Improved Organic Fertilisers Made from Combinations of Compost, Biochar, and Anaerobic Digestate: Evaluation of Maize Growth and Soil Metrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Components Production
2.2. Fertilising Products Description
2.3. Phytotoxicity Test and Toxicity Test on Soil Rhizobacteria
2.4. Microcosm Trial
3. Results
3.1. Phytotoxicity Test and Toxicity Test on Soil Rhizobacteria
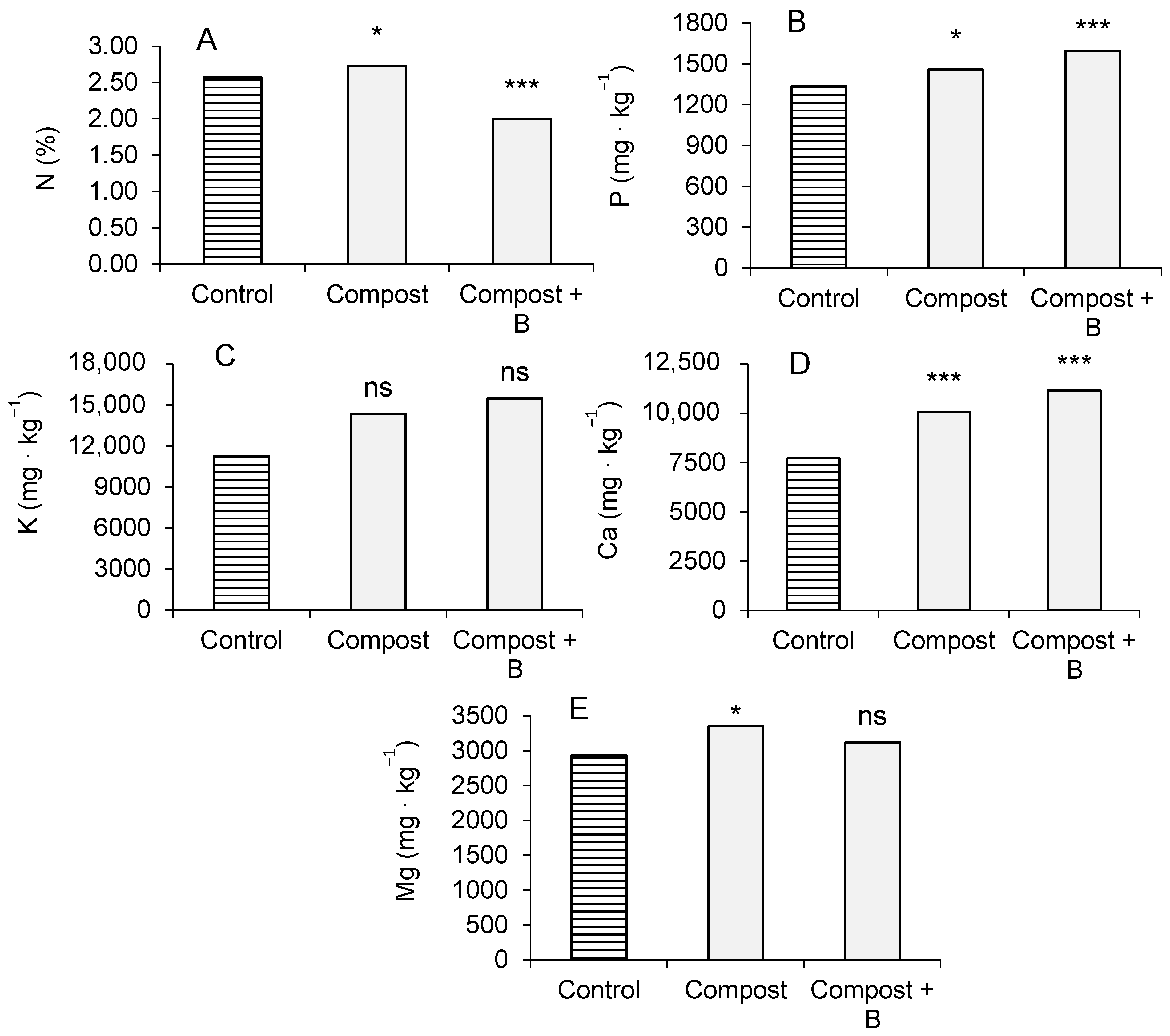
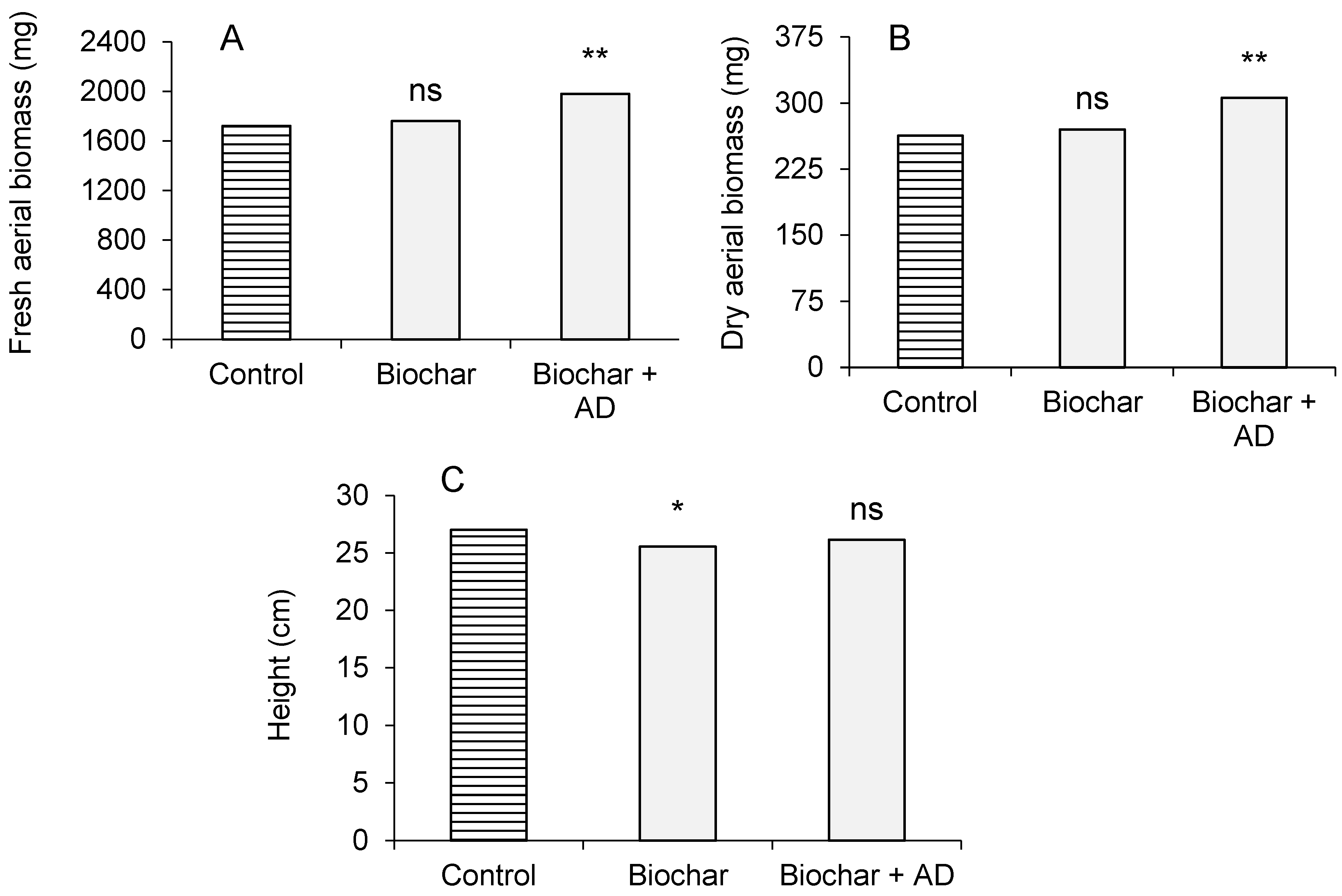
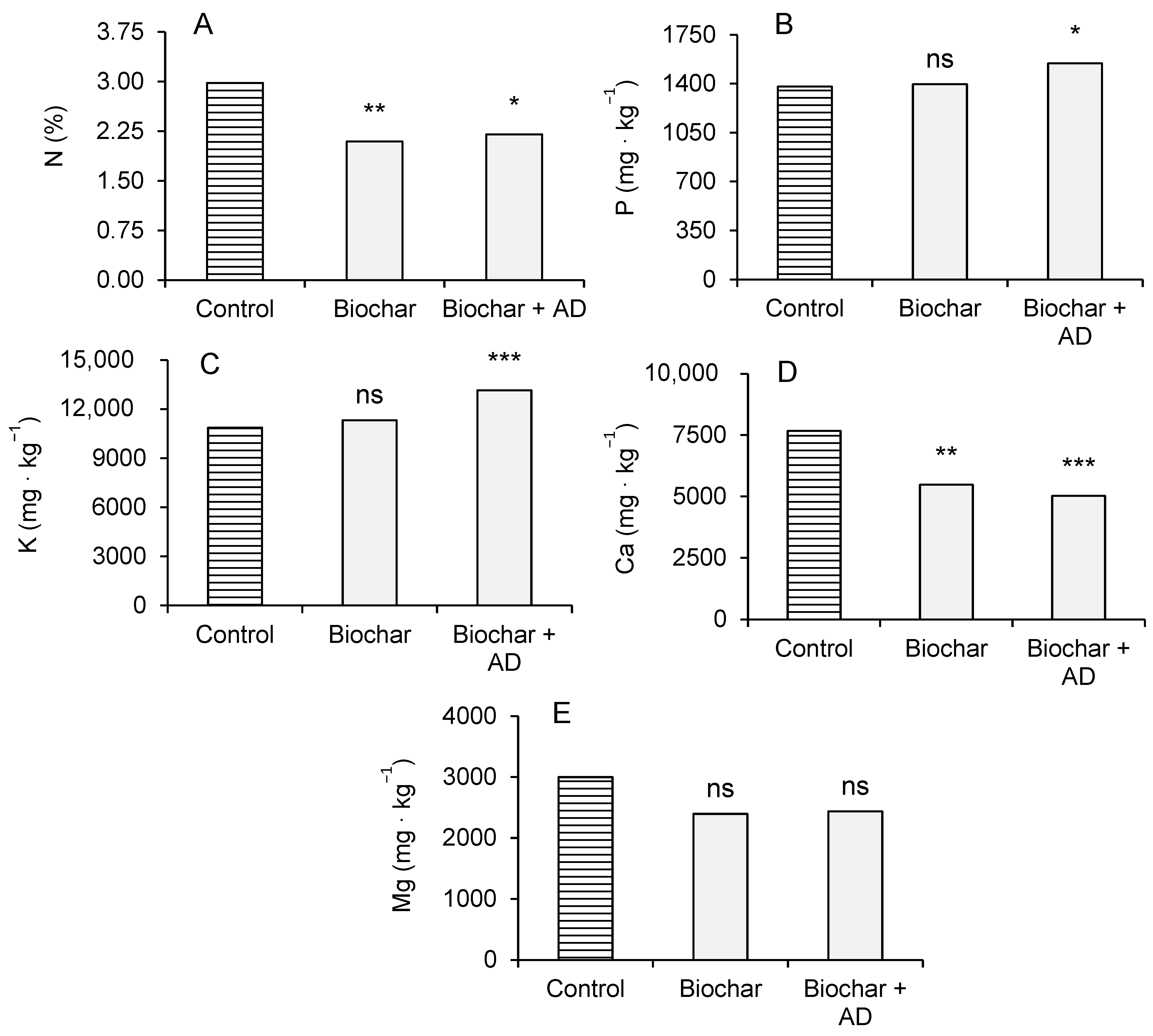
3.2. Plant Parameters in Microcosm Trial: Biomass Production, Height, and Chemical Analysis of Aerial Biomass
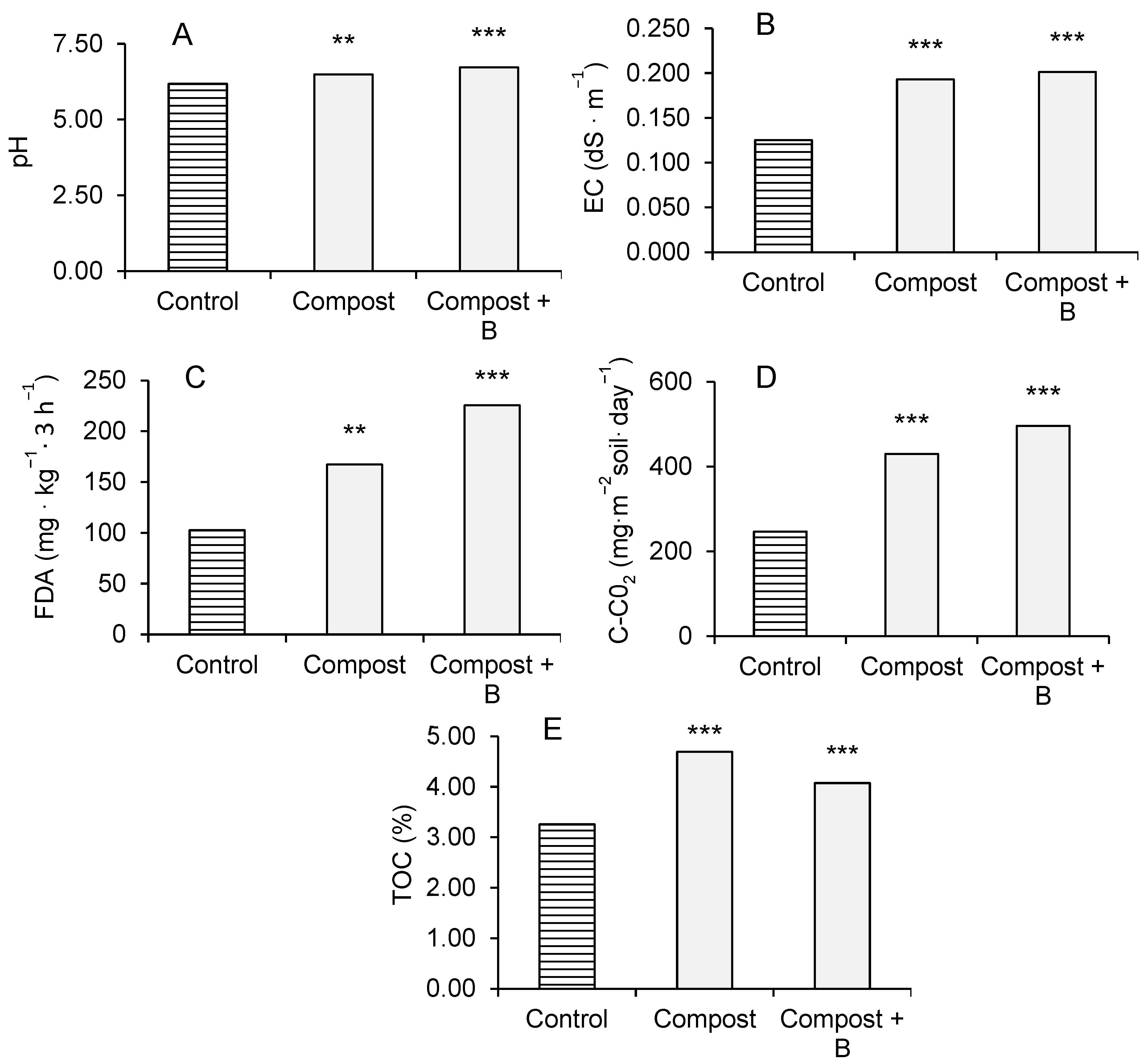
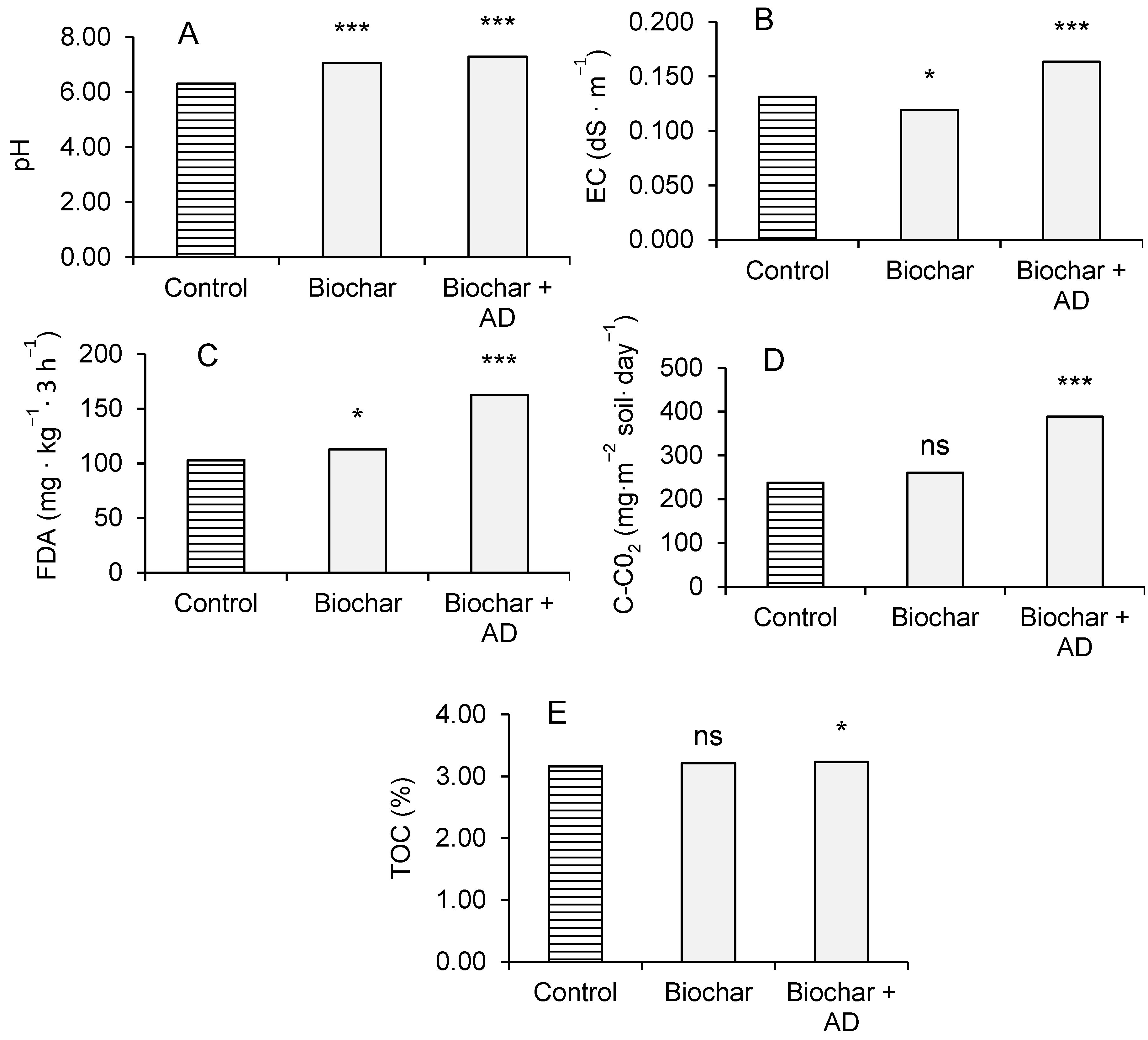
3.3. Soil Chemical and Biological Parameters
4. Discussion
4.1. Phytotoxicity Test and Toxicity Test on Soil Rhizobacteria of the Products Tested
4.2. Plants Growth and Biomass Composition in the Microcosm Trial
4.3. Soil Chemical and Biological Parameters
4.4. Agronomic and Environmental Effects of the Addition of Compost with Biochar and the Use of Biochar as a Carrier for AD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rombel, A.; Krasucka, P.; Oleszczuk, P. Sustainable Biochar-Based Soil Fertilizers and Amendments as a New Trend in Biochar Research. Sci. Total Environ. 2022, 816, 151588. [Google Scholar] [PubMed]
- Czekała, W.; Jezowska, A.; Chełkowski, D. The Use of Biochar for the Production of Organic Fertilizers. J. Ecol. Eng. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Hazra, G. Different Types of Eco-Friendly Fertilizers: An Overview. Sustain. Environ. 2016, 1, 54. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Ok, Y.S.; Usman, A.R.A.; Al-Wabel, M.I.; Oleszczuk, P.; Lee, S.S. The Effects of Biochar Amendment on Soil Fertility. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Wiley: Hoboken, NJ, USA, 2015; pp. 123–144. ISBN 9780891189671. [Google Scholar]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Wezel, A.; Goris, M.; Bruil, J.; Félix, G.F.; Peeters, A.; Bàrberi, P.; Bellon, S.; Migliorini, P. Challenges and Action Points to Amplify Agroecology in Europe. Sustainability 2018, 10, 1598. [Google Scholar] [CrossRef]
- De Corato, U. Towards New Soil Management Strategies for Improving Soil Quality and Ecosystem Services in Sustainable Agriculture: Editorial Overview. Sustainability 2020, 12, 9398. [Google Scholar] [CrossRef]
- Arif, M.S.; Shahzad, S.M.; Riaz, M.; Yasmeen, T.; Shahzad, T.; Akhtar, M.J.; Bragazza, L.; Buttler, A. Nitrogen-Enriched Compost Application Combined with Plant Growth-Promoting Rhizobacteria (PGPR) Improves Seed Quality and Nutrient Use Efficiency of Sunflower. Z. Pflanzenernahr. Bodenkd. 2017, 180, 464–473. [Google Scholar] [CrossRef]
