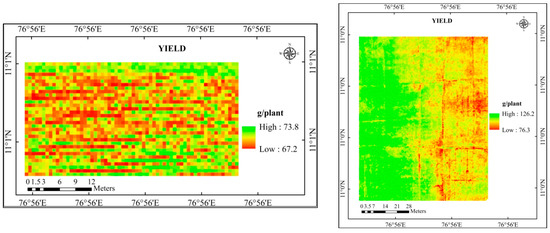
Figure 8.
Predicted yield map of the study area using LAI.
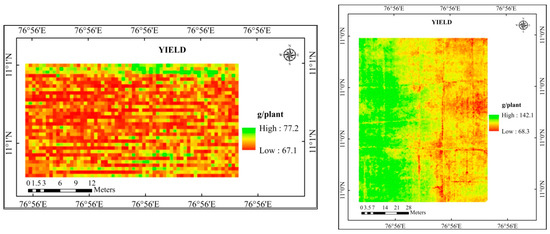
Figure 9.
Predicted yield map of the study area using SPAD chlorophyll.
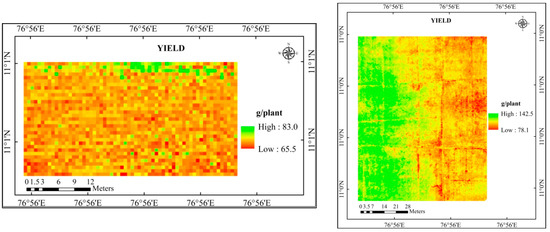
Figure 10.
Predicted yield map of the study area using LAI and SPAD chlorophyll.
The estimated yield for the experimental location was produced by further analyzing the predicted yield maps (LAI, SPAD chlorophyll, and a combination of LAI and SPAD chlorophyll) (Table 10). Higher agreement (92.8%) was found between the yield predicted using LAI and SPAD chlorophyll and the observed yield, which had RMSE and NRMSE of 9.42 kg/ha and 7.25%, respectively.

Table 10.
RMSE, NRMSE, and accuracy validation for actual and predicted yield.
The yield predicted using LAI registered an agreement (88.8 and 77.1) percentage with the observed yield. It recorded an RMSE of 14.57 kg/ha and 917.64 kg/ha and NRMSE of 11.21% and 22.94%. On the other hand, the yield predicted by using SPAD chlorophyll had recorded 90.7 and 77.6% agreement with the observed yield in cotton and rice crops. It recorded an RMSE of 12.05 kg/ha and 895.65 kg/ha with NRMSE of 9.27% and 22.39%.
Furthermore, crop growth status changes directly impact the final yield, which can be monitored using spectral measurements. Drone data collected can be utilized to detect many characteristics, including growth status, canopy height, crop health, disease level, nutrients, and water stress level, and anticipate the final yield using data-driven artificial intelligence algorithms. It can take pictures that can be used to assess the health of crops, the condition of the soil, and other factors that affect how well crops grow. Drone data can also be used to efficiently and quickly speed up the plant phenotypic breeding process. When using satellite images for monitoring larger cropped areas, using these yield prediction equation models for yield prediction in advance will be a very new and promising approach. The farmers will therefore find this early prediction helpful in calculating yield loss or gain to estimate the crop’s market value.
4. Conclusions
Smart agriculture protocols largely depend on real-time measurements of plant biophysical parameters, leading to the precise application of inputs resulting in leveraged productivity and net profit. For commercial fiber crop cotton market information, effective technologies for precise yield estimation before harvest are essential. Currently, agricultural yield estimation using remote sensing methods is accomplished mainly through satellite remote sensing data or unmanned aerial vehicles carrying many sensors. As an important field-scale data collection tool, multispectral UAV remote sensing systems have great application potential in the rapid, accurate, and economic assessment of agricultural crop traits and yields. LAI and chlorophyll, two indicators of plant growth, had a stronger correlation with canopy spectral reflectance. Since crop yield and the presence of photosynthetic tissue are correlated, spectral indices gathered throughout the growing season can also be used to calculate crop yield. Intelligent agricultural decisions are facilitated by the speed and non-destructiveness of these spectral index assessments. Consequently, they can be carried out on a broad scale, in contrast to common agricultural parameter measurements like LAI and chlorophyll.
Author Contributions
P.S.: data processing, analysis, interpretation, and validation of data; S.P.: conceptualization, design, and methodology of the study, investigation, resources, project administration, funding acquisition, and approval of the version of the manuscript to be published and corresponding author; R.K.: conceptualization, design, and methodology of the study, data curation, supervision, visualization, drafting of the manuscript, revising the manuscript critically for important intellectual content, and approval of the version of the manuscript to be published. N.S.S., A.P.S. and S.S.: drafting of the manuscript, revising the manuscript critically for important intellectual content, and approval of the version of the manuscript to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding from GIZ, Germany, Deutsche Gesellschaft für Internationale Zusammenarbeit (Grant number 81278637)/World Bank, Tamil Nadu Irrigated Agriculture Modernization Project (Grant number IFHRMS DPC No. 2415-01-120-PF30903).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All relevant data are included in the manuscript.