- Liu, D.; Ding, Z.; Ali, E.F.; Kheir, A.M.S.; Eissa, M.A.; Ibrahim, O.H.M. Biochar and Compost Enhance Soil Quality and Growth of Roselle (Hibiscus sabdariffa L.) under Saline Conditions. Sci. Rep. 2021, 11, 8739. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.C.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing Straw, Compost, and Biochar Regarding Their Suitability as Agricultural Soil Amendments to Affect Soil Structure, Nutrient Leaching, Microbial Communities, and the Fate of Pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Song, S.; Lim, J.W.; Lee, J.T.E.; Cheong, J.C.; Hoy, S.H.; Hu, Q.; Tan, J.K.N.; Chiam, Z.; Arora, S.; Lum, T.Q.H.; et al. Food-Waste Anaerobic Digestate as a Fertilizer: The Agronomic Properties of Untreated Digestate and Biochar-Filtered Digestate Residue. Waste Manag. 2021, 136, 143–152. [Google Scholar] [CrossRef]
- Smith, P. Soil Carbon Sequestration and Biochar as Negative Emission Technologies. Glob. Chang. Biol. 2016, 22, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Sharma, R.; Kumar, A.; Sepehya, S. Biochar as a Means to Improve Soil Fertility and Crop Productivity: A Review. J. Plant Nutr. 2022, 45, 2380–2388. [Google Scholar] [CrossRef]
- Rosas, J.G.; Gómez, N.; Cara, J.; Ubalde, J.; Sort, X.; Sánchez, M.E. Assessment of Sustainable Biochar Production for Carbon Abatement from Vineyard Residues. J. Anal. Appl. Pyrolysis 2015, 113, 239–247. [Google Scholar] [CrossRef]
- Gonzalez-Gil, G.; Kleerebezem, R.; Lettinga, G. Effects of Nickel and Cobalt on Kinetics of Methanol Conversion by Methanogenic Sludge as Assessed by On-Line CH4 Monitoring. Appl. Environ. Microbiol. 1999, 65, 1789–1793. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; DeBertolli, M. Evaluating Toxicity of Immature Compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Varnero, M.T.; Orellana, R.; Rojas, C.; Santibáñez, C.; Gallardo Lancho, J.F. Evaluación de Especies Sensibles a Metabolitos Fitotóxicos Mediante Bioensayos de Germinación. In Medioambiente en Iberoamérica. Visión Desde la Física y la Química en los Albores del Siglo XXI; Gallardo-Lancho, J.F., Ed.; Diputación provincial de Cáceres: Cáceres, Spain, 2006; Volume III, pp. 363–369. [Google Scholar]
- Mulas-García, D. Caracterización y Selección de Rizobios Que Nodulan Alubia (Phaseolus vulgaris L.) En La IGP “Alubia de La Bañeza-León”. Ph.D. Thesis, University of León, León, Spain, 2010. [Google Scholar]
- Anderson, J.P.E. Soil Respiration. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, Agronomy Monograph; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 831–871. [Google Scholar]
- Alef, K. Soil Respiration. In Methods in Applied Soil Microbiology and Biochemistry; Kassen, A., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 214–219. [Google Scholar]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual, 2nd ed.; International Center for Agricultural Research in the Dry Areas (ICARDA) and the National Agricultural Research Center (NARC): Aleppo, Syria, 2001. [Google Scholar]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for Fluorescein Diacetate Hydrolytic Activity: Optimization for Soil Samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Gulyás, M.; Someus, E.; Klátyik, S.; Fuchs, M.; István Varga, Z.; Dér, S.; Fekete, G.; Czinkota, I.; Székács, A.; Gyuricza, C.; et al. Effects of Combined Application of Solid Pyrolysis Products and Digestate on Selected Soil Properties of Arenoso! And Plant Growth and Composition in Laboratory Experiments. Agronomy 2022, 12, 1440. [Google Scholar] [CrossRef]
- Crespo-Barreiro, A.; Gómez, N.; González-Arias, J.; Ortiz-Liébana, N.; González-Andrés, F.; Cara-Jiménez, J. Scaling-Up of the Production of Biochar from Olive Tree Pruning for Agricultural Use: Evaluation of Biochar Characteristics and Phytotoxicity. Agriculture 2023, 13, 1064. [Google Scholar] [CrossRef]
- Kataki, S.; Hazarika, S.; Baruah, D.C. Investigation on By-Products of Bioenergy Systems (Anaerobic Digestion and Gasification) as Potential Crop Nutrient Using FTIR, XRD, SEM Analysis and Phyto-Toxicity Test. J. Environ. Manag. 2017, 196, 201–216. [Google Scholar] [CrossRef]
- Mclachlan, K.L.; Chong, C.; Voroney, R.P.; Liu, H.W.; Holbein, B.E.; Canada, C. Assessing the Potential Phytotoxicity of Digestates during Processing of Municipal Solid Waste by Anaerobic Digestion: Comparison to Aerobic Composts. In XXVI International Horticultural Congress: Sustainability of Horticultural Systems in the 21st Century 638; ISHS Acta Horticulturae: Leuven, Belgium, 2004; pp. 225–230. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Bartmiński, P.; Rózyło, K.; Debicki, R.; Oleszczuk, P. Ecotoxicological Assessment of Residues from Different Biogas Production Plants Used as Fertilizer for Soil. J. Hazard. Mater. 2015, 298, 195–202. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Tiquia, S. Assessing Toxicity of Spent Pig Litter Using a Seed Germination Technique. Resour. Conserv. Recycl. 1994, 11, 261–274. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the Fertiliser Potential of Digestates from Farm and Agroindustrial Residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Pivato, A.; Vanin, S.; Raga, R.; Lavagnolo, M.C.; Barausse, A.; Rieple, A.; Laurent, A.; Cossu, R. Use of Digestate from a Decentralized On-Farm Biogas Plant as Fertilizer in Soils: An Ecotoxicological Study for Future Indicators in Risk and Life Cycle Assessment. Waste Manag. 2016, 49, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Bueis, R.; Mulas, R.; Gómez, X.; González-Andrés, F. Innovative Liquid Formulation of Digestates for Producing a Biofertilizer Based on Bacillus Siamensis: Field Testing on Sweet Pepper. J. Plant Nutr. Soil Sci. 2017, 180, 748–758. [Google Scholar] [CrossRef]