Acknowledgments
The authors thank the Department of Remote Sensing & GIS, Tamil Nadu Agricultural University, Coimbatore, for extending their guidance and technical assistance in conducting this research work.
Conflicts of Interest
The authors declare no conflict of interest related to this article.
References
- Rani, A.; Chaudhary, A.; Sinha, N.; Mohanty, M.; Chaudhary, R. Drone: The green technology for future agriculture. Har. Dhara 2019, 2, 3–6. [Google Scholar]
- Tahir, M.N.; Naqvi, S.Z.A.; Lan, Y.; Zhang, Y.; Wang, Y.; Afzal, M.; Cheema, M.J.M.; Amir, S. Real time estimation of chlorophyll content based on vegetation indices derived from multispectral UAV in the kinnow orchard. Int. J. Precis. Agric. Aviat. 2018, 1, 24–31. [Google Scholar]
- Li, M.; Wu, J.; Song, C.; He, Y.; Niu, B.; Fu, G.; Tarolli, P.; Tietjen, B.; Zhang, X. Temporal variability of precipitation and biomass of alpine grasslands on the northern Tibetan plateau. Remote Sens. 2019, 11, 360. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X. Unmanned aerial vehicle remote sensing for field-based crop phenotyping: Current status and perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Tsouros, D.C.; Triantafyllou, A.; Bibi, S.; Sarigannidis, P.G. Data acquisition and analysis methods in UAV-based applications for Precision Agriculture. In Proceedings of the 2019 15th International Conference on Distributed Computing in Sensor Systems (DCOSS), Santorini Island, Greece, 29–31 May 2019; pp. 377–384. [Google Scholar]
- Maddikunta, P.K.R.; Hakak, S.; Alazab, M.; Bhattacharya, S.; Gadekallu, T.R.; Khan, W.Z.; Pham, Q.-V. Unmanned aerial vehicles in smart agriculture: Applications, requirements, and challenges. IEEE Sens. J. 2021, 21, 17608–17619. [Google Scholar] [CrossRef]
- Ballesteros, R.; Ortega, J.; Hernández, D.; Moreno, M. Applications of georeferenced high-resolution images obtained with unmanned aerial vehicles. Part I: Description of image acquisition and processing. Precis. Agric. 2014, 15, 579–592. [Google Scholar] [CrossRef]
- Nigon, T.J.; Mulla, D.J.; Rosen, C.J.; Cohen, Y.; Alchanatis, V.; Knight, J.; Rud, R. Hyperspectral aerial imagery for detecting nitrogen stress in two potato cultivars. Comput. Electron. Agric. 2015, 112, 36–46. [Google Scholar] [CrossRef]
- Delavarpour, N.; Koparan, C.; Nowatzki, J.; Bajwa, S.; Sun, X. A technical study on UAV characteristics for precision agriculture applications and associated practical challenges. Remote Sens. 2021, 13, 1204. [Google Scholar] [CrossRef]
- Tsouros, D.C.; Bibi, S.; Sarigiannidis, P.G. A review on UAV-based applications for precision agriculture. Information 2019, 10, 349. [Google Scholar] [CrossRef]
- Chu, T.; Chen, R.; Landivar, J.A.; Maeda, M.M.; Yang, C.; Starek, M.J. Cotton growth modeling and assessment using unmanned aircraft system visual-band imagery. J. Appl. Remote Sens. 2016, 10, 036018. [Google Scholar] [CrossRef]
- Duan, T.; Zheng, B.; Guo, W.; Ninomiya, S.; Guo, Y.; Chapman, S.C. Comparison of ground cover estimates from experiment plots in cotton, sorghum and sugarcane based on images and ortho-mosaics captured by UAV. Funct. Plant Biol. 2016, 44, 169–183. [Google Scholar] [CrossRef]
- Feng, A.; Zhang, M.; Sudduth, K.A.; Vories, E.D.; Zhou, J. Cotton yield estimation from UAV-based plant height. Trans. ASABE 2019, 62, 393–404. [Google Scholar] [CrossRef]
- Huang, Y.; Brand, H.J.; Sui, R.; Thomson, S.J.; Furukawa, T.; Ebelhar, M.W. Cotton yield estimation using very high-resolution digital images acquired with a low-cost small unmanned aerial vehicle. Trans. ASABE 2016, 59, 1563–1574. [Google Scholar]
- Liu, B.; Asseng, S.; Wang, A.; Wang, S.; Tang, L.; Cao, W.; Zhu, Y.; Liu, L. Modelling the effects of post-heading heat stress on biomass growth of winter wheat. Agric. For. Meteorol. 2017, 247, 476–490. [Google Scholar] [CrossRef]