- Luo, X.; Liu, G.; Xia, Y.; Chen, L.; Jiang, Z.; Zheng, H.; Wang, Z. Use of Biochar-Compost to Improve Properties and Productivity of the Degraded Coastal Soil in the Yellow River Delta, China. J. Soils Sediments 2016, 17, 780–789. [Google Scholar] [CrossRef]
- Liao, H.; Zheng, C.; Long, J.; Guzmán, I. Effects of Biochar Amendment on Tomato Rhizosphere Bacterial Communities and Their Utilization of Plant-Derived Carbon in a Calcareous Soil. Geoderma 2021, 396, 115082. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced Growth of Halophyte Plants in Biochar-Amended Coastal Soil: Roles of Nutrient Availability and Rhizosphere Microbial Modulation. Plant Cell Environ. 2018, 41, 517–532. [Google Scholar] [CrossRef]
- Calamai, A.; Palchetti, E.; Masoni, A.; Marini, L.; Chiaramonti, D.; Dibari, C.; Brilli, L. The Influence of Biochar and Solid Digestate on Rose-Scented Geranium (Pelargonium graveolens L’Hér.) Productivity and Essential Oil Quality. Agronomy 2019, 9, 260. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, S.; Quan, C.; Gao, N.; Johnston, C.; Wu, C. One-Pot Synthesis of Digestate-Derived Biochar for Carbon Dioxide Capture. Fuel 2020, 279, 118525. [Google Scholar] [CrossRef]
- Mickan, B.S.; Ren, A.T.; Buhlmann, C.H.; Ghadouani, A.; Solaiman, Z.M.; Jenkins, S.; Pang, J.; Ryan, M.H. Closing the Circle for Urban Food Waste Anaerobic Digestion: The Use of Digestate and Biochar on Plant Growth in Potting Soil. J. Clean. Prod. 2022, 347, 131071. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, K.; Matsumoto, T.; Hatate, Y.; Nishihara, K.; Nakanishi, T. Removal of Nitrate-Nitrogen from Drinking Water Using Bamboo Powder Charcoal. Bioresour. Technol. 2004, 95, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C.; Zhang, Y.; Ma, C. Comparison Study on the Ammonium Adsorption of the Biochars Derived from Different Kinds of Fruit Peel. Sci. Total Environ. 2020, 707, 135544. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, Soil and Land-Use Interactions That Reduce Nitrate Leaching and N2O Emissions: A Meta-Analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. A Wood Based Low-Temperature Biochar Captures NH 3-N Generated from Ruminant Urine-N, Retaining Its Bioavailability. Plant Soil 2012, 353, 73–84. [Google Scholar] [CrossRef]
- Paramashivam, D.; Clough, T.J.; Dickinson, N.M.; Horswell, J.; Lense, O.; Clucas, L.; Robinson, B.H. Effect of Pine Waste and Pine Biochar on Nitrogen Mobility in Biosolids. J. Environ. Qual. 2016, 45, 360–367. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Solid and Modified Biochars Mitigate Root Cell Lignification and Improve Nutrients Uptake in Mint Plants under Fluoride and Cadmium Stresses. Plant Physiol. Biochem. 2023, 200, 107757. [Google Scholar] [CrossRef] [PubMed]
- Nadeem Shah, M.; Wright, D.L.; Hussain, S.; Koutroubas, S.D.; Seepaul, R.; George, S.; Ali, S.; Naveed, M.; Khan, M.; Tanveer Altaf, M.; et al. Organic Fertilizer Sources Improve the Yield and Quality Attributes of Maize (Zea mays L.) Hybrids by Improving Soil Properties and Nutrient Uptake under Drought Stress. J. King Saud Univ.-Sci. 2023, 35, 102570. [Google Scholar] [CrossRef]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More Plant Growth but Less Plant Defence? First Global Gene Expression Data for Plants Grown in Soil Amended with Biochar. GCB Bioenergy 2015, 7, 658–672. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.L.; Bastos, A.C.; van der Velde, M.; Diafas, L. Biochar Application to Soils—A Critical Scientific Review of Effects on Soil Properties, Processes and Functions. Eur. Comm. 2010, 24099, 2183–2207. [Google Scholar] [CrossRef]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini, C. Short Term Effects of Bioenergy By-Products on Soil C and N Dynamics, Nutrient Availability and Biochemical Properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-Mediated Changes in Soil Quality and Plant Growth in a Three Year Field Trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-Chemical Properties and Microbial Responses in Biochar-Amended Soils: Mechanisms and Future Directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Ozdemir, S.; Nuhoglu, N.N.; Dede, O.H.; Yetilmezsoy, K. Mitigation of Soil Loss from Turfgrass Cultivation by Utilizing Poultry Abattoir Sludge Compost and Biochar on Low-Organic Matter Soil. Environ. Technol. 2020, 41, 466–477. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ibrahim, M.; Ali, N.; Ali, H. Effect of Biochar and Compost Amendments on Soil Biochemical Properties and Dry Weight of Canola Plant Grown in Soil Contaminated with Heavy Metals. Commun. Soil Sci. Plant Anal. 2020, 51, 1561–1571. [Google Scholar] [CrossRef]
- Liu, J.; Schulz, H.; Brandl, S.; Miehtke, H.; Huwe, B.; Glaser, B. Short-Term Effect of Biochar and Compost on Soil Fertility and Water Status of a Dystric Cambisol in NE Germany under Field Conditions. J. Plant Nutr. Soil Sci. 2012, 175, 698–707. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ibrahim, M.; Ali, N.; Ali, H. Spectroscopic Analyses to Study the Effect of Biochar and Compost on Dry Mass of Canola and Heavy Metal Immobilization in Soil. Commun. Soil Sci. Plant Anal. 2018, 49, 1990–2001. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, Z.; Lin, X.; Zhao, F.; Wang, B.; Lin, F.; Ge, Y.; Eissa, M.A. Biochar Impacts on NH3-Volatilization Kinetics and Growth of Sweet Basil (Ocimum basilicum L.) under Saline Conditions. Ind. Crops Prod. 2020, 157, 112903. [Google Scholar] [CrossRef]