- Quan, X.; He, B.; Yebra, M.; Yin, C.; Liao, Z.; Zhang, X.; Li, X. A radiative transfer model-based method for the estimation of grassland aboveground biomass. Int. J. Appl. Earth Obs. Geoinf. 2017, 54, 159–168. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, L.; Liu, Y. Inversion of the leaf area index of rice fields using vegetation isoline patterns considering the fraction of vegetation cover. Int. J. Remote Sens. 2021, 42, 1688–1712. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Koti, S. Canopy reflectance in cotton for growth assessment and lint yield prediction. Eur. J. Agron. 2007, 26, 335–344. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Curves: The Functional Approach to Plant Growth Analysis; Edward Arnold Ltd.: London, UK, 1982. [Google Scholar]
- Neupane, K.; Baysal-Gurel, F. Automatic identification and monitoring of plant diseases using unmanned aerial vehicles: A review. Remote Sens. 2021, 13, 3841. [Google Scholar] [CrossRef]
- Cao, J.; Gu, Z.; Xu, J.; Duan, Y.; Liu, Y.; Liu, Y.; Li, D. Sensitivity analysis for leaf area index (LAI) estimation from CHRIS/PROBA data. Front. Earth Sci. 2014, 8, 405–413. [Google Scholar] [CrossRef]
- Shang, J.; Liu, J.; Ma, B.; Zhao, T.; Jiao, X.; Geng, X.; Huffman, T.; Kovacs, J.M.; Walters, D. Mapping spatial variability of crop growth conditions using RapidEye data in Northern Ontario, Canada. Remote Sens. Environ. 2015, 168, 113–125. [Google Scholar] [CrossRef]
- Towers, P.C.; Strever, A.; Poblete-Echeverría, C. Comparison of vegetation indices for leaf area index estimation in vertical shoot positioned vine canopies with and without grenbiule hail-protection netting. Remote Sens. 2019, 11, 1073. [Google Scholar] [CrossRef]
- Bajwa, S.G.; Rupe, J.C.; Mason, J. Soybean disease monitoring with leaf reflectance. Remote Sens. 2017, 9, 127. [Google Scholar] [CrossRef]
- Maresma, Á.; Ariza, M.; Martínez, E.; Lloveras, J.; Martínez-Casasnovas, J.A. Analysis of vegetation indices to determine nitrogen application and yield prediction in maize (Zea mays L.) from a standard UAV service. Remote Sens. 2016, 8, 973. [Google Scholar] [CrossRef]
- Hunt, E.R.; Cavigelli, M.; Daughtry, C.S.; Mcmurtrey, J.E.; Walthall, C.L. Evaluation of digital photography from model aircraft for remote sensing of crop biomass and nitrogen status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Li, W.; Niu, Z.; Chen, H.; Li, D.; Wu, M.; Zhao, W. Remote estimation of canopy height and aboveground biomass of maize using high-resolution stereo images from a low-cost unmanned aerial vehicle system. Ecol. Indic. 2016, 67, 637–648. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Pandit, S.; Tsuyuki, S.; Dube, T. Landscape-scale aboveground biomass estimation in buffer zone community forests of central Nepal: Coupling in situ measurements with Landsat 8 satellite data. Remote Sens. 2018, 10, 1848. [Google Scholar] [CrossRef]
- Lee, D.-H.; Shin, H.-S.; Park, J.-H. Developing a p-NDVI map for highland kimchi cabbage using spectral information from UAVs and a field spectral radiometer. Agronomy 2020, 10, 1798. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Meza, C.; Rivera, J.; Alonso, L.; Moreno, J. A red-edge spectral index for remote sensing estimation of green LAI over agroecosystems. Eur. J. Agron. 2013, 46, 42–52. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Ashapure, A.; Jung, J.; Chang, A.; Oh, S.; Maeda, M.; Landivar, J. A comparative study of RGB and multispectral sensor-based cotton canopy cover modelling using multi-temporal UAS data. Remote Sens. 2019, 11, 2757. [Google Scholar] [CrossRef]
- Shamshiri, R.R.; Mahadi, M.R.; Ahmad, D.; Bejo, S.K.; Aziz, S.A.; Ismail, W.I.W.; Che Man, H. Controller design for an osprey drone to support precision agriculture research in oil palm plantations. In Proceedings of the 2017 ASABE Annual International Meeting, Spokane, DC, USA, 16–19 July 2017; pp. 2–13. [Google Scholar]
- Gitelson, A.A.; Viña, A.; Verma, S.B.; Rundquist, D.C.; Arkebauer, T.J.; Keydan, G.P.; Leavitt, B.; Ciganda, V.S.; Burba, G.; Suyker, A.E.; et al. Relationship between gross primary production and chlorophyll content in crops: Implications for the synoptic monitoring of vegetation productivity. J. Geophys. Res. Atmos. 2006, 111, D8. [Google Scholar] [CrossRef]
- Raper, T.B.; Varco, J.J. Canopy-scale wavelength and vegetative index sensitivities to cotton growth parameters and nitrogen status. Precis. Agric. 2015, 16, 62–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).