- Hosam, B.H. Soil Biological Parameters Inluenced by Various Nitrogen Sources. In Proceedings of the CTEP 2019—International Council on Technologies of Environmental Protection (ICTEP), Stary Smokovec, Slovakia, 23–25 October 2019; pp. 121–127. [Google Scholar]
- Rekaby, S.A.; Awad, M.Y.; Hegab, S.A.; Eissa, M.A. Effect of Some Organic Amendments on Barley Plants under Saline Condition. J. Plant Nutr. 2020, 12, 1840–1851. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Bound, S.A.; Doyle, R.; Bowman, J.P. Effects of Biochar and Compost Amendments on Soil Physico-Chemical Properties and the Total Community within a Temperate Agricultural Soil. Appl. Soil Ecol. 2016, 98, 243–253. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Sudoma, M.; Kintl, A.; Vopravil, J.; Ryant, P.; Skarpa, P.; Radziemska, M.; Latal, O.; Brtnicky, M. Biochar and Sulphur Enriched Digestate: Utilization of Agriculture Associated Waste Products for Improved Soil Carbon and Nitrogen Content, Microbial Activity, and Plant Growth. Agronomy 2021, 11, 2041. [Google Scholar] [CrossRef]
- Jílková, V.; Angst, G. Biochar and Compost Amendments to a Coarse-Textured Temperate Agricultural Soil Lead to Nutrient Leaching. Appl. Soil Ecol. 2022, 173, 104393. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Hussain, Q.; Usman, A.R.A.; Ahmad, M.; Abduljabbar, A.; Sallam, A.S.; Ok, Y.S. Impact of Biochar Properties on Soil Conditions and Agricultural Sustainability: A Review. Land Degrad. Dev. 2018, 29, 2124–2161. [Google Scholar] [CrossRef]
- Nobile, C.; Lebrun, M.; Védère, C.; Honvault, N.; Aubertin, M.L.; Faucon, M.P.; Girardin, C.; Houot, S.; Kervroëdan, L.; Dulaurent, A.M.; et al. Biochar and Compost Addition Increases Soil Organic Carbon Content and Substitutes P and K Fertilizer in Three French Cropping Systems. Agron. Sustain. Dev. 2022, 42, 119. [Google Scholar] [CrossRef]
- Aseri, G.K.; Jain, N.; Panwar, J.; Rao, A.V.; Meghwal, P.R. Biofertilizers Improve Plant Growth, Fruit Yield, Nutrition, Metabolism and Rhizosphere Enzyme Activities of Pomegranate (Punica granatum L.) in Indian Thar Desert. Sci. Hortic. 2008, 117, 130–135. [Google Scholar] [CrossRef]
- Patani, A.; Prajapati, D.; Ali, D.; Kalasariya, H.; Yadav, V.K.; Tank, J.; Bagatharia, S.; Joshi, M.; Patel, A. Evaluation of the Growth-Inducing Efficacy of Various Bacillus Species on the Salt-Stressed Tomato (Lycopersicon esculentum Mill.). Front. Plant Sci. 2023, 14, 1168155. [Google Scholar] [CrossRef]
- Volkogon, V.V.; Potapenko, L.V.; Volkogon, M.V. Vertical Migration of Nutrients and Water-Soluble Organic Matter in the Soil Profile under Pre-Sowing Seed Treatment with Plant Growth Promoting Rhizobacteria. Front. Sustain. Food Syst. 2023, 6, 1054113. [Google Scholar] [CrossRef]

| Product Family | Treatment/ Fertilising Product | Treatment Code | Dose (kg ha−1) of the Fertilising Product | Corresponding Percentage of Biochar in the Final Product (w:w) or AD in the Final Product (v:w) | N-P-K-Ca-Mg Provided by the Fertiliser (Expressed in kg ha−1) |
|---|---|---|---|---|---|
| Compost + biochar | Compost 2 | Compost | 2000 | 0 | 42-9-41-152-12 |
| Compost 5 | Compost | 5000 | 0 | 104-23-103-381-30 | |
| Compost 2 + biochar 3 | Compost + B3 | 2000 | 3 | 42-10-42-154-12 | |
| Compost 2 + biochar 6 | Compost + B6 | 2000 | 6 | 43-10-43-155-13 | |
| Compost 5 + biochar 3 | Compost + B3 | 5000 | 3 | 106-24-106-384-31 | |
| Compost 5 + biochar 6 | Compost + B6 | 5000 | 6 | 107-24-109-387-32 | |
| Biochar + AD | Biochar 250 | Biochar 250 | 250 | 0 | 2.6-0.6-4.7-5.2-1.2 |
| Biochar 500 | Biochar 500 | 500 | 0 | 5.1-1.2-9.3-10.5-2.5 | |
| Biochar 250 + AD 1 | Biochar + AD1 | 250 | 1 | 2.9-0.8-4.9-5.3-1.3 | |
| Biochar 250 + AD 5 | Biochar + AD5 | 250 | 5 | 4.1-1.5-5.9-5.7-1.5 | |
| Biochar 250 + AD 10 | Biochar + AD10 | 250 | 10 | 5.6-2.3-7.2-6.2-1.8 | |
| Biochar 500 + AD 1 | Biochar + AD1 | 500 | 1 | 5.7-1.6-9.8-10.7-2.6 | |
| Biochar 500 + AD 5 | Biochar + AD5 | 500 | 5 | 8.2-2.9-11.9-11.4-3.1 | |
| Biochar 500 + AD 10 | Biochar + AD10 | 500 | 10 | 11.3-4.7-14.4-12.4-3.6 |
| Plant | Compost + B3 | Compost + B6 | Biochar + AD5 | Biochar + AD10 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:5 | 1:10 | 1:25 | 1:5 | 1:10 | 1:25 | 1:5 | 1:10 | 1:25 | 1:5 | 1:10 | 1:25 | |
| Lettuce | 26 | 102 | 479 | 17 | 73 | 309 | 8 | 57 | 132 | 15 | 66 | 154 |
| Tomato | 10 | 76 | 442 | 9 | 67 | 296 | 0 | 52 | 114 | 7 | 63 | 161 |
| Cress | 29 | 110 | 503 | 29 | 95 | 343 | 8 | 71 | 142 | 10 | 84 | 174 |
| Radish | 44 | 115 | 541 | 38 | 104 | 464 | 13 | 82 | 178 | 20 | 89 | 288 |
| Compost Dose (t ha−1) | Biochar Dose (%) | Fresh Aerial Biomass (mg) | Dry Aerial Biomass (mg) | Height (cm) | N (%) | P (mg kg−1) | K (mg kg−1) | Ca (mg kg−1) | Mg (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 0 | 2585 a | 322.4 a | 28.36 a | 2.75 b | 1413 a | 12,969 a | 8921 a | 2961 a |
| 3 | 2982 b | 351.4 ab | 28.52 a | 2.15 a | 1465 ab | 14,082 b | 9571 b | 3204 a | |
| 6 | 3092 b | 381.8 b | 28.83 a | 1.90 a | 1527 b | 14,573 b | 9858 b | 3423 a | |
| 5 | 0 | 3079 a | 373.9 a | 28.87 a | 2.71 b | 1503 a | 15,707 a | 11,247 a | 3091 a |
| 3 | 3113 a | 387.5 a | 29.19 a | 2.09 a | 1645 ab | 16,385 a | 12,225 b | 3216 a | |
| 6 | 3285 a | 401.5 a | 29.55 a | 1.84 a | 1752 b | 16,906 a | 13,019 c | 3279 a |
| Biochar Dose (kg ha−1) | AD Dose (%) | Fresh Aerial Biomass (mg) | Dry Aerial Biomass (mg) | Height (cm) | N (%) | P (mg kg−1) | K (mg kg−1) | Ca (mg kg−1) | Mg (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| 250 | 0 | 1738 a | 266.5 a | 26.23 a | 2.29 a | 1393 a | 11,167 a | 5725 a | 2420 a |
| 1 | 1907 a | 282.5 a | 26.39 a | 2.43 ab | 1468 ab | 12,047 ab | 5688 a | 2500 a | |
| 5 | 1916 a | 297.9 a | 26.41 a | 2.47 ab | 1502 ab | 12,120 ab | 5628 a | 2471 a | |
| 10 | 1964 a | 298.5 a | 26.55 a | 2.52 b | 1587 b | 13,770 b | 5619 a | 2440 a | |
| 500 | 0 | 1791 a | 275.3 a | 25.10 a | 1.90 a | 1400 a | 11,478 a | 5231 b | 2370 a |
| 1 | 1897 a | 294.4 ab | 25.46 a | 1.88 a | 1498 a | 12,057 a | 4971 ab | 2358 a | |
| 5 | 2000 ab | 320.2 bc | 25.90 a | 1.93 a | 1550 a | 12,903 b | 4484 ab | 2399 a | |
| 10 | 2246 b | 335.7 c | 25.95 a | 1.99 a | 1663 a | 16,041 c | 3758 a | 2447 a |
| Compost Dose (t ha−1) | Biochar Dose (%) | pH | EC (dS·m−1) | FDA (mg·kg−1·3 h−1) | C-CO2 (mg·m−2 soil·day−1) | TOC (%) |
|---|---|---|---|---|---|---|
| 2 | 0 | 6.46 a | 0.168 a | 143.8 a | 411.5 a | 4.74 b |
| 3 | 6.63 a | 0.168 a | 179.4 a | 457.2 ab | 4.61 b | |
| 6 | 6.76 a | 0.191 a | 235.9 b | 466.4 b | 4.10 a | |
| 5 | 0 | 6.51 a | 0.218 a | 190.7 a | 448.1 a | 4.65 b |
| 3 | 6.70 a | 0.218 a | 217.6 b | 512.1 b | 3.90 ab | |
| 6 | 6.79 a | 0.228 a | 269.2 c | 548.7 b | 3.68 a |
| Biochar Dose (kg ha−1) | AD Dose (%) | pH | EC (dS∙m−1) | FDA (mg∙kg−1∙3 h−1) | C-CO2 (mg∙m−2 soil∙day−1) | TOC (%) |
|---|---|---|---|---|---|---|
| 250 | 0 | 6.55 a | 0.122 a | 106.5 a | 246.9 a | 3.18 a |
| 1 | 6.63 a | 0.143 b | 117.5 a | 301.8 b | 3.23 b | |
| 5 | 6.75 ab | 0.164 c | 158.4 b | 374.9 c | 3.21 ab | |
| 10 | 6.90 b | 0.173 c | 172.3 c | 438.9 d | 3.18 a | |
| 500 | 0 | 7.57 a | 0.117 a | 119.3 a | 274.3 a | 3.22 a |
| 1 | 7.68 ab | 0.155 b | 124.9 a | 329.2 a | 3.25 a | |
| 5 | 7.82 bc | 0.166 b | 194.6 b | 429.8 b | 3.27 a | |
| 10 | 7.97 c | 0.182 c | 208.6 c | 457.2 b | 3.30 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Liébana, N.; Crespo-Barreiro, A.; Mazuecos-Aguilera, I.; González-Andrés, F. Improved Organic Fertilisers Made from Combinations of Compost, Biochar, and Anaerobic Digestate: Evaluation of Maize Growth and Soil Metrics. Agriculture 2023, 13, 1557. https://doi.org/10.3390/agriculture13081557
Ortiz-Liébana N, Crespo-Barreiro A, Mazuecos-Aguilera I, González-Andrés F. Improved Organic Fertilisers Made from Combinations of Compost, Biochar, and Anaerobic Digestate: Evaluation of Maize Growth and Soil Metrics. Agriculture. 2023; 13(8):1557. https://doi.org/10.3390/agriculture13081557
Chicago/Turabian StyleOrtiz-Liébana, Noemí, Andrea Crespo-Barreiro, Ismael Mazuecos-Aguilera, and Fernando González-Andrés. 2023. "Improved Organic Fertilisers Made from Combinations of Compost, Biochar, and Anaerobic Digestate: Evaluation of Maize Growth and Soil Metrics" Agriculture 13, no. 8: 1557. https://doi.org/10.3390/agriculture13081557
APA StyleOrtiz-Liébana, N., Crespo-Barreiro, A., Mazuecos-Aguilera, I., & González-Andrés, F. (2023). Improved Organic Fertilisers Made from Combinations of Compost, Biochar, and Anaerobic Digestate: Evaluation of Maize Growth and Soil Metrics. Agriculture, 13(8), 1557. https://doi.org/10.3390/agriculture13081557






